Hydrothermal Synthesis of 1T-MoS2/Pelagic Clay Composite and Its Application in the Catalytic Reduction of 4-Nitrophenol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of 1T-MoS2/PC Composites
2.3. Material Characterization
2.4. Catalytic Activity Evaluation
3. Results and Discussion
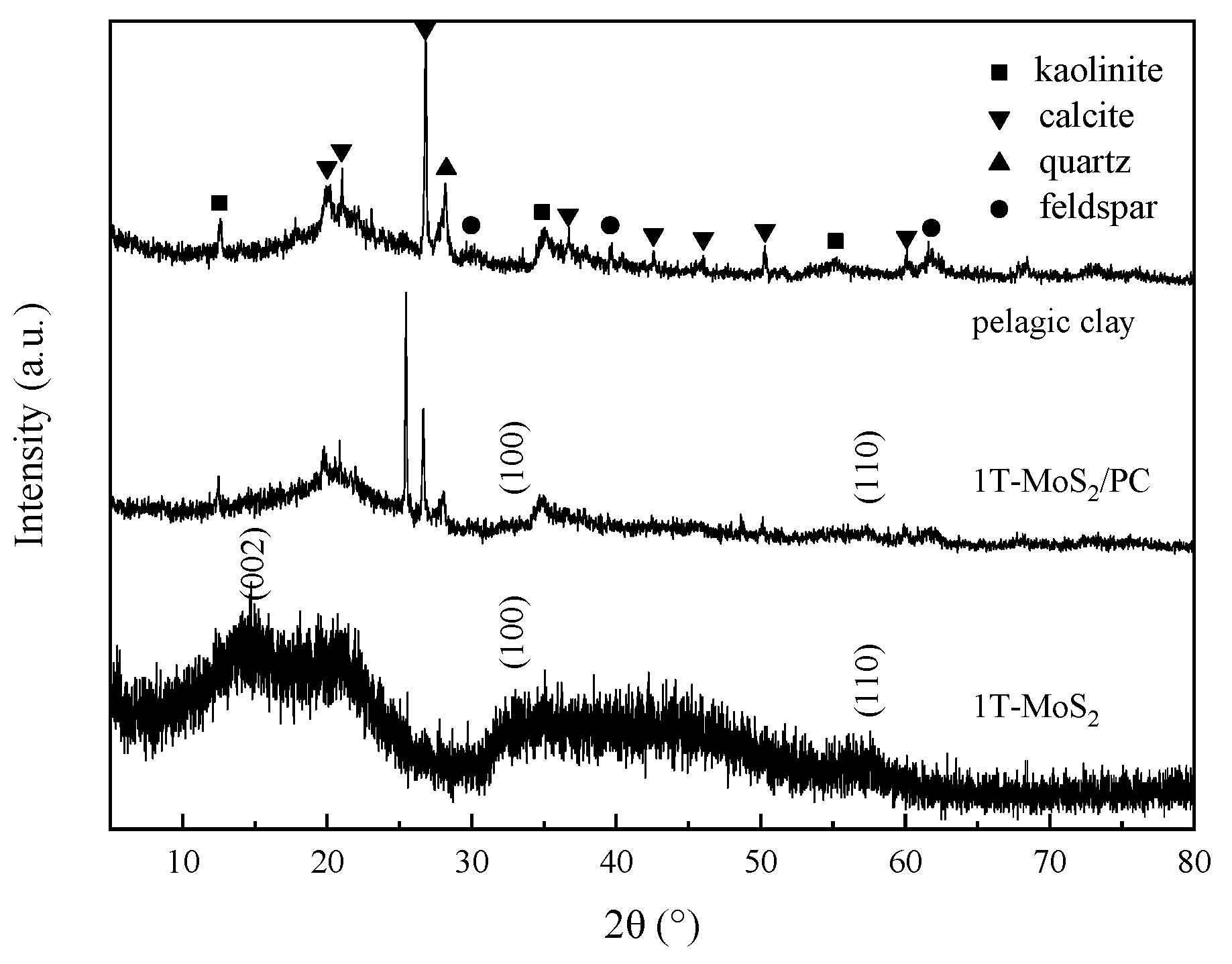
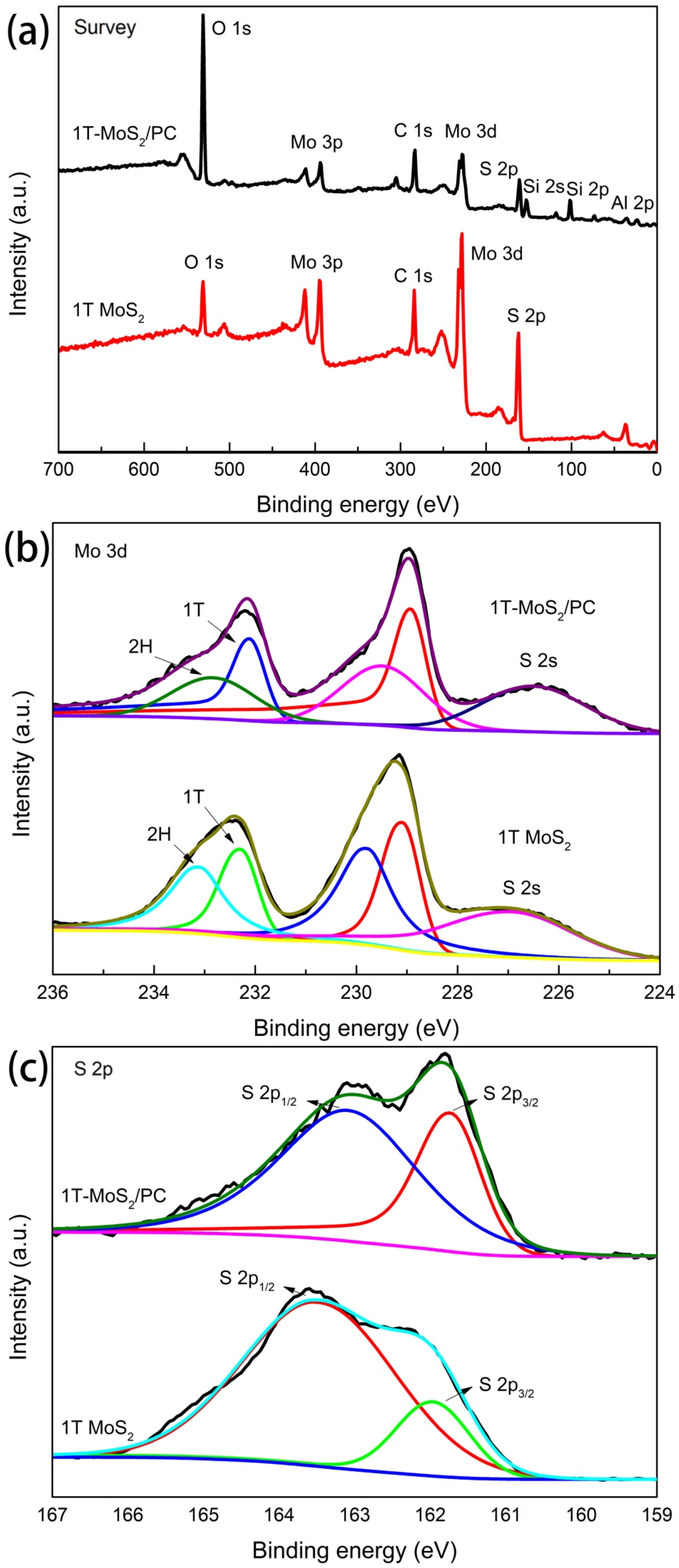
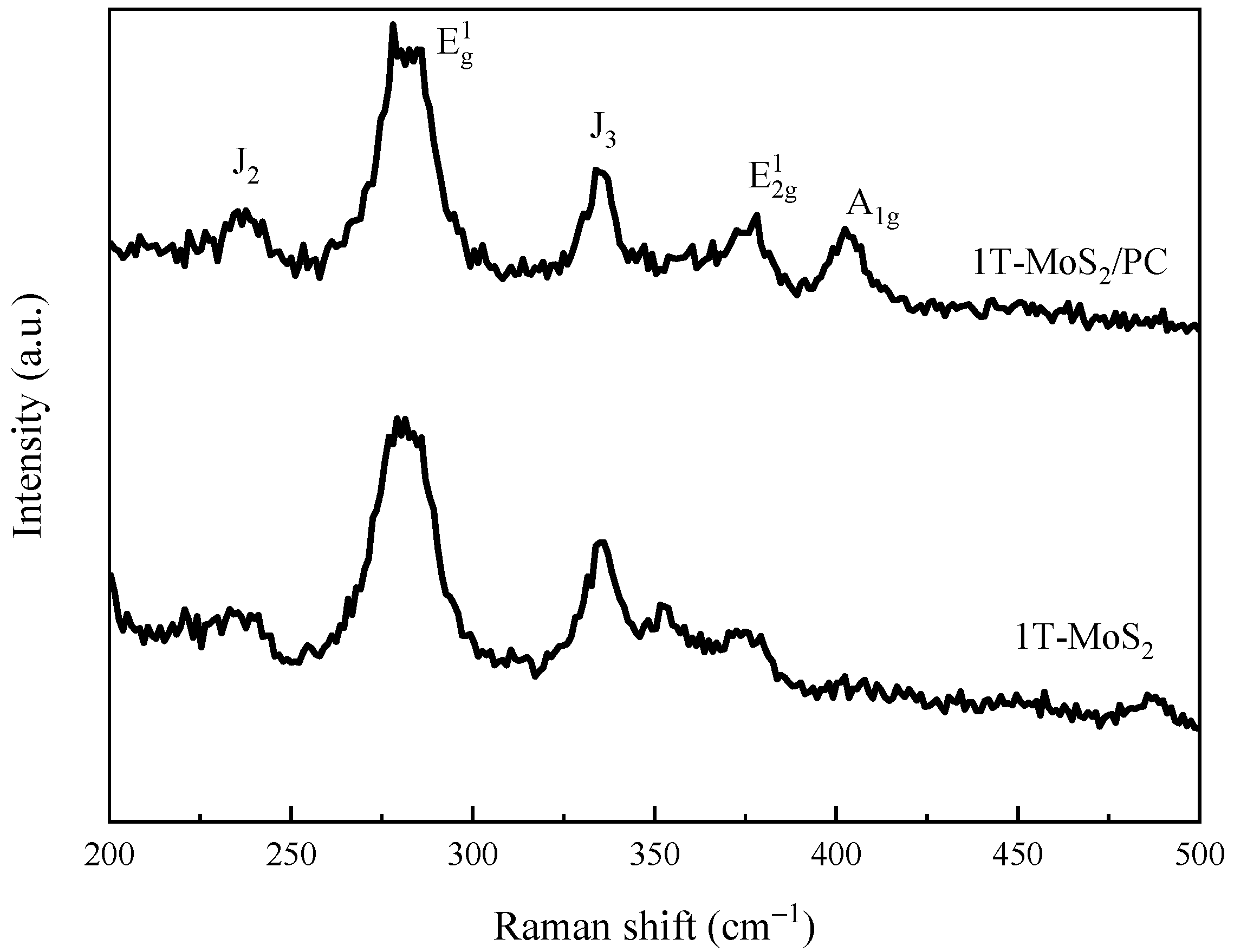
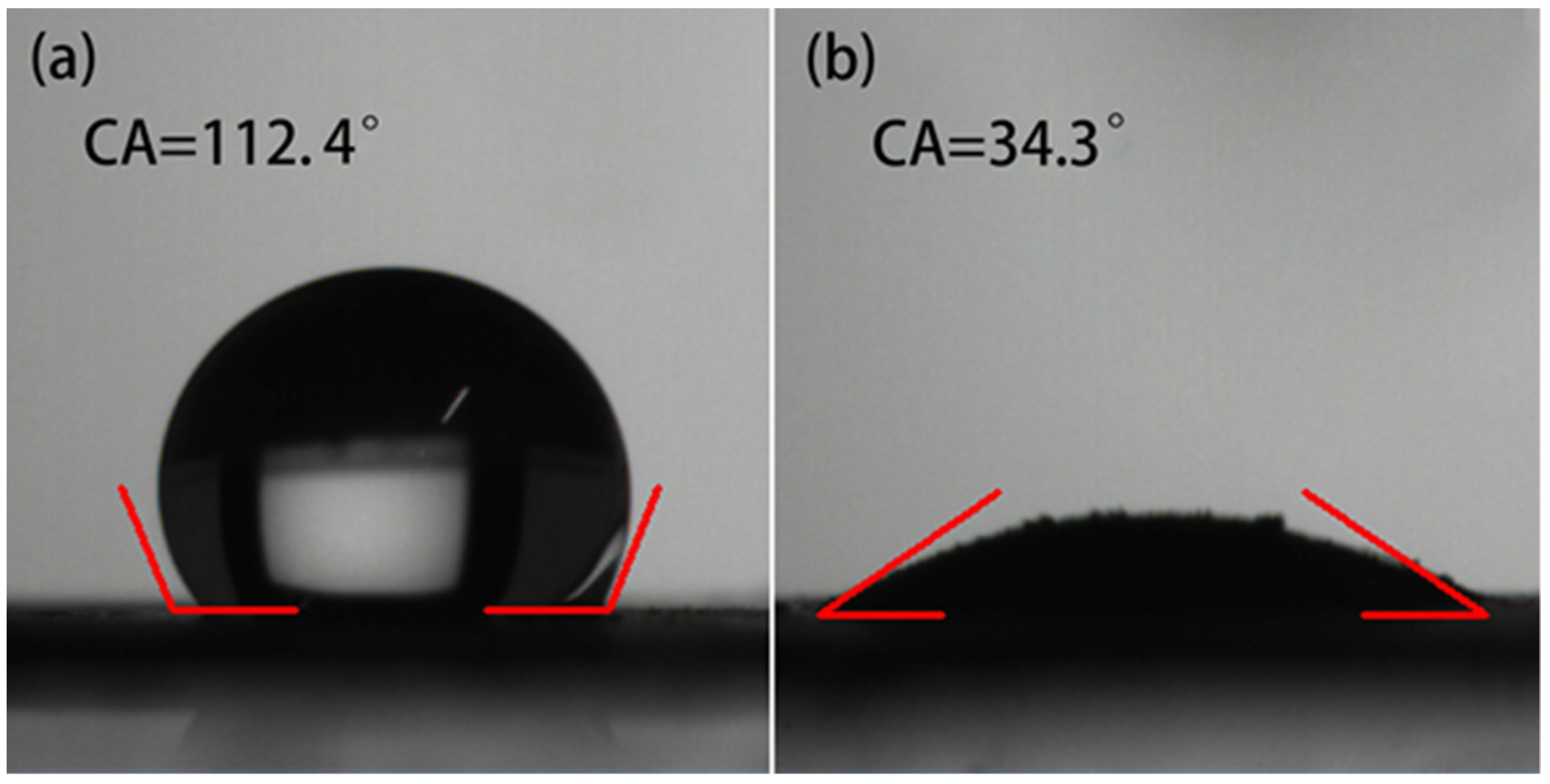
3.1. Structural Characterization
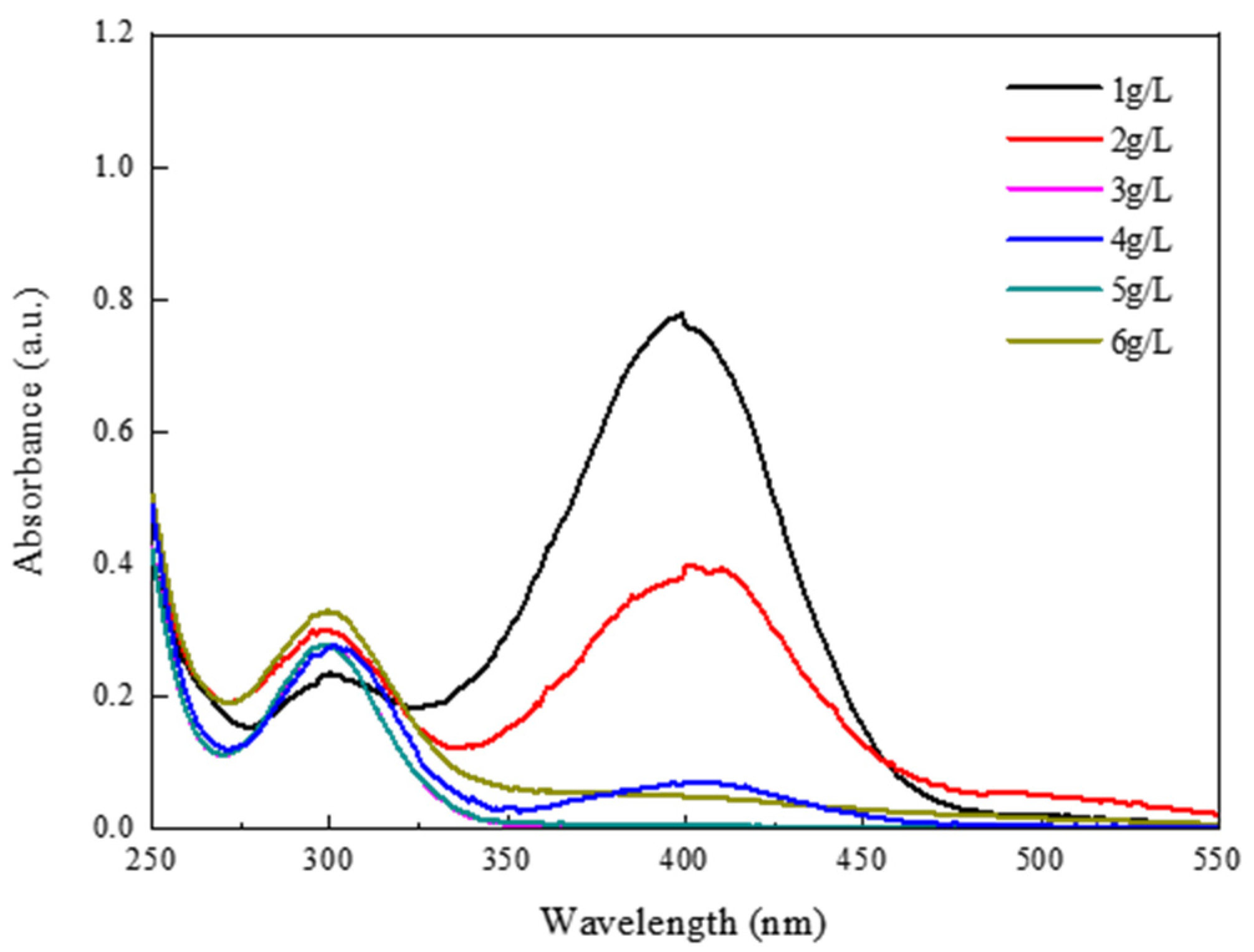
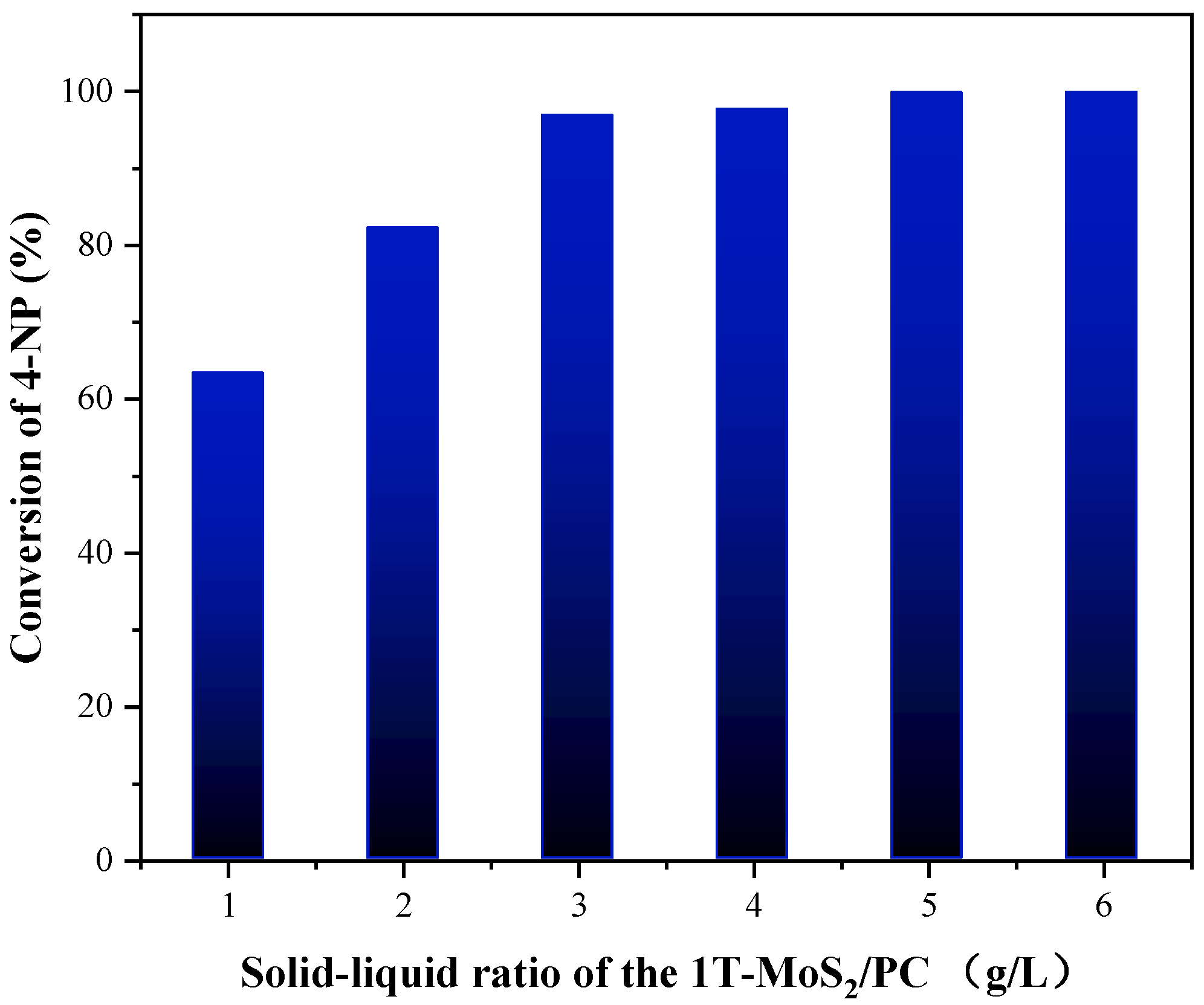
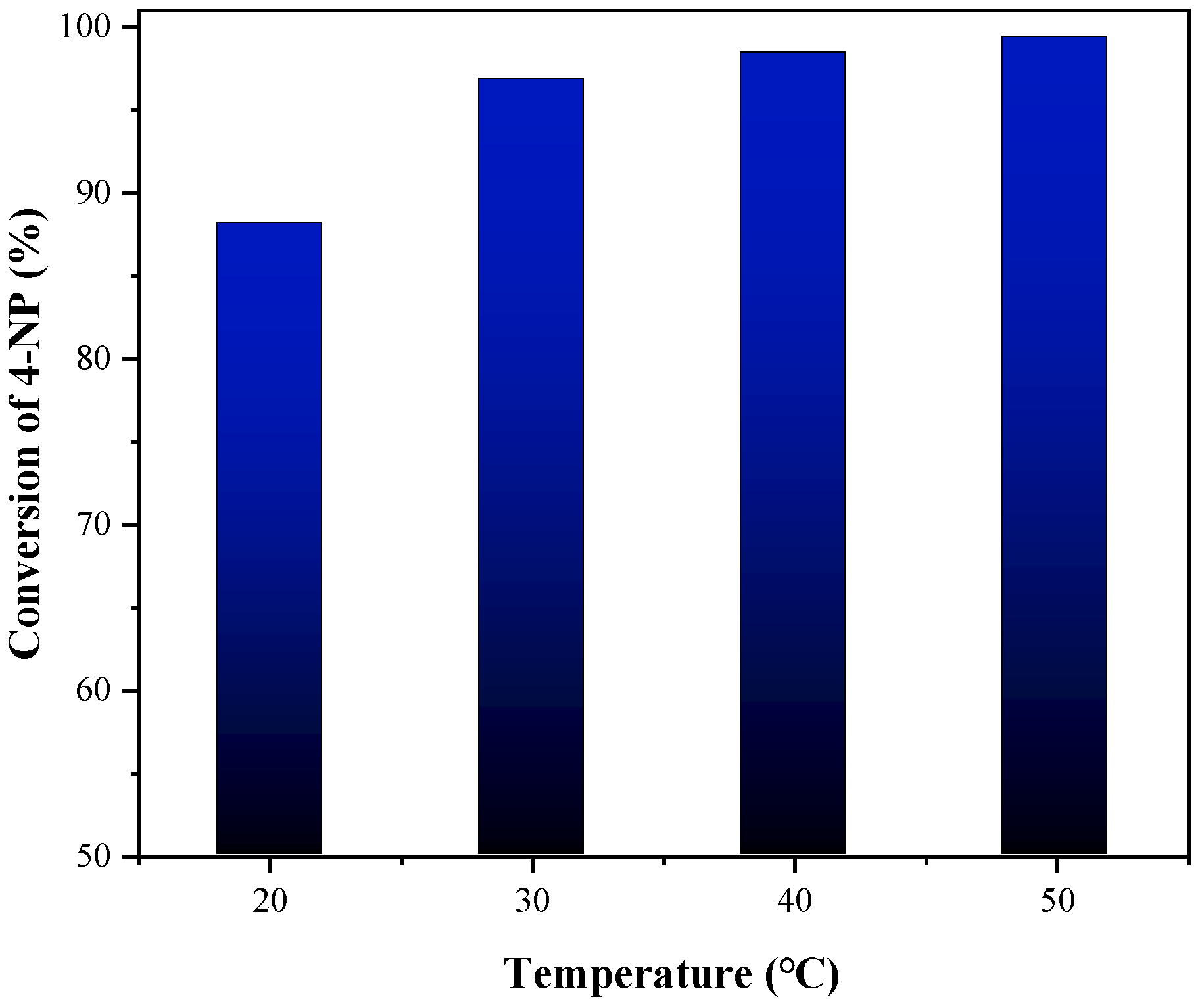
3.2. Testing of the Catalytic Behavior of 1T-MoS2/PC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, K.; Fu, L.; Yang, H.; Ouyang, J.; Tang, A. Hierarchical MoS2 intercalated clay hybrid nanosheets with enhanced catalytic activity. Nano Res. 2017, 10, 570–583. [Google Scholar] [CrossRef]
- Wunder, S.; Polzer, F.; Lu, Y.; Mei, Y.; Ballauff, M. Kinetic Analysis of Catalytic Reduction of 4-Nitrophenol by Metallic Nanoparticles Immobilized in Spherical Polyelectrolyte Brushes. J. Phys. Chem. C 2010, 114, 8814–8820. [Google Scholar] [CrossRef]
- Chi, Y.; Tu, J.; Wang, M.; Li, X.; Zhao, Z. One-pot synthesis of ordered mesoporous silver nanoparticle/carbon composites for catalytic reduction of 4-nitrophenol. J. Colloid Interface Sci. 2014, 423, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, X.-L.; Feng, G.; Chen, X.; Lu, Z.-H. Ag-SiO2 nanocomposites with plum-pudding structure as catalyst for hydrogenation of 4-nitrophenol. Ceram. Int. 2015, 41, 14660–14667. [Google Scholar] [CrossRef]
- Cao, S.-W.; Fang, J.; Shahjamali, M.M.; Wang, Z.; Yin, Z.; Yang, Y.; Boey, F.Y.C.; Barber, J.; Loo, S.C.J.; Xue, C. In situ growth of Au nanoparticles on Fe2O3 nanocrystals for catalytic applications. Crystengcomm 2012, 14, 7229–7235. [Google Scholar] [CrossRef]
- Liang, W.; Lu, Y.; Li, N.; Li, H.; Zhu, F. Microwave-assisted synthesis of magnetic surface molecular imprinted polymer for adsorption and solid phase extraction of 4-nitrophenol in wastewater. Microchem. J. 2020, 159, 105316. [Google Scholar] [CrossRef]
- Moosavifar, M.; Heidari, S.M.; Fathyunes, L.; Ranjbar, M.; Wang, Y.; Arandiyan, H. Photocatalytic Degradation of Dye Pollutant Over FeTPP/NaY Zeolite Nanocomposite. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1621–1628. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, N.; Kumar, M.; Agarwal, A.; Mizaikoff, B. Electrochemical sensing and remediation of 4-nitrophenol using bio-synthesized copper oxide nanoparticles. Chem. Eng. J. 2017, 313, 283–292. [Google Scholar] [CrossRef]
- Jiang, X.-H.; Wang, L.-C.; Yu, F.; Nie, Y.-C.; Xing, Q.-J.; Liu, X.; Pei, Y.; Zou, J.-P.; Dai, W.-L. Photodegradation of Organic Pollutants Coupled with Simultaneous Photocatalytic Evolution of Hydrogen Using Quantum-Dot-Modified g-C3N4 Catalysts under Visible-Light Irradiation. ACS Sustain. Chem. Eng. 2018, 6, 12695–12705. [Google Scholar] [CrossRef]
- Dong, Z.; Le, X.; Li, X.; Zhang, W.; Dong, C.; Ma, J. Silver nanoparticles immobilized on fibrous nano-silica as highly efficient and recyclable heterogeneous catalyst for reduction of 4-nitrophenol and 2-nitroaniline. Appl. Catal. B-Environ. 2014, 158, 129–135. [Google Scholar] [CrossRef]
- Xiong, R.; Lu, C.; Wang, Y.; Zhou, Z.; Zhang, X. Nanofibrillated cellulose as the support and reductant for the facile synthesis of Fe3O4/Ag nanocomposites with catalytic and antibacterial activity. J. Mater. Chem. A 2013, 1, 14910–14918. [Google Scholar] [CrossRef]
- Raza, W.; Ahmad, K.; Kim, H. Fabrication of defective graphene oxide for efficient hydrogen production and enhanced 4-nitro-phenol reduction. Nanotechnology 2021, 32, 495404. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kang, S.-W.; Kim, H.; Fujishima, A.; Terashima, C. Nanoflakes-like nickel cobaltite as active electrode material for 4-nitro-phenol reduction and supercapacitor applications. J. Hazard. Mater. 2021, 419, 126453. [Google Scholar] [CrossRef]
- Akbarzadeh, E.; Bahrami, F.; Gholami, M.R. Au and Pt nanoparticles supported on Ni promoted MoS2 as efficient catalysts for p-nitrophenol reduction. J. Water Process. Eng. 2020, 34, 101142. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; Tian, Y.; Xu, X.; Hou, H.; Wang, M.; Qi, G.; Jin, Y. Long-Range Plasmon Field and Plasmoelectric Effect on Catalysis Revealed by Shell-Thickness-Tunable Pinhole-Free Au@SiO2 Core-Shell Nanoparticles: A Case Study of p-Nitrophenol Reduction. ACS Catal. 2017, 7, 5391–5398. [Google Scholar] [CrossRef]
- Islam, M.R.; Ferdous, M.; Sujan, M.I.; Mao, X.; Zeng, H.; Azam, M.S. Recyclable Ag-decorated highly carbonaceous magnetic nanocomposites for the removal of organic pollutants. J. Colloid Interface Sci. 2020, 562, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Dali, S.; Paredes, J.I.; Villar-Rodil, S.; Martinez-Jodar, A.; Martinez-Alonso, A.; Tascon, J.M.D. Molecular Functionalization of 2H-Phase MoS2 Nanosheets via an Electrolytic Route for Enhanced Catalytic Performance. ACS Appl. Mater. Interfaces 2021, 13, 33157–33171. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zeng, C.; Jiang, J.; Ai, L. Magnetic cobalt nanoparticles embedded in hierarchically porous nitrogen-doped carbon frameworks for highly efficient and well-recyclable catalysis. J. Mater. Chem. A 2016, 4, 7476–7482. [Google Scholar] [CrossRef]
- Wu, K.-L.; Wei, X.-W.; Zhou, X.-M.; Wu, D.-H.; Liu, X.-W.; Ye, Y.; Wang, Q. NiCo2 Alloys: Controllable Synthesis, Magnetic Properties, and Catalytic Applications in Reduction of 4-Nitrophenol. J. Phys. Chem. C 2011, 115, 16268–16274. [Google Scholar] [CrossRef]
- Zhuang, Y.-T.; Zhu, T.-T.; Ruan, M.; Yu, Y.-L.; Wang, J.-H. A 2D porous Fe2O3/graphitic-C3N4/graphene ternary nanocomposite with multifunctions of catalytic hydrogenation, chromium (VI) adsorption and detoxification. J. Mater. Chem. A 2017, 5, 3447–3455. [Google Scholar] [CrossRef]
- Laursen, A.B.; Kegnaes, S.; Dahl, S.; Chorkendorff, I. Molybdenum sulfides-efficient and viable materials for electro—and photoelectrocatalytic hydrogen evolution. Energy Environ. Sci. 2012, 5, 5577–5591. [Google Scholar] [CrossRef]
- Chia, X.; Ambrosi, A.; Sofer, Z.; Luxa, J.; Pumera, M. Catalytic and Charge Transfer Properties of Transition Metal Dichalcogenides Arising from Electrochemical Pretreatment. ACS Nano 2015, 9, 5164–5179. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, Z.; Liu, Y.; Xia, M.; Wang, R.; Li, N. Heterogeneous Nanostructure Based on 1T-Phase MoS2 for Enhanced Electrocatalytic Hydrogen Evolution. ACS Appl. Mater. Interfaces 2017, 9, 25291–25297. [Google Scholar] [CrossRef]
- Ishag, A.; Sun, Y. Recent Advances in Two-Dimensional MoS2 Nanosheets for Environmental Application. Ind. Eng. Chem. Res. 2021, 60, 8007–8026. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Jorgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of active edge sites for electrochemical H-2 evolution from MoS2 nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jorgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Norskov, J.K. Biornimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wen, Z.; Hui, Z.X.; Chen, Z.W.; Yang, C.C.; Jiang, Q. Graphene-MoS2 vertically anchored on an MXene-derived accordion-like TiO2/C skeleton: An ultrastable HER catalyst. J. Mater. Chem. A 2020, 8, 14223–14233. [Google Scholar] [CrossRef]
- Xiang, T.; Fang, Q.; Xie, H.; Wu, C.; Wang, C.; Zhou, Y.; Liu, D.; Chen, S.; Khalil, A.; Tao, S.; et al. Vertical 1T-MoS2 nanosheets with expanded interlayer spacing edged on a graphene frame for high rate lithium-ion batteries. Nanoscale 2017, 9, 6975–6983. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Liu, Y.; Liu, Z.; Huang, G.; Yuan, S.; Yang, G.; Wang, K.; Zhang, P.; Li, N. Symbiotic composite composed of MoS2 and pelagic clay with enhanced disinfection efficiency. RSC Adv. 2021, 11, 9621–9627. [Google Scholar] [CrossRef]
- Unuabonah, E.I.; Ugwuja, C.G.; Omorogie, M.O.; Adewuyi, A.; Oladoja, N.A. Clays for Efficient Disinfection of Bacteria in Water. Appl. Clay Sci. 2018, 151, 211–223. [Google Scholar] [CrossRef]
- Guardia, L.; Paredes, J.I.; Munuera, J.M.; Villar-Rodil, S.; Ayan-Varela, M.; Martinez-Alonso, A.; Tascon, J.M.D. Chemically Exfoliated MoS2 Nanosheets as an Efficient Catalyst for Reduction Reactions in the Aqueous Phase. ACS Appl. Mater. Interfaces 2014, 6, 21702–21710. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Du, K.; Li, Z.; Liu, M.; Ma, Y.; Sun, H.; Zhang, X.; Yang, Y. Co-Doped MoS2 Nanosheets with the Dominant CoMoS Phase Coated on Carbon as an Excellent Electrocatalyst for Hydrogen Evolution. ACS Appl. Mater. Interfaces 2015, 7, 27242–27253. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, H.; Li, S.; Wang, R.; Sun, X.; Zhou, M.; Zhou, J.; Lou, X.W.; Xie, Y. Defect-Rich MoS2 Ultrathin Nanosheets with Additional Active Edge Sites for Enhanced Electrocatalytic Hydrogen Evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, X.; Zheng, L.; Wan, C. Fabrication of TiO2/MoS2 Composite Photocatalyst and Its Photocatalytic Mechanism for Degradation of Methyl Orange under Visible Light. Can. J. Chem. Eng. 2015, 93, 1594–1602. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Sun, Q.; Zhang, P.; Jing, S. Hydrothermal Synthesis of 1T-MoS2/Pelagic Clay Composite and Its Application in the Catalytic Reduction of 4-Nitrophenol. Materials 2021, 14, 7020. https://doi.org/10.3390/ma14227020
Li N, Sun Q, Zhang P, Jing S. Hydrothermal Synthesis of 1T-MoS2/Pelagic Clay Composite and Its Application in the Catalytic Reduction of 4-Nitrophenol. Materials. 2021; 14(22):7020. https://doi.org/10.3390/ma14227020
Chicago/Turabian StyleLi, Nan, Qiwei Sun, Peiping Zhang, and Shubo Jing. 2021. "Hydrothermal Synthesis of 1T-MoS2/Pelagic Clay Composite and Its Application in the Catalytic Reduction of 4-Nitrophenol" Materials 14, no. 22: 7020. https://doi.org/10.3390/ma14227020





