Resistance to Sulfuric Acid Corrosion of Geopolymer Concrete Based on Different Binding Materials and Alkali Concentrations
Abstract
:1. Introduction
2. Experimental Details
2.1. Raw Materials
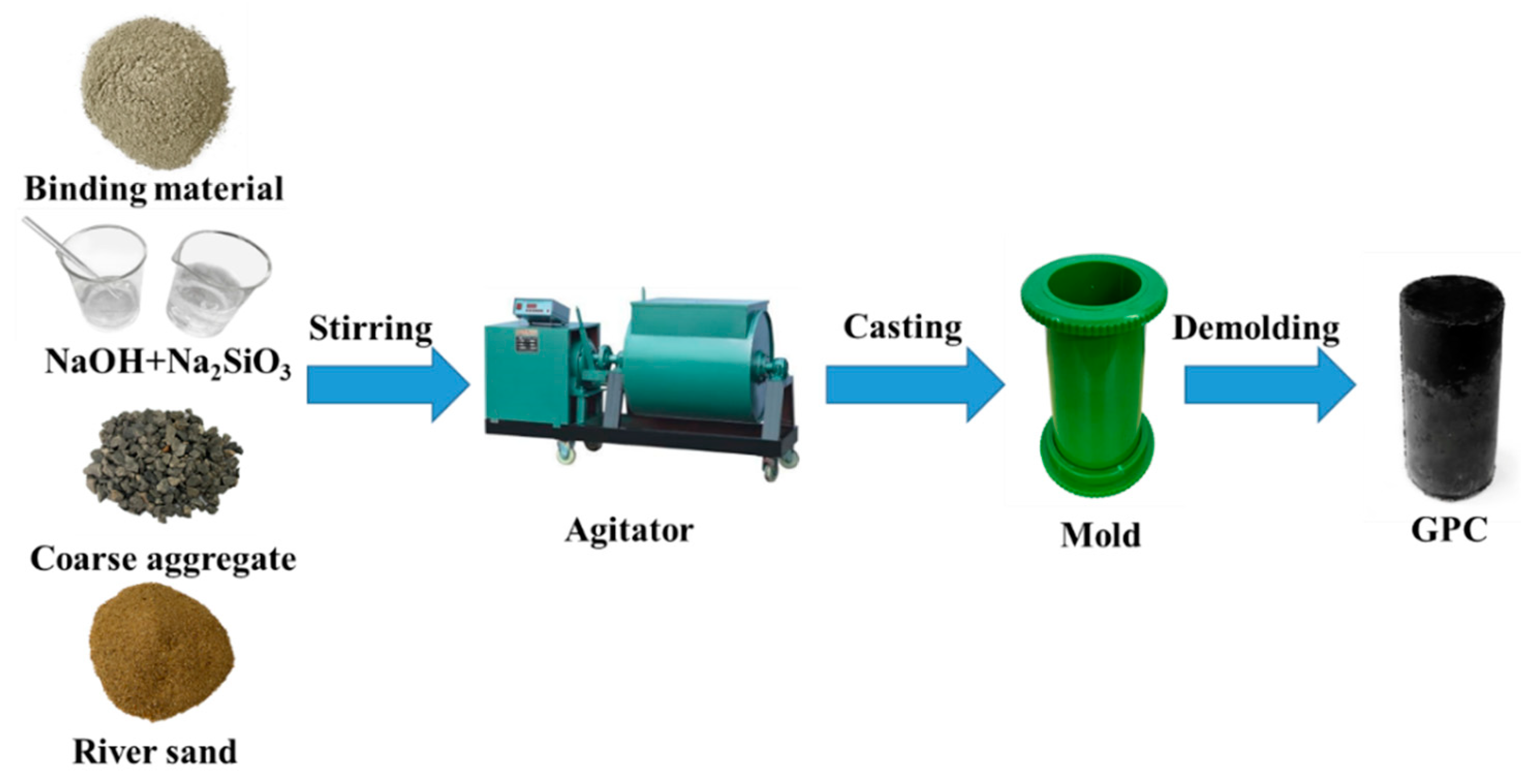
2.2. Mix Proportions and Preparation of Specimens
2.3. Measurements
2.3.1. Sulfuric Acid Corrosion Resistance Test
2.3.2. Microscopic Analysis
3. Results and Discussion
3.1. Macroscopic Properties
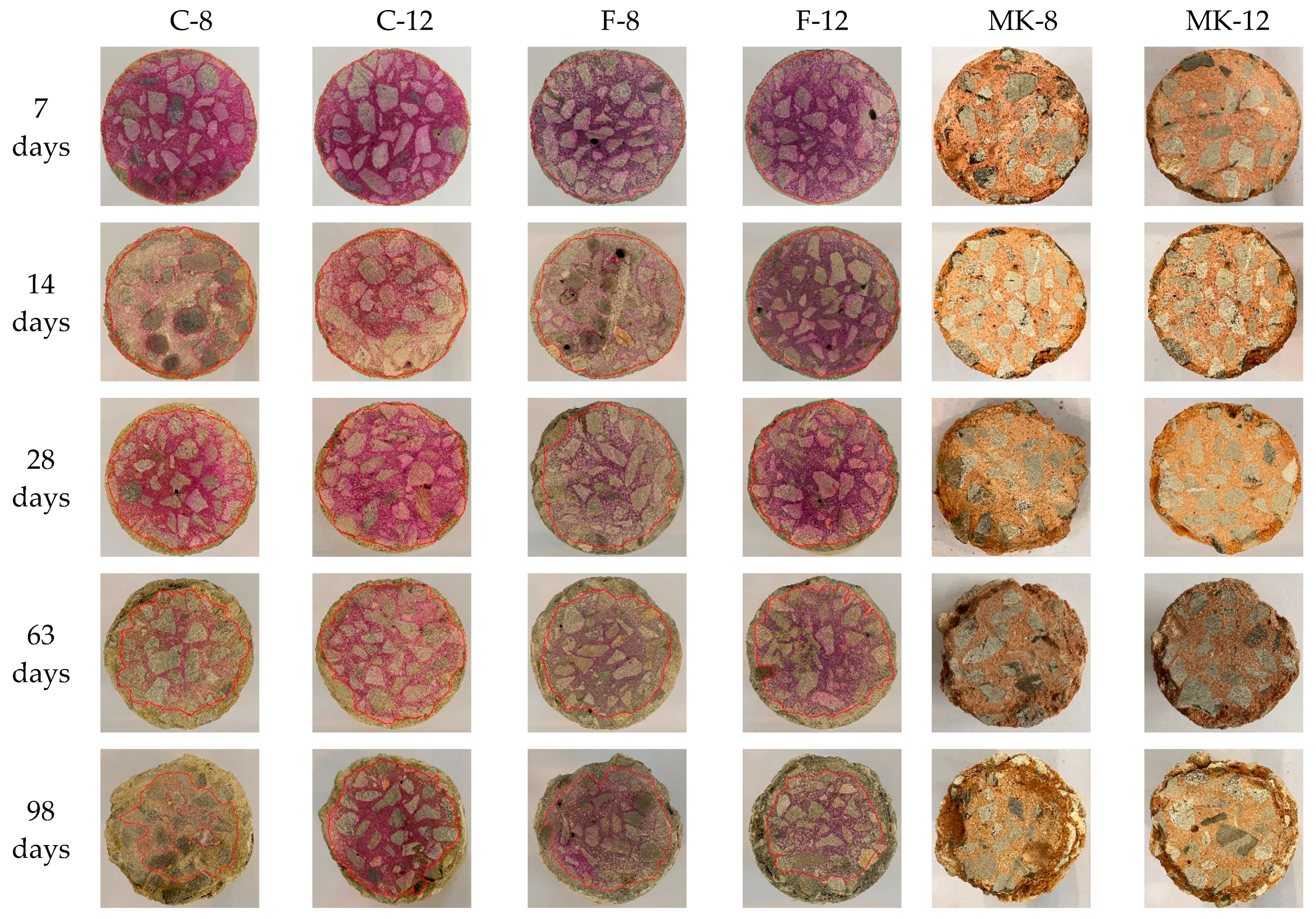
3.1.1. Visual Appearance
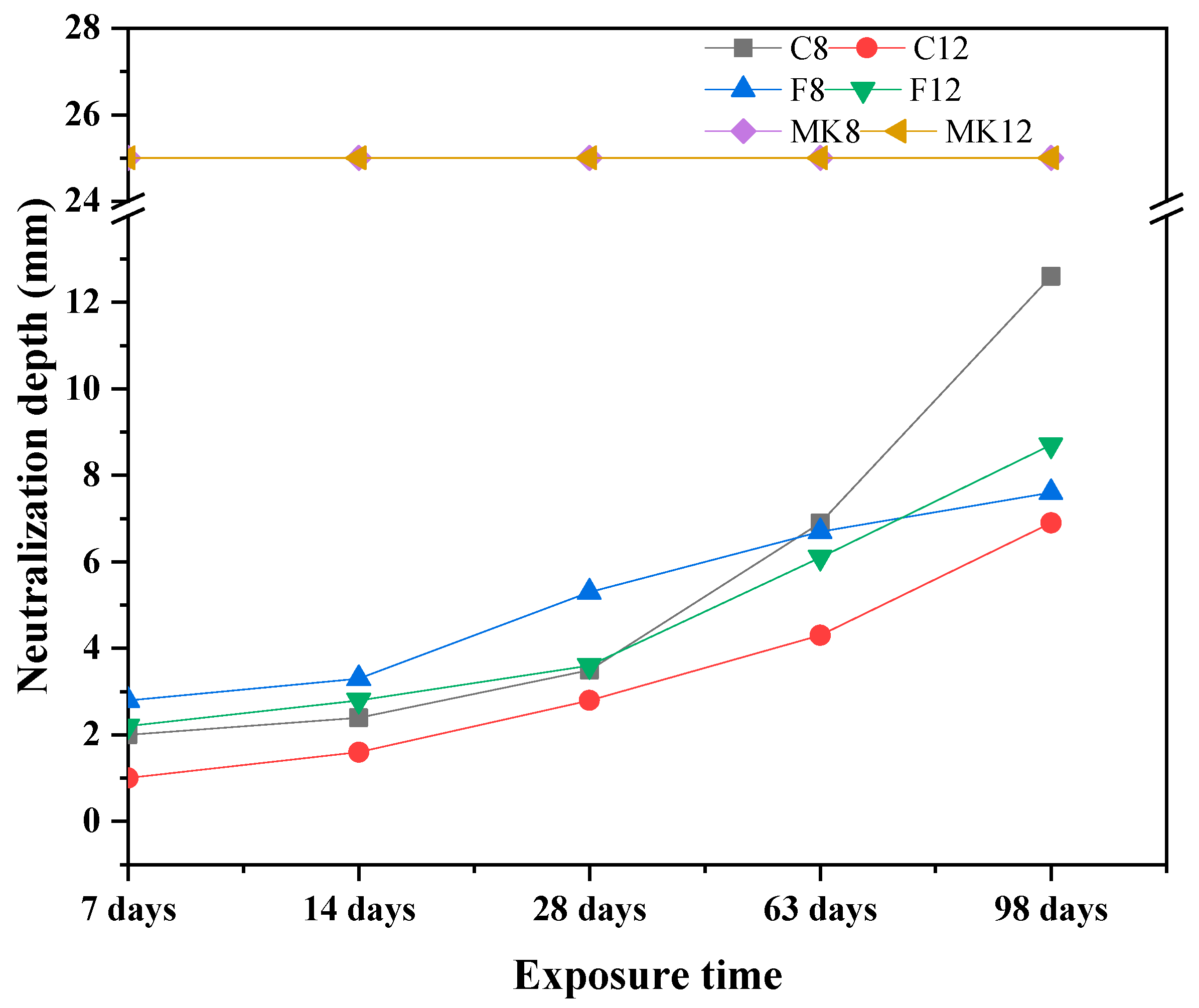
3.1.2. Neutralization Depth
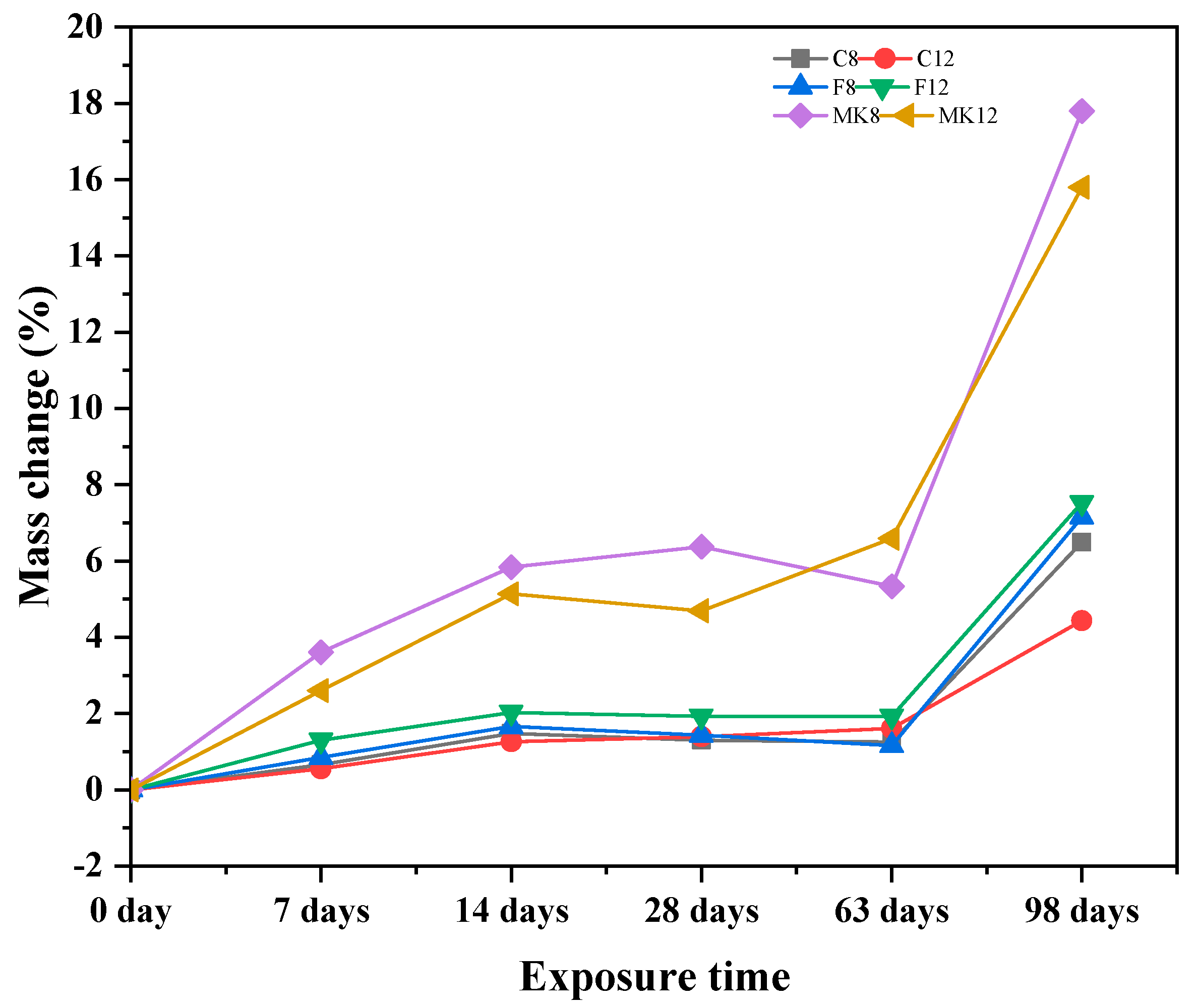
3.1.3. Mass Loss
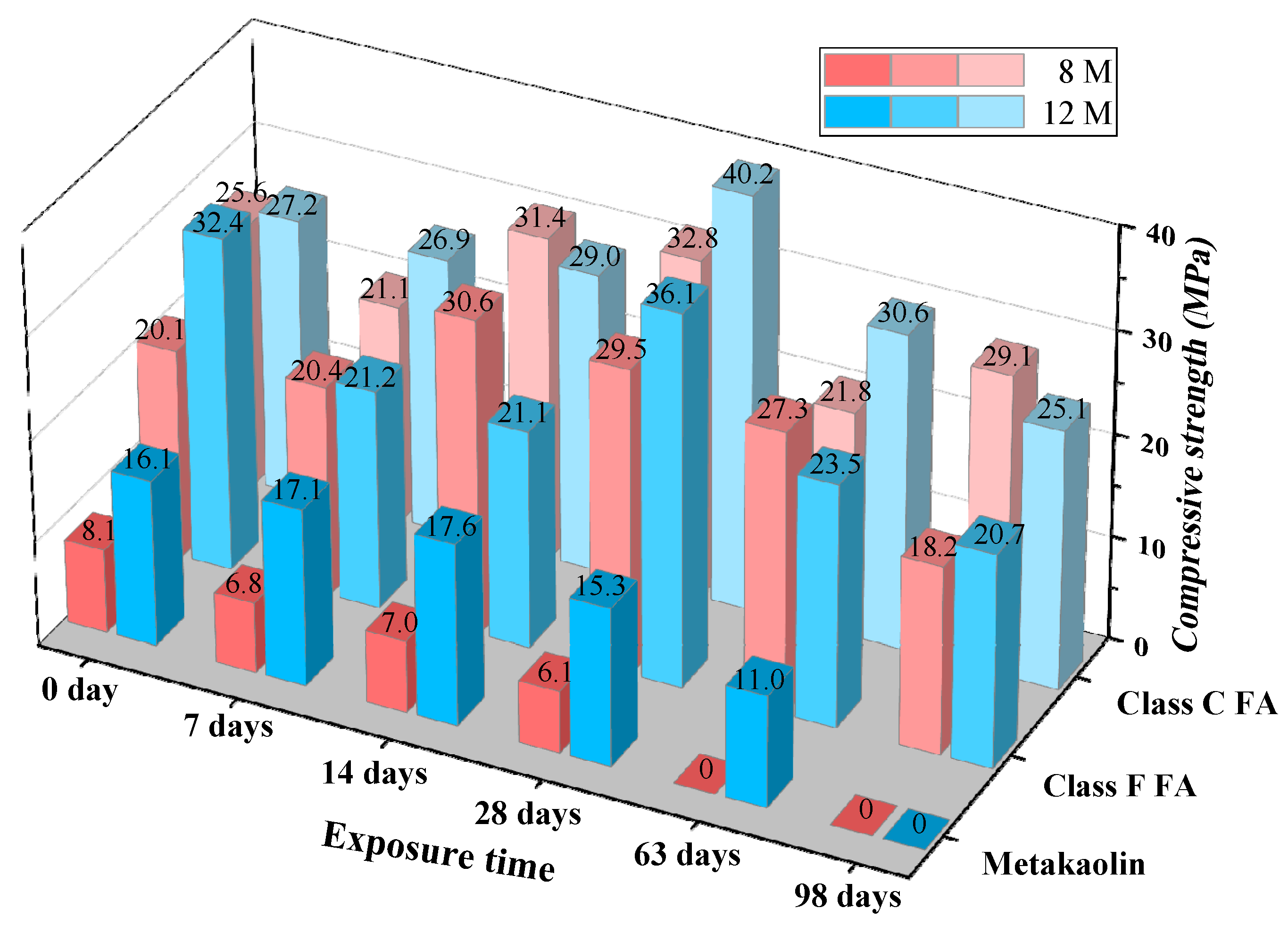
3.1.4. Compressive Strength
3.2. Microstructural Properties
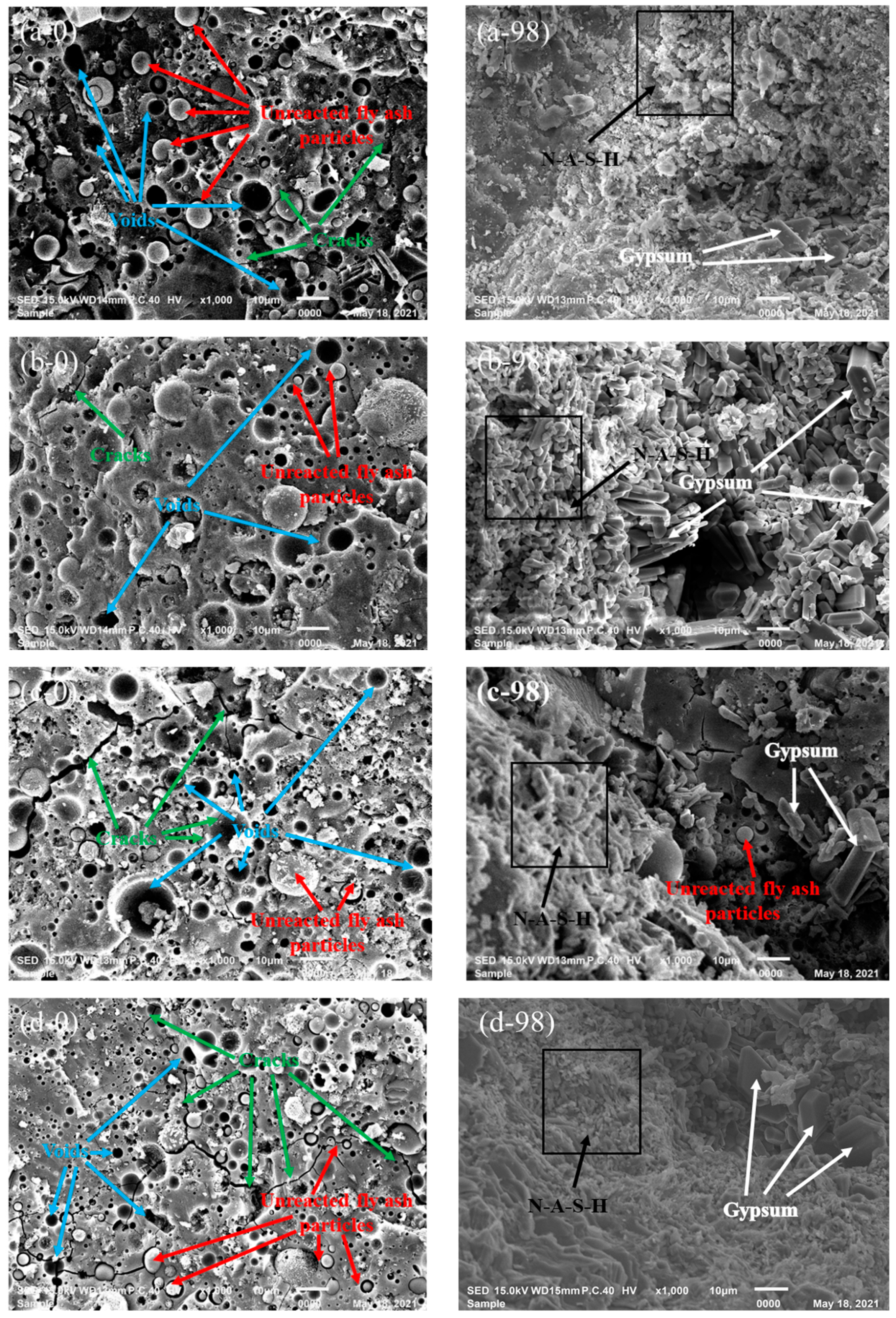
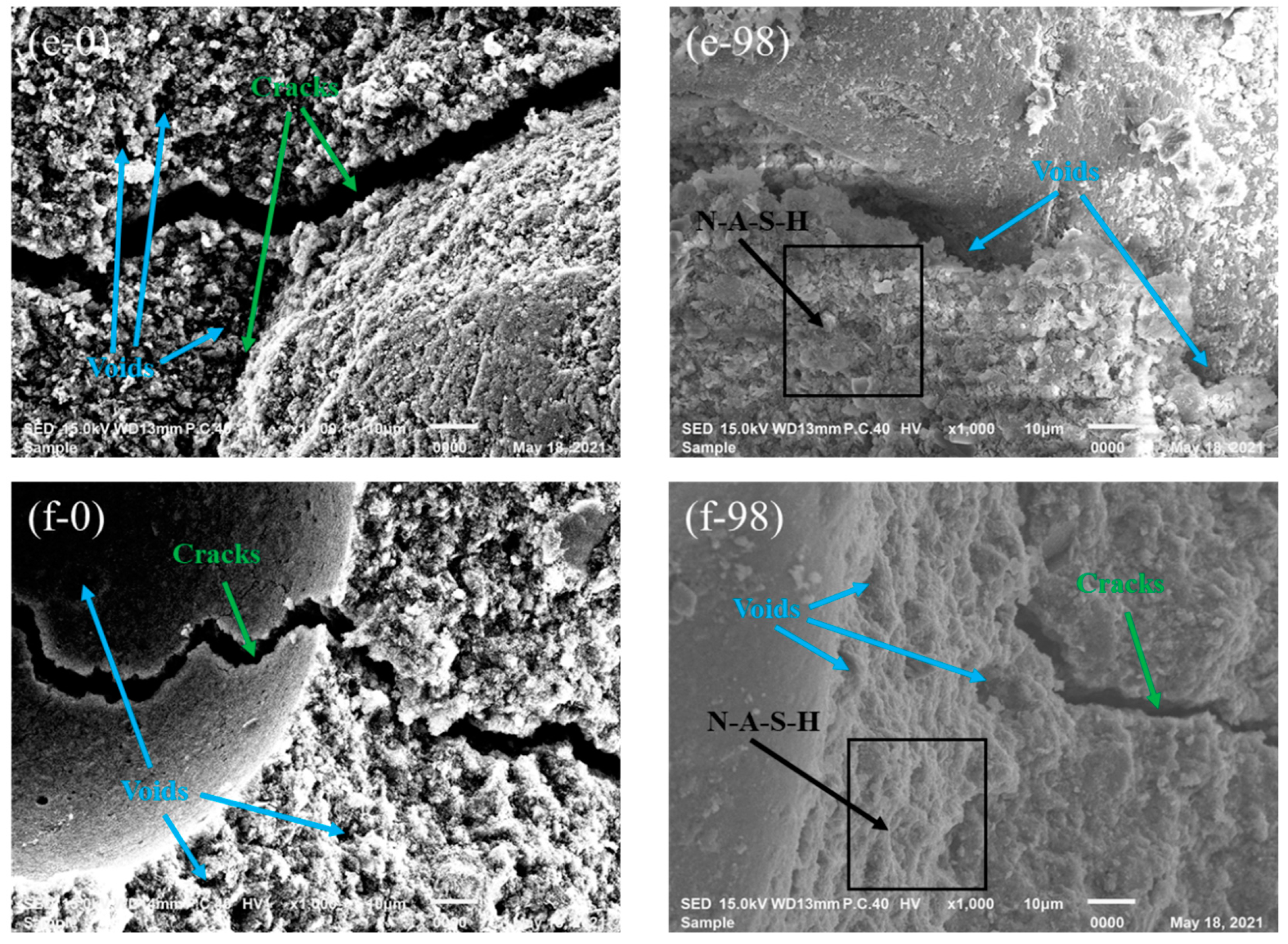
3.2.1. SEM
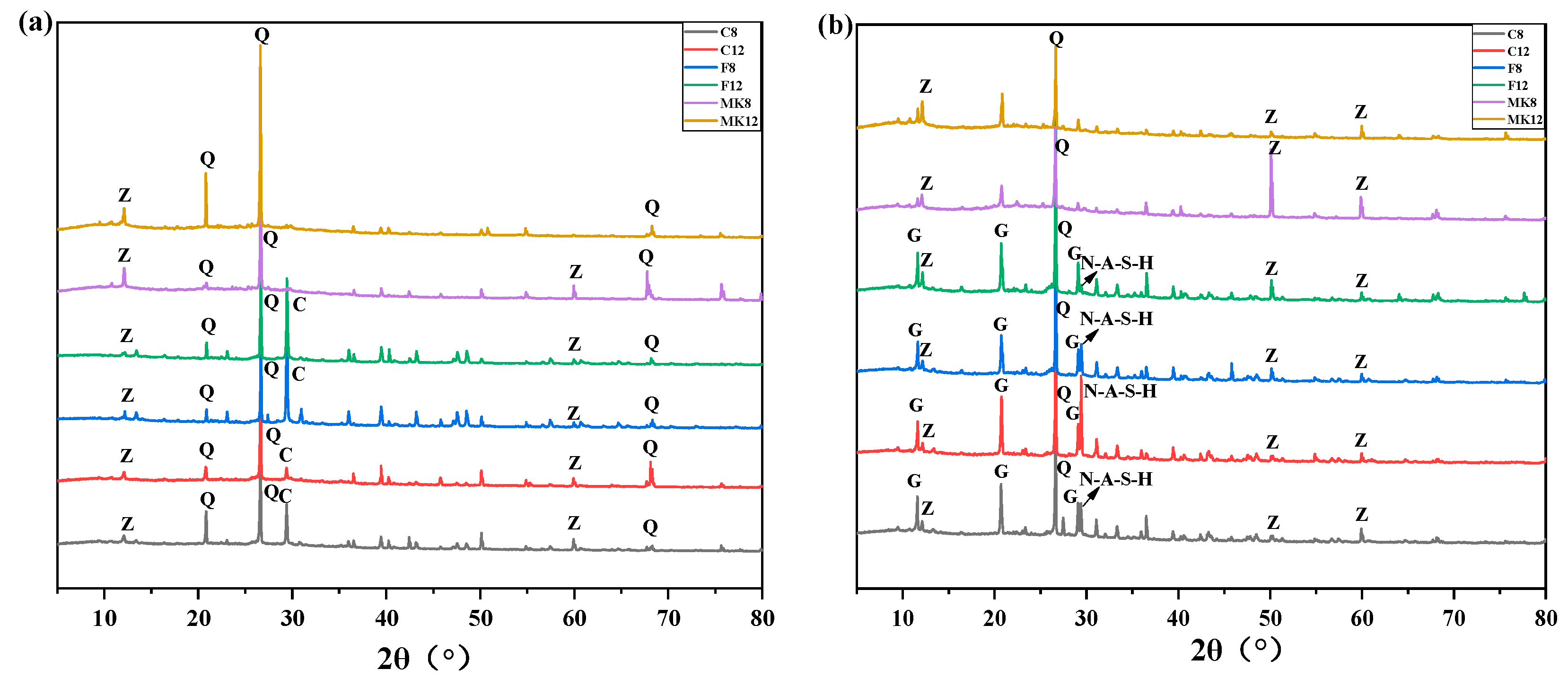
3.2.2. X-ray Diffraction
3.2.3. Fourier Transform Infrared Spectroscopy
4. Conclusions
- (1)
- Among the six kinds of GPC, the compressive strength of GPC activated by 12 M NaOH was higher than the GPC activated by 8 M NaOH, which is owing to the formation of gel depended on the concentration of alkali OH ion. The 8 M concentration of NaOH was so low that it weakened the chemical reaction and reduced the compressive strength.
- (2)
- According to the testing results of GPC unexposed to sulfuric acid, for metakaolin-based GPC, no matter the concentration of NaOH, 8 M or 12 M, the alkali-activator will not stimulate the geopolymerization of GPC completely.
- (3)
- Slight deterioration was observed for specimen C-12 exposed to sulfuric acid solution in terms of visual appearance, neutralization depth, and mass loss. This can be attributed to the fact that gypsum crystals block the pores of specimen and improve the microstructure of GPC, inhibiting further corrosion of sulfuric acid.
- (4)
- From a microscopic perspective, the compressive strength of fly ash-based GPC was superior to that of metakaolin-based GPC, which can be explained from the XRD analysis, i.e., more gismondite than N-A-S-H gel was generated in the geopolymerization of the metakaolin-based geopolymer. In light of the spectra of FT-IR, high-calcium fly ash-based GPC suffering the corrosion of sulfuric acid solution can still keep the obvious peak intensity, illustrating its good crystallinity of N-A-S-H gel and high residual strength. In general, the high-calcium fly ash is more suitable to act as a binding material to prepare GPC for practical engineering.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S.K. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, A.; García-Lodeiro, I.; Palomo, A. Durability of alkali-activated fly ash cementitious materials. J. Mater. Sci. 2007, 42, 3055–3065. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, X.; Meng, Y.; Chen, Z. Durability of concrete containing fly ash and silica fume against combined freezing-thawing and sulfate attack. Constr. Build. Mater. 2017, 147, 398–406. [Google Scholar] [CrossRef]
- Tennakoon, C.; Shayan, A.; Sanjayan, J.G.; Xu, A. Chloride ingress and steel corrosion in geopolymer concrete based on long term tests. Mater. Des. 2017, 116, 287–299. [Google Scholar] [CrossRef]
- Grengg, C.; Mittermayr, F.; Baldermann, A.; Böttcher, M.E.; Leis, A.; Koraimann, G.; Grunert, P.; Dietzel, M. Microbiologically induced concrete corrosion: A case study from a combined sewer network. Cem. Concr. Res. 2015, 77, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Grengg, C.; Mittermayr, F.; Ukrainczyk, N.; Koraimann, G.; Kienesberger, S.; Dietzel, M. Advances in concrete materials for sewer systems affected by microbial induced concrete corrosion: A review. Water Res. 2018, 134, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Castel, A.; Khan, M.S.H. Corrosion investigation of fly ash based geopolymer mortar in natural sewer environment and sulphuric acid solution. Corros. Sci. 2020, 168, 108586. [Google Scholar] [CrossRef]
- Joseph, A.P.; Keller, J.; Bustamante, H.; Bond, P.L. Surface neutralization and H2S oxidation at early stages of sewer corrosion: Influence of temperature, relative humidity and H2S concentration. Water Res. 2012, 46, 4235–4245. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, F.H.A.; Ahmad, R.; Abdullah, M.M.A.; Rahim, S.Z.A.; Yahya, Z.; Li, L.Y.; Ediati, R. Geopolymer as underwater concreting material: A review. Constr. Build. Mater. 2021, 291, 123276. [Google Scholar] [CrossRef]
- Almutairi, A.L.; Tayeh, B.A.; Adesina, A.; Isleem, H.F.; Zeyad, A.M. Potential applications of geopolymer concrete in construction: A review. Case Stud. Constr. Mater. 2021, 15, e00733. [Google Scholar] [CrossRef]
- Mahmood, A.H.; Foster, S.J.; Castel, A. Effects of mixing duration on engineering properties of geopolymer concrete. Constr. Build. Mater. 2021, 303, 124449. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-activated binders: A review. Part 2. About materials and binders manufacture. Constr. Build. Mater. 2008, 22, 1315–1322. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Vollpracht, A. Isothermal calorimetry and in-situ XRD study of the NaOH activated fly ash, metakaolin and slag. Cem. Concr. Res. 2018, 103, 110–122. [Google Scholar] [CrossRef]
- Neupane, K.; Hadigheh, S.A. Sodium hydroxide-free geopolymer binder for prestressed concrete applications. Constr. Build. Mater. 2021, 293, 123397. [Google Scholar] [CrossRef]
- Wan, Q.; Zhang, Y.; Zhang, R. The effect of pore behavior and gel structure on the mechanical property at different initial water content. Constr. Build. Mater. 2021, 309, 125146. [Google Scholar] [CrossRef]
- Hu, S.; Zhong, L.; Yang, X.; Bai, H.; Ren, B.; Zhao, Y.; Zhang, W.; Ju, X.; Wen, H.; Mao, S.; et al. Synthesis of rare earth tailing-based geopolymer for efficiently immobilizing heavy metals. Constr. Build. Mater. 2020, 254, 119273. [Google Scholar] [CrossRef]
- Wan, Q.; Rao, F.; Song, S.; García, R.E.; Estrella, R.M.; Patiño, C.L.; Zhang, Y. Geopolymerization reaction, microstructure and simulation of metakaolin-based geopolymers at extended Si/Al ratios. Cem. Concr. Compos. 2017, 79, 45–52. [Google Scholar] [CrossRef]
- Pather, B.; Ekolu, S.O.; Quainoo, H. Effects of aggregate types on acid corrosion attack upon fly—Ash geopolymer and Portland cement concretes—Comparative study. Constr. Build. Mater. 2021, 313, 125468. [Google Scholar] [CrossRef]
- Fernandes, I.; Pericão, M.; Hagelia, P.; Noronha, F.; Ribeiro, M.A.; Maia, J. Identification of acid attack on concrete of a sewage system. Mater. Struct. Constr. 2012, 45, 337–350. [Google Scholar] [CrossRef]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Ariffin, M.A.M.; Bhutta, M.A.R.; Hussin, M.W.; Tahir, M.M.; Aziah, N. Sulfuric acid resistance of blended ash geopolymer concrete. Constr. Build. Mater. 2013, 43, 80–86. [Google Scholar] [CrossRef]
- Gevaudan, J.P.; Craun, Z.; Srubar, W.V. Sulfuric acid degradation of alkali-activated metakaolin cements supplemented with brucite. Cem. Concr. Compos. 2021, 121, 104063. [Google Scholar] [CrossRef]
- Aiken, T.A.; Kwasny, J.; Sha, W.; Soutsos, M.N. Effect of slag content and activator dosage on the resistance of fly ash geopolymer binders to sulfuric acid attack. Cem. Concr. Res. 2018, 111, 23–40. [Google Scholar] [CrossRef] [Green Version]
- Nuaklong, P.; Sata, V.; Chindaprasirt, P. Properties of metakaolin-high calcium fly ash geopolymer concrete containing recycled aggregate from crushed concrete specimens. Constr. Build. Mater. 2018, 161, 365–373. [Google Scholar] [CrossRef]
- Mehta, A.; Siddique, R. Sulfuric acid resistance of fly ash based geopolymer concrete. Constr. Build. Mater. 2017, 146, 136–143. [Google Scholar] [CrossRef]
- Moghaddam, S.C.; Madandoust, R.; Jamshidi, M.; Nikbin, I.M. Mechanical properties of fly ash-based geopolymer concrete with crumb rubber and steel fiber under ambient and sulfuric acid conditions. Constr. Build. Mater. 2021, 281, 122571. [Google Scholar] [CrossRef]
- Qu, F.; Li, W.; Wang, K.; Zhang, S.; Sheng, D. Performance deterioration of fly ash/slag-based geopolymer composites subjected to coupled cyclic preloading and sulfuric acid attack. J. Clean. Prod. 2021, 321, 128942. [Google Scholar] [CrossRef]
- ASTM. C39/C39M-18 Standard. Test Method Compressive Strength Cylindrical Concrete Specimens. (N.D.). Available online: www.astm.org (accessed on 20 February 2018).
- Khan, H.A.; Khan, M.S.H.; Castel, A.; Sunarho, J. Deterioration of alkali-activated mortars exposed to natural aggressive sewer environment. Constr. Build. Mater. 2018, 186, 577–597. [Google Scholar] [CrossRef]
- Rendell, F.; Jauberthie, R. Deterioration of mortar in sulphate environments. Constr. Build. Mater. 1999, 13, 321–327. [Google Scholar] [CrossRef]
- Lee, N.K.; Lee, H.K. Influence of the slag content on the chloride and sulfuric acid resistances of alkali-activated fly ash/slag paste. Cem. Concr. Compos. 2016, 72, 168–179. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Li, N.; Zhang, Z.; Farzadnia, N. 12—Carbon Dioxide Sequestration by Alkali-Activated Materials. In Carbon Dioxide Sequestration in Cementitious Construction Materials; Pacheco-Torgal, F., Shi, C., Sanchez, A.P., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 279–298. [Google Scholar] [CrossRef]
- Nuaklong, P.; Sata, V.; Chindaprasirt, P. Influence of recycled aggregate on fly ash geopolymer concrete properties. J. Clean. Prod. 2016, 112, 2300–2307. [Google Scholar] [CrossRef]
- Lee, W.K.W.; van Deventer, J.S.J. The effects of inorganic salt contamination on the strength and durability of geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2002, 211, 115–126. [Google Scholar] [CrossRef]
- Xinjie, W.; Wei, Y.; Hui, L.I.U.; Pinghua, Z.H.U.; Ningwen, Z.; Jincai, F. Strength and microstructural analysis of geopolymer prepared with recycled geopolymer powder. J. Wuhan Univ. Technol. Sci. 2021, 36, 439–445. [Google Scholar]
- Gharzouni, A.; Vidal, L.; Essaidi, N.; Joussein, E.; Rossignol, S. Recycling of geopolymer waste: Influence on geopolymer formation and mechanical properties. Mater. Des. 2016, 94, 221–229. [Google Scholar] [CrossRef]
- Rovnaník, P. Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Constr. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Van Deventer, J.S.J.; Provis, J.L.; Duxson, P.; Lukey, G.C. Reaction mechanisms in the geopolymeric conversion of inorganic waste to useful products. J. Hazard. Mater. 2007, 139, 506–513. [Google Scholar] [CrossRef]
- Alonso, S.; Palomo, A. Alkaline activation of metakaolin and calcium hydroxide mixtures: Influence of temperature, activator concentration and solids ratio. Mater. Lett. 2001, 47, 55–62. [Google Scholar] [CrossRef]
- Alzeebaree, R.; Çevik, A.; Nematollahi, B.; Sanjayan, J.; Mohammedameen, A.; Gülşan, M.E. Mechanical properties and durability of unconfined and confined geopolymer concrete with fiber reinforced polymers exposed to sulfuric acid. Constr. Build. Mater. 2019, 215, 1015–1032. [Google Scholar] [CrossRef]
- Chang, J.J.; Yeih, W.; Hung, C.C. Effects of gypsum and phosphoric acid on the properties of sodium silicate-based alkali-activated slag pastes. Cem. Concr. Compos. 2005, 27, 85–91. [Google Scholar] [CrossRef]
- Nematollahi, B.; Qiu, J.; Yang, E.H.; Sanjayan, J. Microscale investigation of fiber-matrix interface properties of strain-hardening geopolymer composite. Ceram. Int. 2017, 43, 15616–15625. [Google Scholar] [CrossRef]
- Williams, R.P.; van Riessen, A. Development of alkali activated borosilicate inorganic polymers (AABSIP). J. Eur. Ceram. Soc. 2011, 31, 1513–1516. [Google Scholar] [CrossRef]
- Allahverdi, A.; Škvára, F. Sulfuric acid attack on hardened paste of geopolymer cements Part 1. Mechanism of corrosion at relatively high concentrations. Ceram. Silik. 2005, 49, 225–229. [Google Scholar]
- Monteny, J.; Vincke, E.; Beeldens, A.; de Belie, N.; Taerwe, L.; van Gemert, D.; Verstraete, W. Chemical, microbiological, and in situ test methods for biogenic sulfuric acid corrosion of concrete. Cem. Concr. Res. 2000, 30, 623–634. [Google Scholar] [CrossRef]
- Xie, Y.; Lin, X.; Ji, T.; Liang, Y.; Pan, W. Comparison of corrosion resistance mechanism between ordinary Portland concrete and alkali-activated concrete subjected to biogenic sulfuric acid attack. Constr. Build. Mater. 2019, 228, 117071. [Google Scholar] [CrossRef]
- Ng, C.; Alengaram, U.J.; Wong, L.S.; Mo, K.H.; Jumaat, M.Z.; Ramesh, S. A review on microstructural study and compressive strength of geopolymer mortar, paste and concrete. Constr. Build. Mater. 2018, 186, 550–576. [Google Scholar] [CrossRef]
- Wan, Q.; Zhang, Y.; Zhang, R. Using mechanical activation of quartz to enhance the compressive strength of metakaolin based geopolymers. Cem. Concr. Compos. 2020, 111, 103635. [Google Scholar] [CrossRef]
- Zhang, W.; Yao, X.; Yang, T.; Zhang, Z. The degradation mechanisms of alkali-activated fly ash/slag blend cements exposed to sulphuric acid. Constr. Build. Mater. 2018, 186, 1177–1187. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Fly ash-based geopolymers: The relationship between composition, pore structure and efflorescence. Cem. Concr. Res. 2014, 64, 30–41. [Google Scholar] [CrossRef]
- Chen, K.; Wu, D.; Xia, L.; Cai, Q.; Zhang, Z. Geopolymer concrete durability subjected to aggressive environments—A review of influence factors and comparison with ordinary Portland cement. Constr. Build. Mater. 2021, 279, 122496. [Google Scholar] [CrossRef]
- Guo, T.; Wu, T.; Gao, L.; He, B.; Ma, F.; Huang, Z.; Bai, X. Compressive strength and electrochemical impedance response of red mud-coal metakaolin geopolymer exposed to sulfuric acid. Constr. Build. Mater. 2021, 303, 124523. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, M.; Zhang, G.; Mann, D.; Lumsden, K.; Tao, M. Durability of red mud-fly ash based geopolymer and leaching behavior of heavy metals in sulfuric acid solutions and deionized water. Constr. Build. Mater. 2016, 124, 373–382. [Google Scholar] [CrossRef] [Green Version]
- Papa, E.; Medri, V.; Kpogbemabou, D.; Morinière, V.; Laumonier, J.; Vaccari, A.; Rossignol, S. Porosity and insulating properties of silica-fume based foams. Energy Build. 2016, 131, 223–232. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Zhang, W.; Li, Z.; Zhang, Y.; Li, Y.; Ren, Y. Effects of Si/Al ratio on the efflorescence and properties of fly ash based geopolymer. J. Clean. Prod. 2020, 244, 118852. [Google Scholar] [CrossRef]
- Wan, Q.; Rao, F.; Song, S.; Cholico-González, D.F.; Ortiz, N.L. Combination formation in the reinforcement of metakaolin geopolymers with quartz sand. Cem. Concr. Compos. 2017, 80, 115–122. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, K.; Mo, B.; Li, X.; Cui, X. Preparation and characterization of a reflective and heat insulative coating based on geopolymers. Energy Build. 2015, 87, 220–225. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.-B. Resistance of alkali-activated slag concrete to acid attack. Cem. Concr. Res. 2003, 33, 1607–1611. [Google Scholar] [CrossRef]


| Chemical Compositions | SiO2 | Al2O3 | Fe2O3 | SO3 | TiO2 | CaO | K2O | MgO | Na2O | LOI a |
|---|---|---|---|---|---|---|---|---|---|---|
| Class F fly ash | 44.94 | 32.15 | 5.14 | 2.07 | 1.49 | 9.90 | 1.13 | 1.04 | 0.81 | 1.33 |
| Class C fly ash | 44.18 | 26.92 | 9.34 | 1.53 | 1.34 | 11.02 | 1.39 | 1.88 | 1.29 | 1.11 |
| Metakaolin | 48.88 | 43.39 | 3.77 | 0.04 | 2.45 | 0.98 | 0.14 | - | - | 0.35 |
| Mixes | Binding Materials | NA | Sand | NaOH | Na2SiO3 | Free Water | |||
|---|---|---|---|---|---|---|---|---|---|
| Class F | Class C | Metakaolin | 8 M | 12 M | |||||
| F-8 | 377 | - | - | 1150 | 500 | 108 | - | 162 | - |
| F-12 | 377 | - | - | 1150 | 500 | - | 108 | 162 | - |
| C-8 | 450 | 1150 | 500 | 108 | - | 162 | - | ||
| C-12 | 450 | 1150 | 500 | - | 108 | 162 | - | ||
| MK-8 | - | - | 399 | 1150 | 500 | 108 | 162 | 60 | |
| MK-12 | - | - | 399 | 1150 | 500 | 108 | 162 | 60 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Zhu, P.; Liu, H.; Wang, X.; Ge, W.; Hua, M. Resistance to Sulfuric Acid Corrosion of Geopolymer Concrete Based on Different Binding Materials and Alkali Concentrations. Materials 2021, 14, 7109. https://doi.org/10.3390/ma14237109
Yang W, Zhu P, Liu H, Wang X, Ge W, Hua M. Resistance to Sulfuric Acid Corrosion of Geopolymer Concrete Based on Different Binding Materials and Alkali Concentrations. Materials. 2021; 14(23):7109. https://doi.org/10.3390/ma14237109
Chicago/Turabian StyleYang, Wei, Pinghua Zhu, Hui Liu, Xinjie Wang, Wei Ge, and Minqi Hua. 2021. "Resistance to Sulfuric Acid Corrosion of Geopolymer Concrete Based on Different Binding Materials and Alkali Concentrations" Materials 14, no. 23: 7109. https://doi.org/10.3390/ma14237109









