Triboluminescence Phenomenon Based on the Metal Complex Compounds—A Short Review
Abstract
:1. Introduction
2. The (Un)known Mechanism of Triboluminescence
3. Synthesis of Triboluminescent Complex Compounds
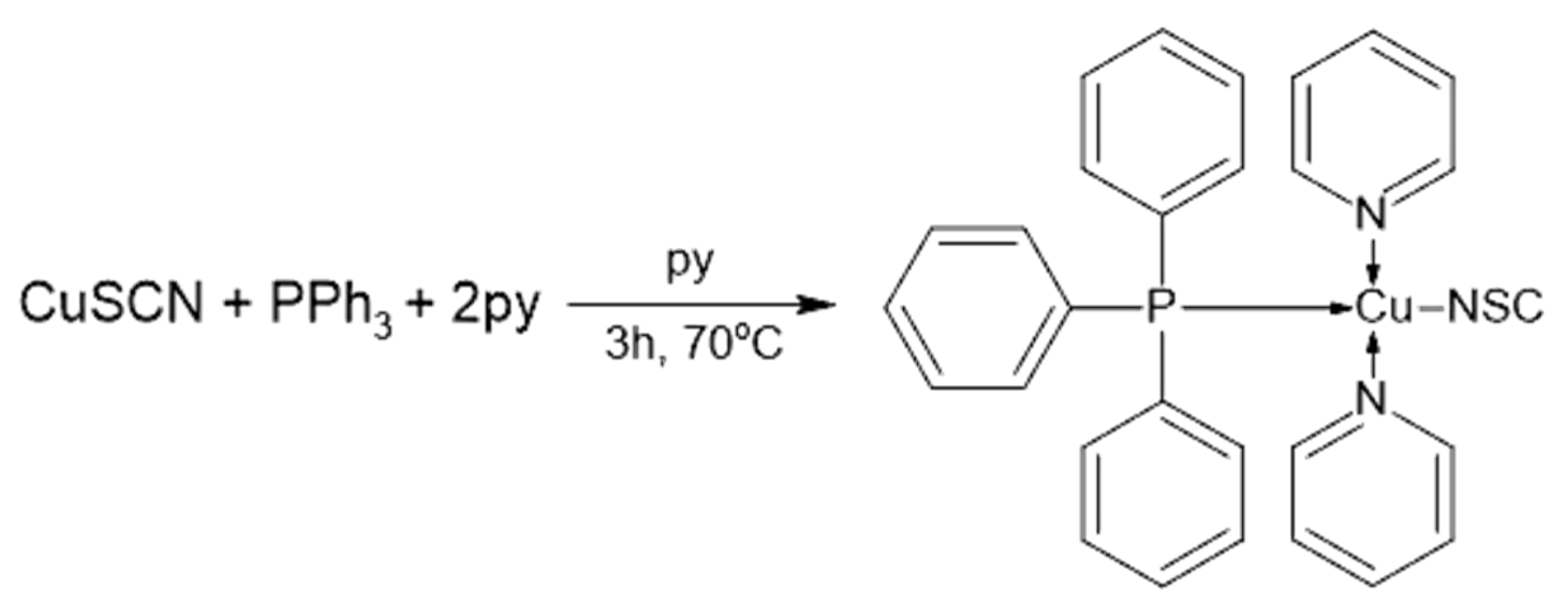
3.1. [Cu(NCS)(py)2(PPh3)] Complex
3.2. EuD4TEA Complex
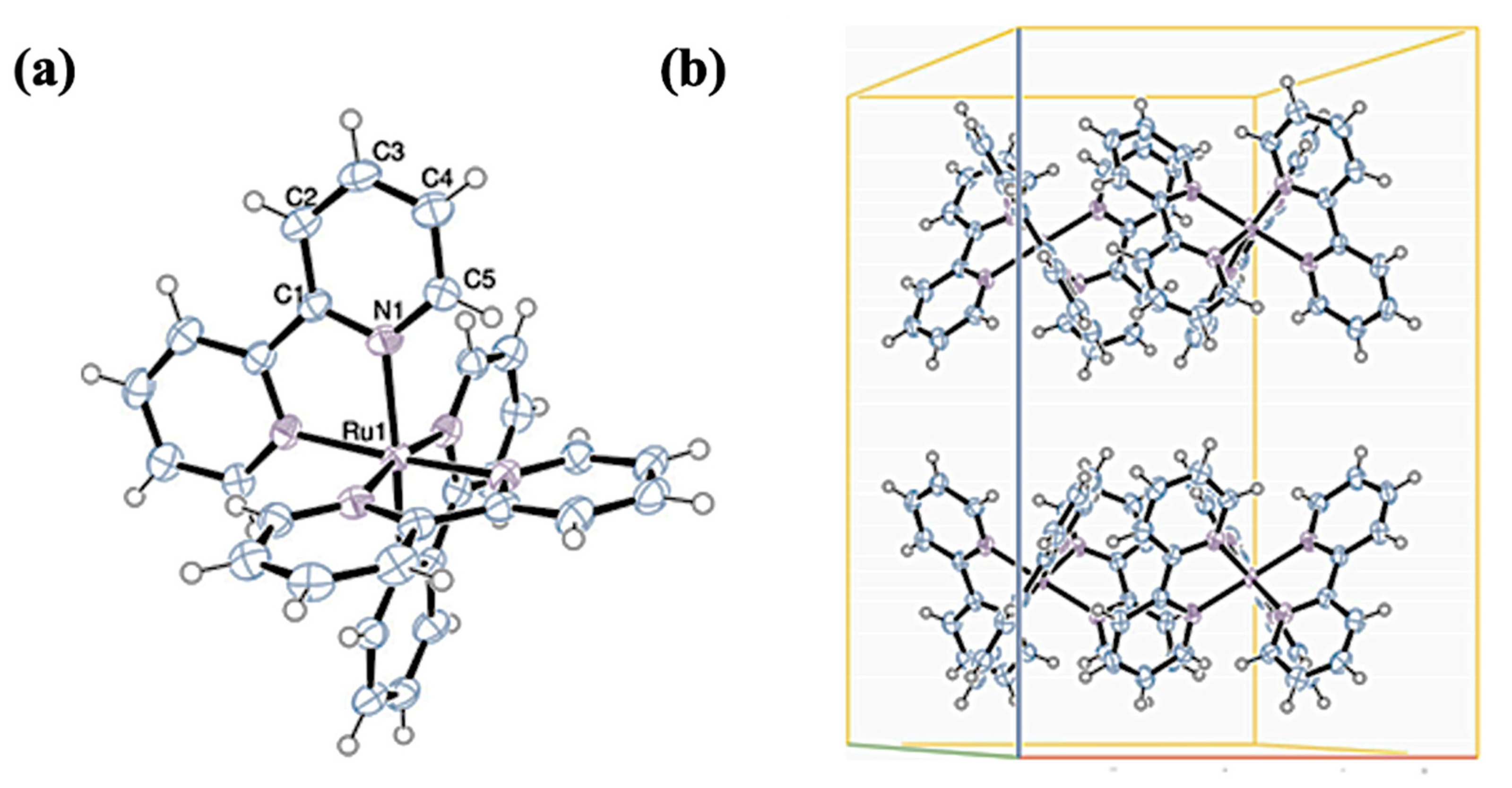
3.3. [Ru(bpy)3]Cl2 Complex
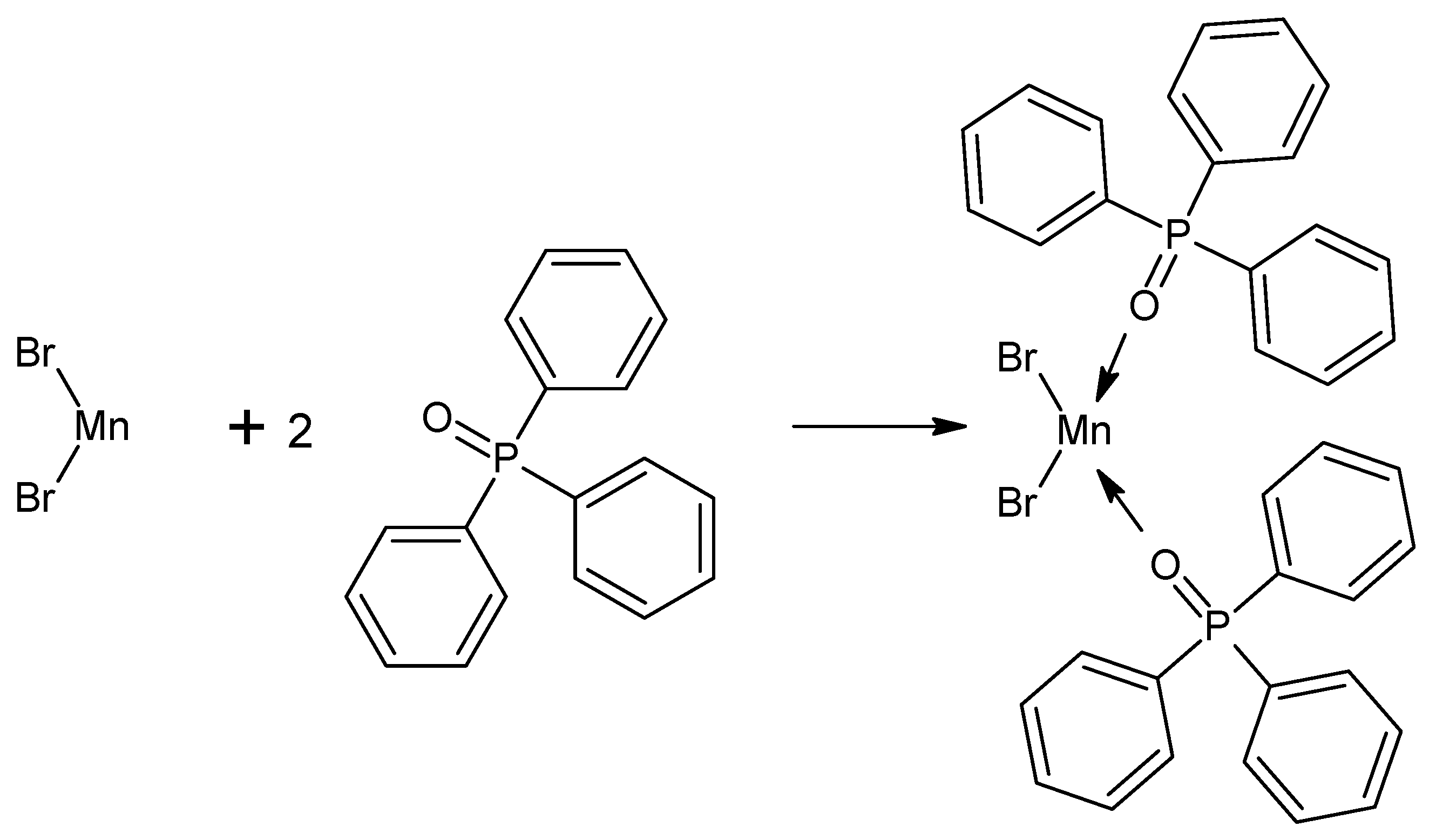
3.4. Mn(Ph3PO)2Br2 Complex
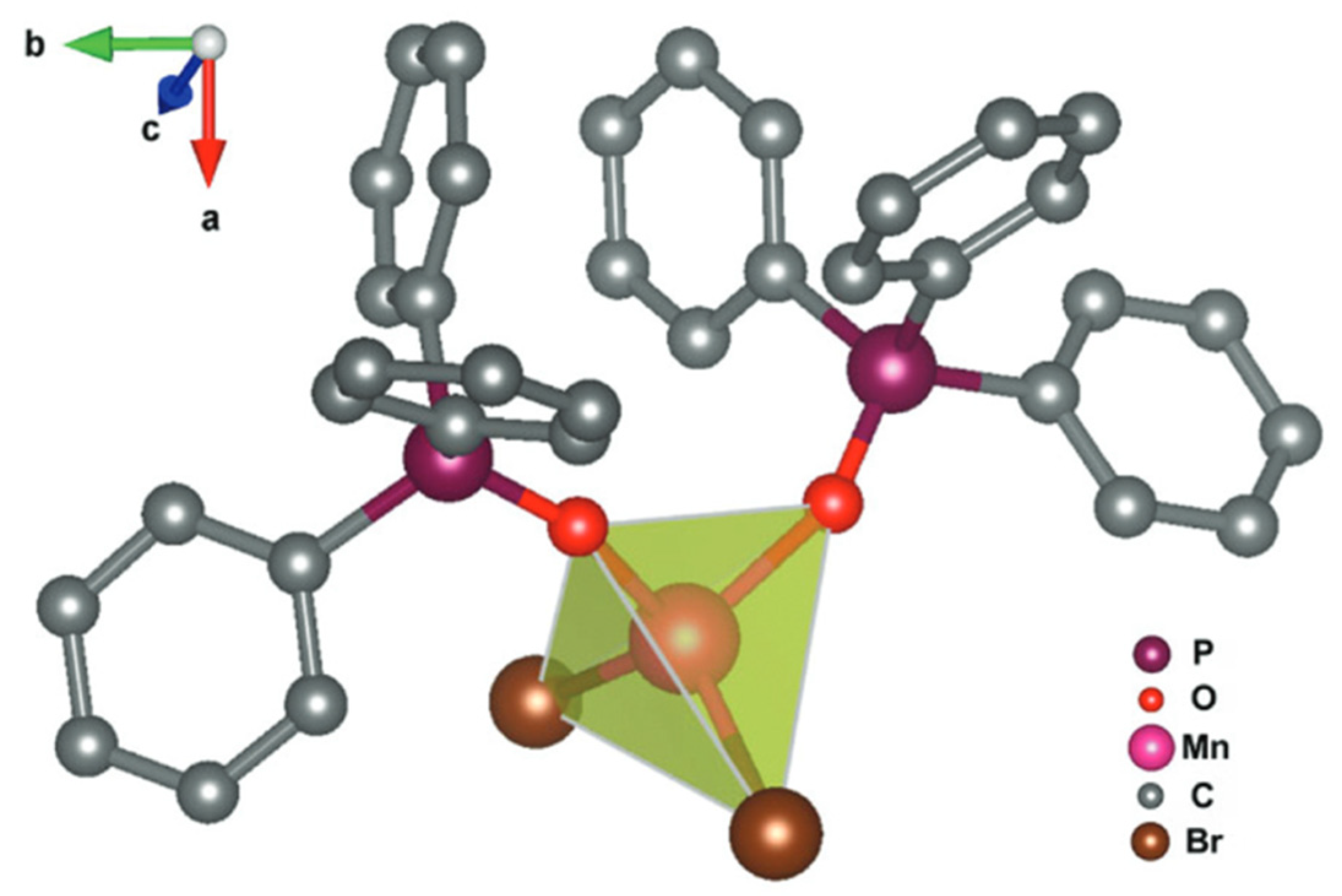
4. Chemical and Structural Analysis
4.1. [Cu(NCS)(py)2(PPh3)] Complex
- d1: 100–25 µm;
- d2: 15–10 µm;
- d3: 40–20 µm [28].
- a = 9.4006(4) Å;
- b = 15.1492(7) Å;
- c = 10.2153(4) Å.
4.2. EuD4TEA Complex
- a = 9.0297(7) Å;
- b = 24.830(3) Å;
- c = 25.203(2) Å [29].
4.3. [Ru(bpy)3]Cl2 Complex
4.4. Mn(Ph3PO)Br2 Complex
- a = 10.013(7) Å;
- b = 10.253(7) Å;
- c = 10.564(6) Å.
5. Photo- and Triboluminescence Phenomena
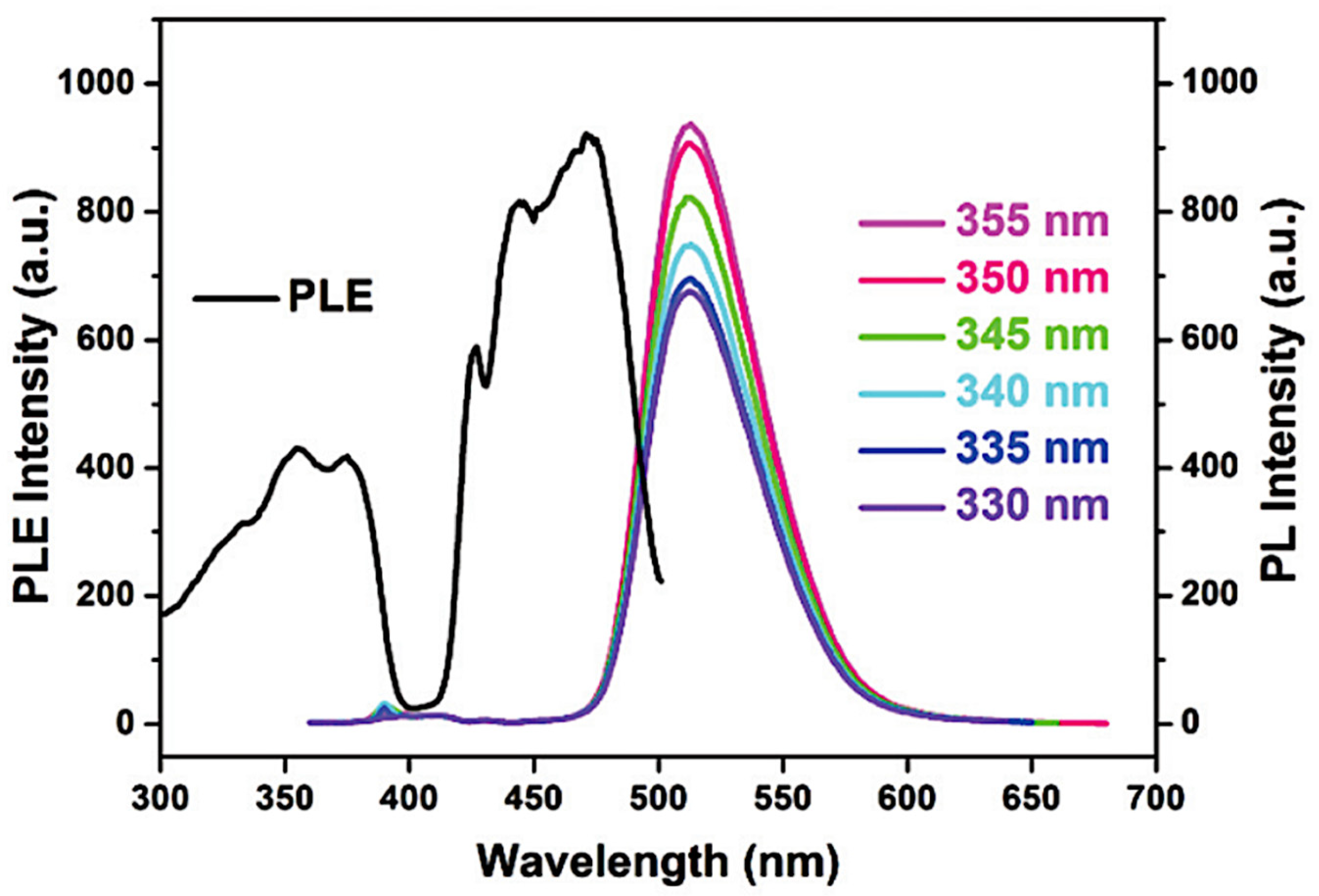
5.1. TL and PL of the [Cu(NCS)(py)2(PPh3)] Complex
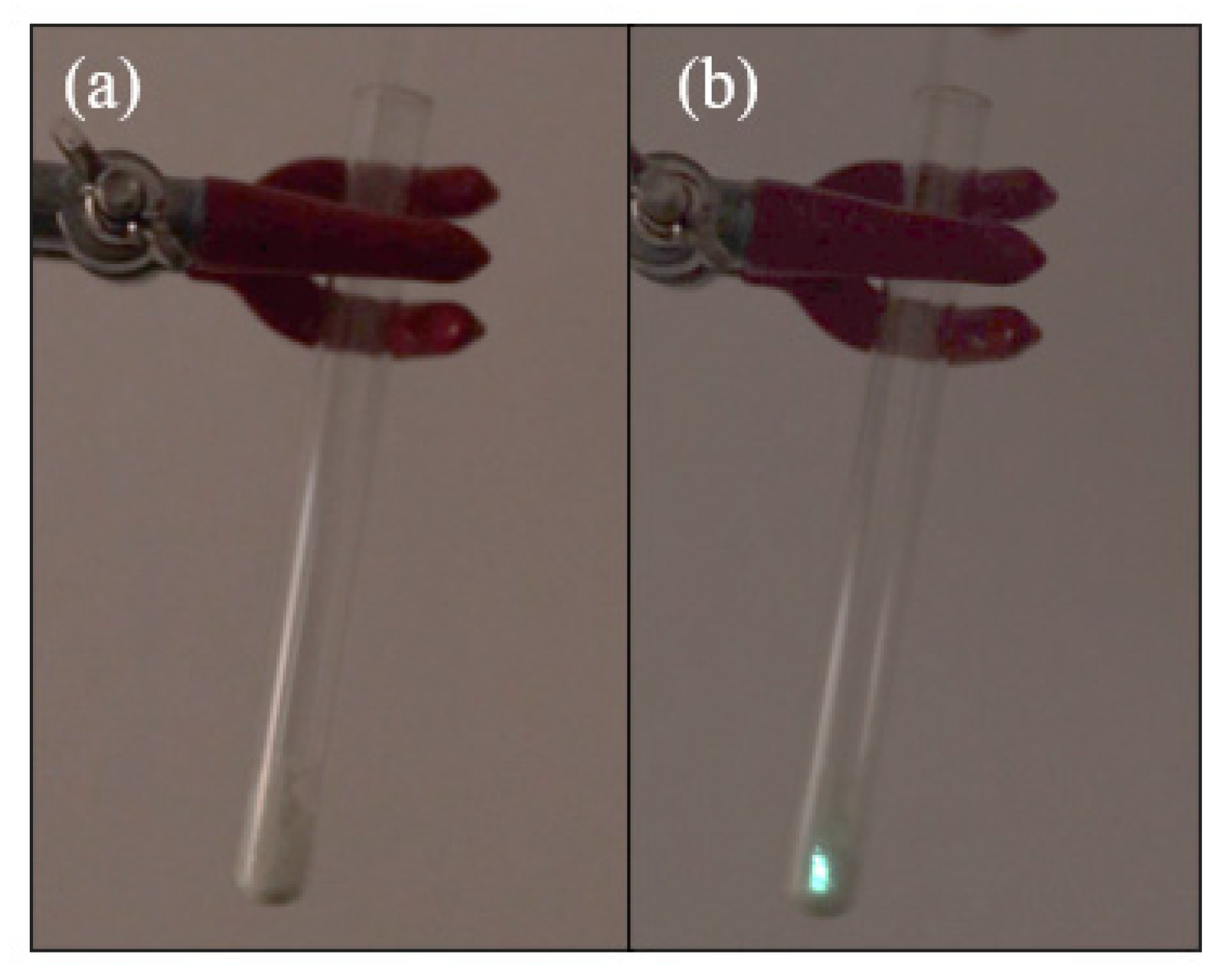
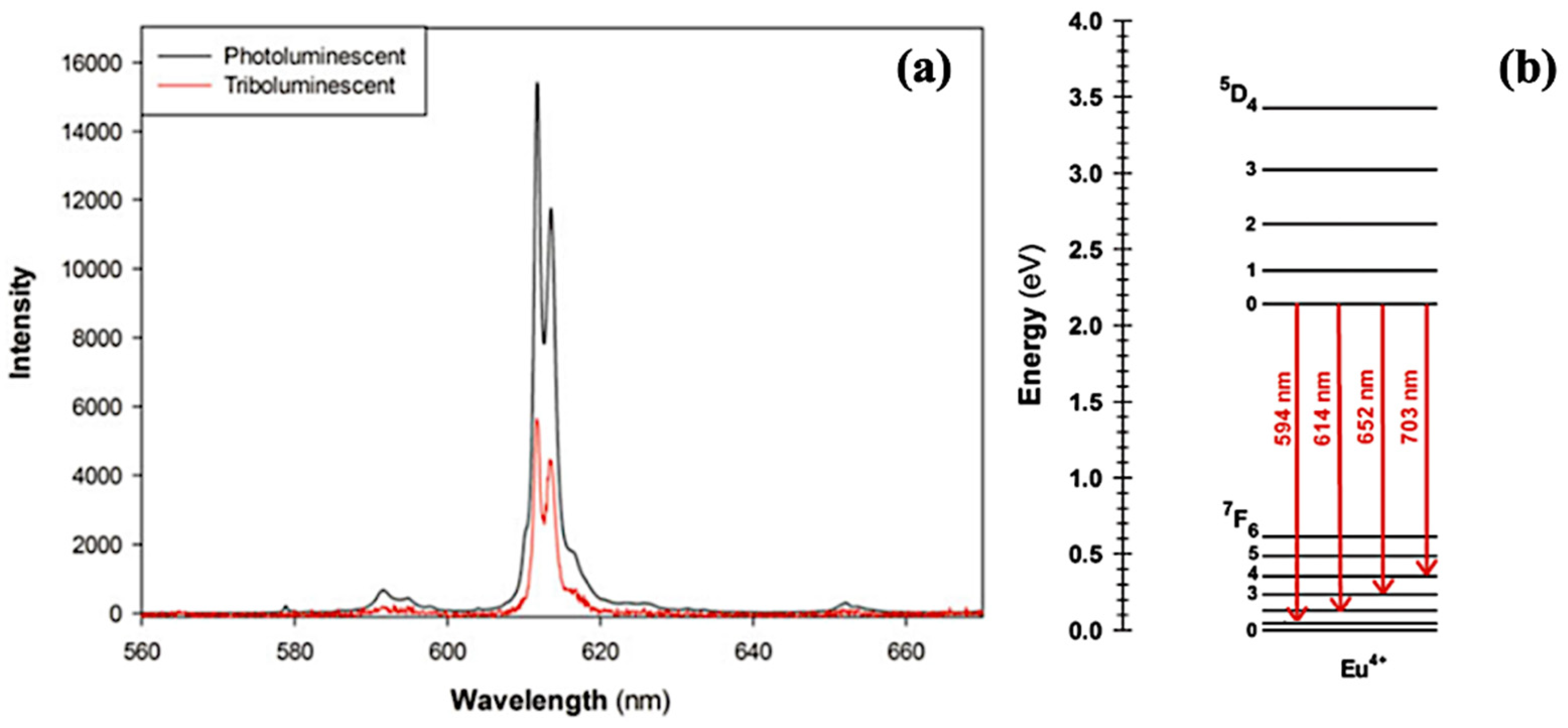
5.2. TL and PL of the Europium Complex
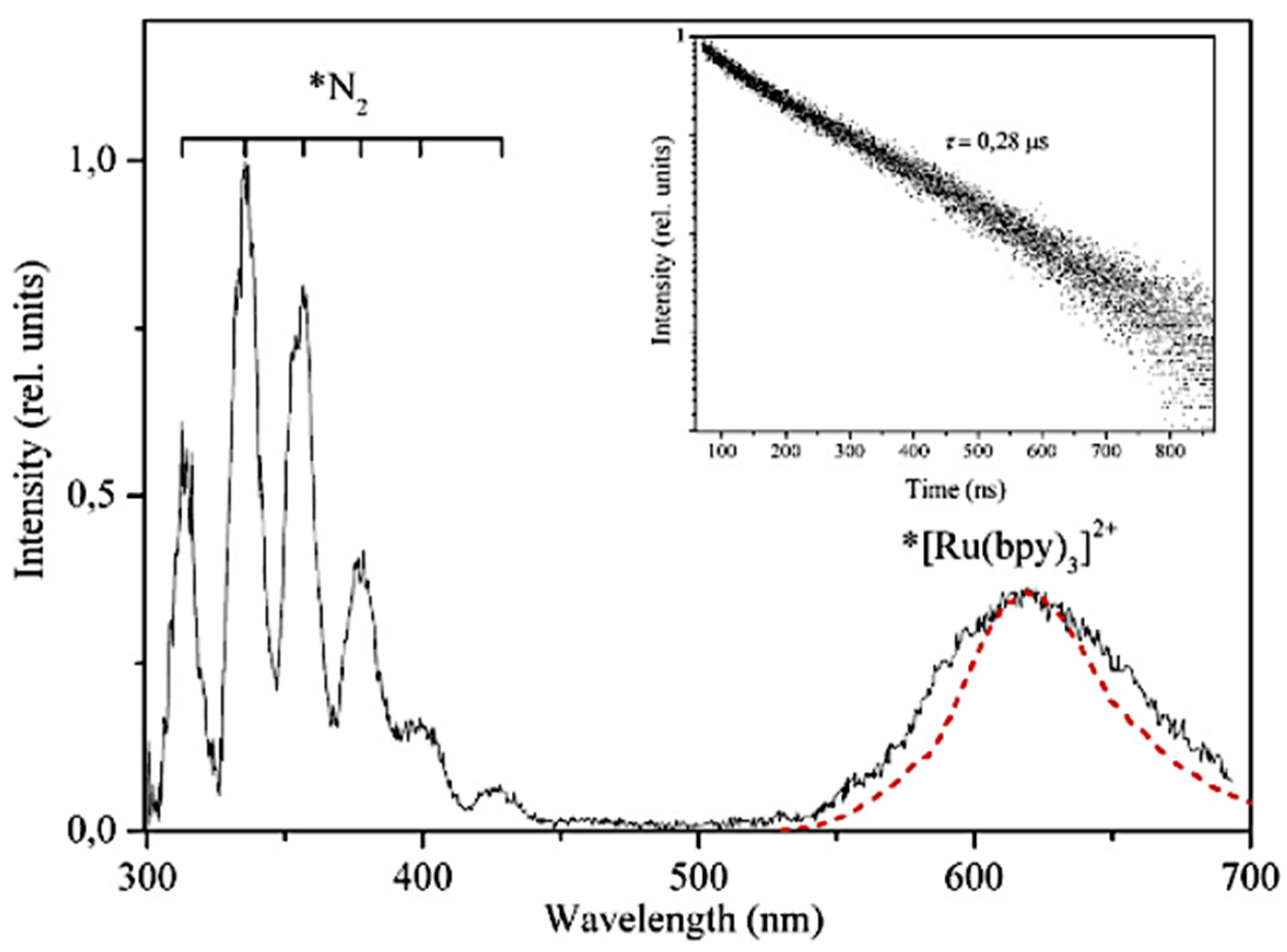
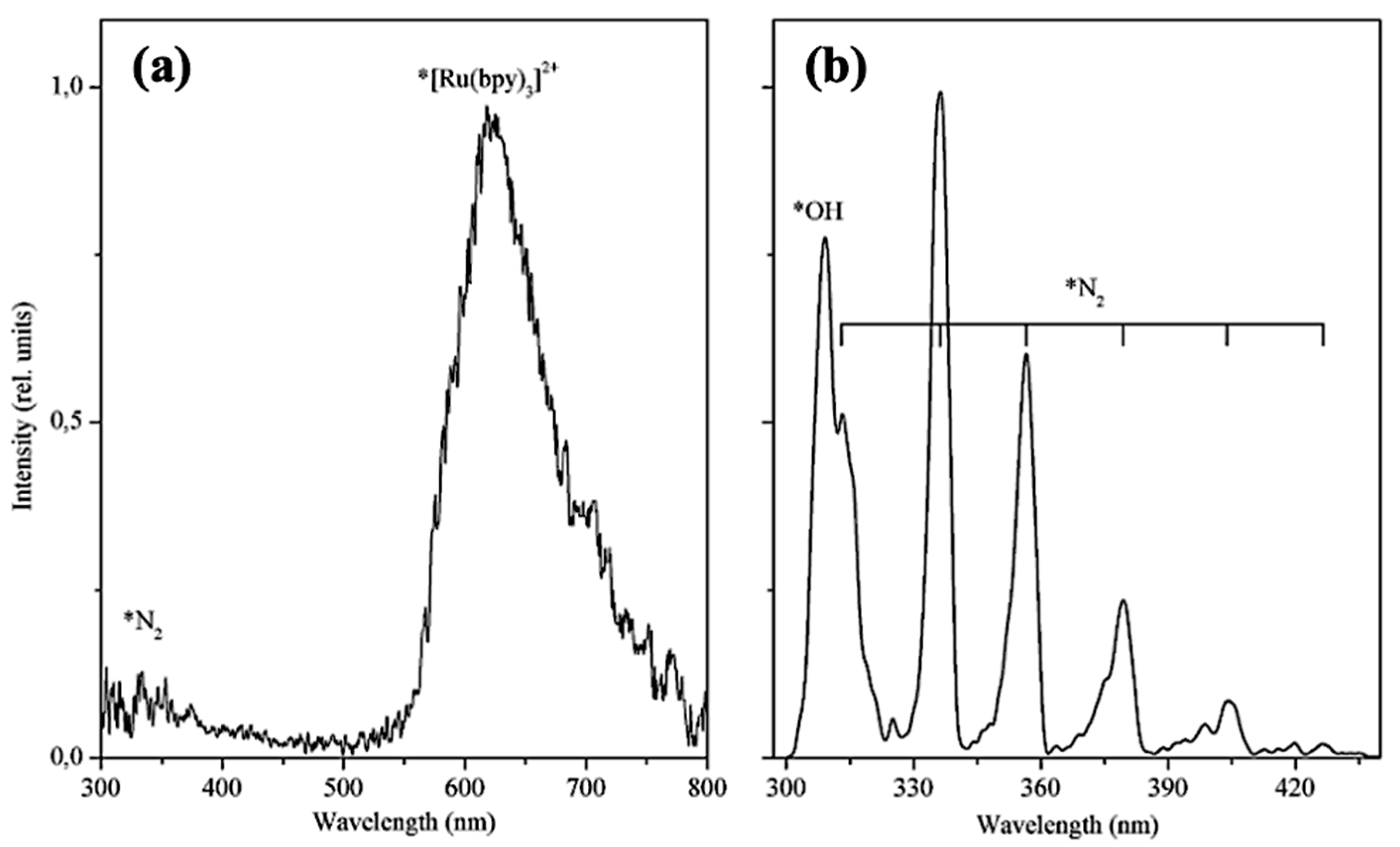
5.3. TL and PL of the [Ru(bpy)3]Cl2 Complex
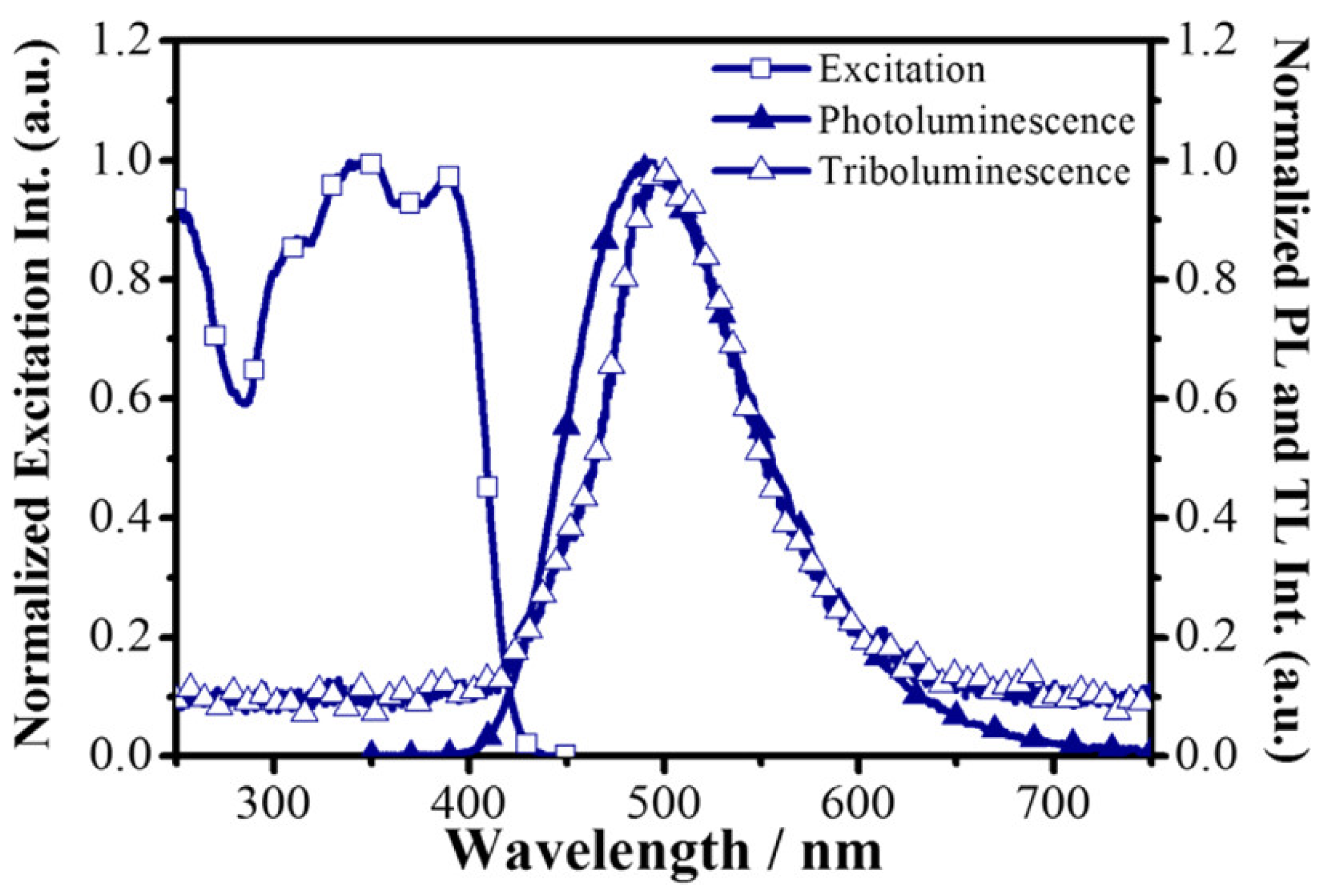
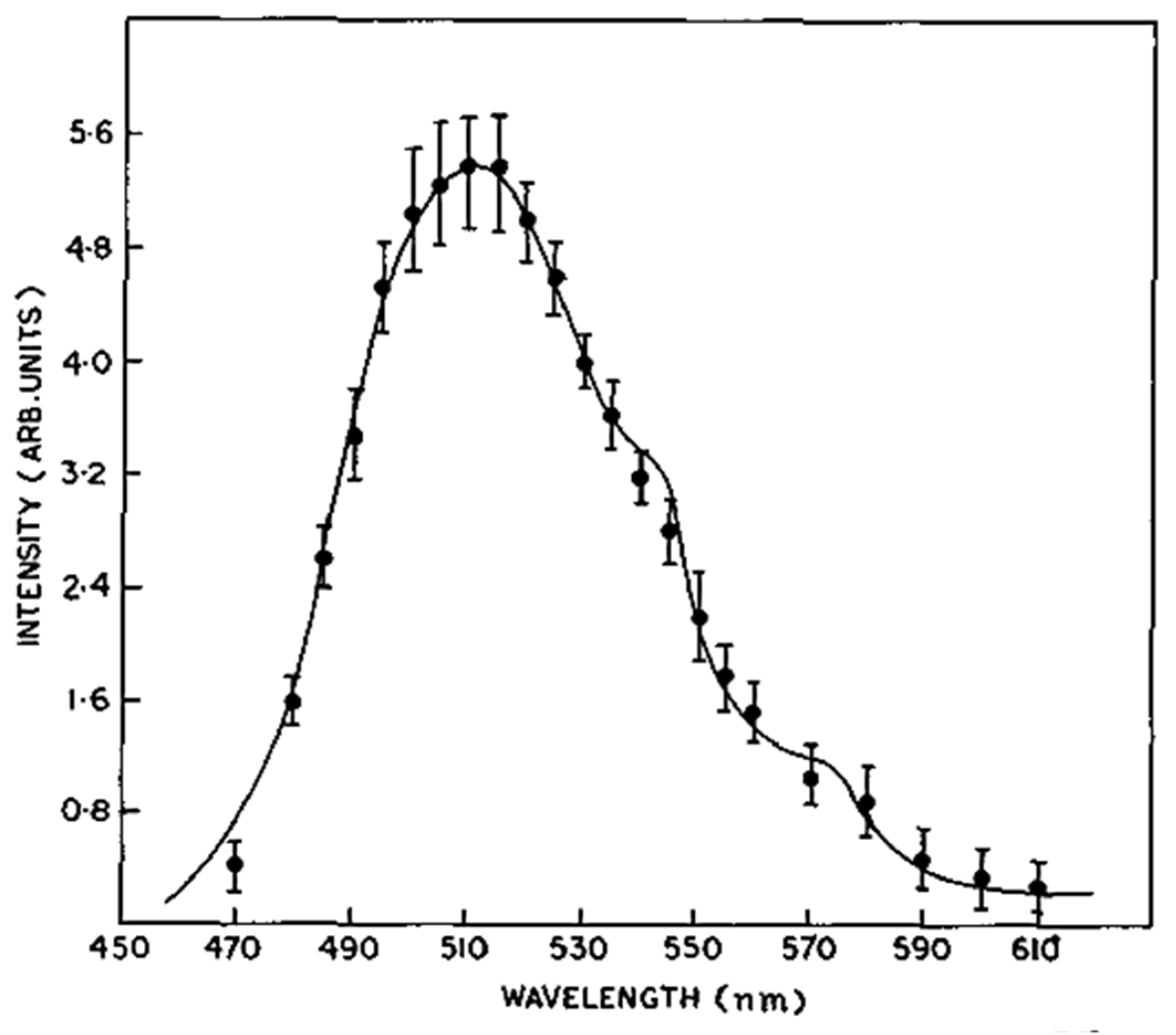
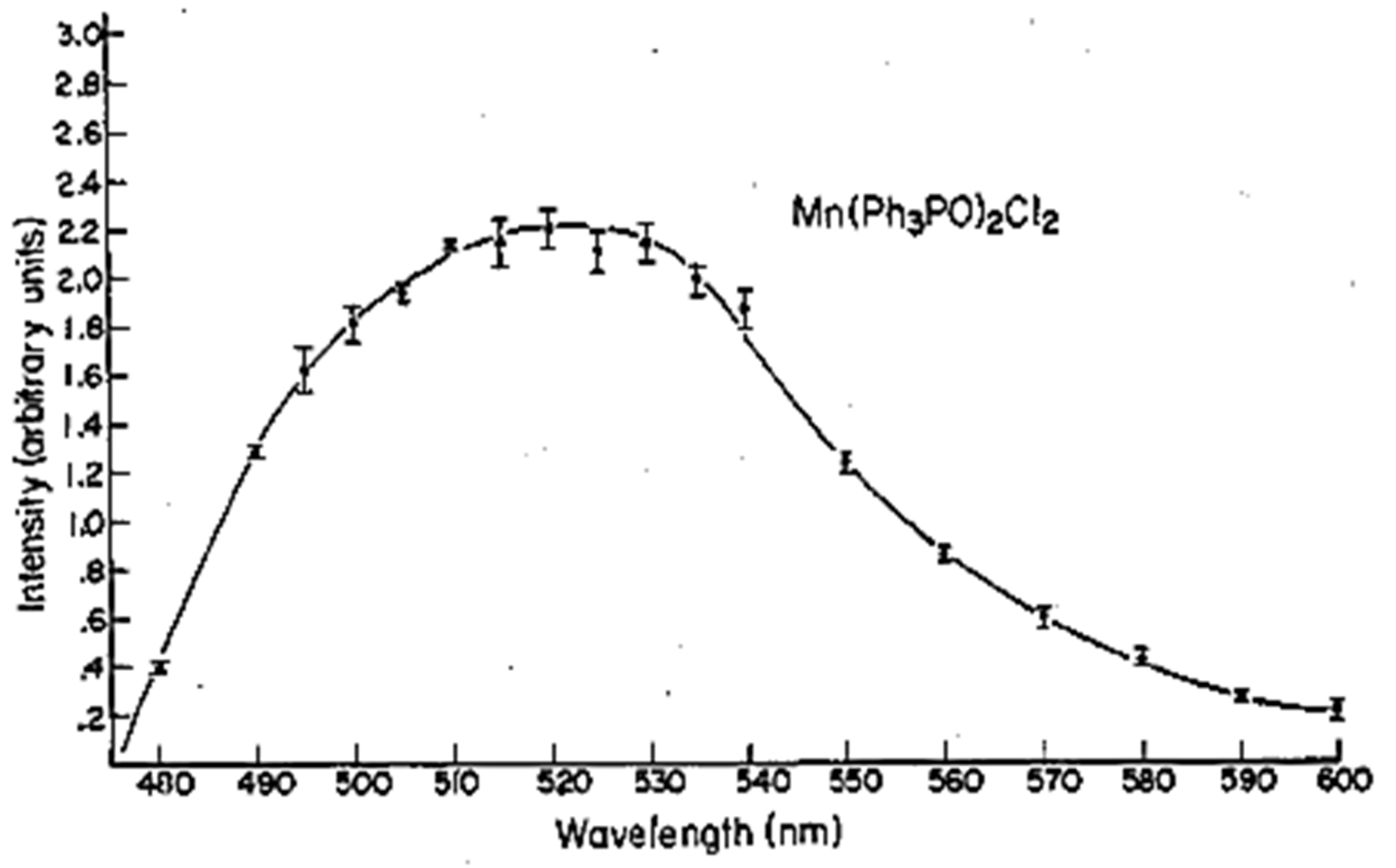
5.4. TL and PL of the Manganese Complex
6. Various Stimuli Affecting the Triboluminescence
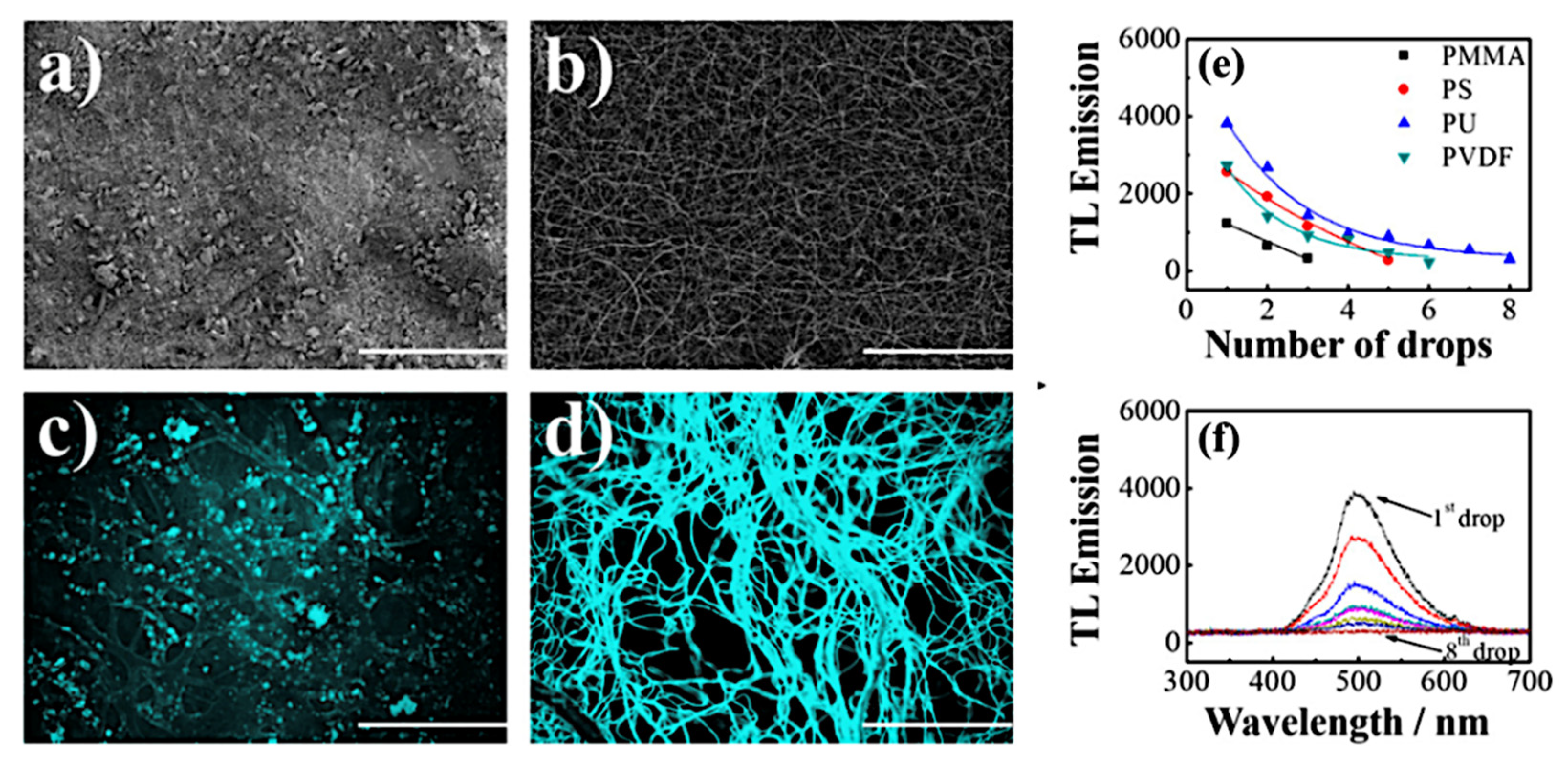
6.1. Polymer Mats Coated with a Triboluminescent Compound
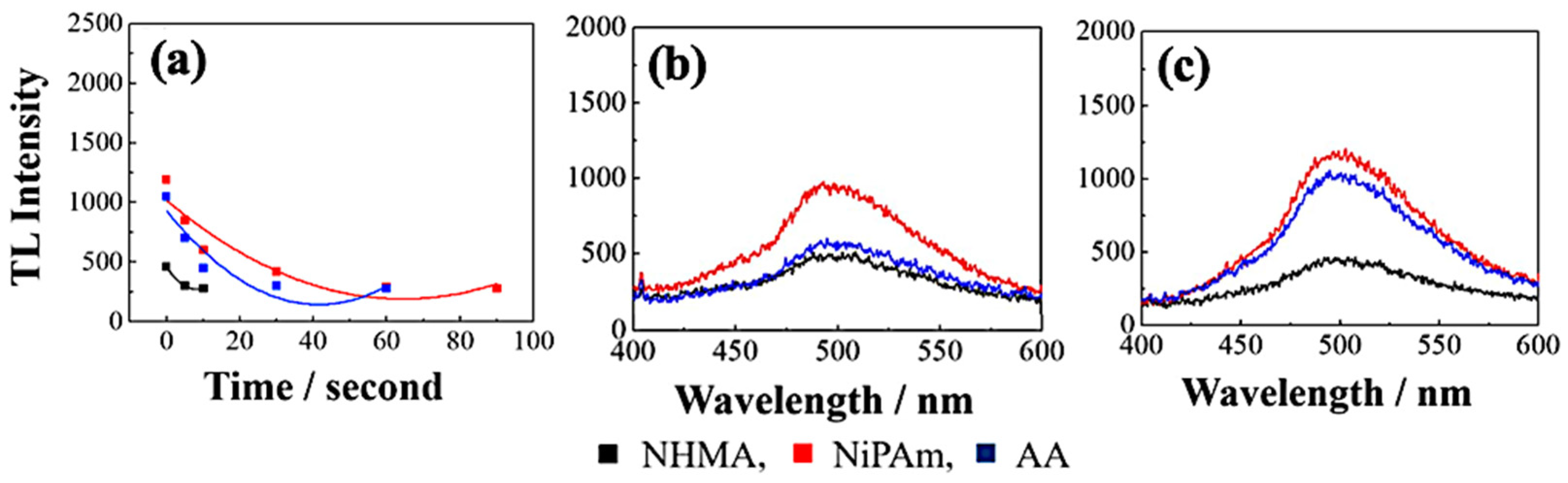
6.2. Dopant Implementation
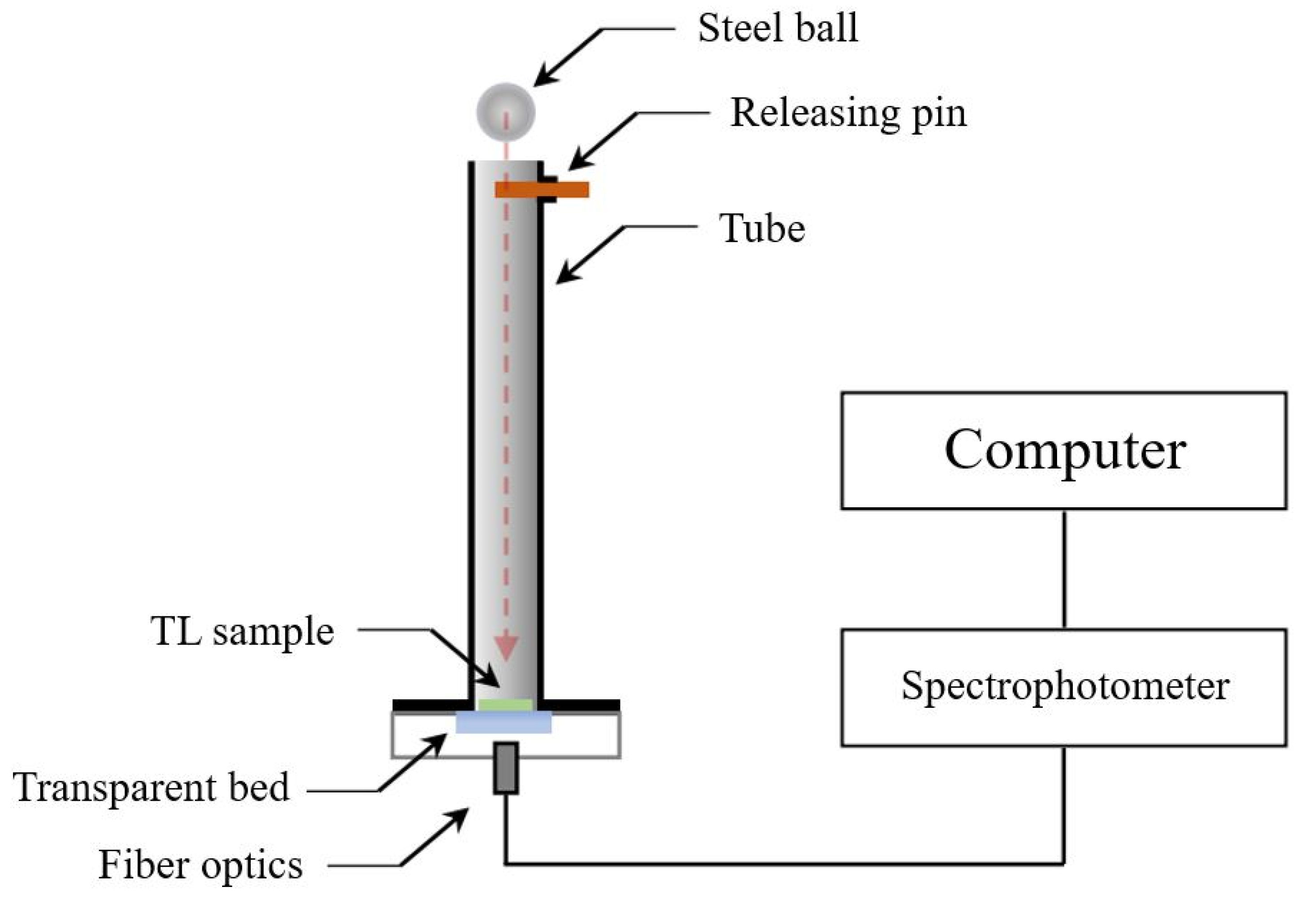
7. Experimental Setup for the Triboluminescence Measurements
8. Perspectives of TL Implementation
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, Y.; Li, Z. Triboluminescence: Recalling Interest and New Aspects. Chem 2018, 4, 943–971. [Google Scholar] [CrossRef] [Green Version]
- Chandra, B.P.; Chandra, V.K.; Jha, P. Models for intrinsic and extrinsic fracto-mechanoluminescence of solids. J. Lumin. 2013, 135, 139–153. [Google Scholar] [CrossRef]
- Zink, J.I.; Hardy, G.E.; Sutton, J.E. Triboluminescence of sugars. J. Phys. Chem. 1976, 80, 248–249. [Google Scholar] [CrossRef]
- Zink, J.I. Triboluminescence of uranyl nitrate. Inorg. Chem. 1975, 14, 555–558. [Google Scholar] [CrossRef]
- Sharma, R.; BiSen, D.P.; Chandra, B.P. Experimental and Theoretical Study of the Mechanoluminescence of ZnS:Mn Nanoparticles. J. Electron. Mater. 2015, 44, 3312–3321. [Google Scholar] [CrossRef]
- Marchetti, F.; Di Nicola, C.; Pettinari, R.; Timokhin, I.; Pettinari, C. Synthesis of a photoluminescent and triboluminescent copper(I) compound: An experiment for an advanced inorganic chemistry laboratory. J. Chem. Educ. 2012, 89, 652–655. [Google Scholar] [CrossRef]
- Hurt, C.R.; McAvoy, N.; Bjorklund, S.; Filipescu, N. High intensity triboluminescence in europium tetrakis (dibenzoylmethide)-triethylammonium. Nature 1966, 212, 179–180. [Google Scholar] [CrossRef]
- Sumana, B.; Rajakumar, A. Ru(II)-Metal complex immobilized mesoporous SBA-15 hybrid for visible light induced photooxidation of chlorophenolic compounds in aqueous medium. Photochem. Photobiol. Sci. 2017, 16, 1290–1300. [Google Scholar]
- Goodgame, D.L.; Cotton, F.A. Phosphine Oxide Complexes. Part V. Tetrahedral Complexes of Manganese containing Triphenylphosphine Oxide, and Triphenylarsine Oxide as Ligands. J. Chem. Soc. 1961, 3735–3741. [Google Scholar] [CrossRef]
- Pettinari, C.; Di Nicola, C.; Marchetti, F.; Pettinari, R.; Skelton, B.W.; Somers, N.; White, A.H.; Robinson, W.T.; Chierotti, M.R.; Gobetto, R.; et al. Synthesis, characterization, spectroscopic and photophysical properties of new [Cu(NCS){(L-N)2 or (L′-N∧N)}(PPh3)] complexes (L-N, L′-N∧N = Aromatic nitrogen base). Eur. J. Inorg. Chem. 2008, 1974–1984. [Google Scholar] [CrossRef]
- Olawale, D.O.; Kliewer, K.; Okoye, A.; Dickens, T.; Uddin, M.J.; Okoli, O.I. Getting light through cementitious composites with in situ triboluminescent damage sensor. Struct. Health Monit. 2014, 13, 177–189. [Google Scholar] [CrossRef]
- Terasaki, N.; Zhang, H.; Imai, Y.; Yamada, H.; Xu, C.N. Hybrid material consisting of mechanoluminescent material and TiO2 photocatalyst. Thin Solid Films 2009, 518, 473–476. [Google Scholar] [CrossRef]
- Terasaki, N.; Yamada, H.; Xu, C.N. Ultrasonic wave induced mechanoluminescence and its application for photocatalysis as ubiquitous light source. Catal. Today 2013, 201, 203–208. [Google Scholar] [CrossRef]
- Yuen, S.; Schreyer, M.; Finlay, W.H.; Löbenberg, R.; Moussa, W. Activation of a photosensitive pharmaceutical agent by a triboluminescent material. Appl. Phys. Lett. 2006, 88, 6–9. [Google Scholar] [CrossRef]
- Walton, A.J. Triboluminescence. Adv. Phys. 1977, 26, 887–948. [Google Scholar] [CrossRef]
- Pettinari, C.; Nicola, C.; Tabacaru, A.; Dinica, R. Synthesis of the [Cu(NCS)(py)2(PPh3)] coordination compound with photoluminescent properties. In Proceedings of the 1st International Symposium on Applied Physics—Materials Science, Environment and Health (ISAP-1), Galati, Romania, 1 November 2009. [Google Scholar]
- Bowmaker, G.A.; Hanna, J.V.; Hart, R.D.; Healy, P.C.; King, S.P.; Marchetti, F.; Pettinari, C.; Skelton, B.W.; Tabacaru, A.; White, A.H. Mechanochemical and solution synthesis, X-ray structure and IR and 31P solid state NMR spectroscopic studies of copper(i) thiocyanate adducts with bulky monodentate tertiary phosphine ligands. Dalt. Trans. 2012, 41, 7513. [Google Scholar] [CrossRef] [PubMed]
- Karimata, A.; Patil, P.H.; Fayzullin, R.R.; Khaskin, E.; Lapointe, S.; Khusnutdinova, J.R. Triboluminescence of a new family of CuI-NHC complexes in crystalline solid and in amorphous polymer films. Chem. Sci. 2020, 11, 10814–10820. [Google Scholar] [CrossRef] [PubMed]
- Fontenot, R.S.; Bhat, K.N.; Hollerman, W.A.; Aggarwal, M.D. Triboluminescent materials for smart sensors. Mater. Today 2011, 14, 292–293. [Google Scholar] [CrossRef]
- Fontenot, R.S.; Hollerman, W.A.; Bhat, K.N.; Aggarwal, M.D. Synthesis and characterization of highly triboluminescent doped europium tetrakis compounds. J. Lumin. 2012, 132, 1812–1818. [Google Scholar] [CrossRef]
- Fontenot, R.S.; Bhat, K.N.; Hollerman, W.A.; Aggarwal, M.D.; Nguyen, K.M. Comparison of the triboluminescent yield and decay time for europium dibenzoylmethide triethylammonium synthesized using different solvents. CrystEngComm 2012, 14, 1382–1386. [Google Scholar] [CrossRef]
- Fontenot, R.S.; Hollerman, W.A.; Bhat, K.N.; Aggarwal, M.D. Effects of added uranium on the triboluminescent properties of europium dibenzoylmethide triethylammonium. J. Lumin. 2013, 134, 477–482. [Google Scholar] [CrossRef]
- Fontenot, R.S.; Bhat, K.N.; Hollerman, W.A.; Aggarwal, M.D. Innovative triboluminescence study of multivitamin doped europium tetrakis. Cryst. Res. Technol. 2012, 47, 573–578. [Google Scholar] [CrossRef]
- Anis-Ul-Haque, K.M.; Clifford, E.W.; Anthony, I.D.; Lynne, W. Interaction of the Large Host Q [10] with Metal Polypyridyl Complexes: Binding Modes and Effects on Luminescence. Inorg. Chem. 2020, 59, 3942–3953. [Google Scholar] [CrossRef] [PubMed]
- Paul, G.B.; Nurit, K.; Boyce, A.H.; Richard, F.D.; William, H.W. Vibrational Spectroscopy of the Electronically Excited State. 5. Time-Resolved Resonance Raman Study of Tris(bipyridine)ruthenium(II) and Related Complexes. Definitive Evidence for the “Localized” MLCT State. J. Am. Chem. Soc. 1981, 103, 7441–7446. [Google Scholar]
- Joyeeta, L.; Apabrita, M.; Soumyajit, R. Light-driven carbon dioxide reduction coupled with conversion of acetylenic group to ketone by a functional Janus catalyst based on keplerate {Mo132}. J. Mater. Chem. A 2018, 6, 20844–20851. [Google Scholar]
- Kaneko, M.; Takabayashi, N.; Yamauchi, Y.; Yamada, A. Water Photolysis by Means of Visible Light with a System Composed of Prussian Blue and the Tris(2,2′-bipyridine)ruthenium(II) Complex. Bull. Chem. Soc. Jpn. 1984, 57, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Incel, A.; Varlikli, C.; McMillen, C.D.; Demir, M.M. Triboluminescent Electrospun Mats with Blue-Green Emission under Mechanical Force. J. Phys. Chem. C 2017, 121, 11709–11716. [Google Scholar] [CrossRef]
- Incel, A.; Emirdag-Eanes, M.; McMillen, C.D.; Demir, M.M. Integration of Triboluminescent EuD4TEA Crystals to Transparent Polymers: Impact Sensor Application. ACS Appl. Mater. Interfaces 2017, 9, 6488–6496. [Google Scholar] [CrossRef]
- Olawale, D.O.; Okoli, O.O.I.; Fontenot, R.S.; Hollerman, W.A. Triboluminescence: Theory, Synthesis, and Application; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 9783319388427. [Google Scholar]
- Feng, W.; Min, W.; Ke, F.; Wen-Jing, L.; Xu-Bing, L.; Bin, C.; Chen-Ho, T.; Li-Zhu, W. Amphiphilic polymeric micelles as microreactors: Improving the photocatalytic hydrogen production of the [FeFe]-hydrogenase mimic in water. Chem. Commun. 2016, 52, 457–460. [Google Scholar]
- Viswanatham, K.; Swapan, K.C.; Brian, T.C. Electrospray Ionization: A New Tool for the Analysis of Ionic Transition-Metal Complexes. J. Am. Chem. Soc. 1990, 112, 5348–5349. [Google Scholar]
- Kian, S.L.; Jacqueline, M.C.; Xiaolan, Z.; Nataliya, Y. Rationalizing the molecular origins of Ru- and Fe based dyes for dye-sensitized solar cells. Acta Cryst. 2012, B68, 137–149. [Google Scholar]
- Tang, Y.Y.; Wang, Z.X.; Li, P.F.; You, Y.M.; Stroppa, A.; Xiong, R.G. Brilliant triboluminescence in a potential organic–inorganic hybrid ferroelectric: (Ph3PO)2MnBr2. Inorg. Chem. Front. 2017, 4, 154–159. [Google Scholar] [CrossRef]
- Fontenot, R.S.; Hollerman, W.A.; Bhat, K.N.; Aggarwal, M.D. Comparison of the triboluminescent properties for europium tetrakis and ZnS:Mn powders. J. Theor. Appl. Phys. 2012, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Fontenot, R.S.; Hollerman, W.A.; Aggarwal, M.D.; Bhat, K.N.; Goedeke, S.M. A versatile low-cost laboratory apparatus for testing triboluminescent materials. Measurement 2012, 45, 431–436. [Google Scholar] [CrossRef]
- Sharipov, G.L.; Tukhbatullin, A.A. Triboluminescence of tris(2,2′-bipyridyl)ruthenium(II) dichloride hexahydrate. J. Lumin. 2019, 215, 116691. [Google Scholar] [CrossRef]
- Kalyanasundaram, K. Photophysics, photochemistry and solar energy conversion with tris(bipyridyl)ruthenium(II) and its analogues. Coord. Chem. Rev. 1982, 46, 159–244. [Google Scholar] [CrossRef]
- Wong, H.Y.; Chan, W.T.K.; Law, G.L. Triboluminescence of Centrosymmetric Lanthanide β-Diketonate Complexes with Aggregation-Induced Emission. Molecules 2019, 24, 662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, B.P.; Kaza, B.R. Mechanoluminescence, electroluminescence and high-pressure photoluminescence of Mn(Ph3PO)2Br2. J. Lumin. 1982, 27, 101–107. [Google Scholar] [CrossRef]
- Hardy, G.E.; Zink, J.I. Triboluminescence and Pressure Dependence of the Photoluminescence of Tetrahedral Manganese(II) Complexes. Inorg. Chem. 1976, 12, 3061–3065. [Google Scholar] [CrossRef]
- İncel, A.; Reddy, S.M.; Demir, M.M. A new method to extend the stress response of triboluminescent crystals by using hydrogels. Mater. Lett. 2017, 186, 210–213. [Google Scholar] [CrossRef]
- Zeng, X.R.; Xiong, R.G.; You, X.Z.; Cheung, K.K. Triboluminescent spectrum and crystal structure of a europate complex with the most intensely triboluminescent emission at ambient temperature. Inorg. Chem. Commun. 2000, 3, 341. [Google Scholar] [CrossRef]
- Bhat, K.N.; Hollerman, W.A.; Fontenot, R.S.; Aggarwal, M. Triboluminescent Research Review of Europium Dibenzoylmethide Triethylammonium (EuD4TEA) and Related Materials. Int. J. Chem. 2012, 1, 100–118. [Google Scholar]
- Walton, A.J.; Botos, P. The application of an image intensifier spectroscope in triboluminescent studies. J. Phys. E 1978, 11, 513–514. [Google Scholar] [CrossRef]
- Jeong, S.M.; Song, S.; Joo, K.I.; Kim, J.; Hwang, S.H.; Jeong, J.; Kim, H. Bright, wind-driven white mechanoluminescence from zinc sulphide microparticles embedded in a polydimethylsiloxane elastomer. Energy Environ. Sci. 2014, 7, 3338–3346. [Google Scholar] [CrossRef] [Green Version]
- Terasaki, N.; Zhang, H.; Yamada, H.; Xu, C.N. Mechanoluminescent light source for a fluorescent probe molecule. Chem. Commun. 2011, 47, 8034–8036. [Google Scholar] [CrossRef] [PubMed]











| Bond Length (Å) | Bond Angle (°) | ||
|---|---|---|---|
| Cu-X | 2.013(2) | X-Cu-N(ar) | 99.58(8) |
| Cu-N(ar) | 2.091(2) | X-Cu-P | 106.70(6) |
| 2.070(1) | P-Cu-N(ar) | 115.34(6) | |
| Cu-P | 2.1974(5) | N(ar)-Cu-N(ar) | 100.37(7) |
| S-C | 1.638(2) | Cu-N-C | 157.4(2) |
| C-N | 1.168(3) | N-C-S | 179.5(2) |
| Bond Length (Å) | Bond Angle (°) | ||
|---|---|---|---|
| Eu1-O1 | 2.365(16) | Eu1-O8 | 2.366(16) |
| Eu1-O3 | 2.369(15) | Eu1-O6 | 2.386(16) |
| Eu1-O2 | 2.385(17) | Eu1-O7 | 2.401(16) |
| Eu1-O4 | 2.402(13) | Eu1-O5 | 2.421(17) |
| O8-Eu1-O6 | 109.7(6) | O3-Eu1-O6 | 70.3(6) |
| O3-Eu1-O7 | 121.5(5) | O8-Eu1-O7 | 72.3(6) |
| O2-Eu1-O4 | 70.2(6) | O1-Eu1-O4 | 75.1(5) |
| O2-Eu1-O5 | 71.2(6) | O1-Eu1-O5 | 71.2(6) |
| Crystal Data | Data Collection | ||
|---|---|---|---|
| Chemical formula | C30H24N6RuCl2∙6H2O | Tmin, Tmax | 0.676, 1.000 |
| Mr | 748.62 | No. of measured, independent and observed [I > 2σ(I)] reflections | 11 174, 1220, 994 |
| a, c (Å) | 13.1383 (12), 20.995 (3) | Rint | 0.084 |
| V (Å3) | 3138.6 (6) | θ values (o) | θmax = 27.5, θmin = 3.7 |
| F(000) | 1536 | (sin θ/λ)max (Å−1) | 0.650 |
| Dx (Mg m−3) | 1.584 | Refinement | |
| µ (mm−1) | 0.72 | R[F2 > 2σ(F2)], wR(F2), S | 0.061, 0.153, 1.09 |
| Crystal size (mm) | 0.21 × 0.16 × 0.12 | No. of reflections | 1220 |
| ∆pmax, ∆pmin (e Å−3) | 0.98, −0.56 | ||
| Bond Length (Å) | Bond Angle (°) | ||
|---|---|---|---|
| O–Mn–O | 101.7(2) | ||
| Mn–O | 2.036(5) | O–Mn–Br | 109.8(2) |
| 2.027(5) | O–Mn–Br | 114.2(2) | |
| Mn–Br | 2.467(1) | O–Mn–Br | 103.7(2) |
| 2.475(1) | O–Mn–Br | 111.6(2) | |
| Br–Mn–Br | 114.95(5) | ||
| [Cu(NCS)(py)2(PPh3)] | FWHMPL | FWHMTL | Eopt (eV) | Φf (%) | |||
|---|---|---|---|---|---|---|---|
| solid | bare | 490 | 113 | 500 | 90 | 2.92 | 98.0 |
| surface-impregnated | PMMA | 490 | 113 | 497 | 87 | 3.06 | 30.8 |
| PS | 490 | 107 | 496 | 85 | 3.05 | 34.7 | |
| PU | 497 | 116 | 498 | 86 | 3.05 | 87.7 | |
| PVDF | 490 | 112 | 496 | 85 | 3.00 | 52.9 | |
| blended | PMMA | 512 | 130 | NA | NA | 3.44 | 0.28 |
| PS | 519 | 127 | NA | NA | 3.53 | 0.50 | |
| PU | 525 | 140 | NA | NA | 3.57 | 1.75 | |
| PVDF | 519 | 143 | NA | NA | 3.55 | 0.86 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szukalski, A.; Kabanski, A.; Goszyk, J.; Adaszynski, M.; Kaczmarska, M.; Gaida, R.; Wyskiel, M.; Mysliwiec, J. Triboluminescence Phenomenon Based on the Metal Complex Compounds—A Short Review. Materials 2021, 14, 7142. https://doi.org/10.3390/ma14237142
Szukalski A, Kabanski A, Goszyk J, Adaszynski M, Kaczmarska M, Gaida R, Wyskiel M, Mysliwiec J. Triboluminescence Phenomenon Based on the Metal Complex Compounds—A Short Review. Materials. 2021; 14(23):7142. https://doi.org/10.3390/ma14237142
Chicago/Turabian StyleSzukalski, Adam, Adam Kabanski, Julia Goszyk, Marek Adaszynski, Milena Kaczmarska, Radoslaw Gaida, Michal Wyskiel, and Jaroslaw Mysliwiec. 2021. "Triboluminescence Phenomenon Based on the Metal Complex Compounds—A Short Review" Materials 14, no. 23: 7142. https://doi.org/10.3390/ma14237142
APA StyleSzukalski, A., Kabanski, A., Goszyk, J., Adaszynski, M., Kaczmarska, M., Gaida, R., Wyskiel, M., & Mysliwiec, J. (2021). Triboluminescence Phenomenon Based on the Metal Complex Compounds—A Short Review. Materials, 14(23), 7142. https://doi.org/10.3390/ma14237142









