Bias-Enhanced Formation of Metastable and Multiphase Boron Nitride Coating in Microwave Plasma Chemical Vapor Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. MPCVD Process
2.2. Characterization Techniques
3. Results
3.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.2. X-ray Photoelectron Spectroscopy
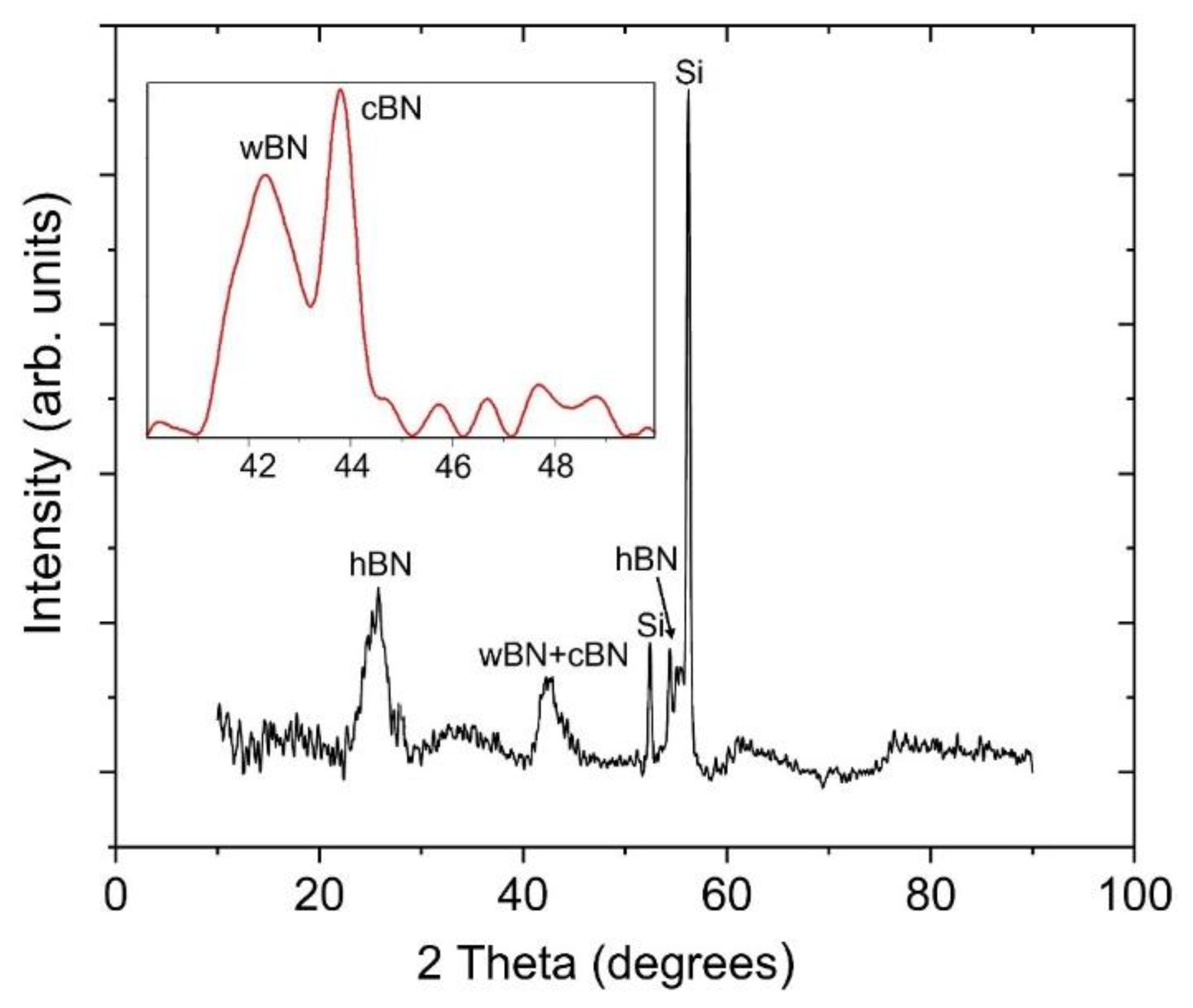
3.3. X-ray Diffraction
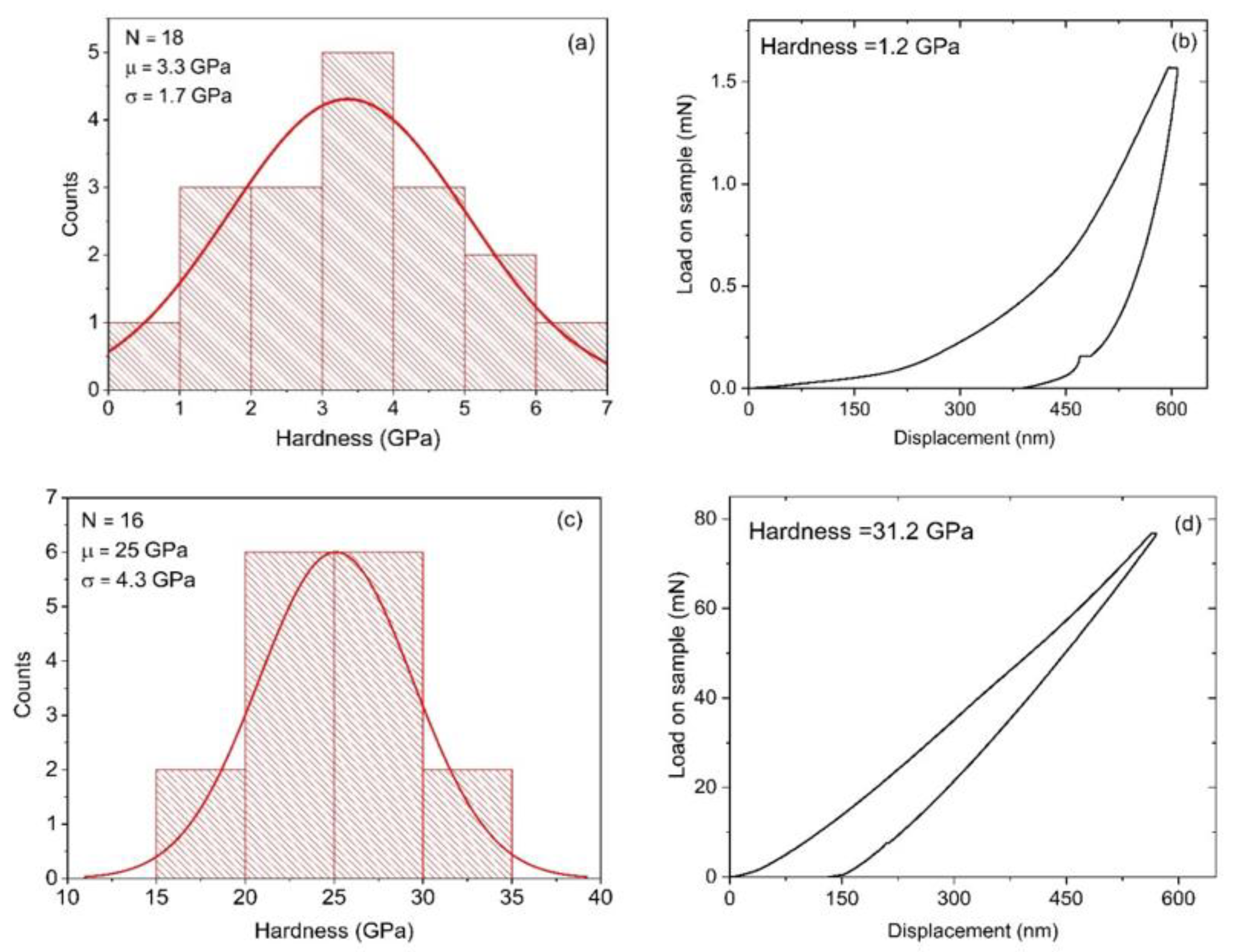
3.4. Nano-Indentation
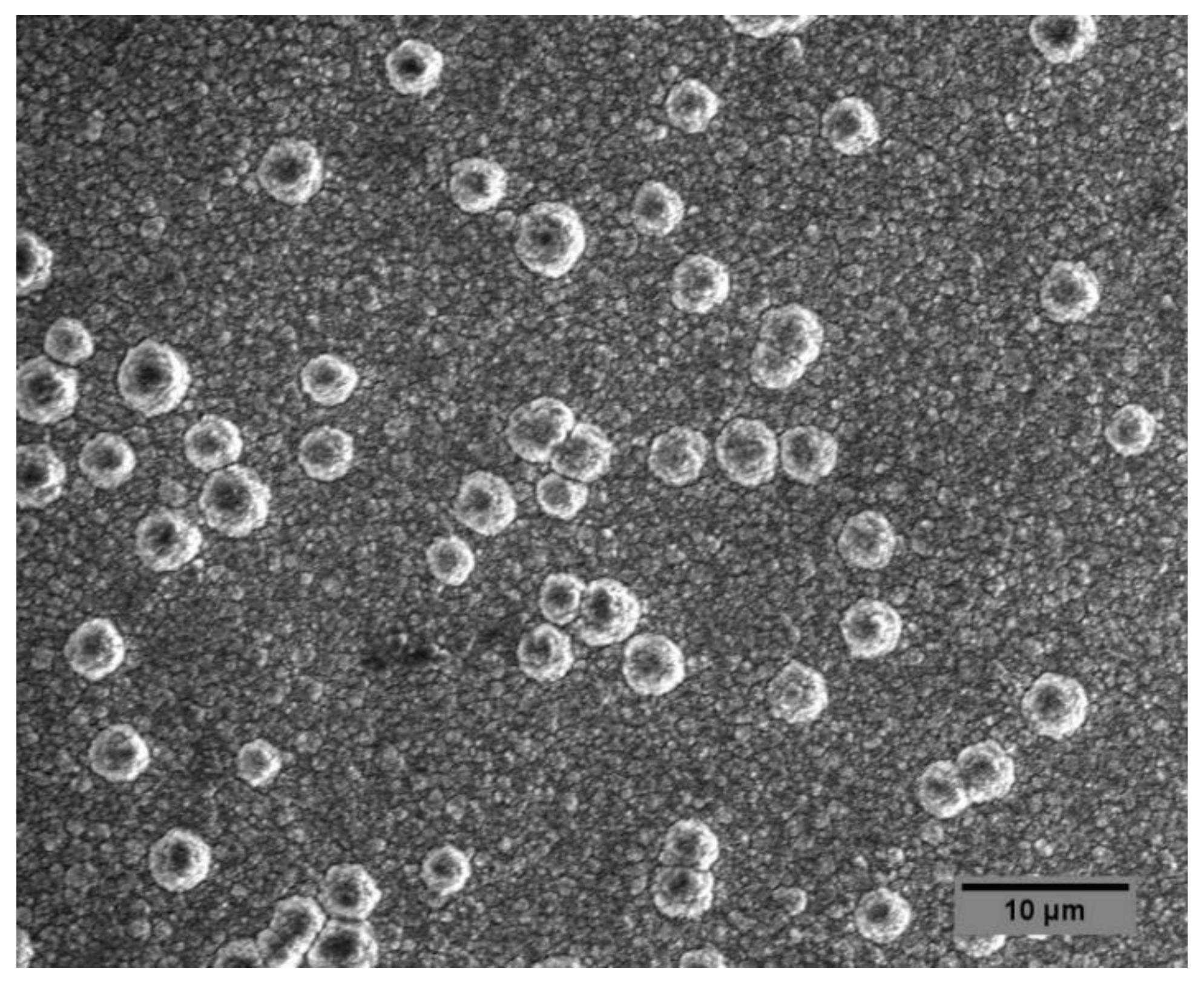
3.5. Scanning Electron Microscopy (SEM)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, X.; Yu, Z.; Chou, K.-C. Facile synthesis of hexagonal boron nitride fibers with uniform morphology. Ceram. Int. 2013, 39, 6427–6431. [Google Scholar] [CrossRef]
- Lipp, A.; Schwetz, K.A.; Hunold, K. Hexagonal boron nitride: Fabrication, properties and applications. J. Eur. Ceram. Soc. 1989, 5, 3–9. [Google Scholar] [CrossRef]
- Samantaray, C.B.; Singh, R.N. Review of synthesis and properties of cubic boron nitride (c-BN) thin films. Int. Mater. Rev. 2013, 50, 313–344. [Google Scholar] [CrossRef]
- Pan, Z.; Sun, H.; Zhang, Y.; Chen, C. Harder than diamond: Superior indentation strength of wurtzite BN and lonsdaleite. Phys. Rev. Lett 2009, 102, 055503. [Google Scholar] [CrossRef] [PubMed]
- Mirkarimi, P.B.; Medlin, D.L.; McCarty, K.F.; Dibble, D.C.; Clift, W.M.; Knapp, J.A.; Barbour, J.C. The synthesis, characterization, and mechanical properties of thick, ultrahard cubic boron nitride films deposited by ion-assisted sputtering. J. Appl. Phys. 1997, 82, 1617–1625. [Google Scholar] [CrossRef]
- Zhang, W.J.; Bello, I.; Lifshitz, Y.; Chan, K.M.; Wu, Y.; Chan, C.Y.; Meng, X.M.; Lee, S.T. Thick and adherent cubic boron nitride films grown on diamond interlayers by fluorine-assisted chemical vapor deposition. Appl. Phys. Lett. 2004, 85, 1344–1346. [Google Scholar] [CrossRef]
- Komatsu, T.; Kakudate, Y.; Fujiwara, S. Heat resistance of a shock-synthesized B–C–N heterodiamond. J. Chem. Soc. Faraday Trans. 1996, 92, 5067–5071. [Google Scholar] [CrossRef]
- Bello, I.; Chan, C.Y.; Zhang, W.J.; Chong, Y.M.; Leung, K.M.; Lee, S.T.; Lifshitz, Y. Deposition of thick cubic boron nitride films: The route to practical applications. Diam. Relat. Mater. 2005, 14, 1154–1162. [Google Scholar] [CrossRef]
- Tasuku Shimizu, T.N.; Shunichi, S. Thin film synthesis of wurtzite boron nitride by femtosecond pulsed laser deposition. In Proceedings of the ROMOPTO 2006: Eighth Conference on Optics, 2006, Sibiu, Romania, 1 August 2007. [Google Scholar]
- Miyata, N.; Moriki, K.; Mishima, O.; Fujisawa, M.; Hattori, T. Optical constants of cubic boron nitride. Phys. Rev. B Condens. Matter 1989, 40, 12028–12029. [Google Scholar] [CrossRef] [PubMed]
- Nose, K.; Oba, H.; Yoshida, T. Electric conductivity of boron nitride thin films enhanced by in situ doping of zinc. Appl. Phys. Lett. 2006, 89, 112124. [Google Scholar] [CrossRef]
- Yin, H.; Pongrac, I.; Ziemann, P. Electronic transport in heavily Si doped cubic boron nitride films epitaxially grown on diamond(001). J. Appl. Phys. 2008, 104, 023703. [Google Scholar] [CrossRef]
- Kojima, K.; Nose, K.; Kambara, M.; Yoshida, T. Effects of magnesium doping on growth and electric conductivity of nanocrystalline cubic boron nitride thin films. J. Phys. D Appl. Phys. 2009, 42, 055304. [Google Scholar] [CrossRef]
- Wenjun Zhang, Y.M.C.; Bin He, I.; Bello, S.-T.L. Cubic Boron Nitride Films: Properties and Applications. Compr. Hard Mater. 2014, 3, 33. [Google Scholar] [CrossRef]
- Yang, J.H.C.; Teii, K.; Chang, C.C.; Matsumoto, S.; Rafailovich, M. Biocompatible Cubic Boron Nitride: A Noncytotoxic Ultrahard Material. Adv. Funct. Mater. 2020, 31, 2005066. [Google Scholar] [CrossRef]
- Sajjad, M.; Morell, G.; Feng, P. Advance in novel boron nitride nanosheets to nanoelectronic device applications. ACS Appl. Mater. Interfaces 2013, 5, 5051–5056. [Google Scholar] [CrossRef]
- Konyashin, I.; Bill, J.; Aldinger, F. Plasma-assisted CVD of cubic boron nitride. Chem. Vap. Depos. 1997, 3, 239–255. [Google Scholar] [CrossRef]
- Saitoh, H.; Yarbrough, W.A. Preparation and characterization of nanocrystalline cubic boron nitride by microwave plasma-enhanced chemical vapor deposition. Appl. Phys. Lett. 1991, 58, 2228–2230. [Google Scholar] [CrossRef]
- Saitoh, H.; Yarbrough, W.A. Growth of cubic boron nitride on diamond particles by microwave plasma enhanced chemical vapor deposition. Appl. Phys. Lett. 1991, 58, 2482–2484. [Google Scholar] [CrossRef]
- Chong, Y.M.; Ma, K.L.; Leung, K.M.; Chan, C.Y.; Ye, Q.; Bello, I.; Zhang, W.; Lee, S.T. Synthesis and Mechanical Properties of Cubic Boron Nitride /Nanodiamond Composite Films. Chem. Vap. Depos. 2006, 12, 33–38. [Google Scholar] [CrossRef]
- Monteiro, S.N.; Skury, A.L.D.; de Azevedo, M.G.; Bobrovnitchii, G.S. Cubic boron nitride competing with diamond as a superhard engineering material—An overview. J. Mater. Res. Technol. 2013, 2, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Kurakevych, O.O.; Solozhenko, V.L. High-Pressure Design of Advanced BN-Based Materials. Molecules 2016, 21, 1399. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Yin, D.; Kato, T.; Taniguchi, T.; Watanabe, K.; Ma, X.; Ye, H.; Ikuhara, Y. Stabilizing the metastable superhard material wurtzite boron nitride by three-dimensional networks of planar defects. Proc. Natl. Acad. Sci. USA 2019, 116, 11181–11186. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Kalita, G.; Mahyavanshi, R.D.; Adhikari, S.; Uchida, H.; Tanemura, M.; Umeno, M.; Kawahara, T. Low temperature wafer-scale synthesis of hexagonal boron nitride by microwave assisted surface wave plasma chemical vapour deposition. AIP Adv. 2019, 9, 035043. [Google Scholar] [CrossRef] [Green Version]
- Nocua, J.E.; Morell, G. Straightforward Deposition of Uniform Boron Nitride Coatings by Chemical Vapor Deposition. MRS Adv. 2018, 3, 191–197. [Google Scholar] [CrossRef]
- Hidalgo, A.; Makarov, V.; Morell, G.; Weiner, B.R. High-Yield Synthesis of Cubic and Hexagonal Boron Nitride Nanoparticles by Laser Chemical Vapor Decomposition of Borazine. Dataset Pap. Nanotechnol. 2013, 2013, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Deura, M.; Kutsukake, K.; Ohno, Y.; Yonenaga, I.; Taniguchi, T. Nanoindentation measurements of a highly oriented wurtzite-type boron nitride bulk crystal. Jpn. J. Appl. Phys. 2017, 56, 030301. [Google Scholar] [CrossRef]
- Chakrabarty, K.; Arnold, I.; Catledge, S.A. Hexagonal boron nitride grown using high atomic boron emission during microwave plasma chemical vapor deposition. J. Vac. Sci. Technol. A 2019, 37, 061507. [Google Scholar] [CrossRef] [Green Version]
- Onodera, A.; Miyazaki, H.; Fujimoto, N. Phase transformation of wurtzite-type boron nitride at high pressures and temperatures. J. Chem. Phys. 1981, 74, 5814–5816. [Google Scholar] [CrossRef]
- Singh, B.P. Characterization of wurtzitic boron nitride compacts. J. Mater. Sci. 1987, 22, 495–498. [Google Scholar] [CrossRef]
- Onodera, A.; Inoue, K.; Yoshihara, H.; Nakae, H.; Matsuda, T.; Hirai, T. Synthesis of cubic boron nitride from rhombohedral form under high static pressure. J. Mater. Sci. 1990, 25, 4279–4284. [Google Scholar] [CrossRef]
- Shtansky, D.V.; Yamada-Takamura, Y.; Yoshida, T.; Ikuhara, Y. Mechanism of nucleation and growth of cubic boron nitride thin films. Sci. Technol. Adv. Mater. 2001, 1, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Ramkorun, B.; Chakrabarty, K.; Catledge, S.A. Effects of direct current bias on nucleation density of superhard boron-rich boron carbide films made by microwave plasma chemical vapor deposition. Mater. Res. Express 2021, 8, 046401. [Google Scholar] [CrossRef]
- Chakrabarty, K.; Chen, W.C.; Baker, P.A.; Vijayan, V.M.; Chen, C.C.; Catledge, S.A. Superhard Boron-Rich Boron Carbide with Controlled Degree of Crystallinity. Materials 2020, 13, 3622. [Google Scholar] [CrossRef]
- Ulrich, S.; Scherer, J.; Schwan, J.; Barzen, I.; Jung, K.; Scheib, M.; Ehrhardt, H. Preparation of cubic boron nitride films by radio frequency magnetron sputtering and radio frequency ion plating. Appl. Phys. Lett. 1996, 68, 909–911. [Google Scholar] [CrossRef]
- Matsuoka, M.; Langhi Jr., M.P.; Isotani, S.; Chubaci, J.F.D. Fourier transform infrared spectroscopy analysis of thin boron nitride films prepared by ion beam assisted deposition. Phys. Status Solidi C 2014, 11, 509–512. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Zhi, J.F.; Wang, P.F.; Chong, Y.M.; Zou, Y.S.; Zhang, W.J.; Lee, S.T. Surface functionalization of cubic boron nitride films for biological sensing applications. Appl. Phys. Lett. 2008, 92, 163105. [Google Scholar] [CrossRef]
- Hu, C.; Kotake, S.; Suzuki, Y.; Senoo, M. Boron nitride thin films synthesized by reactive sputtering. Vacuum 2000, 59, 748–754. [Google Scholar] [CrossRef]
- Kuhr, M.; Reinke, S.; Kulisch, W. Nucleation of cubic boron nitride (c-BN) with ion-induced plasma-enhanced CVD. Diam. Relat. Mater. 1995, 4, 375–380. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, W.; Xu, B.; Li, Y.-A.; Li, H.-D.; Gu, G.-R.; Yin, H. Thick c-BN films deposited by radio frequency magnetron sputtering in argon/nitrogen gas mixture with additional hydrogen gas. Chin. Phys. B 2016, 25, 106801. [Google Scholar] [CrossRef]
- Lili Yu, B.G.; Zhil, C.; Chuantao, S.; Deliang, C.; Chengjian, W.; Qilong, W.; Minhua, J. In situ FTIR investigation on phase transformations in BN nanoparticles. Chin. Sci. Bull. 2005, 50, 2827–2831. [Google Scholar] [CrossRef]
- Lian, G.; Zhang, X.; Tan, M.; Zhang, S.; Cui, D.; Wang, Q. Low-temperature solid state synthesis and in situ phase transformation to prepare nearly pure cBN. Dalton Trans. 2011, 40, 6961–6967. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Lee, D.Y.; Kim, K.J.; Moon, D.W. Observation of a hexagonal BN surface layer on the cubic BN film grown by dual ion beam sputter deposition. Appl. Phys. Lett. 1997, 70, 315–317. [Google Scholar] [CrossRef]
- Jiang, L.; Fitzgerald, A.G.; Rose, M.J.; Lousa, A.; Gimeno, S. Formation of cubic boron nitride films by r.f. magnetron sputtering. Surf. Interface Anal. 2002, 34, 732–734. [Google Scholar] [CrossRef]
- Angleraud, B.; Cahoreau, M.; Jauberteau, I.; Aubreton, J.; Catherinot, A. Nitrogen ion beam-assisted pulsed laser deposition of boron nitride films. J. Appl. Phys. 1998, 83, 3398–3403. [Google Scholar] [CrossRef]
- Moulder, J.F. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics Division; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992; p. 261. [Google Scholar]
- Ji, C.; Levitas, V.I.; Zhu, H.; Chaudhuri, J.; Marathe, A.; Ma, Y. Shear-induced phase transition of nanocrystalline hexagonal boron nitride to wurtzitic structure at room temperature and lower pressure. Proc. Natl. Acad. Sci. USA 2012, 109, 19108–19112. [Google Scholar] [CrossRef] [Green Version]
- Yuan, S.; Journet, C.; Linas, S.; Garnier, V.; Steyer, P.; Benayoun, S.; Brioude, A.; Toury, B. How to Increase the h-BN Crystallinity of Microfilms and Self-Standing Nanosheets: A Review of the Different Strategies Using the PDCs Route. Crystals 2016, 6, 55. [Google Scholar] [CrossRef] [Green Version]
- Luo, K.; Zhang, Y.; Yu, D.; Li, B.; Hu, W.; Liu, Y.; Gao, Y.; Wen, B.; Nie, A.; Zhao, Z.; et al. Small onion-like BN leads to ultrafine-twinned cubic BN. Sci. China Mater. 2019, 62, 1169–1176. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhan, G.D.; Wang, Q.; He, D.; Zhang, J.; Liang, A.; Moellendick, T.E.; Zhao, L.; Li, X. Hardness of Polycrystalline Wurtzite Boron Nitride (wBN) Compacts. Sci. Rep. 2019, 9, 10215. [Google Scholar] [CrossRef] [Green Version]
- Sōma, T.; Sawaoka, A.; Saito, S. Characterization of wurtzite type boron nitride synthesized by shock compression. Mater. Res. Bull. 1974, 9, 755–762. [Google Scholar] [CrossRef]
- Matović, B.; Luković, J.; Nikolić, M.; Babić, B.; Stanković, N.; Jokić, B.; Jelenković, B. Synthesis and characterization of nanocrystaline hexagonal boron nitride powders: XRD and luminescence properties. Ceram. Int. 2016, 42, 16655–16658. [Google Scholar] [CrossRef]
- Rehman, M.M.; Siddiqui, G.U.; Kim, S.; Choi, K.H. Resistive switching effect in the planar structure of all-printed, flexible and rewritable memory device based on advanced 2D nanocomposite of graphene quantum dots and white graphene flakes. J. Phys. D Appl. Phys. 2017, 50, 335104. [Google Scholar] [CrossRef]
- Klett, A.; Freudenstein, R.; Plass, M.F.; Kulisch, W. Stress of c-BN thin films: A parameter investigation. Surf. Coat. Technol. 1999, 116-119, 86–92. [Google Scholar] [CrossRef]
- Zeitler, M.; Sienz, S.; Rauschenbach, B. Study of stress evolution of boron nitride films prepared by ion assisted deposition. J. Vac. Sci. Technol. A Vac. Surf. Film. 1999, 17, 597–602. [Google Scholar] [CrossRef]
- Kioseoglou, J.; Dimitrakopulos, G.P.; Komninou, P.; Polatoglou, H.M.; Serra, A.; Béré, A.; Nouet, G.; Karakostas, T. Junction lines of inversion domain boundaries with stacking faults in GaN. Phys. Rev. B 2004, 70, 115331. [Google Scholar] [CrossRef]
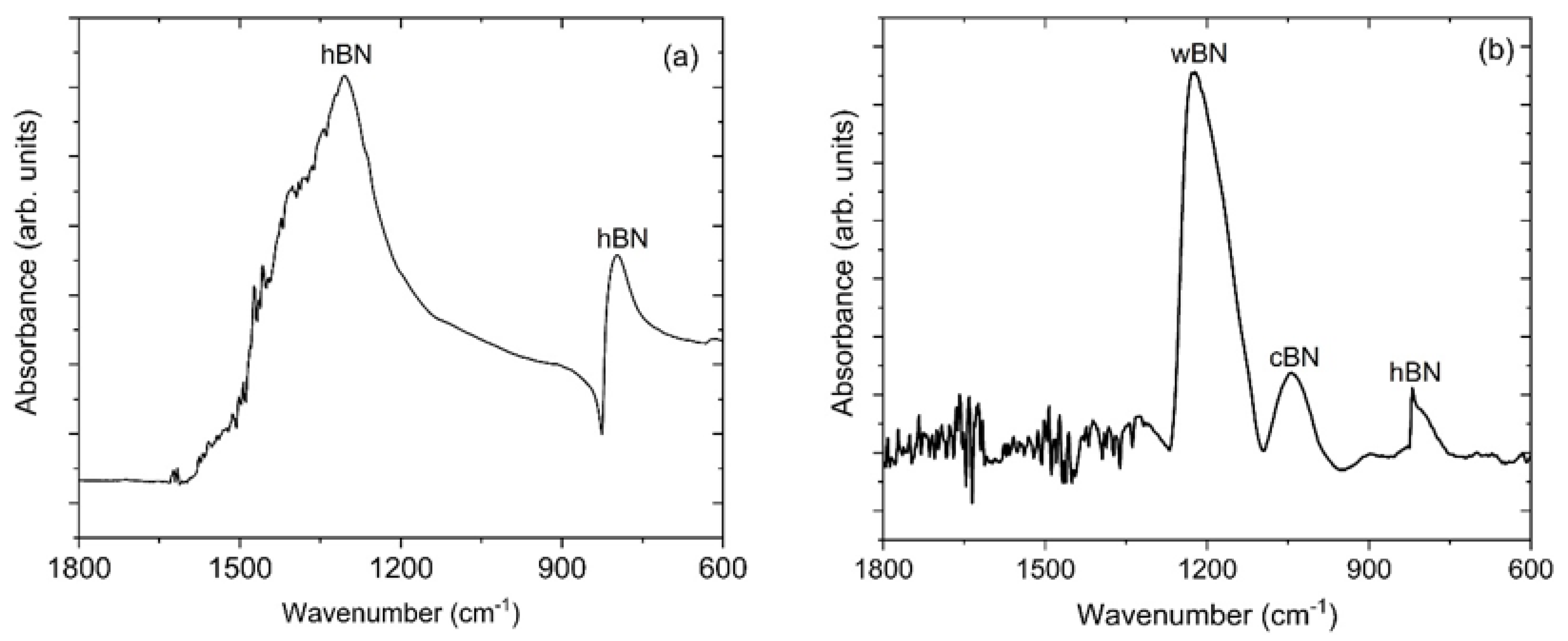

| Transmittance Frequency (cm−1) | Peak Assignment | References |
|---|---|---|
| 786 | Out-of-plane (B-N-B) bending modes of h-BN | [35,36] |
| 1045 | Restrahlen band of c-BN | [37,38] |
| 1224 | Transverse Optical (TO) mode of w-BN | [41] |
| 1307 | In-plane (B-N) stretching modes of h-BN | [35,36] |
| Sample | Peaks | Binding Energy | Peak Area (%) | Assignment | References |
|---|---|---|---|---|---|
| Without Bias BN | B1s | 189.8 | 100 | sp2 BN | [44,45] |
| N1s | 397.2 | 100 | sp2 BN | [44,45] | |
| Bias-Enhanced BN | B1s | 188.0 | 8 | B–B | [34,46] |
| B1s | 190.5 | 62 | sp2 BN | [42,45] | |
| B1s | 191.8 | 30 | sp3 BN | [42,45] | |
| N1s | 398.2 | 65 | sp2 BN | [42,45] | |
| N1s | 399.5 | 35 | sp3 BN | [42,45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakrabarty, K.; Baker, P.A.; Vijayan, V.M.; Catledge, S.A. Bias-Enhanced Formation of Metastable and Multiphase Boron Nitride Coating in Microwave Plasma Chemical Vapor Deposition. Materials 2021, 14, 7167. https://doi.org/10.3390/ma14237167
Chakrabarty K, Baker PA, Vijayan VM, Catledge SA. Bias-Enhanced Formation of Metastable and Multiphase Boron Nitride Coating in Microwave Plasma Chemical Vapor Deposition. Materials. 2021; 14(23):7167. https://doi.org/10.3390/ma14237167
Chicago/Turabian StyleChakrabarty, Kallol, Paul A. Baker, Vineeth M. Vijayan, and Shane A. Catledge. 2021. "Bias-Enhanced Formation of Metastable and Multiphase Boron Nitride Coating in Microwave Plasma Chemical Vapor Deposition" Materials 14, no. 23: 7167. https://doi.org/10.3390/ma14237167
APA StyleChakrabarty, K., Baker, P. A., Vijayan, V. M., & Catledge, S. A. (2021). Bias-Enhanced Formation of Metastable and Multiphase Boron Nitride Coating in Microwave Plasma Chemical Vapor Deposition. Materials, 14(23), 7167. https://doi.org/10.3390/ma14237167







