Stability of Dental Implants and Thickness of Cortical Bone: Clinical Research and Future Perspectives. A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Focus Question
2.3. Eligibility Criteria
2.4. Study Selection and Data Extraction
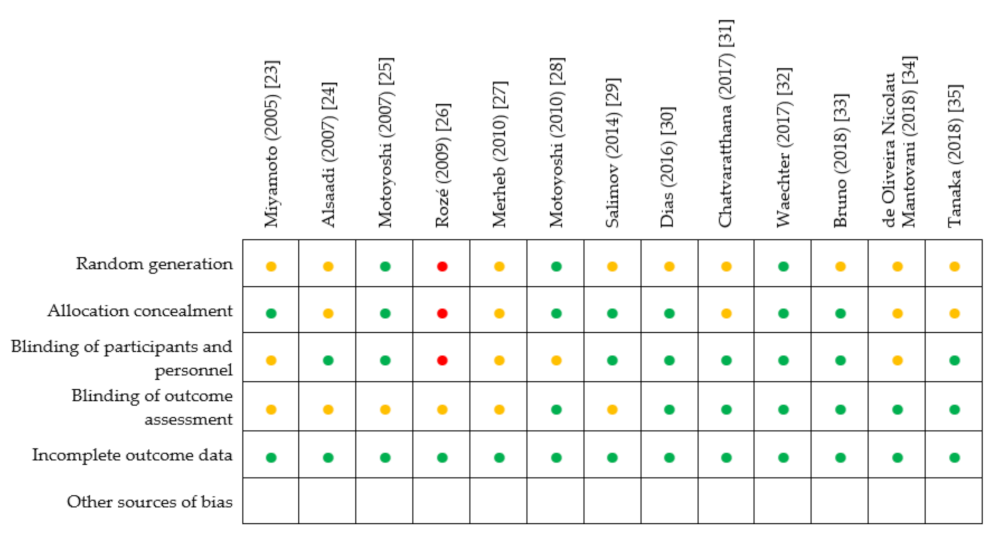
2.5. Risk of Bias Assessment
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias Assessment
4. Measurements for Stability of Dental Implants
4.1. Insertion Torque (IT)
4.2. Implant Stability Quotient (ISQ)
4.3. Periotest™
4.4. Dynamic Parameters of Primary Stability
5. Density and Quality of Bone
Intraoperative Measurement of Bone Density
6. Thickness of Cortical Bone and Implant Stability
6.1. Thickness of Cortical Bone in the Upper and Lower Jaw
6.2. Cortical Bone and Primary Stability of Implants
6.2.1. Regular Implants
6.2.2. Orthodontic Mini-Implants
6.3. Cortical Bone and Secondary Stability of the Implant/MBL
6.4. Cortical Bone and Implant Sizing
6.5. Cortical Bone and Peri-Implantitis
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gehrke, S.A.; Pérez-Díaz, L.; Mazón, P.; De Aza, P.N. Biomechanical effects of a new macrogeometry design of dental implants: An in vitro experimental analysis. J. Funct. Biomater. 2019, 10, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shemtov-Yona, K.; Rittel, D. An overview of the mechanical integrity of dental implants. Biomed. Res. Int. 2015, 2015, 547384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrektsson, T.; Jansson, T.; Lekholm, U. Osseointegrated dental implants. Dent. Clin. N. Am. 1986, 30, 151–174. [Google Scholar]
- Raikar, S.; Talukdar, P.; Kumari, S.; Panda, S.K.; Oommen, V.M.; Prasad, A. Factors affecting the survival rate of dental implants: A retrospective study. J. Int. Soc. Prev. Community Dent. 2017, 7, 351–355. [Google Scholar] [CrossRef]
- Howe, M.S.; Keys, W.; Richards, D. Long-term (10-year) dental implant survival: A systematic review and sensitivity meta-analysis. J. Dent. 2019, 84, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implant. 1986, 1, 11–25. [Google Scholar]
- Misch, C.E.; Perel, M.L.; Wang, H.L.; Sammartino, G.; Galindo-Moreno, P.; Trisi, P. Implant success, survival, and failure: The International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference. Implant Dent. 2008, 17, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Nandal, S.; Ghalaut, P.; Shekhawat, H. A radiological evaluation of marginal bone around dental implants: An in-vivo study. Natl. J. Maxillofac. Surg. 2014, 5, 126–137. [Google Scholar] [CrossRef] [Green Version]
- Sennerby, L.; Roos, J. Surgical determinants of clinical success of osseointegrated oral implants: A review of the literature. Int. J. Prosthodont. 1998, 11, 408–420. [Google Scholar]
- Gómez-Polo, M.; Ortega, R.; Gómez-Polo, C.; Martín, C.; Celemín, A.; Del Río, J. Does length, diameter, or bone quality affect primary and secondary stability in self-tapping dental implants? J. Oral Maxillofac. Surg. 2016, 74, 1344–1353. [Google Scholar] [CrossRef] [Green Version]
- Cobo-Vázquez, C.; Reininger, D.; Molinero-Mourelle, P.; González-Serrano, J.; Guisado-Moya, B.; López-Quiles, J. Effect of the lack of primary stability in the survival of dental implants. J. Clin. Exp. Dent. 2018, 10, e14–e19. [Google Scholar] [CrossRef] [PubMed]
- Ziebart, J.; Fan, S.; Schulze, C.; Kämmerer, P.W.; Bader, R.; Jonitz-Heincke, A. Effects of interfacial micromotions on vitality and differentiation of human osteoblasts. Bone Joint Res. 2018, 7, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Ahmed, H.B.; Crespi, R.; Romanos, G.E. Role of primary stability for successful osseointegration of dental implants: Factors of influence and evaluation. Interv. Med. Appl. Sci. 2013, 5, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Crespi, R.; Capparè, P.; Polizzi, E.M.; Gherlone, E.F. Tissue remodeling after bone expansion in grafted and ungrafted sockets. Int. J. Oral Maxillofac. Implant. 2014, 29, 699–704. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, D.; Sennerby, L.; Jagger, D.; Meredith, N. A comparison of two methods of enhancing implant primary stability. Clin. Implant Dent. Relat. Res. 2004, 6, 48–57. [Google Scholar] [CrossRef]
- Saadoun, A.P.; Le Gall, M.G.; Touati, B. Current trends in implantology: Part 1—Biological response, implant stability, and implant design. Pract. Proced. Aesthet. Dent. 2004, 16, 529–535. [Google Scholar]
- Sevimay, M.; Turhan, F.; Kiliçarslan, M.A.; Eskitascioglu, G. Three-dimensional finite element analysis of the effect of different bone quality on stress distribution in an implant-supported crown. J. Prosthet. Dent. 2005, 93, 227–234. [Google Scholar] [CrossRef]
- Tabassum, A.; Meijer, G.J.; Wolke, J.G.; Jansen, J.A. Influence of the surgical technique and surface roughness on the primary stability of an implant in artificial bone with a density equivalent to maxillary bone: A laboratory study. Clin. Oral. Implant. Res. 2009, 20, 327–332. [Google Scholar] [CrossRef]
- Tabassum, A.; Meijer, G.J.; Wolke, J.G.; Jansen, J.A. Influence of surgical technique and surface roughness on the primary stability of an implant in artificial bone with different cortical thickness: A laboratory study. Clin. Oral Implant. Res. 2010, 21, 213–220. [Google Scholar] [CrossRef]
- Farré-Pagés, N.; Augé-Castro, M.L.; Alaejos-Algarra, F.; Mareque-Bueno, J.; Ferrés-Padró, E.; Hernández-Alfaro, F. Relation between bone density and primary implant stability. Med. Oral Patol. Oral Cir. Bucal 2011, 16, e62–e67. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; The Cochrane Collaboration: London, UK, 2011; Available online: https://www.cochrane-handbook.org (accessed on 10 May 2021).
- Miyamoto, I.; Tsuboi, Y.; Wada, E.; Suwa, H.; Iizuka, T. Influence of cortical bone thickness and implant length on implant stability at the time of surgery—Clinical, prospective, biomechanical, and imaging study. Bone 2005, 37, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Alsaadi, G.; Quirynen, M.; Michiels, K.; Jacobs, R.; Van Steenberghe, D. A biomechanical assessment of the relation between the oral implant stability at insertion and subjective bone quality assessment. J. Clin. Periodontol. 2007, 34, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Motoyoshi, M.; Yoshida, T.; Ono, A.; Shimizu, N. Effect of cortical bone thickness and implant placement torque on stability of orthodontic mini-implants. Int. J. Oral Maxillofac. Implant. 2007, 22, 779–784. [Google Scholar]
- Rozé, J.; Babu, S.; Saffarzadeh, A.; Gayet-Delacroix, M.; Hoornaert, A.; Layrolle, P. Correlating implant stability to bone structure. Clin. Oral Implant. Res. 2009, 20, 1140–1145. [Google Scholar] [CrossRef]
- Merheb, J.; Van Assche, N.; Coucke, W.; Jacobs, R.; Naert, I.; Quirynen, M. Relationship between cortical bone thickness or computerized tomography-derived bone density values and implant stability. Clin. Oral Implant. Res. 2010, 21, 612–617. [Google Scholar] [CrossRef]
- Motoyoshi, M.; Uemura, M.; Ono, A.; Okazaki, K.; Shigeeda, T.; Shimizu, N. Factors affecting the long-term stability of orthodontic mini-implants. Am. J. Orthod. Dentofac. Orthop. 2010, 137, 588.e1–588.e5. [Google Scholar] [CrossRef]
- Salimov, F.; Tatli, U.; Kürkçü, M.; Akoğlan, M.; Oztunç, H.; Kurtoğlu, C. Evaluation of relationship between preoperative bone density values derived from cone beam computed tomography and implant stability parameters: A clinical study. Clin. Oral Implant. Res. 2014, 25, 1016–1021. [Google Scholar] [CrossRef]
- Dias, D.R.; Leles, C.R.; Lindh, C.; Ribeiro-Rotta, R.F. Marginal bone level changes and implant stability after loading are not influenced by baseline microstructural bone characteristics: 1-year follow-up. Clin. Oral Implant. Res. 2016, 27, 1212–1220. [Google Scholar] [CrossRef]
- Chatvaratthana, K.; Thaworanunta, S.; Seriwatanachai, D.; Wongsirichat, N. Correlation between the thickness of the crestal and buccolingual cortical bone at varying depths and implant stability quotients. PLoS ONE 2017, 12, e0190293. [Google Scholar] [CrossRef] [Green Version]
- Waechter, J.; Madruga, M.M.; Carmo Filho, L.C.D.; Leite, F.R.M.; Schinestsck, A.R.; Faot, F. Comparison between tapered and cylindrical implants in the posterior regions of the mandible: A prospective, randomized, split-mouth clinical trial focusing on implant stability changes during early healing. Clin. Implant Dent. Relat. Res. 2017, 19, 733–741. [Google Scholar] [CrossRef]
- Bruno, V.; Berti, C.; Barausse, C.; Badino, M.; Gasparro, R.; Ippolito, D.R.; Felice, P. Clinical relevance of bone density values from CT related to dental implant stability: A retrospective study. Biomed. Res. Int. 2018, 2018, 6758245. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira Nicolau Mantovani, A.K.; de Mattias Sartori, I.A.; Azevedo-Alanis, L.R.; Tiossi, R.; Fontão, F.N.G.K. Influence of cortical bone anchorage on the primary stability of dental implants. Oral Maxillofac. Surg. 2018, 22, 297–301. [Google Scholar] [CrossRef]
- Tanaka, K.; Sailer, I.; Iwama, R.; Yamauchi, K.; Nogami, S.; Yoda, N.; Takahashi, T. Relationship between cortical bone thickness and implant stability at the time of surgery and secondary stability after osseointegration measured using resonance frequency analysis. J. Periodontal Implant Sci. 2018, 48, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.; Strid, K.G. Assessment of bone quality from placement resistance during implant surgery. Int. J. Oral Maxillofac. Implant. 1994, 9, 279–288. [Google Scholar]
- Venkatakrishnan, C.J.; Bhuminathan, S.; Chandran, C.R. Dental implant insertion torque and bone density—Short review. Biomed. Pharmacol. J. 2017, 10, 1305–1309. [Google Scholar] [CrossRef]
- Gahona, O.; Granic, X.; Antunez, C.; Domancic, S.; Díaz-Narváez, V.; Utsman, R. Insertion torque and resonance frequency analysis (ISQ) as predictor methods of implant osseointegration. J. Osseointegr. 2018, 10, 103–107. [Google Scholar]
- Di Stefano, D.A.; Arosio, P.; Perrotti, V.; Iezzi, G.; Scarano, A.; Piattelli, A. Correlation between implant geometry, bone density, and the insertion torque/depth integral: A study on bovine ribs. Dent. J. 2019, 7, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benic, G.I.; Mir-Mari, J.; Hämmerle, C.H. Loading protocols for single implant crowns: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Implant. 2014, 29, 222–238. [Google Scholar] [CrossRef] [Green Version]
- Greenstein, G.; Cavallaro, J. Implant insertion torque: Its role in achieving primary stability of restorable dental implants. Compend. Contin. Educ. Dent. 2017, 38, 88–95. [Google Scholar] [PubMed]
- Trisi, P.; Perfetti, G.; Baldoni, E.; Berardi, D.; Colagiovanni, M.; Scogna, G. Implant micromotion is related to peak insertion torque and bone density. Clin. Oral Implant. Res. 2009, 20, 467–471. [Google Scholar] [CrossRef]
- Barone, A.; Alfonsi, F.; Derchi, G.; Tonelli, P.; Toti, P.; Marchionni, S.; Covani, U. The effect of insertion torque on the clinical outcome of single implants: A randomized clinical trial. Clin. Implant Dent. Relat. Res. 2016, 18, 588–600. [Google Scholar] [CrossRef]
- Baldi, D.; Lombardi, T.; Colombo, J.; Cervino, G.; Perinetti, G.; Di Lenarda, R.; Stacchi, C. Correlation between insertion torque and implant stability quotient in tapered implants with knife-edge thread design. Biomed. Res. Int. 2018, 2018, 7201093. [Google Scholar] [CrossRef]
- Aldahlawi, S.; Demeter, A.; Irinakis, T. The effect of implant placement torque on crestal bone remodeling after 1 year of loading. Clin. Cosmet. Investig. Dent. 2018, 10, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Lemos, C.A.A.; Verri, F.R.; de Oliveira Neto, O.B.; Cruz, R.S.; Luna Gomes, J.M.; da Silva Casado, B.G.; Pellizzer, E.P. Clinical effect of the high insertion torque on dental implants: A systematic review and meta-analysis. J. Prosthet. Dent. 2020, 8, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Roca-Millan, E.; González-Navarro, B.; Domínguez-Mínger, J.; Marí-Roig, A.; Jané-Salas, E.; López-López, J. Implant insertion torque and marginal bone loss: A systematic review and meta-analysis. Int. J. Oral Implantol. 2020, 13, 345–353. [Google Scholar]
- Atieh, M.A.; Baqain, Z.H.; Tawse-Smith, A.; Ma, S.; Almoselli, M.; Lin, L.; Alsabeeha, N.H.M. The influence of insertion torque values on the failure and complication rates of dental implants: A systematic review and meta-analysis. Clin. Implant Dent. Relat. Res. 2021, 23, 341–360. [Google Scholar] [CrossRef] [PubMed]
- Meredith, N. Assessment of implant stability as a prognostic determinant. Int. J. Prosthodont. 1998, 11, 491–501. [Google Scholar] [PubMed]
- Quesada-García, M.P.; Prados-Sánchez, E.; Olmedo-Gaya, M.V.; Muñoz-Soto, E.; González-Rodríguez, M.P.; Valllecillo-Capilla, M. Measurement of dental implant stability by resonance frequency analysis: A review of the literature. Med. Oral Patol. Oral Cir. Bucal 2009, 14, e538–e546. [Google Scholar] [CrossRef] [Green Version]
- Lages, F.S.; Douglas-de Oliveira, D.W.; Costa, F.O. Relationship between implant stability measurements obtained by insertion torque and resonance frequency analysis: A systematic review. Clin. Implant Dent. Relat. Res. 2018, 20, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Degidi, M.; Daprile, G.; Piattelli, A. Determination of primary stabilty: A comparison of the surgeon’s perception and objective measurements. Int. J. Oral Maxillofac. Implant. 2010, 25, 558–561. [Google Scholar]
- Herrero, M.; Albertini, M.; Ríos, J.; Lázaro, P.; Fernández, A.; Bullon, P. Resonance frequency analysis reliability in third generation instruments: Osstell mentor®. Med. Oral Patol. Oral Cir. Bucal 2012, 17, 801–806. [Google Scholar] [CrossRef] [Green Version]
- Levin, B.P. The correlation between immediate implant insertion torque and implant stability quotient. Int. J. Periodontics Restor. Dent. 2016, 36, 833–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tricio, J.; Van Steenberghe, D.; Rosenberg, D.; Duchateau, L. Implant stability related to insertion torque force and bone density: An in vitro study. J. Prosthet. Dent. 1995, 74, 608–612. [Google Scholar] [CrossRef]
- Al-Jetaily, S.; Al-Dosari, A.A. Assessment of Osstell™ and Periotest® systems in measuring dental implant stability (in vitro study). Saudi Dent. J. 2011, 23, 17–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degidi, M.; Daprile, G.; Piattelli, A. Primary stability determination by means of insertion torque and RFA in a sample of 4135 implants. Clin. Implant Dent. Relat. Res. 2012, 14, 501–507. [Google Scholar] [CrossRef]
- Sennerby, L.; Meredith, N. Implant stability measurements using resonance frequency analysis: Biological and biomechanical aspects and clinical implications. Periodontol. 2000 2008, 47, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Degidi, M.; Daprile, G.; Piattelli, A.; Iezzi, G. Development of a new implant primary stability parameter: Insertion torque revisited. Clin. Implant Dent. Relat. Res. 2013, 15, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, S.J.; Cho, I.S.; Kim, S.K.; Kim, T.W. Rotational resistance of surface-treated mini-implants. Angle Orthod. 2009, 79, 899–907. [Google Scholar] [CrossRef] [Green Version]
- Park, K.J.; Kwon, J.Y.; Kim, S.K.; Heo, S.J.; Koak, J.Y.; Lee, J.H.; Lee, S.J.; Kim, T.H.; Kim, M.J. The relationship between implant stability quotient values and implant insertion variables: A clinical study. J. Oral. Rehabil. 2012, 39, 151–159. [Google Scholar] [CrossRef]
- Degidi, M.; Daprile, G.; Piattelli, A. Influence of underpreparation on primary stability of implants inserted in poor quality bone sites: An in vitro study. J. Oral Maxillofac. Surg. 2015, 73, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, D.A.; Arosio, P.; Gastaldi, G.; Gherlone, E. The insertion torque-depth curve integral as a measure of implant primary stability: An in vitro study on polyurethane foam blocks. J. Prosthet. Dent. 2018, 120, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Capparé, P.; Vinci, R.; Di Stefano, D.A.; Traini, T.; Pantaleo, G.; Gherlone, E.F.; Gastaldi, G. Correlation between initial BIC and the insertion torque/depth integral recorded with an instantaneous torque-measuring implant motor: An in vivo study. Clin. Implant Dent. Relat. Res. 2015, 17, e613–e620. [Google Scholar] [CrossRef] [PubMed]
- Iezzi, G.; Di Stefano, D.A.; Arosio, P.; Piattelli, A.; Scarano, A.; Perrotti, V. A site-specific intraoperative measurement of bone-to-implant contact during implant insertion: A study on bovine ribs using a computerized implant motor. J. Dent. Sci. 2015, 10, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Di Stefano, D.A.; Perrotti, V.; Greco, G.B.; Cappucci, C.; Arosio, P.; Piattelli, A.; Iezzi, G. The effect of undersizing and tapping on bone to implant contact and implant primary stability: A histomorphometric study on bovine ribs. J. Adv. Prosthodont. 2018, 10, 227–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makary, C.; Rebaudi, A.; Sammartino, G.; Naaman, N. Implant primary stability determined by resonance frequency analysis: Correlation with insertion torque, histologic bone volume, and torsional stability at 6 weeks. Implant Dent. 2012, 21, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Makary, C.; Menhall, A.; Zammarie, C.; Lombardi, T.; Lee, S.Y.; Stacchi, C.; Park, K.B. Primary stability optimization by using fixtures with different thread depth according to bone density: A clinical prospective study on early loaded implants. Materials 2019, 12, 2398. [Google Scholar] [CrossRef] [Green Version]
- Lekholm, U.; Zarb, G.A. Tissue integrated prostheses: Osseointegration in clinical dentistry. In Patient Selection and Preparation; Branemark, P.I., Zarb, G.A., Albrektsson, T., Eds.; Quintessence Publishing Company: Chicago, IL, USA, 1985; pp. 199–209. [Google Scholar]
- Misch, C.E. Bone density: A key determinant for clinical success. In Contemporary Implant Dentistry, 2nd ed.; Misch, C.E., Ed.; CV Mosby: Saint Louis, MO, USA, 1999; pp. 109–118. [Google Scholar]
- Norton, M.R.; Gamble, C. Bone classification: An objective scale of bone density using the computerized tomography scan. Clin. Oral Implant. Res. 2001, 12, 79–84. [Google Scholar] [CrossRef]
- Turkyilmaz, I.; Tumer, C.; Ozbek, E.N.; Tözüm, T.F. Relations between the bone density values from computerized tomography, and implant stability parameters: A clinical study of 230 regular platform implants. J. Clin. Periodontol. 2007, 34, 716–722. [Google Scholar] [CrossRef]
- Turkyilmaz, I.; Sennerby, L.; McGlumphy, E.A.; Tözüm, T.F. Biomechanical aspects of primary implant stability: A human cadaver study. Clin. Implant Dent. Relat. Res. 2009, 11, 113–119. [Google Scholar] [CrossRef]
- Cehreli, M.C.; Kökat, A.M.; Comert, A.; Akkocaoğlu, M.; Tekdemir, I.; Akça, K. Implant stability and bone density: Assessment of correlation in fresh cadavers using conventional and osteotome implant sockets. Clin. Oral Implant. Res. 2009, 20, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, D.C.; Dial, C.; Mayorga, C. Cone beam CT for pre-surgical assessment of implant sites. J. Calif. Dent. Assoc. 2003, 31, 825–833. [Google Scholar] [PubMed]
- Jacobs, R.; Salmon, B.; Codari, M.; Hassan, B.; Bornstein, M.M. Cone beam computed tomography in implant dentistry: Recommendations for clinical use. BMC Oral Health 2018, 18, 88. [Google Scholar] [CrossRef] [Green Version]
- Mah, P.; Reeves, T.E.; McDavid, W.D. Deriving Hounsfield units using grey levels in cone beam computed tomography. DentoMaxillofac. Radiol. 2010, 39, 323–335. [Google Scholar] [CrossRef]
- Nomura, Y.; Watanabe, H.; Honda, E.; Kurabayashi, T. Reliability of voxel values from cone-beam computed tomography for dental use in evaluating bone mineral density. Clin. Oral Implant. Res. 2010, 21, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Valiyaparambil, J.V.; Yamany, I.; Ortiz, D.; Shafer, D.M.; Pendrys, D.; Freilich, M.; Mallya, S.M. Bone quality evaluation: Comparison of cone beam computed tomography and subjective surgical assessment. Int. J. Oral Maxillofac. Implant. 2012, 27, 1271–1277. [Google Scholar]
- Misch, C.M. Maxillary autogenous bone grafting. Oral Maxillofac. Surg. Clin. N. Am. 2011, 23, 229–238. [Google Scholar] [CrossRef]
- Trisi, P.; Rao, W. Bone classification: Clinical-histomorphometric comparison. Clin. Oral Implant. Res. 1999, 10, 1–7. [Google Scholar]
- Molly, L. Bone density and primary stability in implant therapy. Clin. Oral Implant. Res. 2006, 17, 124–135. [Google Scholar] [CrossRef]
- Linetskiy, I.; Demenko, V.; Linetska, L.; Yefremov, O. Impact of annual bone loss and different bone quality on dental implant success—A finite element study. Comput. Biol. Med. 2017, 91, 318–325. [Google Scholar] [CrossRef]
- Johansson, B.; Bäck, T.; Hirsch, J.M. Cutting torque measurements in conjunction with implant placement in grafted and nongrafted maxillas as an objective evaluation of bone density: A possible method for identifying early implant failures? Clin. Implant Dent. Relat. Res. 2004, 6, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Friberg, B.; Sennerby, L.; Roos, J.; Johansson, P.; Strid, C.G.; Lekholm, U. Evaluation of bone density using cutting resistance measurements and microradiography: An in vitro study in pig ribs. Clin. Oral Implant. Res. 1995, 6, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Friberg, B.; Sennerby, L.; Roos, J.; Lekholm, U. Identification of bone quality in conjunction with insertion of titanium implants. A pilot study in jaw autopsy specimens. Clin. Oral Implants Res. 1995, 6, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, D.A.; Arosio, P. Correlation Between bone density and instantaneous torque at implant site preparation: A validation on polyurethane foam blocks of a device assessing density of jawbones. Int. J. Oral Maxillofac. Implant. 2016, 31, e128–e135. [Google Scholar] [CrossRef] [PubMed]
- Iezzi, G.; Scarano, A.; Di Stefano, D.A.; Arosio, P.; Doi, K.; Ricci, L.; Piattelli, A.; Perrotti, V. Correlation between the bone density recorded by a computerized implant motor and by a histomorphometric analysis: A preliminary in vitro study on bovine ribs. Clin. Implant. Dent. Relat. Res. 2015, 17, e35–e44. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, D.A.; Arosio, P.; Pagnutti, S. A possible novel objective intraoperative measurement of maxillary bone density. Minerva Stomatol. 2013, 62, 259–265. [Google Scholar]
- Di Stefano, D.A.; Arosio, P.; Piattelli, A.; Perrotti, V.; Iezzi, G. A torque-measuring micromotor provides operator independent measurements marking four different density areas in maxillae. J. Adv. Prosthodont. 2015, 7, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Di Stefano, D.A.; Arosio, P.; Pagnutti, S.; Vinci, R.; Gherlone, E.F. Distribution of trabecular bone density in the maxilla and mandible. Implant Dent. 2019, 28, 340–348. [Google Scholar] [CrossRef]
- Ribeiro-Rotta, R.F.; de Oliveira, R.C.; Dias, D.R.; Lindh, C.; Leles, C.R. Bone tissue microarchitectural characteristics at dental implant sites part 2: Correlation with bone classification and primary stability. Clin. Oral Implant. Res. 2014, 25, e47–e53. [Google Scholar] [CrossRef]
- Barewal, R.M.; Stanford, C.; Weesner, T.C. A randomized controlled clinical trial comparing the effects of three loading protocols on dental implant stability. Int. J. Oral Maxillofac. Implant. 2012, 27, 945–956. [Google Scholar]
- Anitua, E.; Alkhraisat, M.H.; Piñas, L.; Orive, G. Efficacy of biologically guided implant site preparation to obtain adequate primary implant stability. Ann. Anat. 2015, 199, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, F.; Hasan, I.; Bourauel, C.; Biffar, R.; Mundt, T. Bone stability around dental implants: Treatment related factors. Ann. Anat. 2015, 199, 3–8. [Google Scholar] [CrossRef]
- Schwartz-Dabney, C.L.; Dechow, P.C. Edentulation alters material properties of cortical bone in the human mandible. J. Dent. Res. 2002, 81, 613–617. [Google Scholar] [CrossRef]
- Katranji, A.; Misch, K.; Wang, H.L. Cortical bone thickness in dentate and edentulous human cadavers. J. Periodontol. 2007, 78, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, T.; Nasu, M.; Murakami, K.; Yabuuchi, T.; Kamioka, H.; Takano-Yamamoto, T. Quantitative evaluation of cortical bone thickness with computed tomographic scanning for orthodontic implants. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 721.e7–721.e12. [Google Scholar] [CrossRef] [PubMed]
- Ono, A.; Motoyoshi, M.; Shimizu, N. Cortical bone thickness in the buccal posterior region for orthodontic mini-implants. Int. J. Oral Maxillofac. Surg. 2008, 37, 334–340. [Google Scholar] [CrossRef]
- Ko, Y.C.; Huang, H.L.; Shen, Y.W.; Cai, J.Y.; Fuh, L.J.; Hsu, J.T. Variations in crestal cortical bone thickness at dental implant sites in different regions of the jawbone. Clin. Implant Dent. Relat. Res. 2017, 19, 440–446. [Google Scholar] [CrossRef]
- Gupta, A.; Rathee, S.; Agarwal, J.; Pachar, R.B. Measurement of crestal cortical bone thickness at implant site: A cone beam computed tomography study. J. Contemp. Dent. Pract. 2017, 18, 785–789. [Google Scholar] [CrossRef]
- Ohiomoba, H.; Sonis, A.; Yansane, A.; Friedland, B. Quantitative evaluation of maxillary alveolar cortical bone thickness and density using computed tomography imaging. Am. J. Orthod. Dentofac. Orthop. 2017, 151, 82–91. [Google Scholar] [CrossRef]
- Stocchero, M.; Toia, M.; Cecchinato, D.; Becktor, J.P.; Coelho, P.G.; Jimbo, R. Biomechanical, biologic, and clinical outcomes of undersized implant surgical preparation: A systematic review. Int. J. Oral Maxillofac. Implant. 2016, 31, 1247–1263. [Google Scholar] [CrossRef] [Green Version]
- Trisi, P.; Todisco, M.; Consolo, U.; Travaglini, D. High versus low implant insertion torque: A histologic, histomorphometric, and biomechanical study in the sheep mandible. Int. J. Oral Maxillofac. Implant. 2011, 26, 837–849. [Google Scholar]
- Ahn, S.J.; Leesungbok, R.; Lee, S.W.; Heo, Y.K.; Kang, K.L. Differences in implant stability associated with various methods of preparation of the implant bed: An in vitro study. J. Prosthet. Dent. 2012, 107, 366–372. [Google Scholar] [CrossRef]
- Coelho, P.G.; Marin, C.; Teixeira, H.S.; Campos, F.E.; Gomes, J.B.; Guastaldi, F.; Anchieta, R.B.; Silveira, L.; Bonfante, E.A. Biomechanical evaluation of undersized drilling on implant biomechanical stability at early implantation times. J. Oral Maxillofac. Surg. 2013, 71, e69–e75. [Google Scholar] [CrossRef]
- Campos, F.E.; Jimbo, R.; Bonfante, E.A.; Barbosa, D.Z.; Oliveira, M.T.; Janal, M.N.; Coelho, P.G. Are insertion torque and early osseointegration proportional? A histologic evaluation. Clin. Oral Implant. Res. 2015, 26, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Jimbo, R.; Tovar, N.; Anchieta, R.B.; Machado, L.S.; Marin, C.; Teixeira, H.S.; Coelho, P.G. The combined effects of undersized drilling and implant macrogeometry on bone healing around dental implants: An experimental study. Int. J. Oral Maxillofac. Surg. 2014, 43, 1269–1275. [Google Scholar] [CrossRef]
- Kim, W.H.; Song, E.S.; Ju, K.W.; Lee, J.H.; Kim, M.Y.; Lim, D.; Kim, B. Finite element analysis of novel separable fixture for easy retrievement in case with peri-implantitis. Materials 2019, 12, 235. [Google Scholar] [CrossRef] [Green Version]
- Ayangco, L.; Sheridan, P.J. Development and treatment of retrograde peri-implantitis involving a site with a history of failed endodontic and apicoectomy procedures: A series of reports. Int. J. Oral Maxillofac. Implant. 2001, 16, 412–417. [Google Scholar]
- Smeets, R.; Henningsen, A.; Jung, O.; Heiland, M.; Hammächer, C.; Stein, J.M. Definition, etiology, prevention and treatment of peri-implantitis—A review. Head Face Med. 2014, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Bogovič, V.; Svete, A.; Bajsić, I. Effects of a drill diameter on the temperature rise in a bone during implant site preparation under clinical conditions. Proc. Inst. Mech. Eng. H 2016, 230, 907–917. [Google Scholar] [CrossRef]
- Flanagan, D. Implant placement in failed endodontic sites: A review. J. Oral Implantol. 2016, 42, 224–230. [Google Scholar] [CrossRef]


| First Author (Publication Year) | Study Design | Intervention | Outcomes | ||
|---|---|---|---|---|---|
| Evaluation of Cortical Bone | Evaluation of Dental ImplantStability | Statistical Correlation | |||
| Miyamoto (2005) [23] | Prospective clinical study | 225 dental implants (diameter, 3.5 mm; length, 8-9-11-13-15 or 17 mm); (maxilla 98, mandible 127) | Preoperative CT scans | Primary stability measured by RFA (ISQ) | Yes (r = 0.84, p < 0.0001) |
| Alsaadi (2007) [24] | Retrospective clinical study | 761 Mark III TiUnite™ implants(maxilla 386, mandible 334) | Tactile sensations during high-speed drilling | Primary stability measured by IT, ISQ and PTV | Yes Significant relationship between ISQ, PTV and cortical bone grades (p = 0.02 and p < 0.0001, respectively) |
| Motoyoshi (2007) [25] | Prospective clinical study | 87 mini-implants (1.6 mm wide and 8 mm long) placed in the posterior alveolar bone | Preoperative CT scans | Primary stability measured by IT | No Cortical bone thickness of at least 1.0 mm and IT up to 10 Ncm improve implant success rate |
| Rozé (2009) [26] | Cadaver study | 22 implants into maxillary and mandibular sites | CT | Primary stability measured by RFA (ISQ) | Yes (p = 0.003) |
| Merheb (2010) [27] | Prospective clinical study | 136 dental implants into the upper jaw (diameter, 3.3 or 4.1 mm; length, 6, 8, 10, 12 or 14 mm) | Preoperative CT scans | Primary stability measured by RFA and PTV | Yes (p < 0.05) |
| Motoyoshi (2010) [28] | Prospective clinical study | 134 mini-implants placed into posterior maxillary and mandibular sites (diameter, 1.6 mm; length, 8 mm) | CT | Primary stability measured by IT upon implant placement and removal | YesSignificant correlation between cortical bone thickness and placement torque in the upper jaw (r = 0.392, p < 0.05) |
| Salimov (2014) [29] | Prospective clinical study | 65 dental implants (diameter, 3.4, 3.8 or 4.3 mm; length, 12 mm) (maxilla 44, mandible 21) | CBCT Tactile sensations during high-speed drilling | Primary stability measured by IT, RFA (ISQ) | YesSignificant correlation between IT, ISQ and cortical bone density (r = 0.935, p < 0.001 and r = 0.888, p < 0.001, respectively) |
| Dias (2016) [30] | Prospective clinical study | 57 dental implants (maxilla 22, mandible 35) | CT images | Implant stability measured by RFA (ISQ) MBL measured by periapical radiographs at the 1-year follow-up | No No significant relationship between MBL changes and cortical thickness (r = −0.029; p = 0.832) and between cortical thickness and ISQ (r = 0.145; p = 0.292) |
| Chatvaratthana (2017) [31] | Prospective clinical study | 19 implants (diameter, 5 mm; length, 9 mm) inserted into posterior maxillary and mandibular sites | CBCT | Primary stability measured by RFA (ISQ) | Yes (p < 0.001) |
| Waechter (2017) [32] | Prospective RCT | 20 tapered implants (diameter, 4.6 mm; length, 10 mm) and 20 cylindrical implants (diameter, 4 mm; length, 10 mm) into the posterior mandible | Tactile sensations during high-speed drilling | Primary stability measured by IT and ISQ | Yes IT was directly related to cortical bone height (r = 0.32; p = 0.0441). ISQ seems to be dependent on cancellous bone availability (r = 0.32; p = 0.0471). |
| Bruno (2018) [33] | Retrospective clinical study | 269 implants (mean diameter, 4.36 ± 0.64 mm; mean length, 13.08 ± 1.71 mm) (maxilla 149, mandible 120) | CT | Primary stability measured by IT and ISQ | YesPositive correlation between IT and cortical bone thickness at the middle of the ridge (ρ = 0.196; p = 0.032) |
| de Oliveira Nicolau Mantovani (2018) [34] | Retrospective clinical study | 97 implants into mandibular sites, divided into 3 classes: (1) with apical cortical bone contact; (2) with bicortical bone contact; (3) with cervical cortical bone contact | CBCT | Primary stability measured by IT and ISQ | Yes IT values and ISQ are influenced by cortical bone anchorage (i.e., bicortical bone anchorage led to higher IT and ISQ, p < 0.05) |
| Tanaka (2018) [35] | Retrospective clinical study | 229 dental implants (diameter range, 3.0–5.0 mm; length range, 6–13 mm) (maxilla, 111; mandible, 118) | CT | Primary and secondary implant stability measured by RFA (ISQ) | Yes Weak positive correlation between cortical bone thickness and primary/secondary implant stability (p < 0.01) |
| Bone Density | Description | Tactile Analog | Location |
|---|---|---|---|
| D1 | Dense cortical bone | Oak wood | Anterior lower jaw |
| D2 | Porous cortical bone and dense trabecular bone | Spruce wood | Anterior lower jaw Posterior lower jaw Anterior upper jaw |
| D3 | Thin and porous cortical bone and thin trabecular bone | Balsa wood | Posterior lower jaw Anterior upper jaw Posterior upper jaw |
| D4 | Thin trabecular bone | Styrofoam™ | Posterior upper jaw |
| D5 | Non-mineralized bone (unsuitable for implantation) | - | - |
| Bone Density | CT Evaluation | Description |
|---|---|---|
| D1 | >1250 HU | Dense cortical bone of the anterior lower jaw |
| D2 | 850–1250 HU | Porous cortical and coarse trabecular bone in the anterior/posterior mandible and anterior upper jaw |
| D3 | 350–850 HU | Thin cortical and fine trabecular bone of the posterior lower jaw and anterior/posterior upper jaw |
| D4 | 150–350 HU | Fine trabecular bone of the posterior upper jaw |
| D5 | <150 HU | Immature non-mineralized bone |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Stefano, D.A.; Arosio, P.; Capparè, P.; Barbon, S.; Gherlone, E.F. Stability of Dental Implants and Thickness of Cortical Bone: Clinical Research and Future Perspectives. A Systematic Review. Materials 2021, 14, 7183. https://doi.org/10.3390/ma14237183
Di Stefano DA, Arosio P, Capparè P, Barbon S, Gherlone EF. Stability of Dental Implants and Thickness of Cortical Bone: Clinical Research and Future Perspectives. A Systematic Review. Materials. 2021; 14(23):7183. https://doi.org/10.3390/ma14237183
Chicago/Turabian StyleDi Stefano, Danilo Alessio, Paolo Arosio, Paolo Capparè, Silvia Barbon, and Enrico Felice Gherlone. 2021. "Stability of Dental Implants and Thickness of Cortical Bone: Clinical Research and Future Perspectives. A Systematic Review" Materials 14, no. 23: 7183. https://doi.org/10.3390/ma14237183
APA StyleDi Stefano, D. A., Arosio, P., Capparè, P., Barbon, S., & Gherlone, E. F. (2021). Stability of Dental Implants and Thickness of Cortical Bone: Clinical Research and Future Perspectives. A Systematic Review. Materials, 14(23), 7183. https://doi.org/10.3390/ma14237183








