Self-Healing Products of Cement Pastes with Supplementary Cementitious Materials, Calcium Sulfoaluminate and Crystalline Admixtures
Abstract
:1. Introduction
2. Materials and Experiments
2.1. Materials
2.2. Mixture Proportions
2.3. Test Methods
3. Results and Discussion
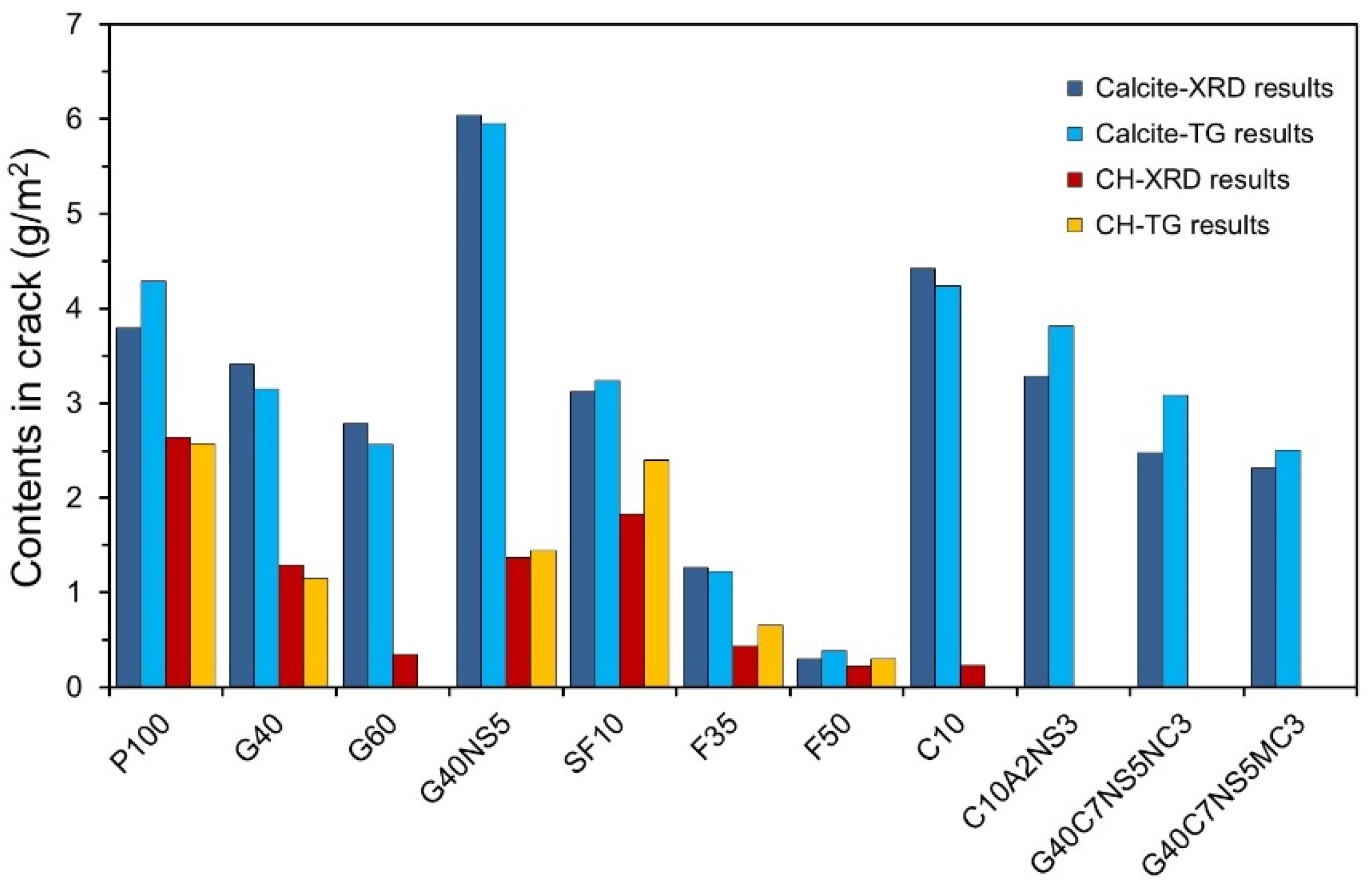
3.1. Quantitative Analysis of Hydration Products in Cracks
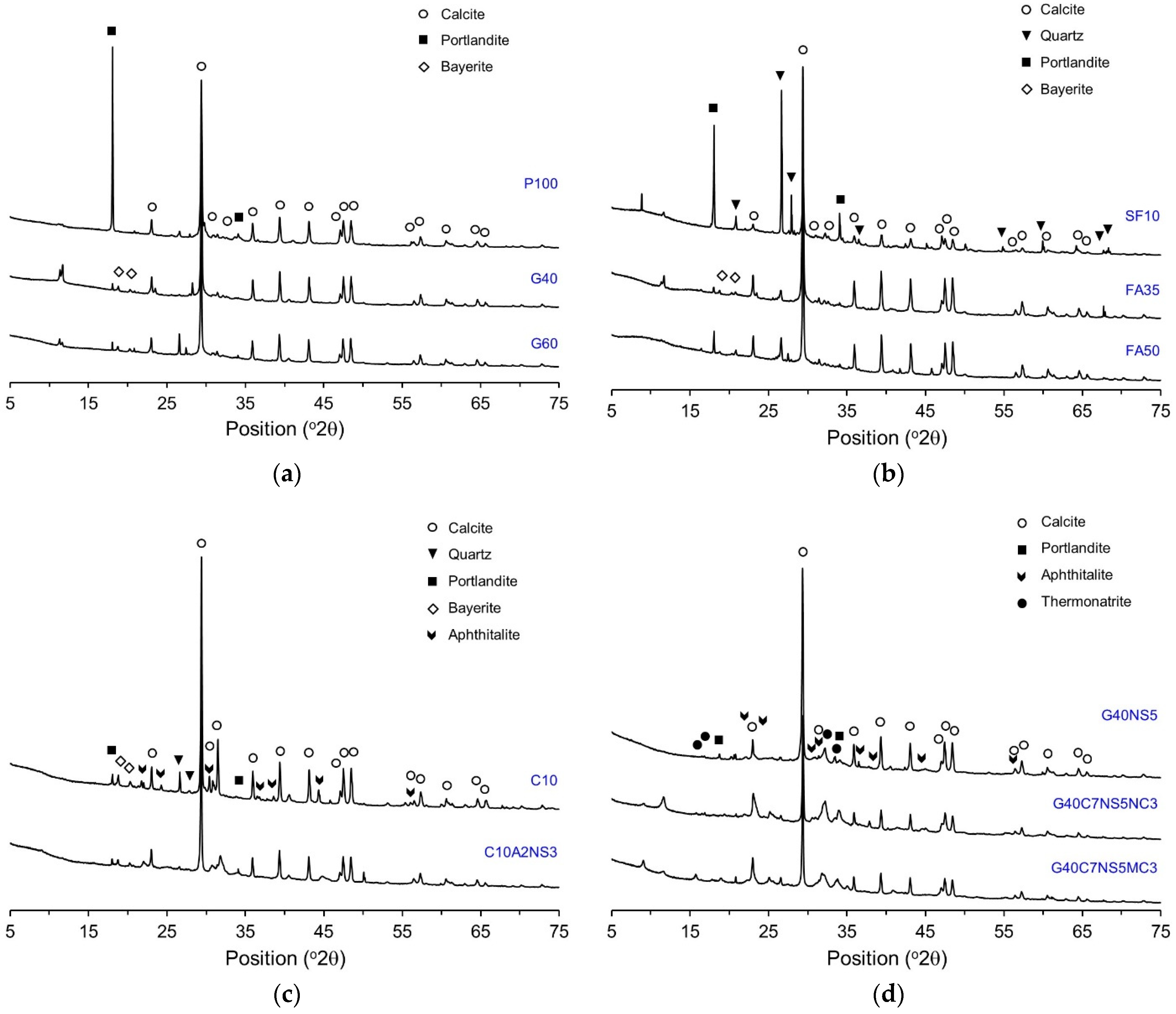
3.2. XRD Analysis
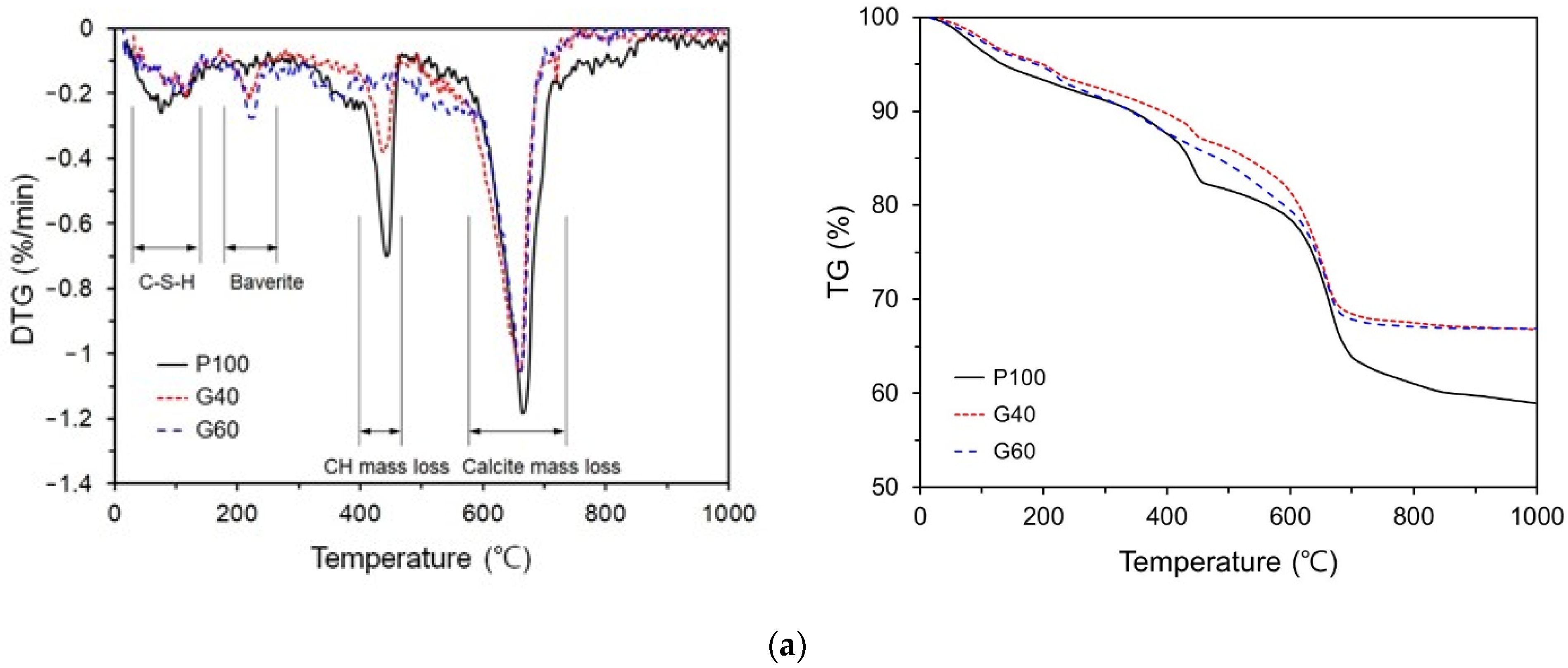
3.3. TG/DTG Analysis
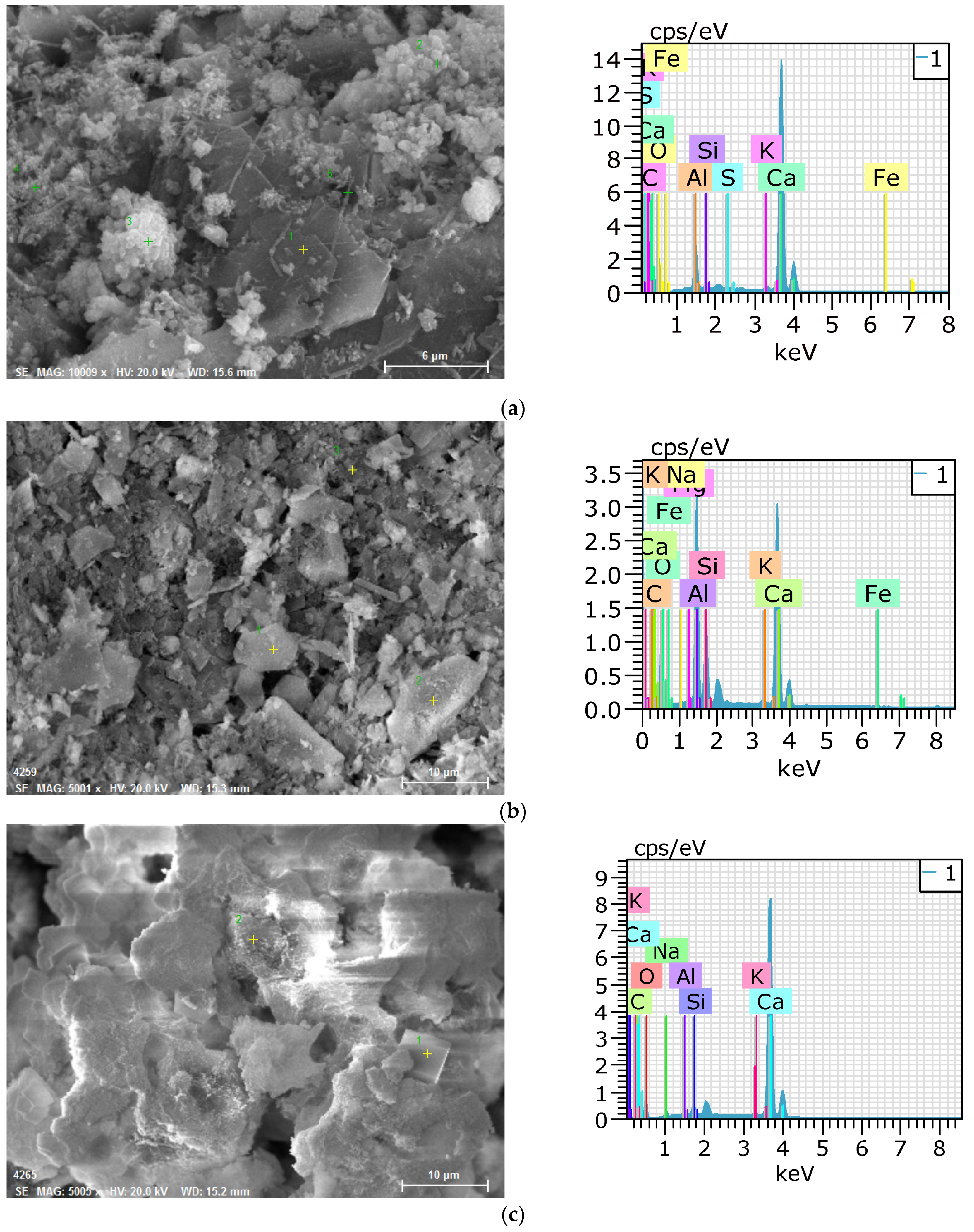
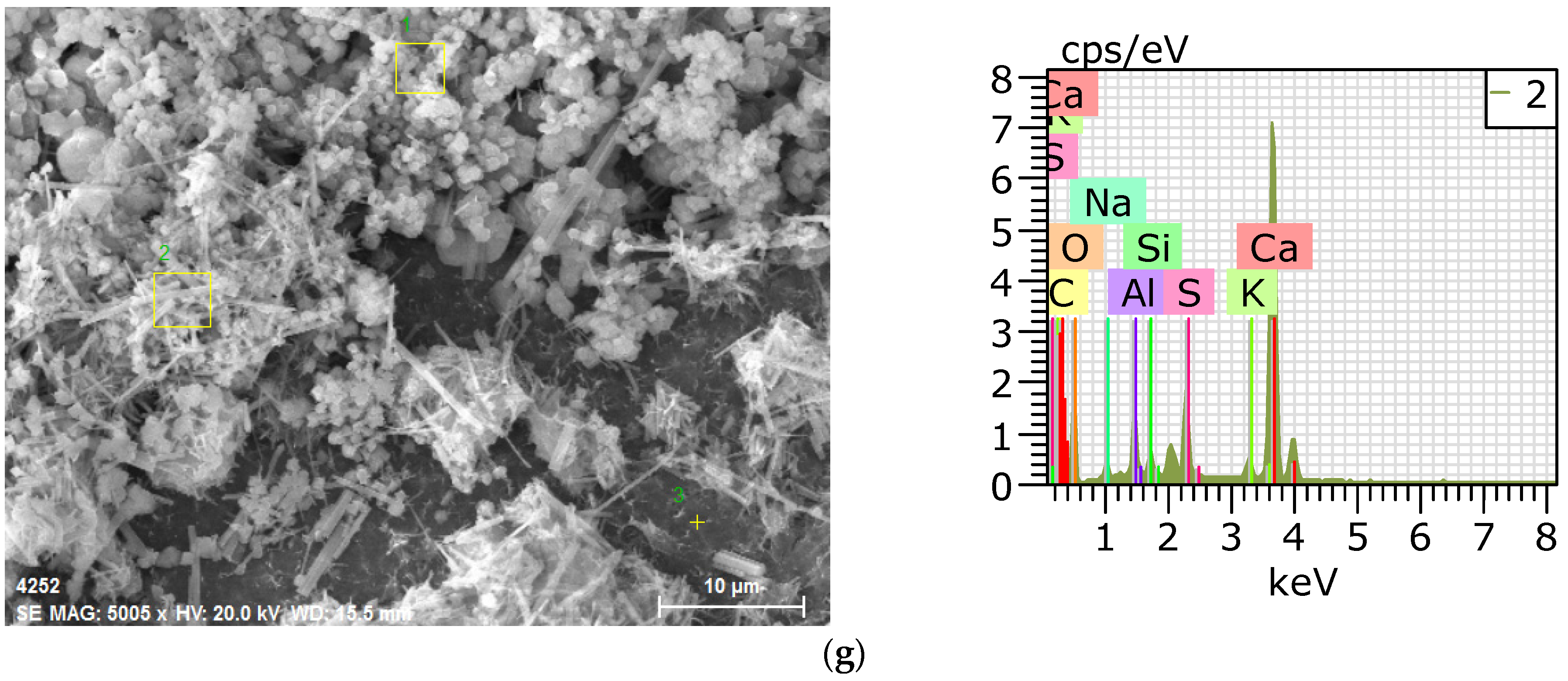
3.4. SEM–EDS Analysis
4. Conclusions
- (1)
- The amount of self-healing products decreased when SCMs were used, whereas it increased when SCMs were used along with CAs. G40NS5 showed the highest content of self-healing products.
- (2)
- The XRD/Rietveld refinement of the phase composition of the self-healing products showed that calcite was the main compound, irrespective of the mixture proportions. The portlandite content decreased when SCMs and crystalline additives were mixed. When CAs were mixed, alkali sulfate and alkali carbonate appeared. This indicates that the phase composition of self-healing products varies with the mixture proportions.
- (3)
- The TG/DTG analysis results showed that the addition of GGBFS and FA significantly decreased the portlandite content in the self-healing products. In every specimen, the weight change due to the decomposition of calcite was the highest, and the calcite content decreased in the G40NS5, G40C7NS5NC3, and G40C7NS5MC3 with crystalline additives.
- (4)
- The quantitative analysis results of calcite and portlandite obtained using the XRD/Rietveld refinement and TG/DTG analysis methods were compared with the results of existing studies. The calcite and portlandite contents were not found to be proportional to the autogenous healing performance. This is believed to be due to the effect of different types of phase compositions in the self-healing products and the crack geometry.
- (5)
- The SEM–EDS analysis results showed that portlandite and calcite were mainly observed in P100 and SF10. When SF was replaced with 10%, the self-healing products were similar to the case using OPC alone. When GGBFS and FA were added, portlandite did not appear, and C-A-H and C-S-H mainly appeared. When the CSA was used, plate-type monosulfate and acicular ettringite appeared in the self-healing products, whereas plate-type and hexahedral hydrates mainly appeared in the G40NS5.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vantadori, S.; Carpinteri, A.; Guo, L.P.; Ronchei, C.; Zanichelli, A. Synergy assessment of hybrid reinforcements in concrete. Compos. Part B Eng. 2018, 147, 197–206. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, S.H.; Shi, Y.; Tang, S.W.; Chen, E. Effect of silica fume and PVA fiber on the abrasion resistance and volume stability of concrete. Compos. Part B Eng. 2017, 130, 28–37. [Google Scholar] [CrossRef]
- Uno, P.J. Plastic shrinkage cracking and evaporation formulas. ACI Mater. J. 1998, 95, 365–375. [Google Scholar]
- Wang, L.; Yang, H.Q.; Zhou, S.H.; Chen, E.; Tang, S.W. Mechanical properties, long-term hydration heat, shrinkage behavior and crack resistance of dam concrete designed with low heat Portland (LHP) cement and fly ash. Constr. Build. Mater. 2018, 187, 1073–1091. [Google Scholar] [CrossRef]
- Li, K.; Li, L. Crack-altered durability properties and performance of structural concretes. Cem. Concr. Res. 2019, 124, 105811. [Google Scholar] [CrossRef]
- Djerbi, A.; Bonnet, S.; Khelidj, A.; Baroghel-Bouny, V. Influence of traversing crack on chloride diffusion into concrete. Cem. Concr. Res. 2008, 38, 877–883. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zheng, Q.; Ashour, A.; Han, B. Self-healing cement concrete composites for resilient infrastructures: A Review. Compos. Part B Eng. 2020, 189, 107892. [Google Scholar] [CrossRef]
- Vijay, K.; Murmu, M.; Deo, S.V. Bacteria based self-healing concrete–A review. Constr. Build. Mater. 2017, 152, 1008–1014. [Google Scholar] [CrossRef]
- Wu, M.; Johannesson, B.; Geiker, M. A review: Self-healing in cementitious materials and engineered cementitious composite as a self-healing material. Constr. Build. Mater. 2012, 28, 571–583. [Google Scholar] [CrossRef]
- Van Belleghem, B.; Zaccardi, Y.V.; Van den Heede, P.; Van Tittelboom, K.; De Belie, N. Evaluation and comparison of traditional methods and electron probe micro analysis (EPMA) to determine the chloride ingress perpendicular to cracks in self-healing concrete. Constr. Build. Mater. 2019, 227, 116789. [Google Scholar] [CrossRef]
- Van Belleghem, B.; Kessler, S.; Van den Heede, P.; Van Tittelboom, K.; De Belie, N. Chloride induced reinforcement corrosion behavior in self-healing concrete with encapsulated polyurethane. Cem. Concr. Res. 2018, 113, 130–139. [Google Scholar] [CrossRef]
- Gilabert, F.A.; Van Tittelboom, K.; Tsangouri, E.; Van Hemelrijck, D.; De Belie, N.; Van Paepegem, W. Determination of strength and debonding energy of a glass-concrete interface for encapsulation-based self-healing concrete. Cem. Concr. Compos. 2017, 79, 76–93. [Google Scholar] [CrossRef] [Green Version]
- Maes, M.; Snoeck, D.; De Belie, N. Chloride penetration in cracked mortar and the influence of autogenous crack healing. Constr. Build. Mater. 2016, 115, 114–124. [Google Scholar] [CrossRef]
- Huang, H.; Ye, G.; Qian, C.; Schlangen, E. Self-healing in cementitious materials: Materials, methods and service conditions. Mater. Des. 2016, 92, 499–511. [Google Scholar] [CrossRef]
- Van Tittelboom, K.; De Belie, N. Self-healing in cementitious materials—A review. Materials 2013, 6, 2182–2217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinhardt, H.W.; Jooss, M. Permeability and self-healing of cracked concrete as a function of temperature and crack width. Cem. Concr. Res. 2003, 33, 981–985. [Google Scholar] [CrossRef]
- Abdel-Jawad, Y.; Dehn, F. Self-Healing of Self-Compacting Concrete; SCC2005: Orlando, FL, USA, 2005; pp. 1023–1029. [Google Scholar]
- Granger, S.; Loukili, A.; Pijaudier-Cabot, G.; Chanvillard, G. Experimental characterization of the self-healing of cracks in an ultra-high performance cementitious material: Mechanical tests and acoustic emission analysis. Cem. Concr. Res. 2007, 37, 519–527. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Ye, G. Simulation of self-healing by further hydration in cementitious materials. Cem. Concr. Compos. 2012, 34, 460–467. [Google Scholar] [CrossRef]
- Ahn, T.H.; Kishi, T. Crack self-healing behavior of cementitious composites incorporating various mineral admixtures. J. Adv. Concr. Technol. 2010, 8, 171–186. [Google Scholar] [CrossRef] [Green Version]
- Van Tittelboom, K.; Gruyaert, E.; Rahier, H.; Belie, N.D. Influence of mix composition on the extent of autogenous crack healing by continued hydration or calcium carbonate formation. Constr. Build. Mater. 2013, 37, 349–359. [Google Scholar] [CrossRef]
- Termkhajornkit, P.; Nawa, T.; Yamashiro, Y.; Saito, T. Self-healing ability of fly ash–cement systems. Cem. Concr. Compos. 2009, 31, 195–203. [Google Scholar] [CrossRef]
- Sisomphon, K.; Copuroglu, O.; Koenders, E.A. Self-healing of surface cracks in mortars with expansive additive and crystalline additive. Cem. Concr. Compos. 2012, 34, 566–574. [Google Scholar] [CrossRef]
- Jaroenratanapirom, D.; Sahamitmongkol, R. Self-crack closing ability of mortar with different additives. J. Met. Mater. Miner. 2011, 21, 9–17. [Google Scholar]
- Roig-Flores, M.; Moscato, S.; Serna, P.; Ferrara, L. Self-healing capability of concrete with crystalline admixtures in different environments. Constr. Build. Mater. 2015, 86, 1–11. [Google Scholar] [CrossRef]
- Azarsa, P.; Gupta, R.; Biparva, A. Assessment of self-healing and durability parameters of concretes incorporating crystalline admixtures and Portland limestone cement. Cem. Concr. Compos. 2019, 99, 17–31. [Google Scholar] [CrossRef]
- Sahmaran, M.; Yildirim, G.; Erdem, T.K. Self-healing capability of cementitious composites incorporating different supplementary cementitious materials. Cem. Concr. Compos. 2013, 35, 89–101. [Google Scholar] [CrossRef] [Green Version]
- Hung, C.C.; Su, Y.F. Medium-term self-healing evaluation of engineered cementitious composites with varying amounts of fly ash and exposure durations. Constr. Build. Mater. 2016, 118, 194–203. [Google Scholar] [CrossRef]
- Şahmaran, M.; Yildirim, G.; Noori, R.; Ozbay, E.; Lachemi, M. Repeatability and pervasiveness of self-healing in engineered cementitious composites. ACI Mater. J. 2015, 112, 513–522. [Google Scholar]
- Yıldırım, G.; Khiavi, A.H.; Yeşilmen, S.; Şahmaran, M. Self-healing performance of aged cementitious composites. Cem. Concr. Compos. 2018, 87, 172–186. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, W.; Yuan, Z. Influence of mineral additives and environmental conditions on the self-healing capabilities of cementitious materials. Cem. Concr. Compos. 2015, 57, 116–127. [Google Scholar] [CrossRef]
- Lefever, G.; Aggelis, D.G.; De Belie, N.; Raes, M.; Hauffman, T.; Van Hemelrijck, D.; Snoeck, D. The Influence of Superabsorbent Polymers and Nanosilica on the Hydration Process and Microstructure of Cementitious Mixtures. Materials 2020, 13, 5194. [Google Scholar] [CrossRef] [PubMed]
- Snoeck, D.; Pel, L.; Belie, N.D. Autogenous Healing in Cementitious Materials with Superabsorbent Polymers Quantified by Means of NMR. Sci. Rep. 2020, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Snoeck, D.; Belie, N.D. Repeated Autogenous Healing in Strain-Hardening Cementitious Composites by Using Superabsorbent Polymers. J. Mater. Civ. Eng. 2016, 28, 04015086. [Google Scholar] [CrossRef] [Green Version]
- Snoeck, D.; Tittelboom, K.V.; Steuperaert, S.; Dubruel, P.; Belie, N.D. Self-healing cementitious materials by the combination of microfibres and superabsorbent polymers. J. Intell. Mater. Syst. Struct. 2014, 25, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Ye, G.; Damidot, D. Effect of blast furnace slag on self-healing of microcracks in cementitious materials. Cem. Concr. Res. 2014, 60, 68–82. [Google Scholar] [CrossRef]
- Li, G.; Liu, S.; Niu, M.; Liu, Q.; Yang, X.; Deng, M. Effect of granulated blast furnace slag on the self-healing capability of mortar incorporating crystalline admixture. Constr. Build. Mater. 2020, 239, 117818. [Google Scholar] [CrossRef]
- Park, B.; Choi, Y.C. Effect of healing products on the self-healing performance of cementitious materials with crystalline admixtures. Constr. Build. Mater. 2021, 270, 121389. [Google Scholar] [CrossRef]
- Hemalatha, T.; Ramaswamy, A. A review on fly ash characteristics e towards promoting high volume utilization in developing sustainable concrete. J. Clean. Prod. 2017, 147, 546–559. [Google Scholar] [CrossRef]
- Wang, H.Y. The effects of elevated temperature on cement paste containing GGBS. Cem. Concr. Compos. 2008, 30, 992–999. [Google Scholar] [CrossRef]
- Jia, Z.; Chen, C.; Shi, J.; Zhang, Y.; Sun, Z.; Zhang, P. The microstructural change of C-S-H at elevated temperature in Portland cement/GGBS blended system. Cem. Concr. Res. 2019, 123, 105773. [Google Scholar] [CrossRef]
- Mota, B.; Matschei, T.; Scrivener, K. Impact of NaOH and Na2SO4 on the kinetics and microstructural development of white cement hydration. Cem. Concr. Res. 2018, 108, 172–185. [Google Scholar] [CrossRef]
- Rodriguez, E.T.; Garbev, K.; Merz, D.; Black, L.; Richardson, I.G. Thermal stability of C-S-H phases and applicability of Richardson and Groves’ and Richardson C-(A)-S-H(I) models to synthetic C-S-H. Cem. Concr. Res. 2017, 93, 45–46. [Google Scholar] [CrossRef]
- Du, X.; Su, X.; Wang, Y.; Li, J. Thermal decomposition of grinding activated bayerite. Mater. Res. Bull. 2009, 44, 660–665. [Google Scholar] [CrossRef]
- Park, B.; Moon, E.J.; Choi, Y.C. Investigation of microstructure and mechanical performance of carbon-capture binder using AOD stainless steel slag. Constr. Build. Mater. 2020, 242, 118174. [Google Scholar] [CrossRef]
- Choi, Y.C.; Park, B. Enhanced autogenous healing of ground granulated blast furnace slag blended cements and mortars. J. Mater. Res. Technol. 2019, 8, 3443–3452. [Google Scholar] [CrossRef]
- Park, B.; Choi, Y.C. Prediction of self-healing potential of cementitious materials incorporating crystalline admixture by isothermal calorimetry. Int. J. Concr. Struct. Mater. 2019, 13, 36. [Google Scholar] [CrossRef]
- Park, B.; Choi, Y.C. Investigating a new method to assess the autogenous healing performance of hardened cement pastes containing supplementary cementitious materials and crystalline admixtures. J. Mater. Res. Technol. 2019, 8, 6058–6073. [Google Scholar]
- Qiu, J.; Tan, H.S.; Yang, E.H. Coupled effects of crack width, slag content, and conditioning alkalinity on autogenous healing of engineered cementitious composites. Cem. Concr. Compos. 2016, 73, 203–212. [Google Scholar] [CrossRef]
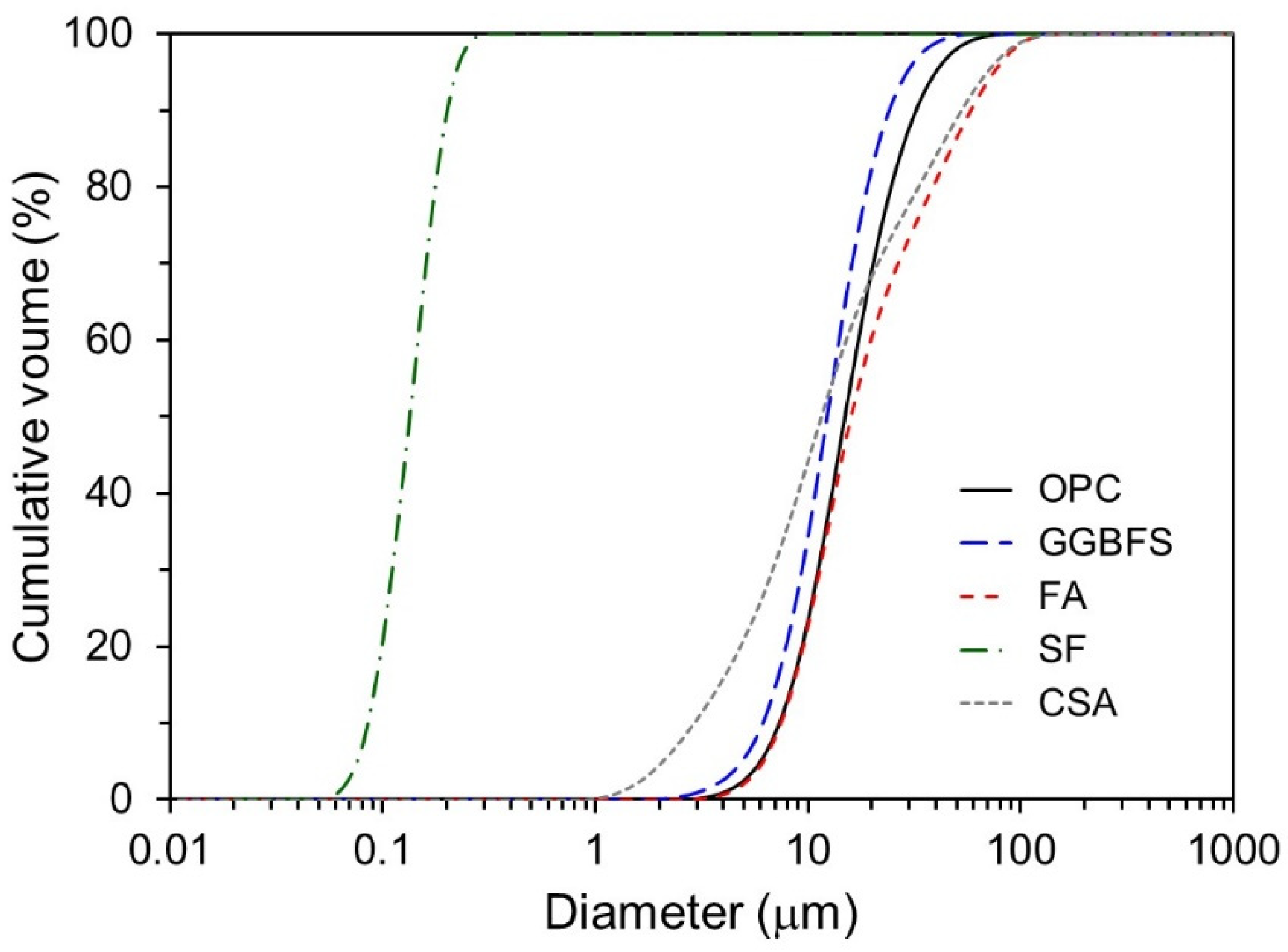
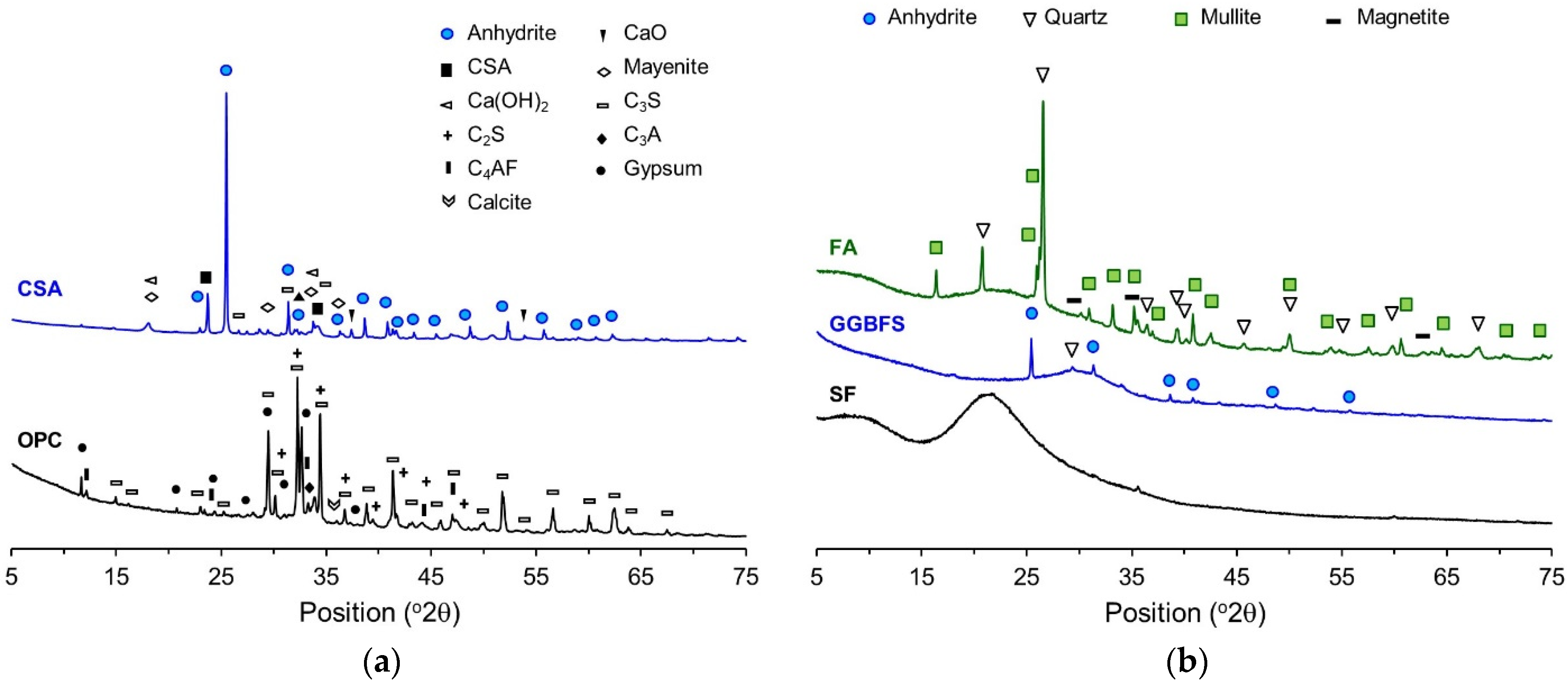
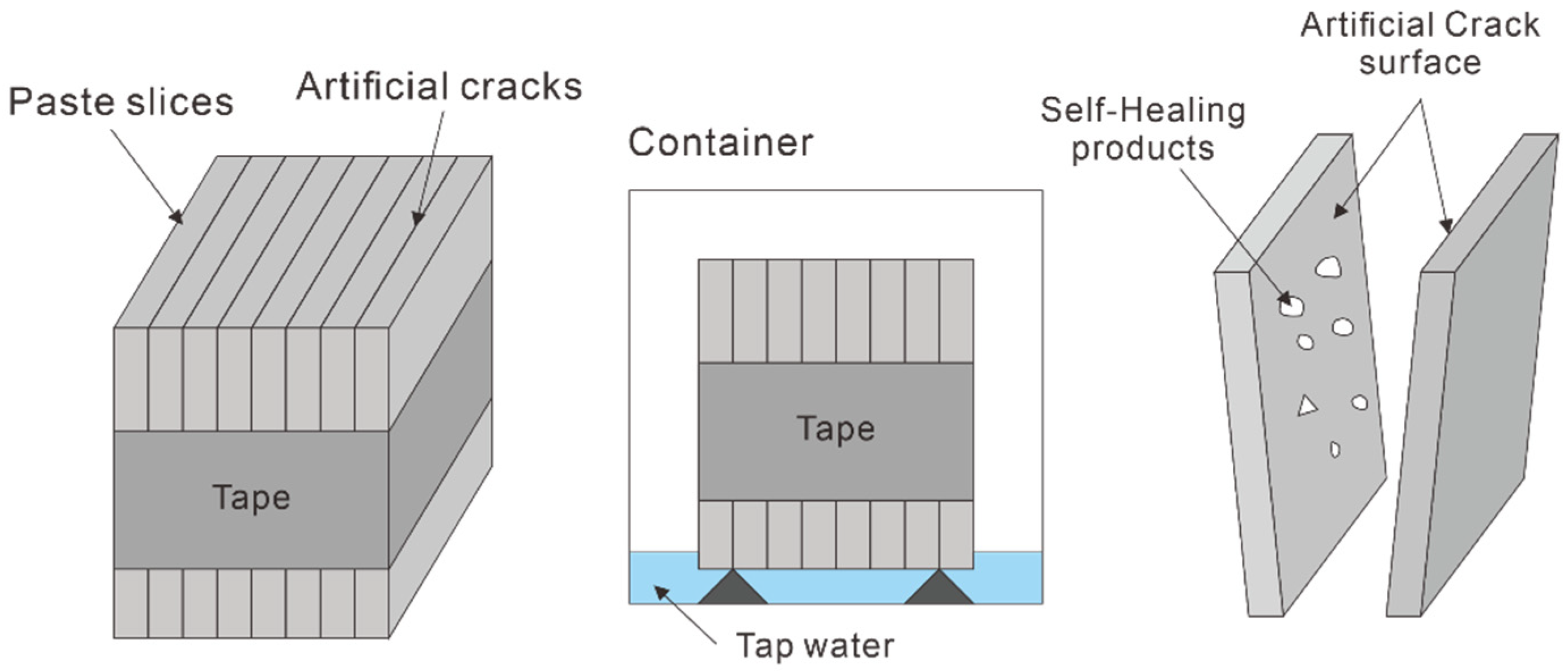

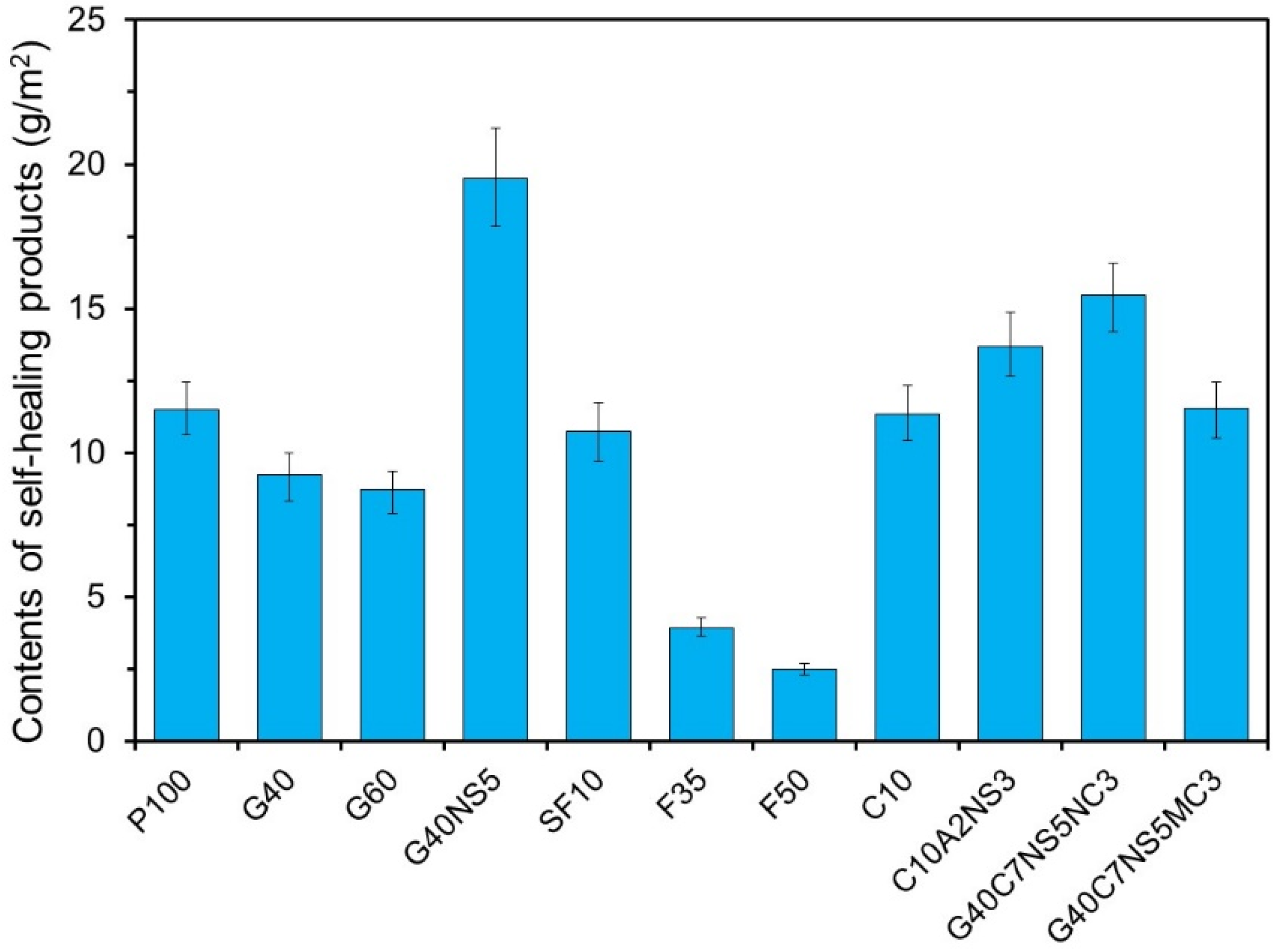


| Chemical Compositions (wt. %) | |||||
|---|---|---|---|---|---|
| OPC | GGBFS | FA | SF | CSA | |
| CaO | 63.13 | 45.2 | 5.24 | 0.17 | 3.6 |
| SiO2 | 21.05 | 29.3 | 52.4 | 91.76 | 10.23 |
| Al2O3 | 4.71 | 13.8 | 24.3 | 0.39 | 0.74 |
| Fe2O3 | 3.23 | 0.53 | 6.96 | 0.9 | 53.74 |
| MgO | 3.06 | 4.13 | 1.46 | 1.23 | 0.78 |
| K2O | 1.67 | 0.45 | 1.56 | 0.97 | 0.2 |
| Na2O | 0.17 | 0.28 | 0.98 | 0.77 | 0.03 |
| SO3 | 1.05 | 3.59 | 2.15 | 0.41 | 0.48 |
| Physical property | |||||
| Density (g/cm3) | 3.14 | 2.9 | 2.15 | 2.35 | 2.73 |
| Blaine fineness (m2/kg) | 376 | 424 | 328 | 17,400 1 | 708 |
| Mean diameter (μm) | 17.47 | 13.16 | 31.96 | 0.15 | 20.45 |
| W/B | Binder (wt. %) | Crystalline Additive (wt. %) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OPC | GGBFS | FA | SF | CSA | Anhydrite | Na2SO4 | Na2CO3 | MgCO3 | ||
| P100 | 0.4 | 100 | - | - | - | - | - | - | - | - |
| G40 | 0.4 | 60 | 40 | - | - | - | - | - | - | - |
| G60 | 0.4 | 40 | 60 | - | - | - | - | - | - | - |
| G40NS5 | 0.4 | 55 | 40 | - | - | - | - | 5 | - | - |
| SF10 | 0.4 | 90 | - | - | 10 | - | - | - | - | - |
| F35 | 0.4 | 65 | - | 35 | - | - | - | - | - | - |
| F50 | 0.4 | 50 | - | 50 | - | - | - | - | - | - |
| C10 | 0.4 | 90 | - | - | - | 10 | - | - | - | - |
| C10A2NS3 | 0.4 | 85 | - | - | - | 10 | 2 | 3 | - | |
| G40C7NS5NC3 | 0.4 | 45 | 40 | - | - | 7 | - | 5 | 3 | - |
| G40C7NS5MC3 | 0.4 | 45 | 40 | - | - | 7 | - | 5 | - | 3 |
| Self-Healing Products (wt. %) | ||||||
|---|---|---|---|---|---|---|
| Amorphous | Calcium Carbonate (CaCO3) | Portlandite (Ca(OH)2) | Alkali Sulfate (K,Na)3Na(SO4)2 | Bayerite (Al(OH)3) | Alkali Carbonate (Na2CO3·H2O) | |
| P100 | 44 | 33 | 23 | - | - | - |
| G40 | 47 | 37 | 14 | - | 2 | - |
| G60 | 63 | 32 | 4 | - | 1 | - |
| G40NS5 | 60 | 31 | 7 | 1 | <1 | 1 |
| SF10 | 54 | 29 | 17 | - | - | |
| F35 | 57 | 32 | 11 | - | - | |
| F50 | 79 | 12 | 9 | - | - | - |
| C10 | 47 | 39 | 2 | 9 | 3 | - |
| C10A2NS3 | 66 | 24 | - | 8 | 2 | - |
| G40C7NS5NC3 | 63 | 16 | - | 18 | - | 3 |
| G40C7NS5MC3 | 73 | 20 | - | 7 | - | <1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, B.; Choi, Y.-C. Self-Healing Products of Cement Pastes with Supplementary Cementitious Materials, Calcium Sulfoaluminate and Crystalline Admixtures. Materials 2021, 14, 7201. https://doi.org/10.3390/ma14237201
Park B, Choi Y-C. Self-Healing Products of Cement Pastes with Supplementary Cementitious Materials, Calcium Sulfoaluminate and Crystalline Admixtures. Materials. 2021; 14(23):7201. https://doi.org/10.3390/ma14237201
Chicago/Turabian StylePark, Byoungsun, and Young-Cheol Choi. 2021. "Self-Healing Products of Cement Pastes with Supplementary Cementitious Materials, Calcium Sulfoaluminate and Crystalline Admixtures" Materials 14, no. 23: 7201. https://doi.org/10.3390/ma14237201






