Synthesis of Mg-Al Hydrotalcite Clay with High Adsorption Capacity
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Mg-Al Metal Oxides Materials
2.3. Characterization of Materials
2.4. Adsorption Experiment
3. Results and Discussion
3.1. Structural Characterization
3.1.1. SEM Analysis
3.1.2. Specific Surface Area and Porosity Analysis
3.1.3. XRD Analysis
3.1.4. FTIR Analysis
3.2. Adsorption Performance
3.2.1. Comparison of Adsorbents
3.2.2. Effect of Precursor Concentration, Reaction Time, Calcination Temperatures, Adsorbent Dosage, and pH
3.2.3. Kinetics of Adsorption
3.2.4. Adsorption Isotherms
3.2.5. Adsorption Thermodynamics
3.2.6. Desorption and Regeneration
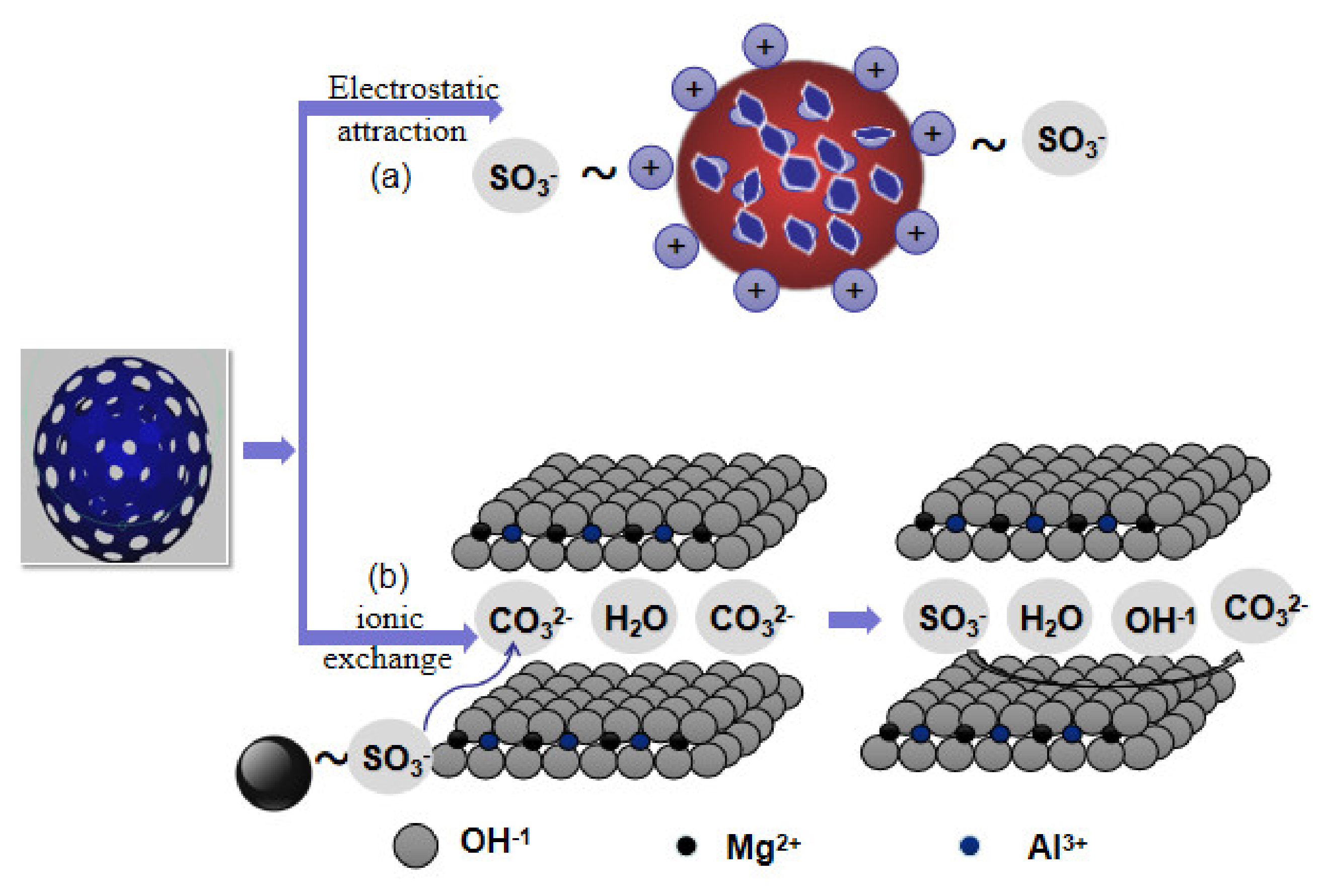
3.3. Formation Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, R.; Wang, B.; Ma, H. Studies on Chromium (VI) adsorption on sulfonated lignite. Desalination 2010, 255, 61–66. [Google Scholar] [CrossRef]
- Can, O. COD removal from fruit-juice production wastewater by electrooxidation electrocoagulation and electro-Fenton processes. Desalin. Water. Treat. 2014, 52, 65–73. [Google Scholar] [CrossRef]
- Xia, T.; Li, Q.; Liu, X.; Meng, A.J.; Cao, X. Morphology-Controllable Synthesis and Characterization of Single-Crystal Molybdenum Trioxide. J. Phys. Chem. B 2006, 110, 2006–2012. [Google Scholar] [CrossRef]
- Sun, M.-H.; Huang, S.-Z.; Chen, L.-H.; Li, Y.; Yang, X.-Y.; Yuan, Z.-Y.; Su, B.-L. ChemInform Abstract: Applications of Hierarchically Structured Porous Materials from Energy Storage and Conversion, Catalysis, Photocatalysis, Adsorption, Separation, and Sensing to Biomedicine. ChemInform Soc. Rev. 2016, 47, 3479–3563. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, G.; Yang, W.; Yang, J.; Li, J. Biotemplated synthesis of hierarchically porous ZnAl-CLDH/FeWO4 for effective removal of dyes from water. Water Air. Soil. 2019, 230, 1–13. [Google Scholar] [CrossRef]
- Wong, W.T.; Chen, B.H.; Maggay, I.V.B.; Liu, C.; Duh, J.G.; Liu, W.R. Inside cover: Synthesis and electrochemical properties of hierarchically porous Zn(Co1−x Mnx)2O4 anodes for Li-ion batteries. Energy Technol. 2017, 9, 1516. [Google Scholar] [CrossRef][Green Version]
- Li, H.; Li, J.; Yang, P.; Ng, D.H.; Cui, X.; Ji, F. Three-dimensional biogenic C-doped Bi2MoO6/In2O3-ZnO Z-scheme hetero-junctions derived from a layered precursor. J. Environ. Sci. 2018, 79, 54–66. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, V.W.; Cai, Y.; Berrigan, J.D.; Marder, S.R.; Perry, J.W.; Sandhage, K.H. Berrigan, Biologically Enabled Syntheses of freestanding metallic structures pos-sessing subwavelength pore arrays for extraordinary (surface plasmon-mediated) infrared transmission. Adv. Funct. Mater. 2012, 22, 2550–2559. [Google Scholar] [CrossRef]
- Matovic, B.; Babic, B.; Egelja, A.; Radosavljevic-Mihajlovic, A.; Logar, V.; Saponjic, A.; Boskovic, S. Preparation of Porous Silica Ceramics Using the Wood Template. Mater. Manuf. Process. 2009, 24, 1109–1113. [Google Scholar] [CrossRef]
- Gao, L.; Li, Q.; Hu, X.; Wang, X.; Song, H.; Yan, L.; Xiao, H. One-pot synthesis of biomorphic Mg-Al mixed metal oxides with enhanced methyl orange adsorption properties. Appl. Clay Sci. 2016, 126, 299–305. [Google Scholar] [CrossRef]
- Pandoli, O.G.; Neto, R.J.; Oliveira, N.R.; Fingolo, A.C.; Corrêa, C.C.; Ghavami, K.; Santhiago, M. Ultra-highly conductive hollow channels guided by a bamboo bio-template for electric and electrochemical devices. J. Mater. Chem. A 2020, 8, 4030–4039. [Google Scholar] [CrossRef]
- Wen-kun, Z.; Xue-gang, L.; Chi, Z.; Tao, D.; Jian, Z. Regulation of Microstructure of Calcium Carbonate Crystals by Egg White Protein. Chem. Res. Chin. Univ. 2021, 2, 180–185. [Google Scholar]
- Sun, R.-Q.; Sun, L.-B.; Chun, Y.; Xu, Q.-H.; Wu, H. Synthesizing nanocrystal-assembled mesoporous magnesium oxide using cotton fibres as exotemplate. Microporous Mesoporous Mater. 2008, 111, 314–322. [Google Scholar] [CrossRef]
- Liu, X.; Gu, Y.; Huang, J. Hierarchical, Titania-Coated, Carbon Nanofibrous Material Derived from a Natural Cellulosic Substance. Chem. A Eur. J. 2010, 16, 7730–7740. [Google Scholar] [CrossRef] [PubMed]
- Parfen’Eva, L.S.; Orlova, T.S.; Smirnov, B.I.; Smirnov, I.A.; Misiorek, H.; Mucha, J.; Jezowski, A.; Rodríguez, R.C.; Ramirez-Rico, J. Thermal conductivity of high-porosity heavily doped biomorphic silicon carbide prepared from sapele wood biocarbon. Phys. Solid State 2012, 54, 1732–1739. [Google Scholar] [CrossRef]
- Dias, J.M.; Alvim-Ferraz, M.C.; Almeida, M.F.; Rivera-Utrilla, J.; Sanchez-Polo, M. Waste materials for activated carbon preparation and its use in aqueous-phase treatment: A review. J. Environ. Manag. 2008, 85, 833–846. [Google Scholar] [CrossRef]
- Xu, Z.P.; Zhang, J.; Adebajo, M.; Zhang, H.; Zhou, C. Catalytic applications of layered double hydroxides and derivatives. Appl. Clay Sci. 2011, 53, 139–150. [Google Scholar] [CrossRef]
- Yu, J.; Lu, L.; Li, J.; Song, P. Biotemplated hierarchical porous-structure of ZnAl-LDH/ZnCo2O4 composites with enhanced adsorption and photocatalytic performance. RSC Adv. 2018, 6, 12797–12808. [Google Scholar] [CrossRef]
- Yu, J.; Lu, L.; Li, J.; Song, P. Magnetic calcinated cobalt ferrite/magnesium aluminum hydrotalcite composite for enhanced adsorption of methyl orange-sciencedirect. J. Alloy. Compd. 2016, 688, 101–112. [Google Scholar]
- Kim, K.C.; Yoon, T.-U.; Bae, Y.-S. Applicability of using CO2 adsorption isotherms to determine BET surface areas of microporous materials. Microporous Mesoporous Mater. 2016, 224, 294–301. [Google Scholar] [CrossRef]
- Kumar, K.Y.; Muralidhara, H.; Nayaka, Y.A.; Balasubramanyam, J.; Hanumanthappa, H. Low-cost synthesis of metal oxide nanoparticles and their application in adsorption of commercial dye and heavy metal ion in aqueous solution. Powder Technol. 2013, 246, 125–136. [Google Scholar] [CrossRef]
- Tian, P.; Han, X.-Y.; Ning, G.-L.; Fang, H.-X.; Ye, J.-W.; Gong, W.-T.; Lin, Y. Synthesis of Porous Hierarchical MgO and Its Superb Adsorption Properties. ACS Appl. Mater. Interfaces 2013, 5, 12411–12418. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, M.; Markovits, A.; Ménétrey, M.; Mguig, B.; Minot, C. Adsorption on perfect and reduced surfaces of metal oxides. Catal. Today 2003, 85, 125–143. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, Y.; Wang, J.; Zhou, C.; Tang, Q.; Rao, X. Calcined graphene/MgAl-layered double hydroxides for enhanced Cr(VI) removal. Chem. Eng. J. 2013, 221, 204–213. [Google Scholar] [CrossRef]
- Duan, N.; Li, Q.; Liu, J.; Xiao, H. Enhanced adsorption performance of CuO-Al2O3 composite derived from cotton template. Can. J. Chem. Eng. 2015, 93, 2015–2023. [Google Scholar] [CrossRef]
- Yuan, D.; Zhou, L.; Fu, D. Adsorption of methyl orange from aqueous solutions by calcined ZnMgAl hydrotalcite. Appl. Phys. A 2017, 123, 146. [Google Scholar] [CrossRef]
- Bing, X.; Li, J.; Liu, J.; Cui, X.; Ji, F. Biomimetic synthesis of Bi2O3/Bi2WO6/MgAl-CLDH hybrids from lotus pollen and their enhanced adsorption and photocatalysis performance. J. Photoch. Photobio. A 2018, 364, 449–460. [Google Scholar] [CrossRef]
- Li, H.-Q.; Huang, G.-H.; An, C.-J.; Zhang, W.-X. Kinetic and equilibrium studies on the adsorption of calcium lignosulfonate from aqueous solution by coal fly ash. Chem. Eng. J. 2012, 200-202, 275–282. [Google Scholar] [CrossRef]
- Ho, Y. The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Lorenc-Grabowska, E.; Gryglewicz, G. Adsorption of lignite-derived humic acids on coal-based mesoporous activated carbons. J. Colloid Interface Sci. 2005, 284, 416–423. [Google Scholar] [CrossRef]
- Guo, Y.; Zhu, Z.; Qiu, Y.; Zhao, J. Enhanced adsorption of acid brown 14 dye on calcined Mg/Fe layered double hydroxide with memory effect. Chem. Eng. J. 2013, 219, 69–77. [Google Scholar] [CrossRef]
- Gupta, V.K.; Pathania, D.; Sharma, S.; Singh, P. Preparation of bio-based porous carbon by microwave assisted phosphoric acid activation and its use for adsorption of Cr(VI). J. Colloid Interface Sci. 2013, 401, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Tanzifi, M.; Hosseini, S.H.; Kiadehi, A.D.; Olazar, M.; Karimipour, K.; Rezaiemehr, R.; Ali, D.I. Artificial neural network optimization for methyl orange adsorption onto polyaniline nano-adsorbent: Kinetic, isotherm and thermodynamic studies. J. Mol. Liq. 2017, 244, 189–200. [Google Scholar] [CrossRef]
- Lv, L.; He, J.; Wei, M.; Evans, D.G.; Duan, X. Uptake of chloride ion from aqueous solution by calcined layered double hy-droxides: Equilibrium and kinetic studies. Water Res. 2006, 40, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Irving, L. Adsorption of gases on glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 361–1403. [Google Scholar]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Liu, Z.; Chen, G.; Xu, L.; Hu, F.; Duan, X. Removal of Cr(VI) from wastewater by a novel adsorbent of magnetic goethite: Ad-sorption performance and adsorbent characterisation. Chemistry. Select. 2019, 47, 13817–13827. [Google Scholar] [CrossRef]
- Ma, Y.-Z.; Zheng, D.-F.; Mo, Z.-Y.; Dong, R.-J.; Qiu, X.-Q. Magnetic lignin-based carbon nanoparticles and the adsorption for removal of methyl orange. Colloids Surf. A: Physicochem. Eng. Asp. 2018, 559, 226–234. [Google Scholar] [CrossRef]
- Yamaguchi, N.U.; Bergamasco, R.; Hamoudi, S. Magnetic MnFe2O4 –graphene hybrid composite for efficient removal of glyphosate from water. Chem. Eng. J. 2016, 295, 391–402. [Google Scholar] [CrossRef]
- Tan, X.; Liu, S.; Liu, Y.; Gu, Y.; Zeng, G.; Cai, X.; Chen, B. One-pot synthesis of carbon supported calcined-Mg/Al layered double hydroxides for antibiotic removal by slow pyrolysis of biomass waste. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]















| Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| Filter paper-templated Mg-Al metal oxides | 178.84 | 0.3177 | 7.10 |
| Without biotemplate | 117.04 | 0.1879 | 6.42 |
| Concentration | Pseudo-First-Order Model | Pseudo-Second-Order Model | |||||
|---|---|---|---|---|---|---|---|
| C0 | qe,exp | qe,cal | K1min−1 | R2 | qe,cal | K2min−1 | R2 |
| 100 | 249.88 | 131.63 | 0.0089 | 0.817 | 268.82 | 0.912 × 10−4 | 0.997 |
| 150 | 373.43 | 193.33 | 0.01054 | 0.8325 | 400.00 | 0.678 × 10−4 | 0.994 |
| 200 | 498.43 | 264.36 | 0.01231 | 0.925 | 520.83 | 0.886 × 10−4 | 0.999 |
| Concentration | Intra-Particle Diffusion Model | |||||
|---|---|---|---|---|---|---|
| C0 | Kd1 | Kd2 | C1 | C2 | R12 | R22 |
| 100 | 5.742 | 1.378 | 127.84 | 217.33 | 0.516 | 0.436 |
| 150 | 3.204 | 0.490 | 251.42 | 361.91 | 0.809 | 0.698 |
| 200 | 10.110 | 1.257 | 312.66 | 469.61 | 0.995 | 0.698 |
| Temperature | Langmuir Isotherm Model | Freundlich Isotherm Model | |||||
|---|---|---|---|---|---|---|---|
| T (°C) | qmax (mg/g) | KL (L/mg) | RL | R2 | KF (mg/g)(L/mg)1/n | 1/n | R2 |
| 25 | 1139.56 | 0.34 | 0.0083–0.0149 | 0.997 | 523 | 0.2039 | 0.992 |
| 30 | 1189.29 | 0.38 | 0.0075–0.0129 | 0.99 | 527.63 | 0.2301 | 0.953 |
| Temperature (K) | ΔG (KJ·mol−1) | ΔH (KJ·mol−1) | ΔS (J·mol−1·K−1) |
|---|---|---|---|
| 298 | −8.51 | 179.43 | 630.67 |
| 303 | −11.66 | ||
| 308 | −14.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhang, J.; Qu, C.; Tang, Y.; Slaný, M. Synthesis of Mg-Al Hydrotalcite Clay with High Adsorption Capacity. Materials 2021, 14, 7231. https://doi.org/10.3390/ma14237231
Li Z, Zhang J, Qu C, Tang Y, Slaný M. Synthesis of Mg-Al Hydrotalcite Clay with High Adsorption Capacity. Materials. 2021; 14(23):7231. https://doi.org/10.3390/ma14237231
Chicago/Turabian StyleLi, Zhaoyi, Jie Zhang, Chengtun Qu, Ying Tang, and Michal Slaný. 2021. "Synthesis of Mg-Al Hydrotalcite Clay with High Adsorption Capacity" Materials 14, no. 23: 7231. https://doi.org/10.3390/ma14237231
APA StyleLi, Z., Zhang, J., Qu, C., Tang, Y., & Slaný, M. (2021). Synthesis of Mg-Al Hydrotalcite Clay with High Adsorption Capacity. Materials, 14(23), 7231. https://doi.org/10.3390/ma14237231







