Experimental Study on the Corrosion of Carbon Steel and Aluminum Alloy in Firefighting Protein Foam Concentrates
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Long Exposure Study by Weight Losses
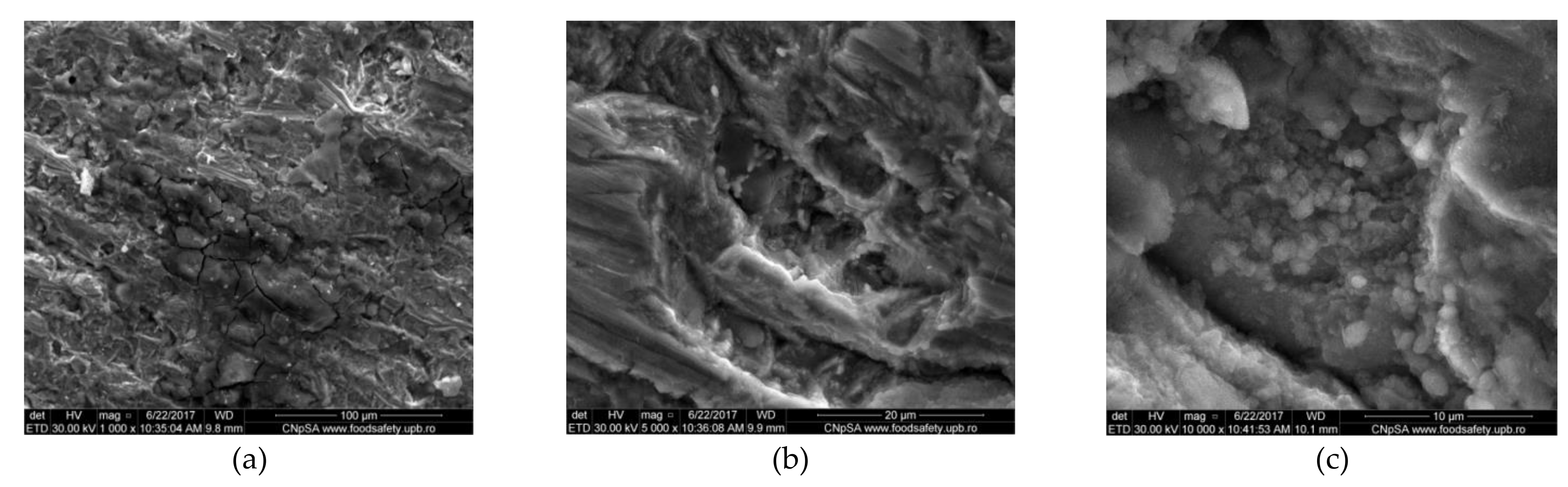
3.1.1. Corrosion of Carbon Steel
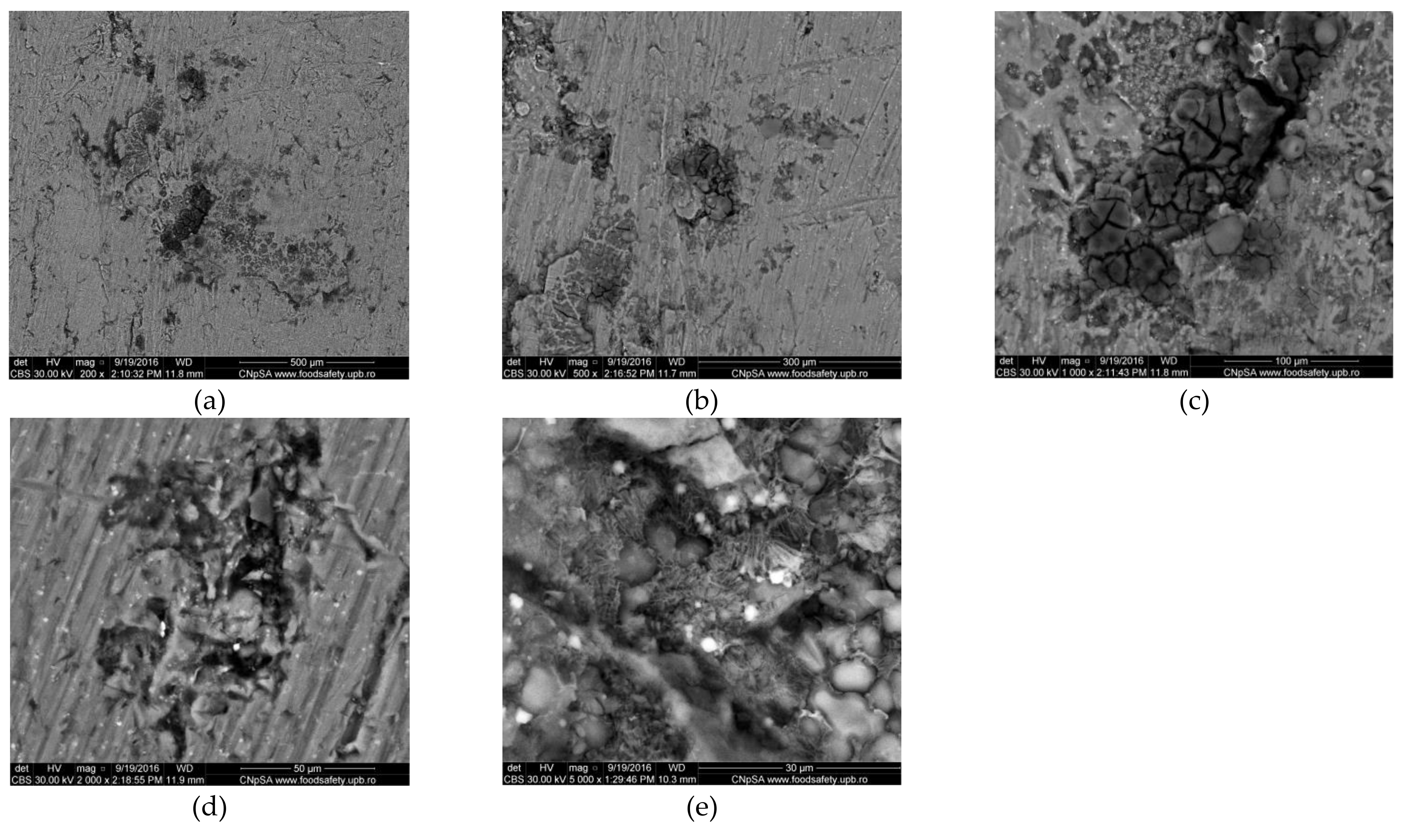
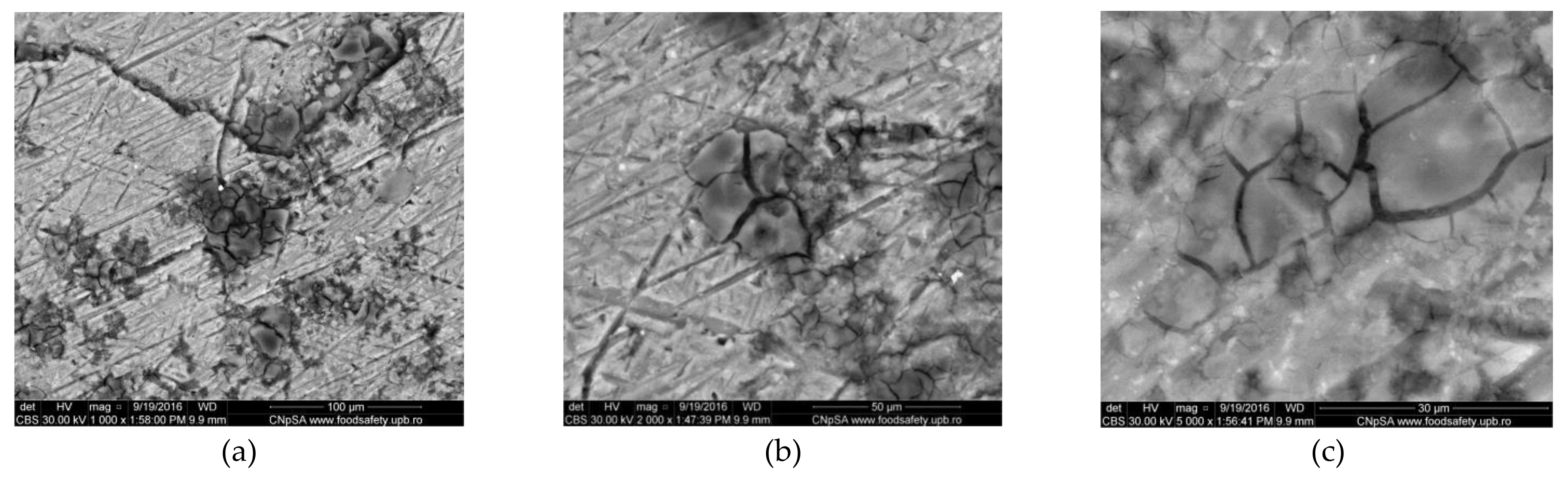
3.1.2. Corrosion of Al Alloy (AlSiCuMg Type)
3.2. Studies of the Accelerated Corrosion Using Electrochemical Methods
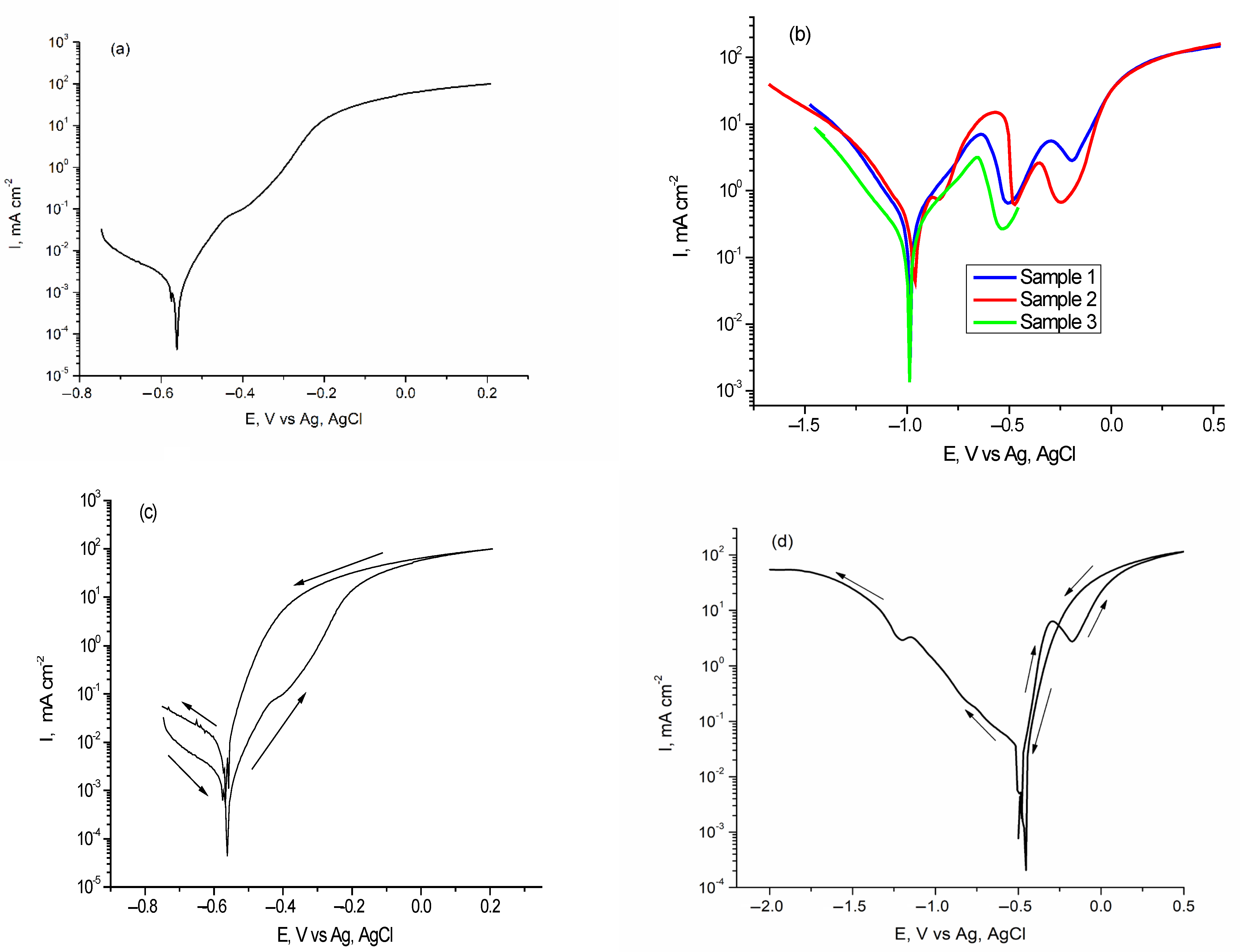
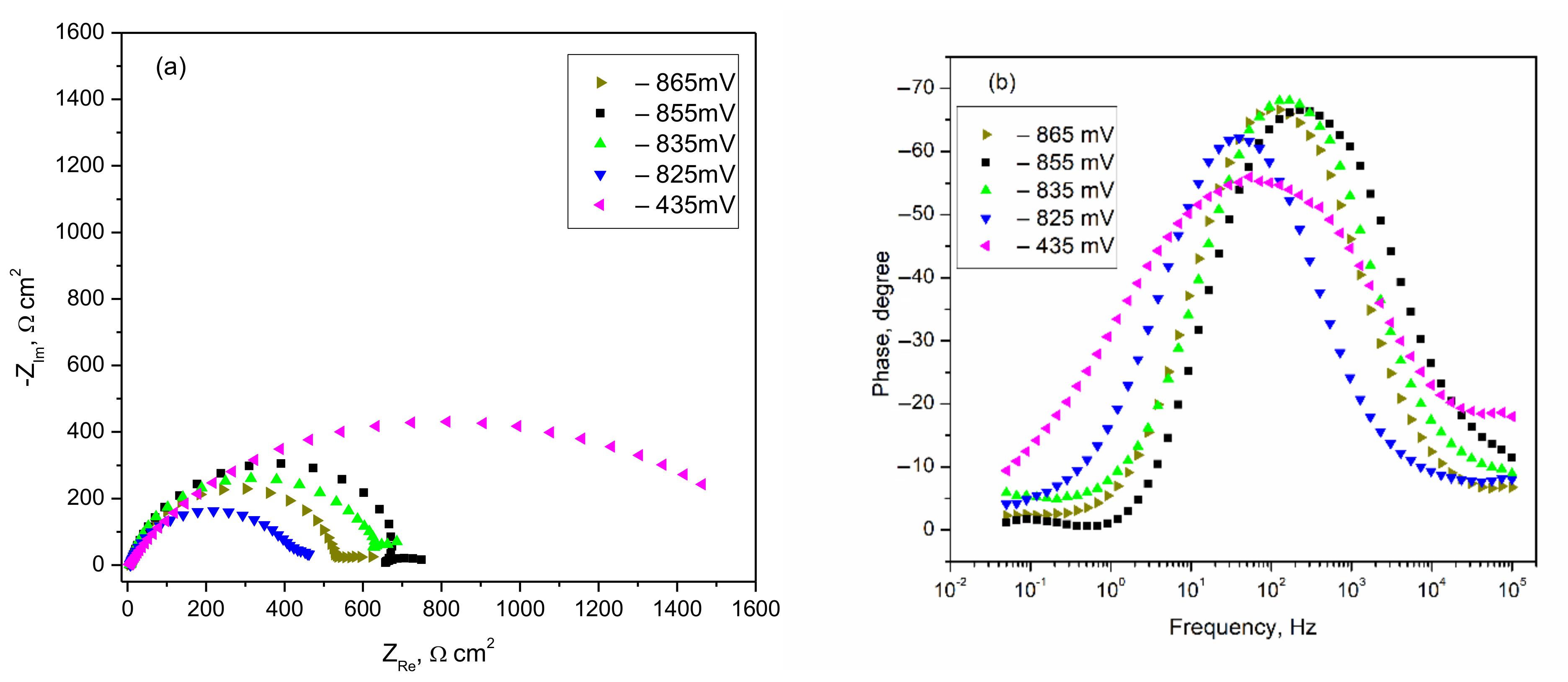
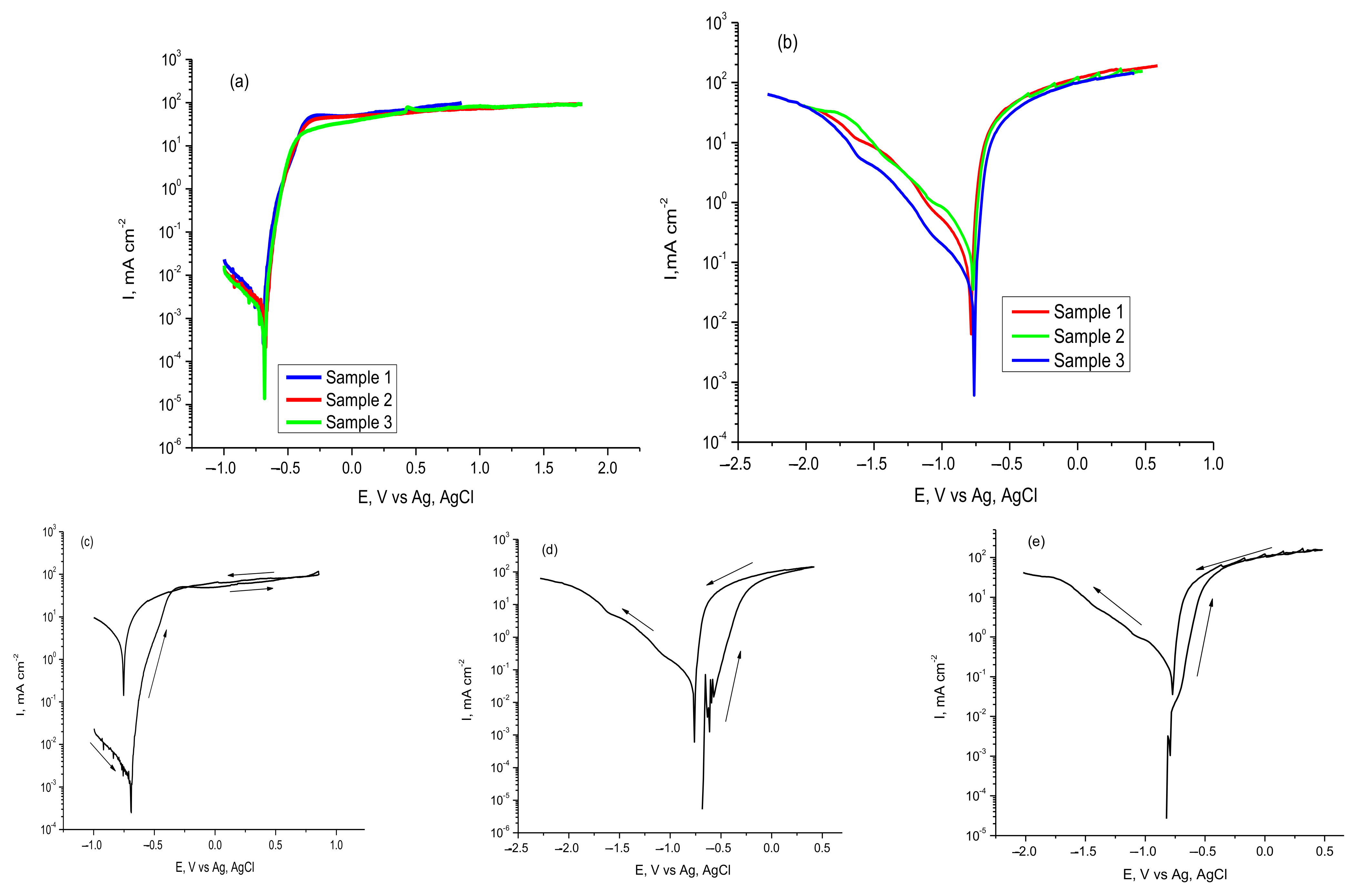
3.2.1. Polarization Curves and EIS Spectra of Carbon Steel
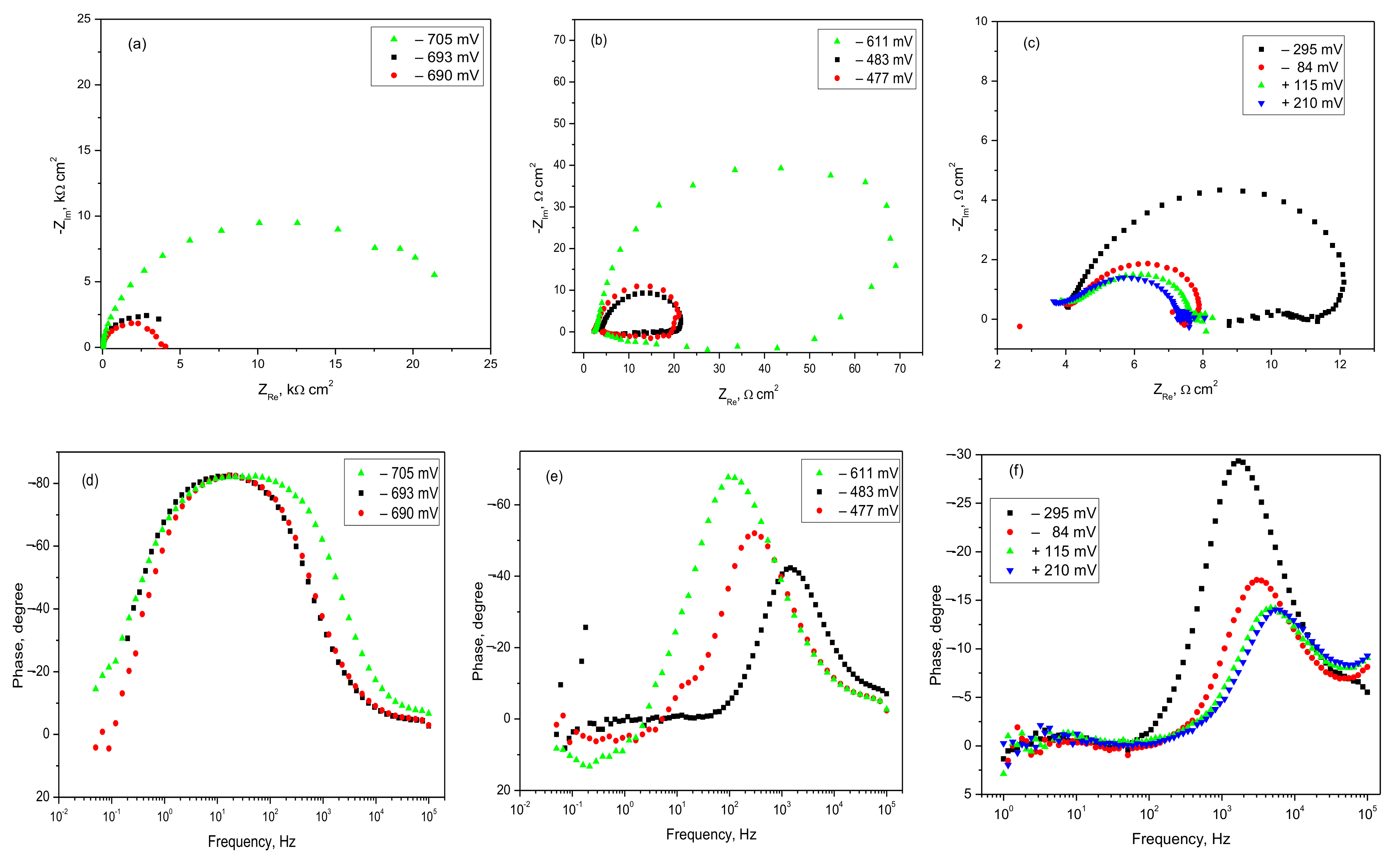
3.2.2. Polarization Curves and EIS Spectra of AlSiCuMg Alloy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foorginezhad, S.; Mohseni-Dargah, M.; Firoozirad, K.; Aryai, V.; Razmjou, A.; Abbassi, R.; Garaniya, V.; Beheshti, A.; Asadnia, M. Recent advances in sensing and assessment of corrosion in sewage pipelines. Process Saf. Environ. Prot. 2021, 147, 192–213. [Google Scholar] [CrossRef]
- Bertschy, A.V.; Peterson, H.B. Corrosion Resistance of Some Common Metals to Concentrated and 6% Solutions of Light Water Fire-Extinguishing Agent; NRL Report 6932; Naval Research Laboratory: Washington, DC, USA, 1969. [Google Scholar]
- Scheffey, J.L.; Wright, J.A. Analysis of Test Criteria for Specifying Foam Firefighting Agents for Aircraft Rescue and Firefighting. Appendix A.; Report DOT/ FAA/CT-94-04; FAA Technical Center: Atlantic City, NJ, USA, 1994. [Google Scholar]
- Su, P.; Fuller, D.B. Corrosion and Corrosion Mitigation in Fire Protection Systems; Res Tech Report, 0003040794; FM Global: Johnston, RI, USA, 2014. [Google Scholar]
- Radwan, K.; Rakowska, J.; Ślosorz, Z. Impact of surfactants used in extinguishing agent to corrosiveness of firefighting equipment, chapter 27. In Advances and Trends in Engineering Sciences and Technologies; Al Ali, M., Platko, P., Eds.; CRC Press/Balkema: London, UK, 2015; pp. 159–164. [Google Scholar]
- Tihen, J. Corrosion in Fire Sprinkler Systems. Potter Signal Electric Co. 2014. Available online: https://www.abspk.org.br/CBSpk/Apresentacoes/Dia05-15%20-%20Painel%203%20-%20JOSH%20TIHEN.pdf?pdf=PDF-05-15 (accessed on 4 August 2018).
- Ryan Fireprotection Inc. The Solution for Corrosion in a Fire Sprinkler System. 2014. Available online: https://www.ryanfp.com/solution-for-corrosion/ (accessed on 4 August 2018).
- FM Global. Corrosion in Automatic Sprinkler Systems. In FM Global Property Loss Prevention Data Sheets 2-1. 2016, pp. 1–19. Available online: https://unitedfiresystems.net/wp-content/uploads/2018/11/01-Loss-Prevention-Data-Sheet-2-1-Corrosion-in-Automatic-Sprinkler-Systems-FM-Global-Oct-20161.pdf (accessed on 4 August 2018).
- Merwin, J. Corrosion in Fire Sprinkler Systems Increasing Your Liability, Increasing Your Costs, and Shortening System Life. Potter/Corrosion Solutions:1–5. 2016. Available online: www.ifma-afc.org/docs/submissions/20160718_AFC_PotterCorrosionSolutions.pdf (accessed on 4 August 2018).
- O’Connor, J. Inhibiting sprinkler pipe corrosion with nitrogen generation technology. Int. Fire Prot. 2017, 98–100. [Google Scholar]
- Knurek, M. The Problem of Corrosion in Metal Fire Sprinkler Systems. Gulf Fire 5. 2017. Available online: https://gulffire.mdmpublishing.com/the-problem-of-corrosion-in-metal-fire-sprinkler-systems/ (accessed on 4 August 2018).
- Broadley, S. Corrosion in Fire Sprinkler Systems. Potter/Corrosion Solutions. 2017. Available online: https://www.fgsikring.no/.../stephen-broadley---potter-electric-signal-company.pdf (accessed on 4 August 2018).
- Hopkins, M. An Introduction to Corrosion in Sprinkler Systems: Its Identification and Mitigation; National Fire Sprinkler Association (NFSA): Anne Arundel, MD, USA, 2018; p. 2019. [Google Scholar]
- Quick Response Fire Supply. #223, Fire Sprinkler System Pipe Material: NFPA Requirements and the Pros and Cons of Steel; QRFS: Ashland, VA, USA, 2019. [Google Scholar]
- Carpen, L.; Ohligschläger, T. A case study on corrosion of stainless steel in firewater mains. In Proceedings of the 6th European Stainless Steel Conference—Science and Market, Helsinki, Finland, 10–13 June 2008; pp. 129–135. [Google Scholar]
- Carpen, L. Corrosion of Stainless Steel in Fire Protection Systems; Res Report VTT-R-01556-08; Technical Research Centre of Finland: Espoo, Finland, 2008. [Google Scholar]
- Su, P.; Doerr, W.W. Fire protection sprinkler system for extremely corrosive industrial duct environments. Process Saf. Prog. 2010, 29, 70–2978. [Google Scholar] [CrossRef]
- Su, P.; Tatar, F.W.; Chivukula, S.; Doerr, W.W. Weld Seam Corrosion of Steel Sprinkler Pipe; NACE Int Conf: Orlando, FL, USA, 2013; p. 2140. [Google Scholar]
- Su, P.; Fuller, D.B. Steel piping material corrosion: Dry and pre-action fire protection systems. Sprink. Age 2013, 22–26. [Google Scholar]
- Su, P.; Chivukula, S.; Tatar, F.W.; Swinnerton, B. Corrosion of Sprinkler Piping under Compressed Nitrogen and Air Supervision; NACE Int Conf: Dallas, TX, USA, 2015; p. 5548. [Google Scholar]
- Laundess, A.J.; Rayson, M.S.; Dlugogorski, B.Z.; Kennedy, E.M. Small-scale test protocol for firefighting foams DEF(AUST)5706: Effect of bubble size distribution and expansion ratio. Fire Technol. 2011, 47, 149–162. [Google Scholar] [CrossRef] [Green Version]
- Moe, M.K.; Huber, S.; Svenson, J.; Hagenaars, A.; Pabon, M.; Trümper, M.; Berger, U.; Knapen, D.; Herzke, D. The structure of the fire fighting foam surfactant Forafac® 1157 and its biological and photolytic transformation products. Chemosphere 2012, 89, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Laundess, A.J.; Rayson, M.S.; Dlugogorski, B.Z.; Kennedy, E.M. Suppression performance comparison for aspirated, compressed-air and in situ chemically generated class B foams. Fire Technol. 2012, 48, 625–640. [Google Scholar] [CrossRef]
- Castro, J. Foam concentrates for the 21st century. Ind. Fire J. 2015, 36–41. [Google Scholar]
- Wang, Q.; Shao, G.; Duan, Q.; Chen, M.; Li, Y.; Wu, K.; Liu, B.; Peng, P.; Sun, J. The efficiency of heptafluoropropane fire extinguishing agent on suppressing the lithium titanate battery fire. Fire Technol. 2016, 52, 387–396. [Google Scholar] [CrossRef]
- Scheffey, J.L. Foam agents and AFFF system design considerations, chapter 47. In SFPE Handbook of Fire Protection Engineering, 5th ed.; Hurley, M., Ed.; Springer: Cham, Switzerland, 2016; pp. 1646–1706. [Google Scholar]
- Korzeniowski, S.H.; Buck, R.C.; Kempisty, D.M.; Pabon, M. Fluorosurfactants in firefighting foams: Past and present, chapter 1. In Perfluoroalkyl Substances in the Environment: Theory, Practice and Innovation; Kempisty, D.M., Xing, Y., Racz, L.A., Eds.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Zaggia, A.; Conte, L.; Padoan, G.; Bertani, R. Synthesis and application of perfluoroalkyl quaternary ammonium salts in protein-based fire-fighting foam concentrates. J. Surfact. Deterg. 2010, 13, 33–40. [Google Scholar] [CrossRef]
- Zhao, M.; Ni, X.; Zhang, S.; Cao, W.; Guan, Y.; Liang, C.; Wang, X.; Zhang, H. Improving the performance of fluoroprotein foam in extinguishing gasoline pool fires with addition of bromofluoropropene. Fire Mater. 2016, 40, 261–272. [Google Scholar] [CrossRef]
- Kang, W.; Yan, L.; Ding, F.; Guo, X.; Xu, Z. Experimental study on fire-extinguishing efficiency of protein foam in diesel pool fire. Case Stud. Therm. Eng. 2019, 16, 100557. [Google Scholar] [CrossRef]
- Hinnant, K.M.; Conroy, M.W.; Ananth, R. Influence of fuel on foam degradation for fluorinated and fluorine-free foams. Colloids Surf. A 2017, 522, 1–17. [Google Scholar] [CrossRef]
- Hinnant, K.M.; Giles, S.L.; Ananth, R. Measuring fuel transport through fluorocarbon and fluorine-free firefighting foams. Fire Saf. J. 2017, 91, 653–661. [Google Scholar]
- Hinnant, K.M.; Giles, S.L.; Snow, A.W.; Farley, J.P.; Fleming, J.W.; Ananth, R. An analytically defined fire-suppressing foam formulation for evaluation of fluorosurfactant replacement. J. Surfact. Deterg. 2018, 21, 711–722. [Google Scholar] [CrossRef]
- Giles, S.L.; Snow, A.W.; Hinnant, K.M.; Ananth, R. Modulation of fluorocarbon surfactant diffusion with diethylene glycol butyl ether for improved foam characteristics and fire suppression. Colloids Surf. A 2019, 579, 123660. [Google Scholar] [CrossRef]
- Ananth, R.; Snow, A.W.; Hinnant, K.M.; Giles, S.L.; Farley, J.P. Synergisms between siloxane-polyoxyethylene and alkyl polyglycoside surfactants in foam stability and pool fire extinction. Colloids Surf. A 2019, 579, 123686. [Google Scholar] [CrossRef]
- Hinnant, K.M.; Giles, S.L.; Smith, E.P.; Snow, A.W.; Ananth, R. Characterizing the role of fluorocarbon and hydrocarbon surfactants in firefighting-foam formulations for fire-suppression. Fire Technol. 2020, 56, 1413–1441. [Google Scholar] [CrossRef]
- Schaefer, T.H.; Dlugogorski, B.Z.; Kennedy, E.M. Sealability properties of fluorine-free fire-fighting foams (FfreeF). Fire Technol. 2008, 44, 297–309. [Google Scholar] [CrossRef]
- Wang, P. Application of green surfactants developing environment friendly foam extinguishing agent. Fire Technol. 2014, 51, 503–511. [Google Scholar] [CrossRef]
- Sheng, Y.; Jiang, N.; Sun, X.; Lu, S.; Li, C. Experimental study on effect of foam stabilizers on aqueous film-forming foam. Fire Technol. 2018, 54, 211–228. [Google Scholar] [CrossRef]
- Sheng, Y.; Jiang, N.; Lu, S.; Wang, Q.; Zhao, Y.; Liu, X. Study of environmental-friendly firefighting foam based on the mixture of hydrocarbon and silicone surfactants. Fire Technol. 2020, 56, 1059–1075. [Google Scholar] [CrossRef]
- Wood Comp; Ramboll Comp; COWI Group. The Use of PFAS and Fluorine-Free Alternatives in Fire-Fighting Foams—Final Report; Report for the European Commission DG Environment and European Chemicals Agency. 2020. Available online: https://echa.europa.eu/documents/10162/28801697/pfas_flourine-free_alternatives_fire_fighting_en.pdf/d5b24e2a-d027-0168-cdd8-f723c675fa98 (accessed on 1 September 2018).
- Solberg Company Bergen, Norway Re-Healing Foam; Technical Reference Guide, Section 2. In Foam Systems Design and Application Manual. 2017, p. 11. Available online: https://ufppro.com/wp-content/uploads/2018/03/Kopiya-SOLBERG-Design-Application-Manual-F-2013014-6_EN-1.pdf (accessed on 1 September 2018).
- Tyco Fire Protection Products, Lansdale, PA, US, Acceptable Materials of Construction for Use with Firefighting Foam Concentrates. Techn. Bull. T-2016111. 2016. Available online: https://www.williamsfire.com/uploads/media/T-2016111_-_Acceptable_Materials_of_Construction.pdf (accessed on 1 September 2018).
- Safyari, M.; Hojo, T.; Moshtaghi, M. Effect of environmental relative humidity on hydrogen-induced mechanical degradation in an Al–Zn–Mg–Cu alloy. Vacuum 2021, 192, 110489. [Google Scholar] [CrossRef]
- Moshtaghi, M.; Safyari, M.; Kuramoto, S.; Hojo, T. Unraveling the effect of dislocations and deformation-induced boundaries on environmental hydrogen embrittlement behavior of a cold-rolled Al-Zn-Mg-Cu alloy. Int. J. Hydrog. Energy 2021, 46, 8285–8299. [Google Scholar] [CrossRef]
- Peterson, H.B.; Burnett, J.C. The Corrosive Effects of Protein-Type Foam-Forming Concentrates on Common Metals and Dissimilar Metal Couples; NRL Report 4947; Naval Research Laboratory: Washington, DC, USA, 1957. [Google Scholar]
- Rakowska, J.; Ślosorz, Z. Corrosion of fire estinguishing systems and firefighting fittings. Saf. Fire Tech. 2011, 24, 113–120. [Google Scholar]
- Radwan, K.; Rakowska, J.; Slosorz, Z. Corrosion of fire fighting equipment caused by surfactants used in extinguishing agent. In Proceedings of the International Conference in Engineering Sciences and Technologies (ESaT 2015), Tatranska Strba, High Tatras Mountains, Slovakia, 27–29 May 2015. [Google Scholar]
- Kostyaev, A.A.; Balmasov, A.V.; Inasaridze, L.N. Effect of inhibitors on carbon steel corrosion in foaming solutions. Russ. J. Gen. Chem. 2016, 86, 429–433. [Google Scholar] [CrossRef]
- Elahresh, N.; Jewilli, F. Evaluation of fire extinguishing foam concentrate for 304 stainless steel. In Proceedings of the 4th International Technology of Oil and Gas, TOG 2008, Tripoli, Libya, 21–23 October 2008; p. TOG-78. [Google Scholar]
- De la Fuente, D.; Díaz, I.; Alcantara, J.; Chico, B.; Simancas, J.; Llorente, I.; García-Delgado, A.; Jimenez, J.A.; Adeva, P.; Morcillo, M. Corrosion mechanisms of mild steel in chloride-rich atmospheres. Mater. Corros. 2016, 67, 227–238. [Google Scholar] [CrossRef]
- Huang, W.H.; Yen, H.W.; Lee, Y.L. Corrosion behavior and surface analysis of 690 Mpa-grade offshore steels in chloride media. J. Mater. Res. Technol. 2019, 8, 1476–1485. [Google Scholar] [CrossRef]
- Xiao, H.; Ye, W.; Song, X.; Ma, Y.; Li, Y. Evolution of akaganeite in rust layers formed on steel submitted to wet/dry cyclic tests. Materials 2017, 10, 1262. [Google Scholar] [CrossRef] [Green Version]
- Kocijan, A.; Milosev, I.; Pihlar, B. The influence of complexing agent and proteins on the corrosion of stainless steels and their metal components. J. Mater. Sci. Mater. Med. 2003, 14, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Rojas, C.R.; Lago, M.E. Effects of proteins in corrosion of stainless steel used in traumatology. Corros. Rev. 2005, 23, 445–459. [Google Scholar] [CrossRef]
- Geringer, J.; Pellier, J.; Taylor, M.L.; Macdonald, D.D. Fretting corrosion with proteins: The role of organic coating on the synergistic mechanisms. Thin Solid Films 2013, 528, 123–129. [Google Scholar] [CrossRef]
- Hedberg, Y.S. Role of proteins in the degradation of relatively inert alloys in the human body. Mater. Degrad. 2018, 2, 26–31. [Google Scholar] [CrossRef]
- Atapour, M.; Wallinder, I.O.; Hedberg, Y. Stainless steel in simulated milk and whey protein solutions—Influence of grade on corrosion and metal release. Electrochim. Acta 2020, 331, 135428. [Google Scholar] [CrossRef]
- Wagener, V.; Faltz, A.-S.; Killian, M.S.; Schmuki, P.; Virtanen, S. Protein interactions with corroding metal surfaces: Comparison of Mg and Fe. Faraday Discuss. 2015, 180, 347–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salem, Y.; Oudbashi, O.; Eid, D. Characterization of the microstructural features and the rust layers of an archaeological iron sword in the Egyptian Museum in Cairo (380–500 A.D.). Heritage Sci. 2019, 7, 19. [Google Scholar] [CrossRef]
- Mobin, M.; Aslam, R. Experimental and theoretical study on corrosion inhibition performance of environmentally benign non-ionic surfactants for mild steel in 3.5% NaCl solution. Process Saf. Environ. Prot. 2018, 114, 279–295. [Google Scholar] [CrossRef]
- Rabizadeh, T.; Asl, S.K. Casein as a natural protein to inhibit the corrosion of mild steel in HCl solution. J. Mol. Liq. 2019, 276, 694–704. [Google Scholar] [CrossRef]
- Hendy, A.; El-Nady, J.; Hassan, N.; Tamer, T.M.; Ahmed, A.S.I.; Abou Shahba, R.M. Corrosion inhibition of casein as a natural protein for 316L stainless steel in simulated body fluid. Al-Azhar Bull. Sci. 2020, 31, 11–18. [Google Scholar] [CrossRef]
- Clark, G.C.F.; Williams, D.F. The effects of proteins on metallic corrosion. J. Biomed. Mater. Res. A 1982, 16, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Mueller, H.J. Effects of proteins on metal corrosion. In Proceeding of the International Metallographic Society Technical Meeting—IMS Technical Meeting, ASM International, Ottawa, ON, Canada, 1–4 November 1999. [Google Scholar]
- Boag, A.; Hughes, A.E.; Glenn, A.M.; Muster, T.H.; McCulloch, D. Corrosion of AA2024-T3 Part I: Localised corrosion of isolated IM particles. Corros. Sci. 2011, 53, 17–26. [Google Scholar] [CrossRef]


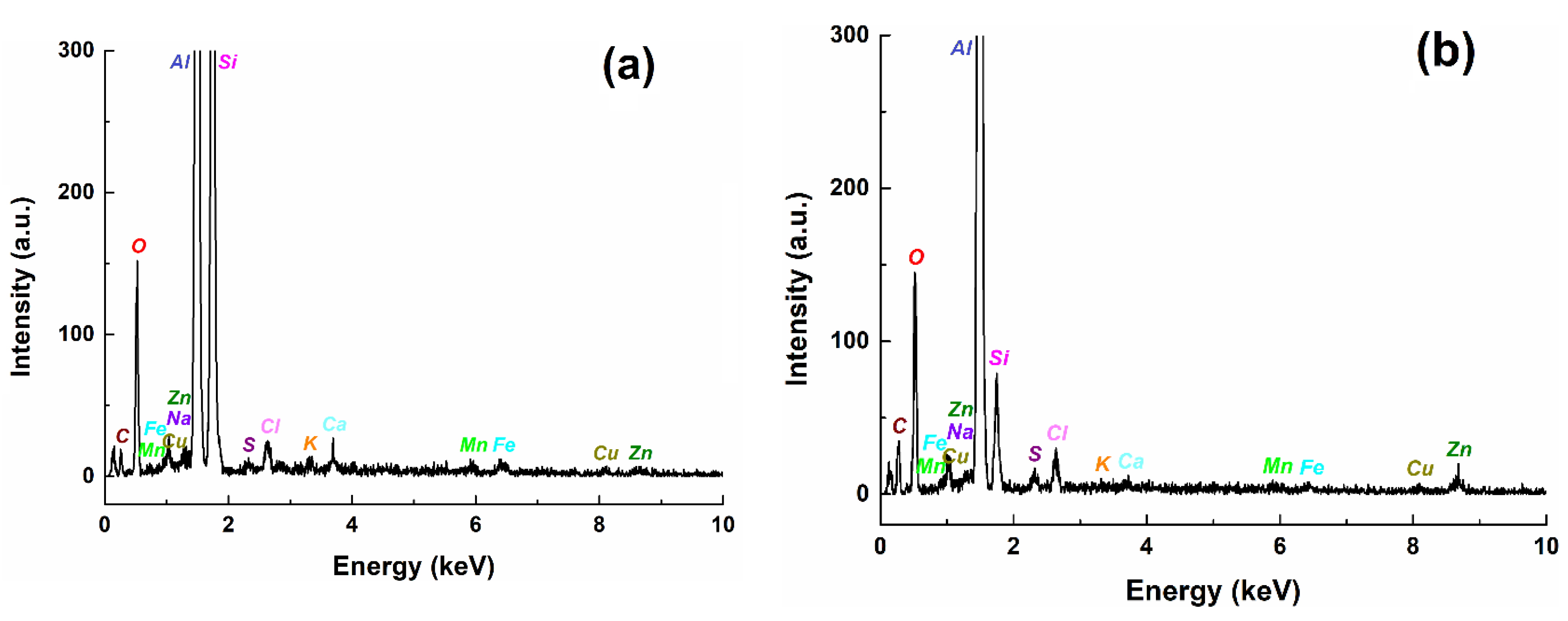

| Steel Sample | kg (mdd) after Different Immersion Periods of Time. in Brackets: Penetration Rate (mipy) | ||||
|---|---|---|---|---|---|
| 29 Days | 63 Days | 81 Days | 232 Days | 348 Days | |
| Fomtec P 6% concentrate | |||||
| 1 | 2.551 (0.466) | 3.233 (0.591) | 3.278 (0.599) | 2.990 (0.547) | 2.962 (0.543) |
| 2 | 2.654 (0.484) | 3.190 (0.583) | 3.204 (0.587) | 3.396 (0.622) | 3.672 (0.673) |
| 3 | 3.552 (0.650) | 4.258 (0.779) | 4.236 (0.776) | 5.568 (1.016) | 5.594 (1.020) |
| Average | 2.919 ± 0.550 (0.533 ± 0.060) | 3.560 ± 0.604 (0.651 ± 0.111) | 3.573 ± 0.576 (0.654 ± 0.106) | 3.985 ± 1.386 (0.728 ± 0.252) | 4.076 ± 1.362 (0.745 ± 0.247) |
| 6% P Profoam 806 concentrate | |||||
| 4 | 7.174 (1.311) | 9.602 (1.756) | 11.424 (2.087) | 8.693 and 7.226 * (1.589 and 1.311 *) | 10.205 (1.866) |
| 5 | 8.798 (1.610) | 13.994 (2.559) | 14.304 (2.614) | 11.194 and 9.523 * (2.047 and 1.740 *) | 12.180 (2.228) |
| 6 | 7.411 (1.354) | 9.890 (1.807) | 11.513 (2.106) | 9.103 and 7.810 * (1.665 and 1.429 *) | 10.586 (1.937) |
| Average | 7.794 ± 0.877 (1.425 ± 0.162) | 11.162 ± 2.457 (2.041 ± 0.446) | 12.414 ± 1.482 (2.269 ± 0.299) | 9.663 ± 1.341 (1.767 ± 0.245) | 10.990 ± 1.012 (2.010 ± 0.192) |
| Chemical Composition | Elements | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Mn | S | Ca | Na | K | C | O | N | Cl | |
| wt% | 5.65 | 1.05 | 0.67 | 0.48 | 5.48 | 0.20 | 36.98 | 34.69 | 10.72 | 4.08 |
| at% | 1.55 | 0.25 | 0.32 | 0.18 | 3.65 | 0.08 | 47.22 | 33.25 | 11.74 | 1.76 |
| Chem. Compos. | Elements | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Si | Cu | Fe | Mn | Zn | Ca | Na | K | S | C | O | Cl | |
| Corroded Al alloy in Fomtec | |||||||||||||
| wt% | 35.25 | 33.92 | 0.35 | 0.54 | 0.39 | 0.46 | 0.51 | 0.49 | 0.26 | 0.34 | - | 26.20 | 1.28 |
| at% | 30.61 | 28.29 | 0.13 | 0.23 | 0.17 | 0.17 | 0.30 | 0.50 | 0.16 | 0.25 | - | 38.37 | 0.85 |
| Corroded Al alloy in Profoam | |||||||||||||
| wt% | 40.90 | 4.40 | 0.47 | 0.33 | 0.48 | 1.28 | - | 0.33 | - | 0.47 | 20.95 | 29.42 | 0.98 |
| at% | 28.31 | 2.93 | 0.14 | 0.11 | 0.16 | 0.37 | - | 0.27 | - | 0.28 | 32.58 | 34.34 | 0.52 |
| Corrosion System: Steel/Foam Agent | OCP, mV | Ecorr vs. Ag/AgCl, mV | Icorr, μA/cm2 | CR, mipy |
|---|---|---|---|---|
| Sample 1 in Fomtec | −526 | −561 | 43.5 | 19.887 |
| Sample 2 in Fomtec (cyclic) | −538 | −566 | 48.2 | 22.036 |
| Sample 3 in Fomtec (cyclic) | −501 | −538 | 64.6 | 29.533 |
| Average: | 52.1 ± 10.98 | 23.818 ± 5.06 | ||
| Sample 1 in Profoam | −862 | −984 | 194 | 88.690 |
| Sample 2 in Profoam | −887 | −929 | 238 | 108.806 |
| Sample 3 in Profoam | −865 | −987 | 172 | 78.633 |
| Average: | 201.3 ± 33.60 | 92.043 ± 15.36 |
| Anodic Polarization, mV | Rs, Ωcm2 | CPE−T, μF/cm2 | CPE1−p Exponent | Rp, Ωcm2 |
|---|---|---|---|---|
| −865 | 7.2 | 48.54 | 0.875 | 550 |
| −855 | 6.8 | 23.33 | 0.862 | 699 |
| −845 | 6.4 | 29.83 | 0.890 | 526 |
| −835 | 6.4 | 126.49 | 0.746 | 605 |
| −825 | 7.7 | 177.33 | 0.825 | 439 |
| −795 | 5.9 | 41.29 | 0.852 | 1180 |
| −435 | 5.1 | 204.44 | 0.664 | 1558 |
| Corrosion System: Al Alloy/Foam Agent | OCP, mV | Ecorr vs. Ag/AgCl, mV | Icorr, μA/cm2 | CR, Mipy |
|---|---|---|---|---|
| Sample 1 in Fomtec | −705 | −695 | 1.45 | 0.606 |
| Sample 2 in Fomtec | −680 | −677 | 1.93 | 0.806 |
| Sample 3 in Fomtec | −676 | −677 | 1.32 | 0.551 |
| Average: | 1.57 ± 0.321 | 0.654 ± 0.134 | ||
| Sample 1 in Profoam | −745 | −747 | 10.0 | 4.178 |
| Sample 2 in Profoam | −729 | −713 | 7.1 | 2.966 |
| Sample 3 in Profoam | −776 | −781 | 9.1 | 3.802 |
| Sample 4 in Profoam | −705 | −702 | 6.3 | 2.632 |
| Average: | 8.12 ± 1.717 | 3.394 ± 0.718 |
| Anodic Polarization, mV | Rs, Ωcm2 | CPE-T, μF/cm2 | CPE-p | Rp, Ωcm2 |
|---|---|---|---|---|
| −705 | 3.3 | 111.98 | 0.939 | 25352 |
| −693 | 3.2 | 97.01 | 0.948 | 5933 |
| −690 | 4.4 | 28.60 | 0.926 | 2831 |
| −611 | 3.0 | 125.47 | 0.926 | 78 |
| −483 | 4.1 | 10.95 | 1.010 | 17 |
| −477 | 2.8 | 99.39 | 0.938 | 16 |
| −295 | 4.2 | 13.58 | 1.018 | 7.4 |
| −84 | 4.2 | 13.06 | 1.016 | 3.5 |
| +115 | 4.2 | 24.50 | 0.920 | 3.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patrascu, M.T.; Busuioc, A.D.; Busuioc, C.; Cotarta, A.; Cojocaru, A.; Visan, T.; Vaireanu, D.I. Experimental Study on the Corrosion of Carbon Steel and Aluminum Alloy in Firefighting Protein Foam Concentrates. Materials 2021, 14, 7259. https://doi.org/10.3390/ma14237259
Patrascu MT, Busuioc AD, Busuioc C, Cotarta A, Cojocaru A, Visan T, Vaireanu DI. Experimental Study on the Corrosion of Carbon Steel and Aluminum Alloy in Firefighting Protein Foam Concentrates. Materials. 2021; 14(23):7259. https://doi.org/10.3390/ma14237259
Chicago/Turabian StylePatrascu, Marina Teodora, Andrei Dan Busuioc, Cristina Busuioc, Adina Cotarta, Anca Cojocaru, Teodor Visan, and Danut Ionel Vaireanu. 2021. "Experimental Study on the Corrosion of Carbon Steel and Aluminum Alloy in Firefighting Protein Foam Concentrates" Materials 14, no. 23: 7259. https://doi.org/10.3390/ma14237259