Dilatometric Analysis and Kinetics Research of Martensitic Transformation under a Temperature Gradient and Stress
Abstract
1. Introduction
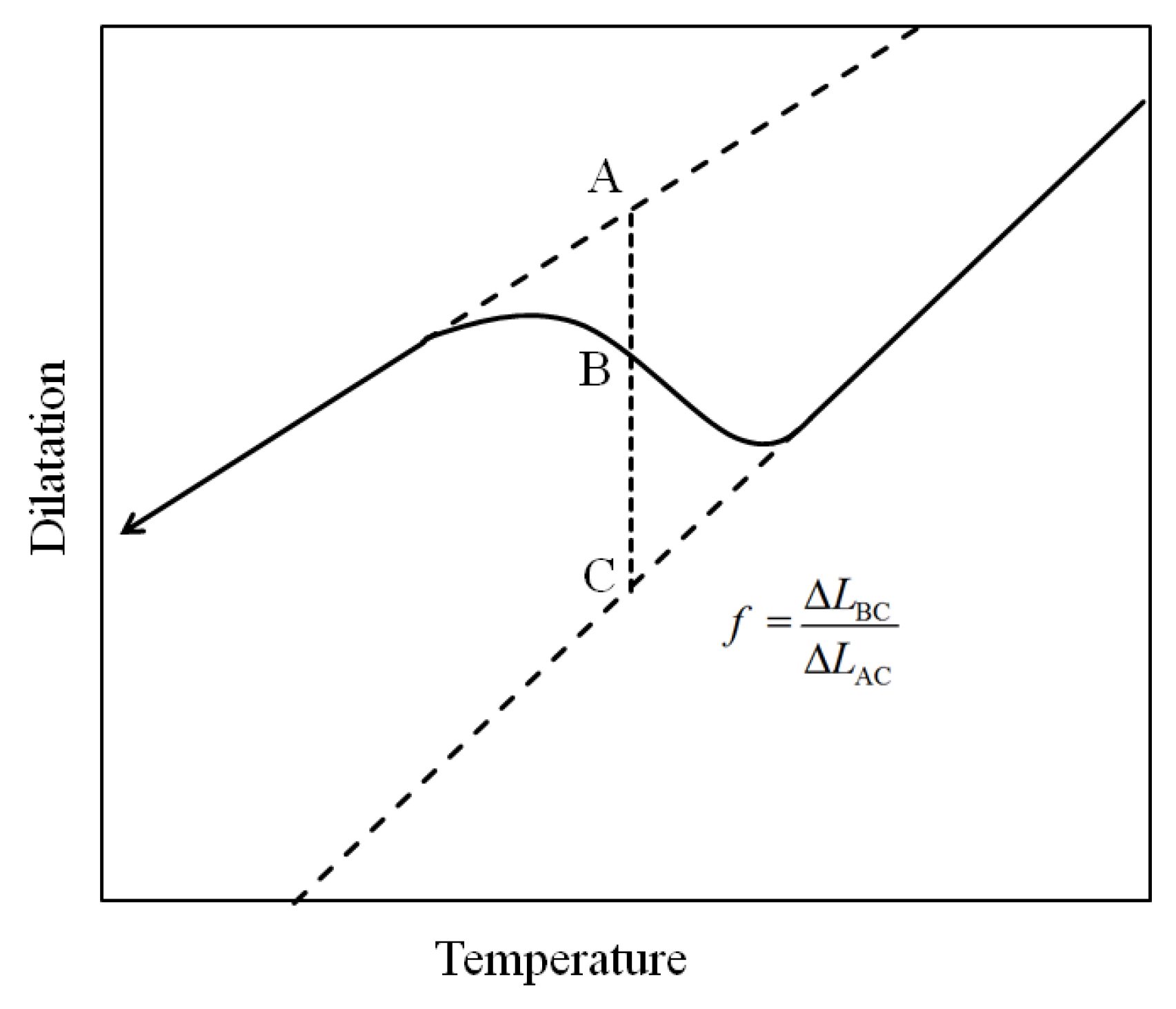
- (1)
- The transformation is essentially complete when the maximum strain of the dilatometric curve is reached, usually at room temperature.
- (2)
- The lever rule can only be applied to single-phase transformation or to multiple phase transformations if they can be considered to be in sequence, with no overlaps.
- (3)
- The lever rule is only valid for a transformation without repartition of alloy elements.
2. Models
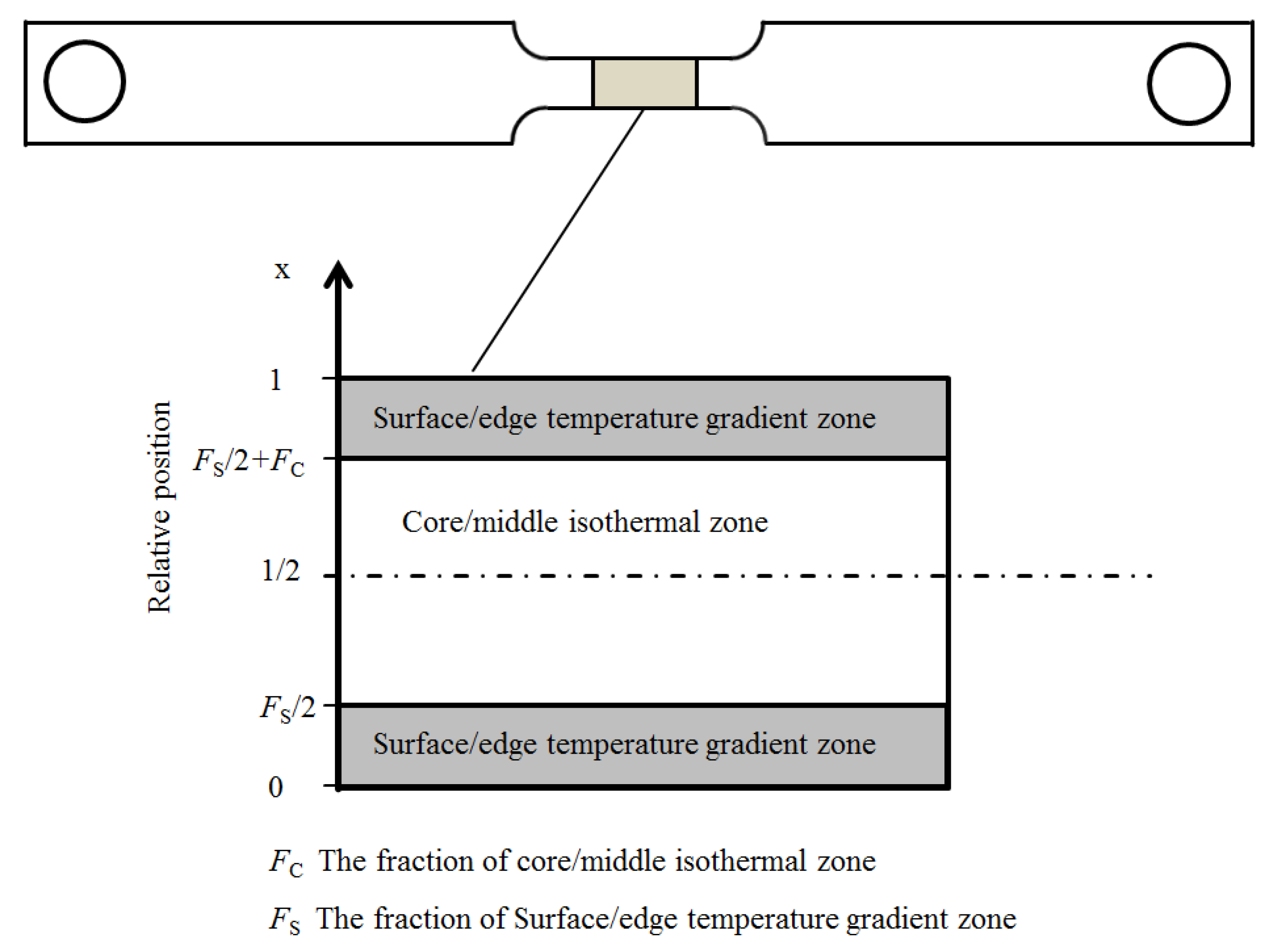
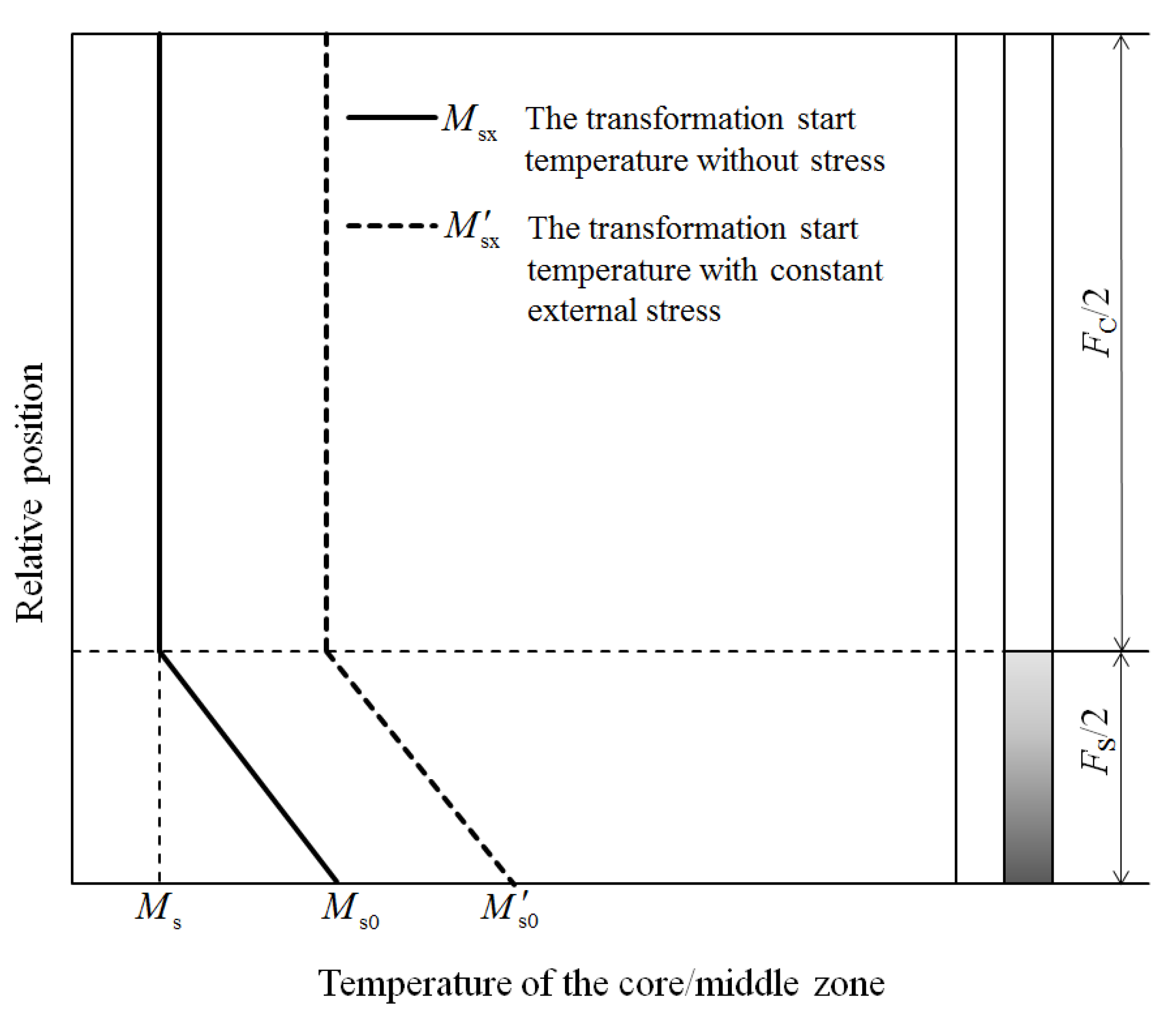
2.1. The Temperature Field and the Martensite-Start Temperature in the Specimen
2.2. Extracting the Model of the Martensitic Kinetics Curve under a Temperature Gradient and Stress
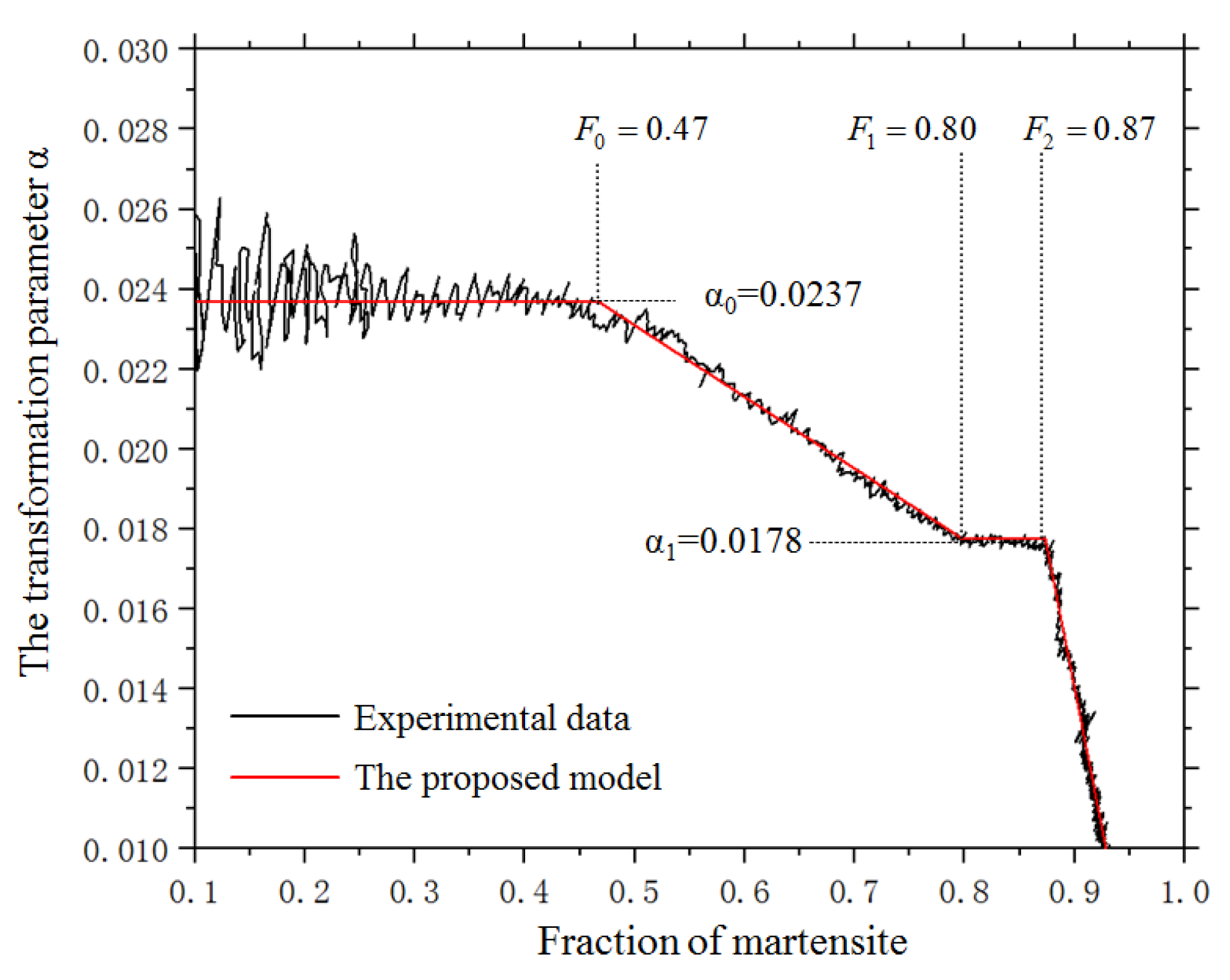
2.3. The Determine of the Transformation Parameter α and the Martensite Start Temperature
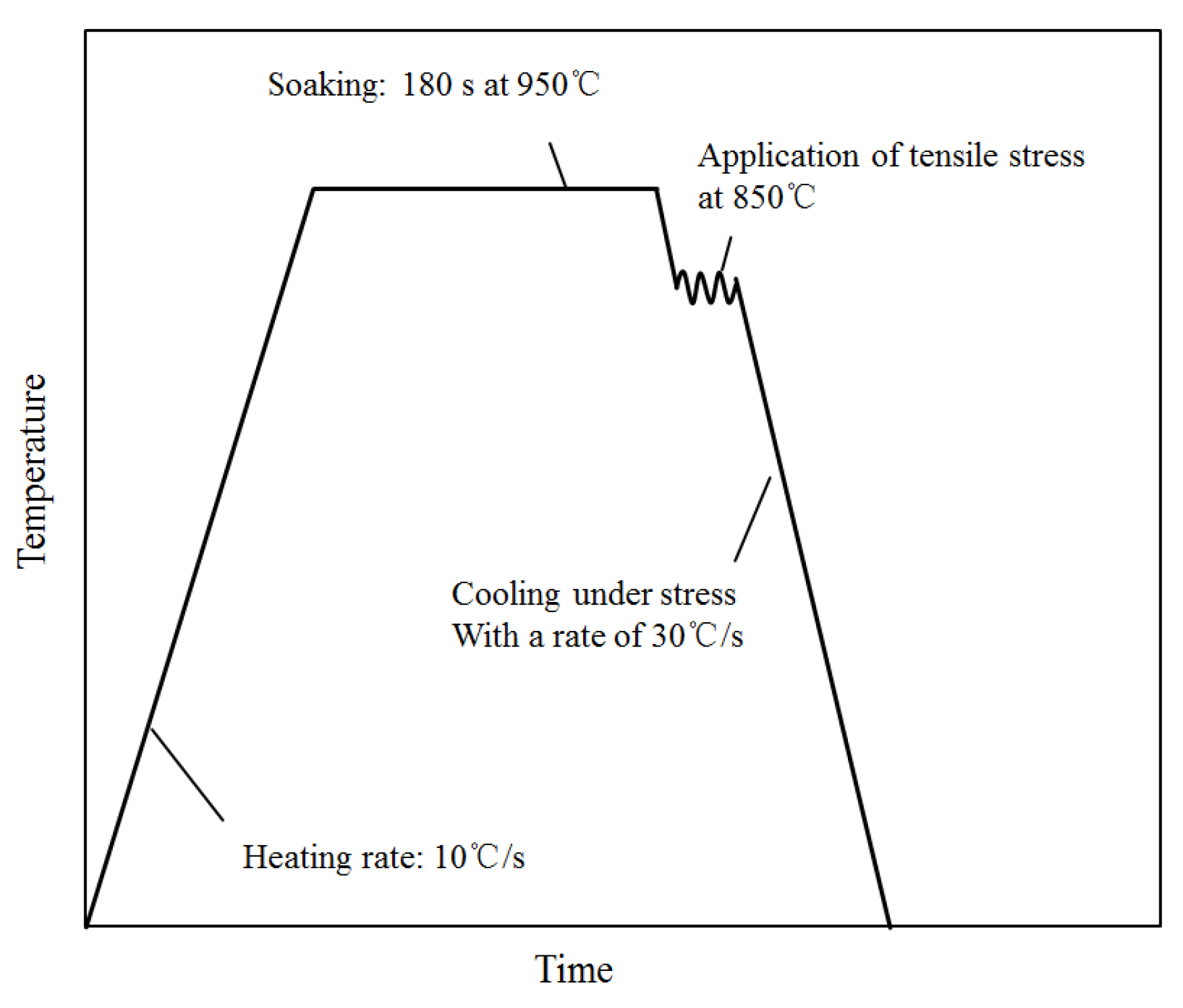
3. Experimental Procedure
4. Results and Discussion
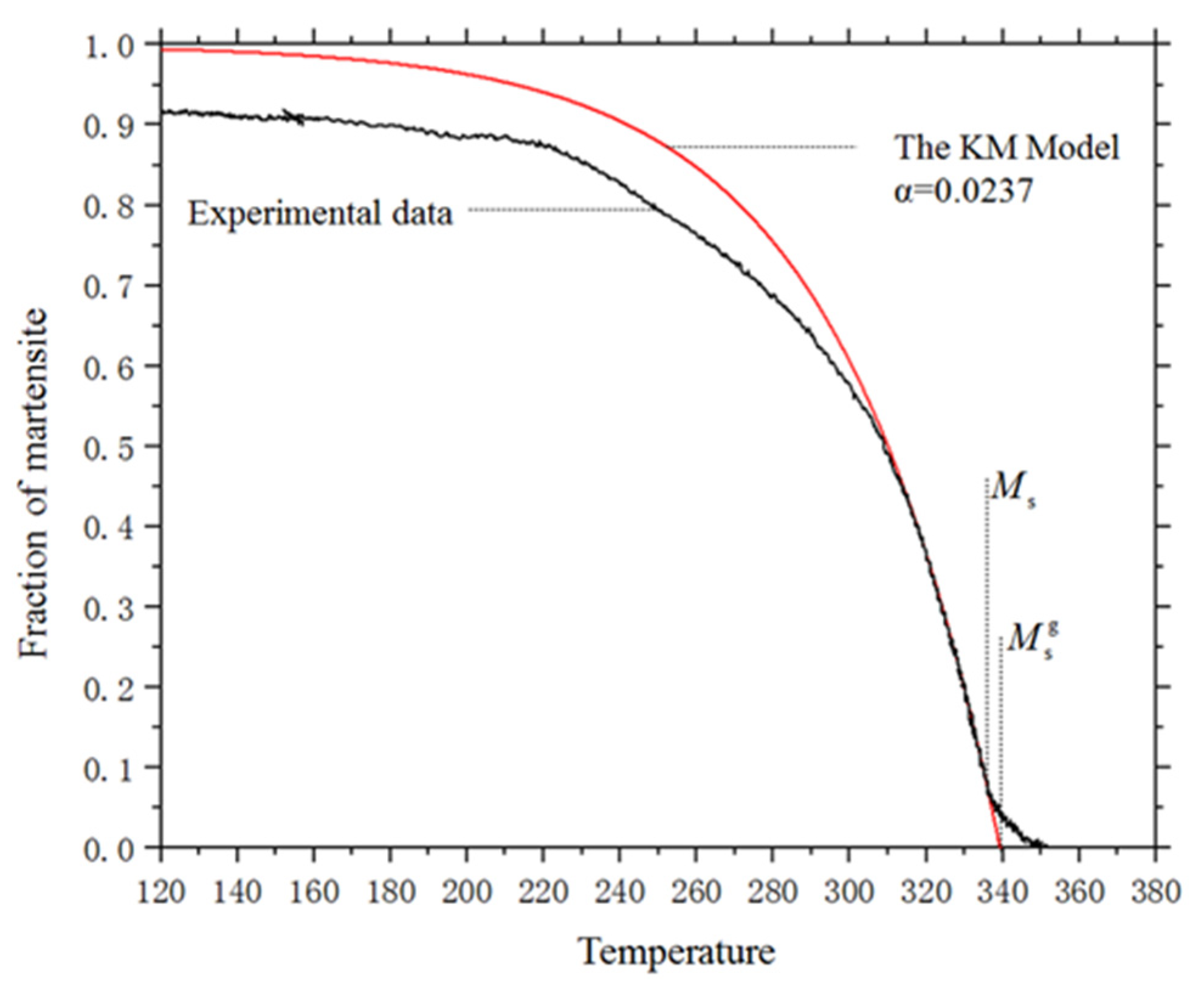
4.1. The Kinetics of Martensitic Transformation without External Stress
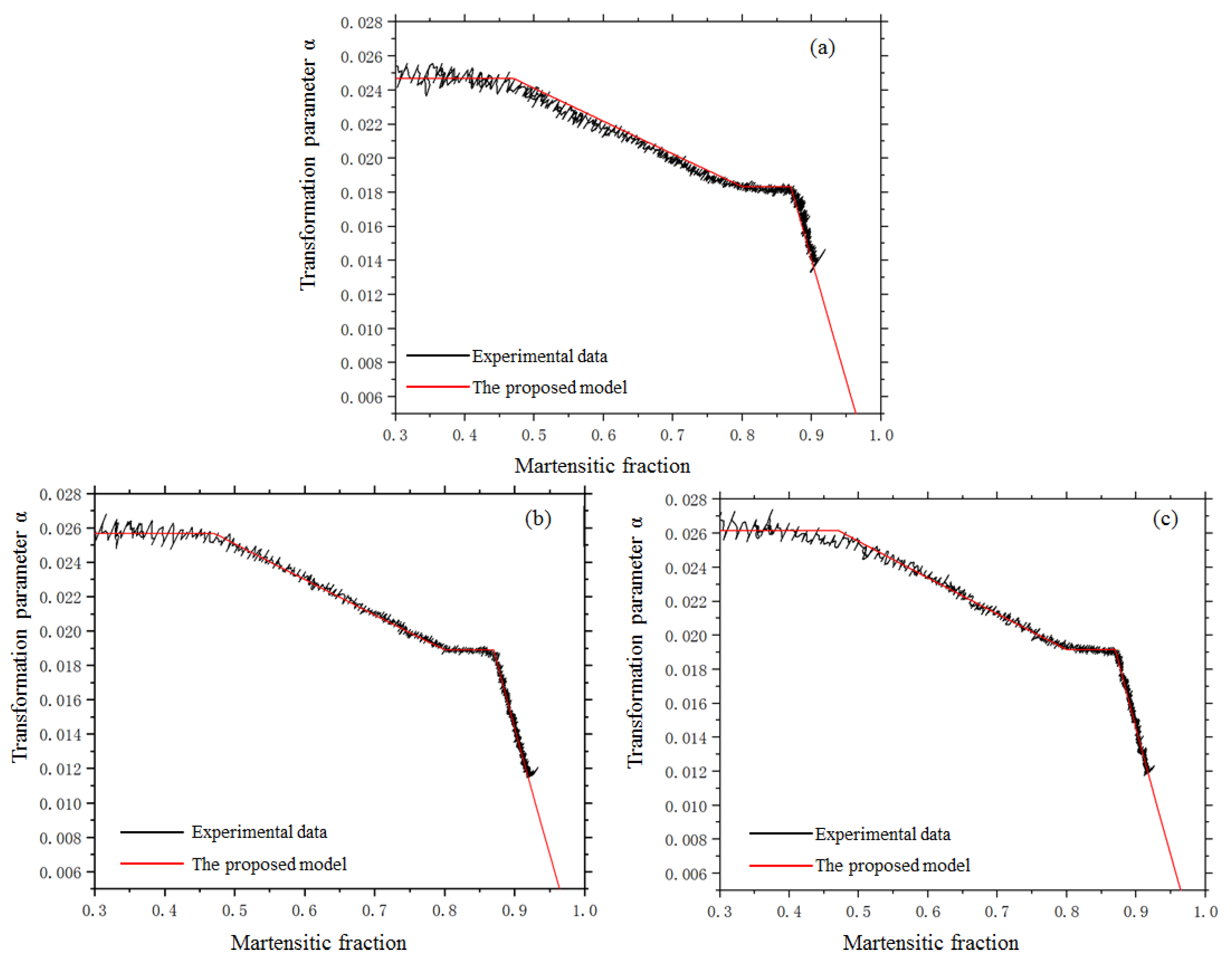
4.2. The Kinetics of Martensitic Transformation under Stress
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kop, T.A.; Sietsma, J.; Van Der Zwaag, S. Dilatometric analysis of phase transformations in hypo-eutectoid steels. J. Mater. Sci. 2001, 36, 519–526. [Google Scholar] [CrossRef]
- Salari, S.; Naderi, M.; Prahl, U.; Bleck, W. Quantification of Phase Transformation Kinetics under Thermomechanical Conditions Using Dilatometry Data. Adv. Materials Research 2012, 622–623, 581–584. [Google Scholar] [CrossRef]
- Morawiec, M.; Skowronek, A.; Król, M.; Grajcar, A. Dilatometric Analysis of the Austenite Decomposition in Undeformed and Deformed Low-Carbon Structural Steel. Materials 2020, 13, 5443. [Google Scholar] [CrossRef]
- Suh, D.-W.; Oh, C.-S.; Han, H.N.; Kim, S.-J. Dilatometric analysis of austenite decomposition considering the effect of non-isotropic volume change. Acta Materialia 2007, 55, 2659–2669. [Google Scholar] [CrossRef]
- ASTM International. Standard Practice for Quantitative Measurement and Reporting of Hypoeutectoid Carbon and Low-Alloy Steel Phase Transformations; A1033-10; ASTM: West Conshohocken, PA, USA, 2013; Volume 1, pp. 1–14. [Google Scholar]
- Kamyabi-Gol, A.; Clark, S.J.; Gibbs, J.W.; Sridhar, S.; Mendez, P.F. Quantification of evolution of multiple simultaneous phase transformations using dilation curve analysis (DCA). Acta Materialia 2016, 102, 231–240. [Google Scholar] [CrossRef]
- Warke, V.S.; Sisson, R.D.; Makhlouf, M.M. A Model for Converting Dilatometric Strain Measurements to the Fraction of Phase Formed during the Transformation of Austenite to Martensite in Powder Metallurgy Steels. Metall. Mater. Trans. A 2009, 40, 569–572. [Google Scholar] [CrossRef]
- Takahashi, M.; Bhadeshia, H.K.D.H. The interpretation of dilatometric data for transformations in steels. J. Mater. Sci. Lett. 1989, 8, 477–478. [Google Scholar] [CrossRef]
- Onink, M.; Brakman, C.M.; Tichelaar, F.D.; Mittemeijer, E.J.; van der Zwaag, S.; Root, J.H.; Konyer, N.B. The lattice parameters of austenite and ferrite in Fe–C alloys as functions of carbon concentration and temperature. Scr. Metallurgica et Materialia 1993, 29, 1011–1016. [Google Scholar] [CrossRef]
- Onink, M.; Tichelaar, F.D.; Brakman, C.M.; Mittemeijer, E.J.; Van der Zwaag, S. Quantitative analysis of the dilatation by decomposition of Fe-C austenites; Calculation of volume change upon transformation. Int. J. Mater. Res. 1996, 87, 24–32. [Google Scholar] [CrossRef]
- Zhao, J.Z.; Mesplont, C.; De Cooman, B.C. Kinetics of Phase Transformations in Steels: A New Method for Analysing Dilatometric Results. ISIJ Int. 2001, 41, 492–497. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, G.; Mao, X.; Zhao, G.; Bao, S. Method to Evaluate the Kinetics of Bainite Transformation in Low-Temperature Nanobainitic Steel Using Thermal Dilatation Curve Analysis. Metals 2017, 7, 330. [Google Scholar] [CrossRef]
- Li, C.M.; Sommer, F.; Mittemeijer, E.J. Quantitative dilatometric analysis of the isothermal decomposition of Fe-C austenite. Z. Für Metallkunde 2001, 91, 1957–1960. [Google Scholar]
- Li, C.M.; Sommer, F.; Mittemeijer, E.J. Carbon composition and temperature dependence of relative change in length during isothermal decomposition of Fe–C austenite. Mater. Sci. Technol. 2000, 16, 625–629. [Google Scholar]
- Lee, S.J.; Tyne, C. Prediction of Martensite Volume Fraction in Fe–Cr–Ni Alloys. Isij International 2011, 51, 169–171. [Google Scholar] [CrossRef][Green Version]
- Sangwoo; C. Model for estimation of transformation kinetics from the dilatation data during a cooling of hypoeutectoid steels. Mater. Sci. Eng. A 2003, 363, 72–80. [Google Scholar] [CrossRef]
- De Andrés, C.G.; Capdevila, C.; Caballero, F.; Bhadeshia, H. Modelling of isothermal ferrite formation using an analytical treatment of soft impingement in 0.37C–1.45Mn–0.11V microalloyed steel. Scr. Mater. 1998, 39, 853–859. [Google Scholar] [CrossRef]
- Suh, D.-W.; Oh, C.-S.; Han, H.N.; Kim, S.-J. Dilatometric Analysis of Phase Fraction during Austenite Decomposition into Banded Microstructure in Low-Carbon Steel. Met. Mater. Trans. A 2007, 38, 2963–2973. [Google Scholar] [CrossRef]
- Knorovsky, G.A.; Robino, C.V.; Dykhuizen, R.C.; Maccallum, D.O. Dilatometry in the Gleeble: What did you really measure? In Proceedings of the 5th International Conference on Trends in Welding Research, Pine Mountain, GA, USA, 1–5 June 1998. [Google Scholar]
- Gür, C.H.; Pan, J. Handbook of Thermal Process Modeling Steels, 1st ed.; CRC Press: Boca Raton, FL, USA, 2009; Volume 13, pp. 1–2. [Google Scholar]
- Patel, J.; Cohen, M. Criterion for the action of applied stress in the martensitic transformation. Acta Met. 1953, 1, 531–538. [Google Scholar] [CrossRef]
- Song, K.; Wei, Y.; Dong, Z.; Ma, R.; Zhan, X.; Zheng, W.; Fang, K. Constitutive model coupled with mechanical effect of volume change and transformation induced plasticity during solid phase transformation for TA15 alloy welding. Appl. Math. Model. 2015, 39, 2064–2080. [Google Scholar] [CrossRef]
- Liu, C.; Yao, K.-F.; Liu, Z.; Gao, G. Study of the effects of stress and strain on martensite transformation: Kinetics and transformation plasticity*. J. Comput. Mater. Des. 2000, 7, 63–69. [Google Scholar] [CrossRef]
- Schuh, C.; Dunand, D.C. Non-isothermal transformation-mismatch plasticity: Modeling and experiments on Ti–6Al–4V. Acta Materialia 2001, 49, 199–210. [Google Scholar] [CrossRef]
- Koistinen, D.; Marburger, R. A general equation prescribing the extent of the austenite-martensite transformation in pure iron-carbon alloys and plain carbon steels. Acta Met. 1959, 7, 59–60. [Google Scholar] [CrossRef]
- Christian, J.W. The Theory of Transformations in Metals and Alloys, 3rd ed.; Elsevier Science Ltd.: Kidlington, Oxford, UK, 2002; p. 1073. [Google Scholar]
- Magee, C.L. The nucleation of martensite. In Phase Transformations; ASM: Cleveland, OH, USA, 1970; p. 115. [Google Scholar]


| C | Mn | Si | B | Cr | Ti | P | Ni | Al | Cu | Mo | Co | S |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.360 | 1.240 | 0.232 | 0.002 | 0.116 | 0.031 | 0.012 | 0.018 | 0.016 | 0.011 | 0.005 | 0.005 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Guo, B. Dilatometric Analysis and Kinetics Research of Martensitic Transformation under a Temperature Gradient and Stress. Materials 2021, 14, 7271. https://doi.org/10.3390/ma14237271
Liu L, Guo B. Dilatometric Analysis and Kinetics Research of Martensitic Transformation under a Temperature Gradient and Stress. Materials. 2021; 14(23):7271. https://doi.org/10.3390/ma14237271
Chicago/Turabian StyleLiu, Liheng, and Bin Guo. 2021. "Dilatometric Analysis and Kinetics Research of Martensitic Transformation under a Temperature Gradient and Stress" Materials 14, no. 23: 7271. https://doi.org/10.3390/ma14237271
APA StyleLiu, L., & Guo, B. (2021). Dilatometric Analysis and Kinetics Research of Martensitic Transformation under a Temperature Gradient and Stress. Materials, 14(23), 7271. https://doi.org/10.3390/ma14237271





