Colloidal Synthesis of MoSe2/WSe2 Heterostructure Nanoflowers via Two-Step Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MoSe2, WSe2, and MoSe2/WSe2 Heterostructures
2.3. Characterization
2.4. Electrochemical Measurements
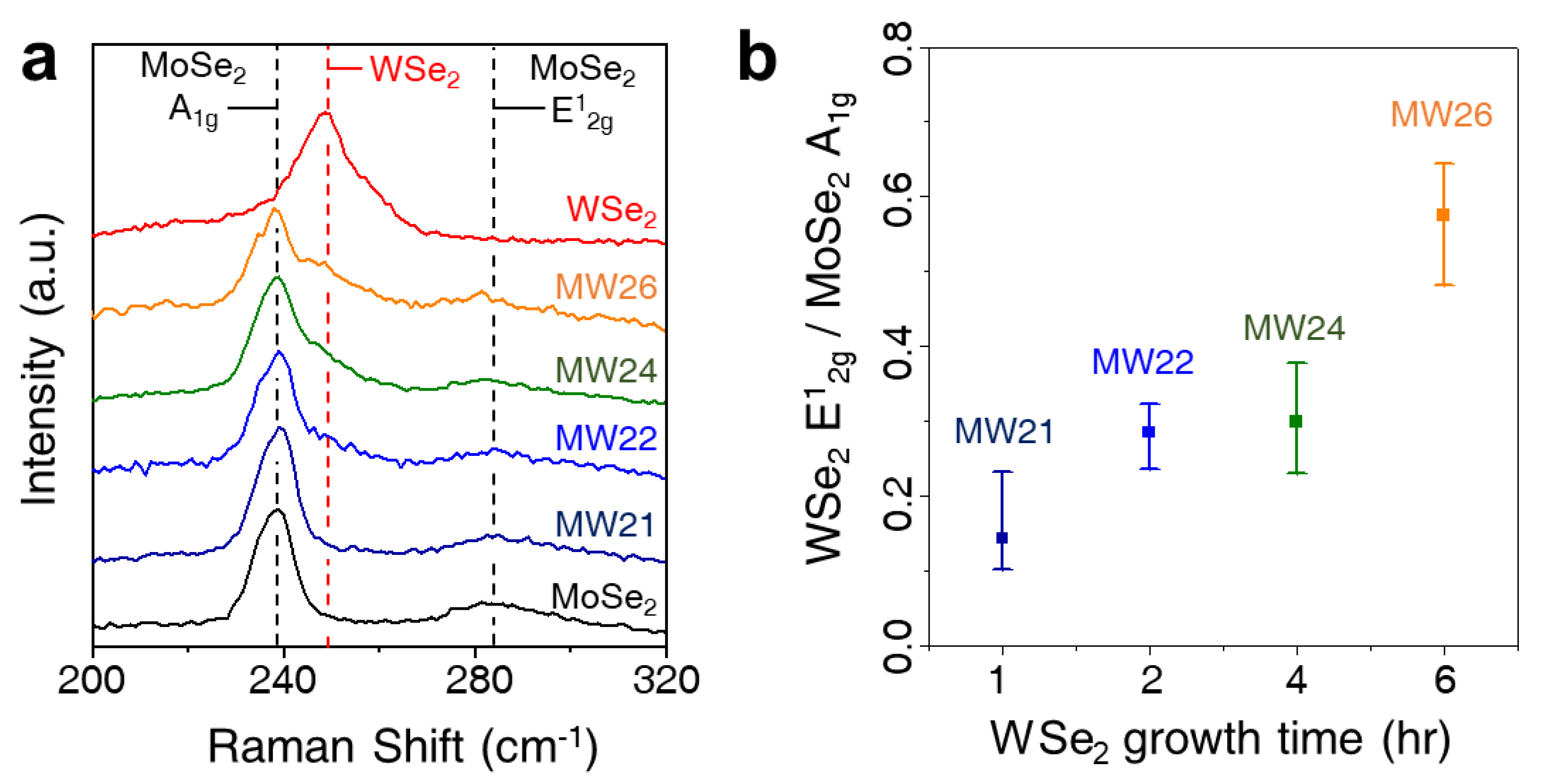
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, W.; Choudhary, N.; Han, G.H.; Park, J.; Akinwande, D.; Lee, Y.H. Recent Development of Two-Dimensional Transition Metal Dichalcogenides and Their Applications. Mater. Today 2017, 20, 116–130. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kwak, J.; Ciobanu, C.V.; Kwon, S.Y. Recent Developments in Controlled Vapor-Phase Growth of 2D Group 6 Transition Metal Dichalcogenides. Adv. Mater. 2019, 31, 1804939. [Google Scholar] [CrossRef]
- Huang, Q.; Li, X.; Sun, M.; Zhang, L.; Song, C.; Zhu, L.; Chen, P.; Xu, Z.; Wang, W.; Bai, X. The Mechanistic Insights into the 2H-1T Phase Transition of MoS2 upon Alkali Metal Intercalation: From the Study of Dynamic Sodiation Processes of MoS2 Nanosheets. Adv. Mater. Interfaces 2017, 4, 1700171. [Google Scholar] [CrossRef]
- Fan, S.; Zou, X.; Du, H.; Gan, L.; Xu, C.; Lv, W.; He, Y.B.; Yang, Q.H.; Kang, F.; Li, J. Theoretical Investigation of the Intercalation Chemistry of Lithium/Sodium Ions in Transition Metal Dichalcogenides. J. Phys. Chem. C 2017, 121, 13599–13605. [Google Scholar] [CrossRef]
- Cho, S.; Kim, S.; Kim, J.H.; Zhao, J.; Seok, J.; Keum, D.H.; Baik, J.; Choe, D.; Chang, K.J.; Suenaga, K.; et al. Phase Patterning for Ohmic Homojunction Contact in MoTe2. Science 2015, 349, 625–628. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.S.; Park, B.; Oh, S.H.; Roy, S.; Kim, J.; Choi, W. Composition-Tunable Synthesis of Large-Scale Mo1−xWxS2 Alloys with Enhanced Photoluminescence. ACS Nano 2018, 12, 6301–6309. [Google Scholar] [CrossRef]
- Susarla, S.; Kutana, A.; Hachtel, J.A.; Kochat, V.; Apte, A.; Vajtai, R.; Idrobo, J.C.; Yakobson, B.I.; Tiwary, C.S.; Ajayan, P.M. Quaternary 2D Transition Metal Dichalcogenides (TMDs) with Tunable Bandgap. Adv. Mater. 2017, 29, 1702457. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, M.; Chen, X.; Yang, S.; Wu, H.; Pan, J.; Xiong, X. CTAB-Assisted Synthesis of Novel Ultrathin MoSe2 Nanosheets Perpendicular to Graphene for the Adsorption and Photodegradation of Organic Dyes under Visible Light. Nanoscale 2016, 8, 440–450. [Google Scholar] [CrossRef]
- Jung, Y.; Shen, J.; Liu, Y.; Woods, J.M.; Sun, Y.; Cha, J.J. Metal Seed Layer Thickness-Induced Transition from Vertical to Horizontal Growth of MoS2 and WS2. Nano Lett. 2014, 14, 6842–6849. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki, A.; Shokouh, S.H.H.; Nazari, T.; Oh, K.; Im, S. Electric and Photovoltaic Behavior of a Few-Layer α-MoTe2/MoS2 Dichalcogenide Heterojunction. Adv. Mater. 2016, 28, 3216–3222. [Google Scholar] [CrossRef]
- Rigosi, A.F.; Hill, H.M.; Li, Y.; Chernikov, A.; Heinz, T.F. Probing Interlayer Interactions in Transition Metal Dichalcogenide Heterostructures by Optical Spectroscopy: MoS2/WS2 and MoSe2/WSe2. Nano Lett. 2015, 15, 5033–5038. [Google Scholar] [CrossRef]
- Vajtai, R.; He, Y.; Liu, Z.; Lei, S.; Zhang, X.; Zhou, W.; Ye, G.; Gong, Y.; Lou, J.; Wang, Q.; et al. Two-Step Growth of Two-Dimensional WSe2 /MoSe2 Heterostructures. Nano Lett. 2015, 15, 6135–6141. [Google Scholar] [CrossRef]
- Gong, Y.; Lin, J.; Wang, X.; Shi, G.; Lei, S.; Lin, Z.; Zou, X.; Ye, G.; Vajtai, R.; Yakobson, B.I.; et al. Vertical and In-Plane Heterostructures from WS2/MoS2 Monolayers. Nat. Mater. 2014, 13, 1135–1142. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Jiang, J.; Ding, T.; Zhang, J.; Pan, B.; Zuo, J.; Yang, Q. Fast Colloidal Synthesis of Scalable Mo-Rich Hierarchical Ultrathin MoSe2−x nanosheets for High-Performance Hydrogen Evolution. Nanoscale 2014, 6, 11046–11051. [Google Scholar] [CrossRef]
- Sun, D.; Feng, S.; Terrones, M.; Schaak, R.E. Formation and Interlayer Decoupling of Colloidal MoSe2 Nanoflowers. Chem. Mater. 2015, 27, 3167–3175. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, Q.; Zhou, X.; Li, L.; Su, J.; Wang, F.; Zhai, T. Self-Powered Photovoltaic Photodetector Established on Lateral Monolayer MoS2-WS2 Heterostructures. Nano Energy 2018, 51, 45–53. [Google Scholar] [CrossRef]
- Kim, J.E.; Kang, W.T.; Tu Vu, V.; Kim, Y.R.; Shin, Y.S.; Lee, I.; Won, U.Y.; Lee, B.H.; Kim, K.; Phan, T.L.; et al. Ideal PN Photodiode Using Doping Controlled WSe2-MoSe2 Lateral Heterostructure. J. Mater. Chem. C 2021, 9, 3504–3512. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, G.H.; Van Der Zande, A.M.; Chen, W.; Li, Y.; Han, M.; Cui, X.; Arefe, G.; Nuckolls, C.; Heinz, T.F.; et al. Atomically Thin P-n Junctions with van Der Waals Heterointerfaces. Nat. Nanotechnol. 2014, 9, 676–681. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Chen, Y.; Wang, L.; Xu, J.; Zeng, D.; Peng, D.L. Colloidal Synthesis of MoSe2 Nanonetworks and Nanoflowers with Efficient Electrocatalytic Hydrogen-Evolution Activity. Electrochim. Acta 2017, 231, 69–76. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, H.; Li, N.; Zhang, Y.; Zhang, X.; Du, Y. Assembled 3D Electrocatalysts for Efficient Hydrogen Evolution: WSe2 layers Anchored on Graphene Sheets. Inorg. Chem. Front. 2016, 3, 313–319. [Google Scholar] [CrossRef]
- Abdallah, W.A.; Nelson, A.E. Characterization of MoSe2 (0001) and Ion-Sputtered MoSe2 by XPS. J. Mater. Sci. 2005, 40, 2679–2681. [Google Scholar] [CrossRef]
- Boscher, N.D.; Carmalt, C.J.; Parkin, I.P. Atmospheric Pressure Chemical Vapor Deposition of WSe2 Thin Films on Glass—Highly Hydrophobic Sticky Surfaces. J. Mater. Chem. 2006, 16, 122–127. [Google Scholar] [CrossRef]
- Ambrosi, A.; Sofer, Z.; Pumera, M. 2H → 1T Phase Transition and Hydrogen Evolution Activity of MoS2, MoSe2, WS2 and WSe2 Strongly Depends on the MX2 composition. Chem. Commun. 2015, 51, 8450–8453. [Google Scholar] [CrossRef]
- Liu, X.Y. Heterogeneous nucleation or homogeneous nucleation? J. Chem. Phys. 2000, 112, 9949–9955. [Google Scholar] [CrossRef]
- Viksman, G.S.; Gardienko, S.P.; Klochkov, L.A.; Yanaki, A.A. Dissociation of molybdenum and tungsten selenides. Sov. Powder Met. Met. Ceram. 1986, 25, 64–66. [Google Scholar] [CrossRef]
- Gradečak, S.; Garaj, S.; Bogaert, K.; Titow, D.; Liu, S.; Chesin, J. Diffusion-Mediated Synthesis of MoS2/WS2 Lateral Heterostructures. Nano Lett. 2016, 16, 5129–5134. [Google Scholar] [CrossRef]
- Zeng, H.; Liu, G.B.; Dai, J.; Yan, Y.; Zhu, B.; He, R.; Xie, L.; Xu, S.; Chen, X.; Yao, W.; et al. Optical Signature of Symmetry Variations and Spin-Valley Coupling in Atomically Thin Tungsten Dichalcogenides. Sci. Rep. 2013, 3, 4908–4916. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Wu, J.; Zhu, Y.; Dumcenco, D.O.; Hong, J.; Mao, N.; Deng, S.; Chen, Y.; Yang, Y.; Jin, C.; et al. Two-Dimensional Molybdenum Tungsten Diselenide Alloys: Photoluminescence, Raman Scattering, and Electrical Transport. ACS Nano 2014, 8, 7130–7137. [Google Scholar] [CrossRef]
- Lei, Z.; Xu, S.; Wu, P. Ultra-Thin and Porous MoSe2 nanosheets: Facile Preparation and Enhanced Electrocatalytic Activity towards the Hydrogen Evolution Reaction. Phys. Chem. Chem. Phys. 2016, 18, 70–74. [Google Scholar] [CrossRef]
- Cui, Y. Synthesis of MoS2 and MoSe2 Films with Vertically Aligned Layers. Nano Lett. 2013, 13, 1341–1347. [Google Scholar] [CrossRef]
- Gong, Q.; Cheng, L.; Liu, C.; Zhang, M.; Feng, Q.; Ye, H.; Zeng, M.; Xie, L.; Liu, Z.; Li, Y. Ultrathin MoS2(1−x)Se2x Alloy Nanoflakes for Electrocatalytic Hydrogen Evolution Reaction. ACS Catal. 2015, 5, 2213–2219. [Google Scholar] [CrossRef]







| Materials | Loading | η at j = 10 mA/cm2 (mV) | Tafel Slope (mV/decade) | Ref. |
|---|---|---|---|---|
| MoSe2 nanoflowers | 0.28 mg cm−2 on GC | 340 | 105 | This work |
| MW22 nanoflowers | 410 | 150 | ||
| MW24 nanoflowers | 580 | 238 | ||
| WSe2 nanoflowers | 650 | 178 | ||
| Bulk MoSe2 | 0.28 mg cm−2 on GC | >600 | 346 | [14] |
| Porous MoSe2 nanosheets | 0.47 mg cm−2 on GC | 150 | 80 | [29] |
| Vertically aligned MoSe2 layers | 0.0135 mg cm−2 on GC | −450 | 105–120 | [30] |
| Hierarchical MoSe2−x nanosheets | 0.28 mg cm−2 on GC | 288 | 98 | [14] |
| MoSe2 nanoflakes (2:1 ratio to Ketjen black) | 0.28 mg cm−2 on GC | 181 ± 1 | 45 ± 1 | [31] |
| MoSe2 nanonetworks (8:1 ratio to Ketjen black) | 0.28 mg cm−2 on GC | 302 | 109 | [19] |
| MoSe2 nanoflowers (8:1 ratio to Ketjen black) | 0.28 mg cm−2 on GC | 233 | 89 | [19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, Y.; Shin, N. Colloidal Synthesis of MoSe2/WSe2 Heterostructure Nanoflowers via Two-Step Growth. Materials 2021, 14, 7294. https://doi.org/10.3390/ma14237294
Hwang Y, Shin N. Colloidal Synthesis of MoSe2/WSe2 Heterostructure Nanoflowers via Two-Step Growth. Materials. 2021; 14(23):7294. https://doi.org/10.3390/ma14237294
Chicago/Turabian StyleHwang, Yunjeong, and Naechul Shin. 2021. "Colloidal Synthesis of MoSe2/WSe2 Heterostructure Nanoflowers via Two-Step Growth" Materials 14, no. 23: 7294. https://doi.org/10.3390/ma14237294
APA StyleHwang, Y., & Shin, N. (2021). Colloidal Synthesis of MoSe2/WSe2 Heterostructure Nanoflowers via Two-Step Growth. Materials, 14(23), 7294. https://doi.org/10.3390/ma14237294






