Preparation and Flame Retardant Properties of Calcium–Aluminium Hydrotalcite with Root Cutting Silicate Layers as Bamboo Flame Retardants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
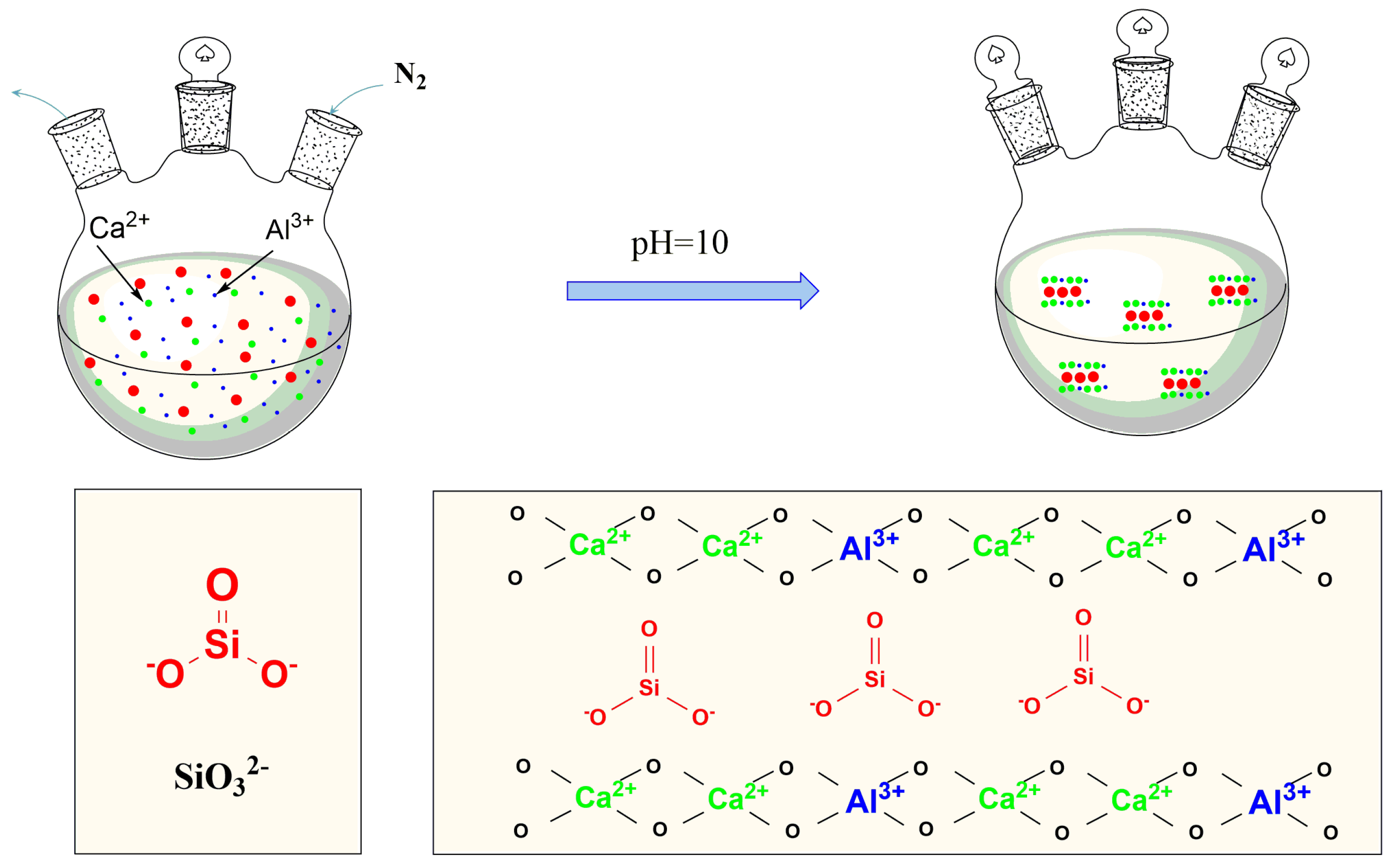
2.2. Preparation of The CaAl–SiO3–LDHs
2.3. CaAl–SiO3–LDHs Flame-Retardant Treated Bamboo
2.4. Analysis and Testing
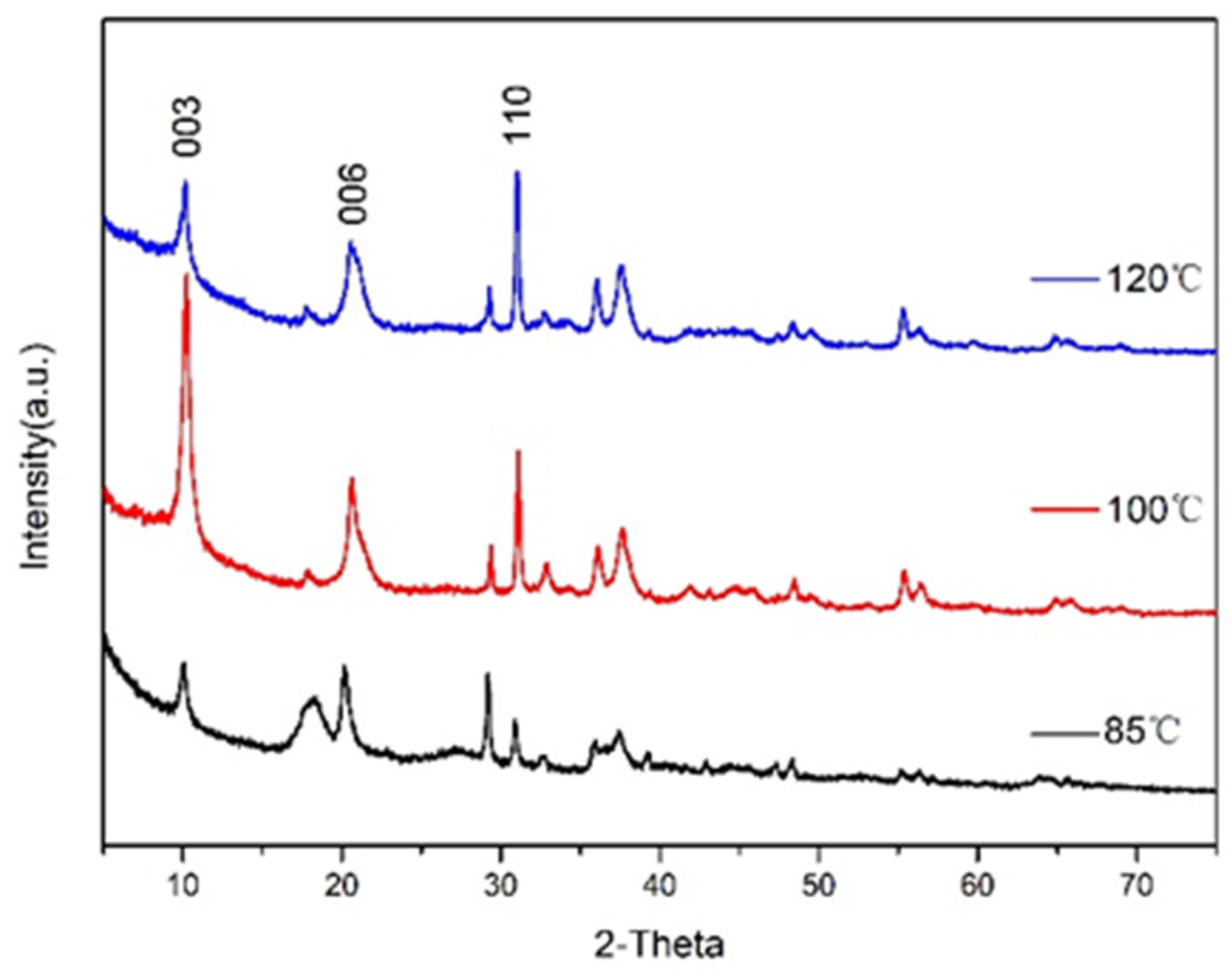
2.4.1. X-ray Diffraction (XRD) Analysis
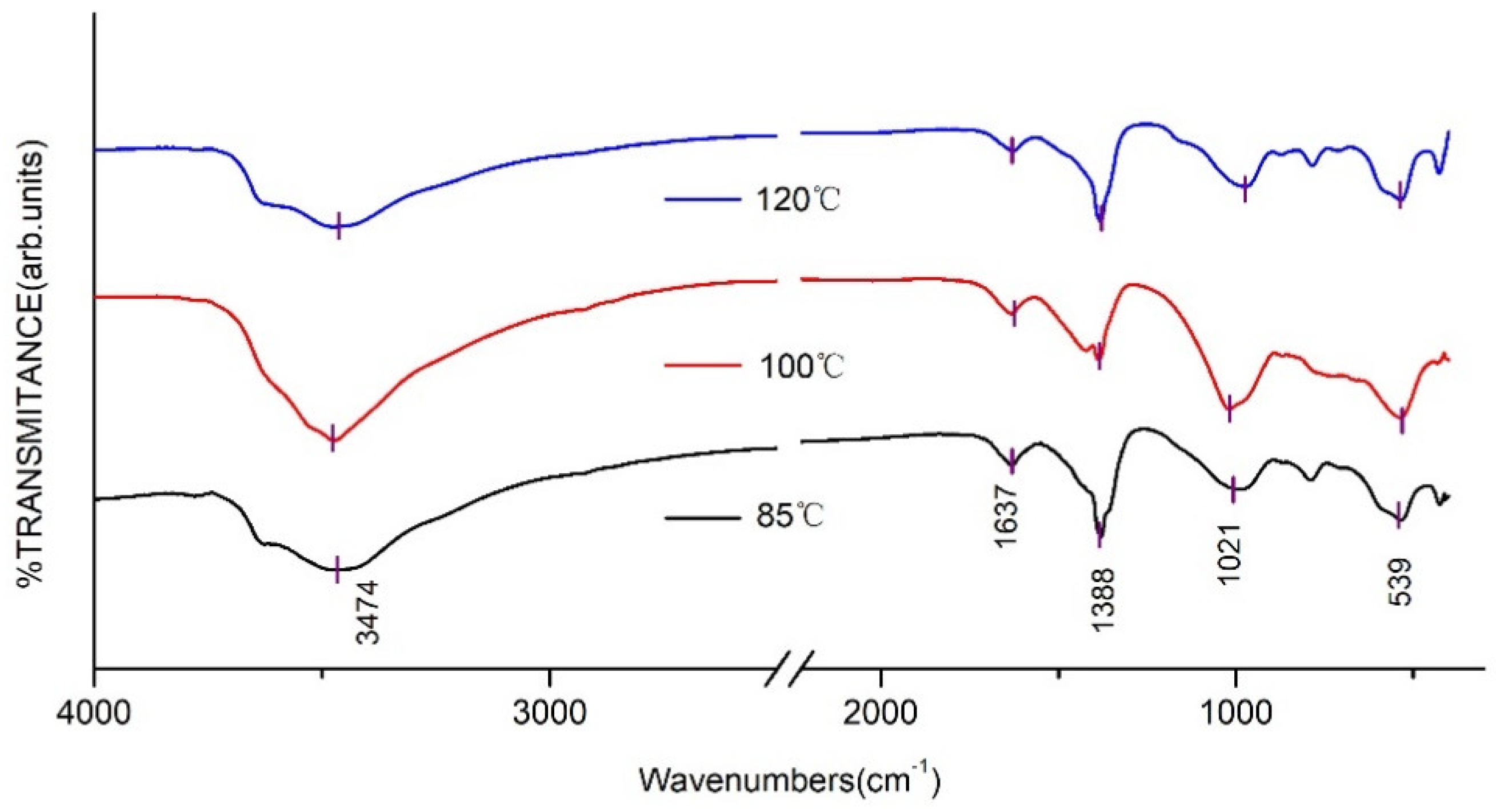
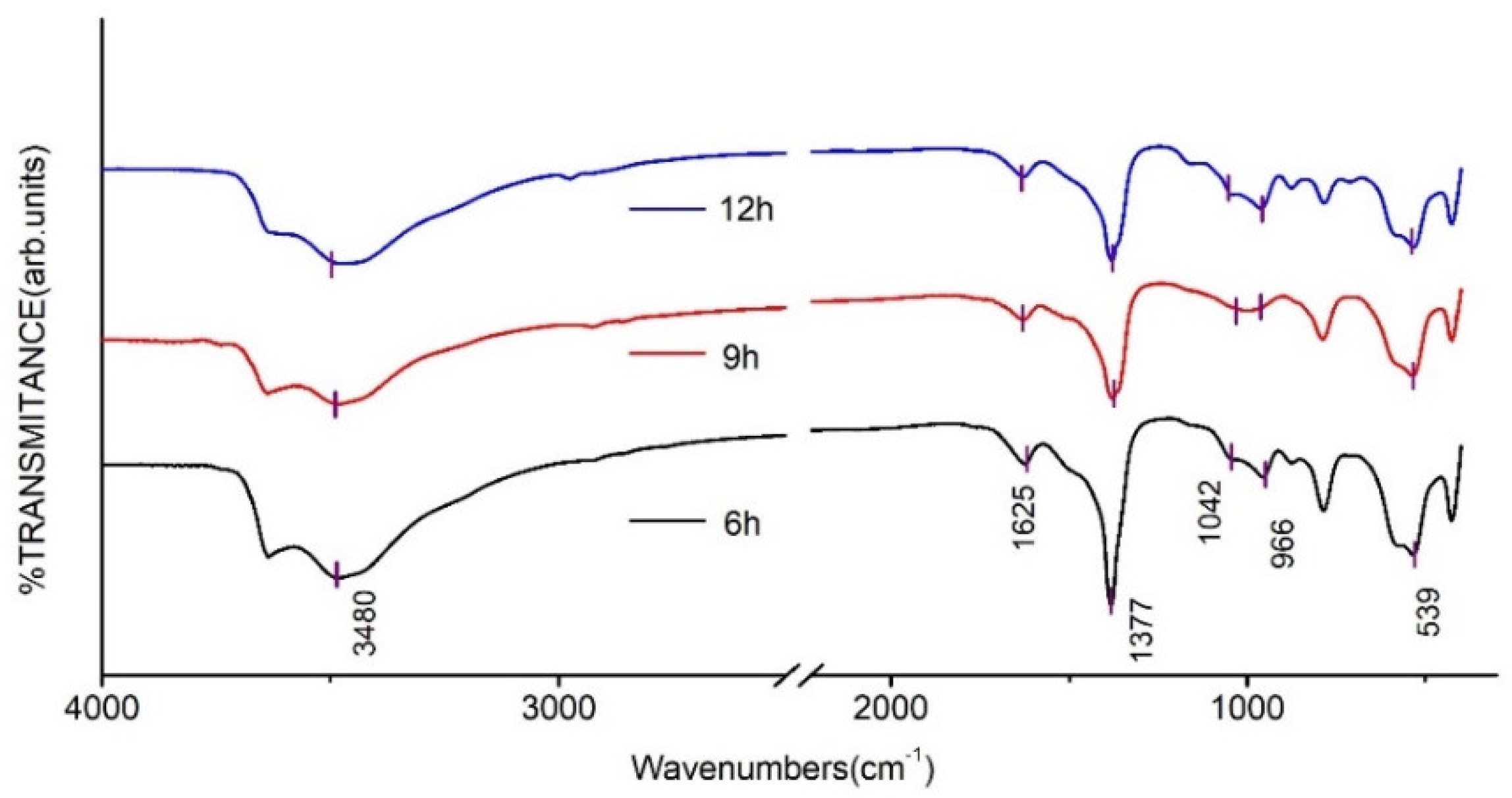
2.4.2. Fourier Infrared Spectrum (FT-IR) Analysis
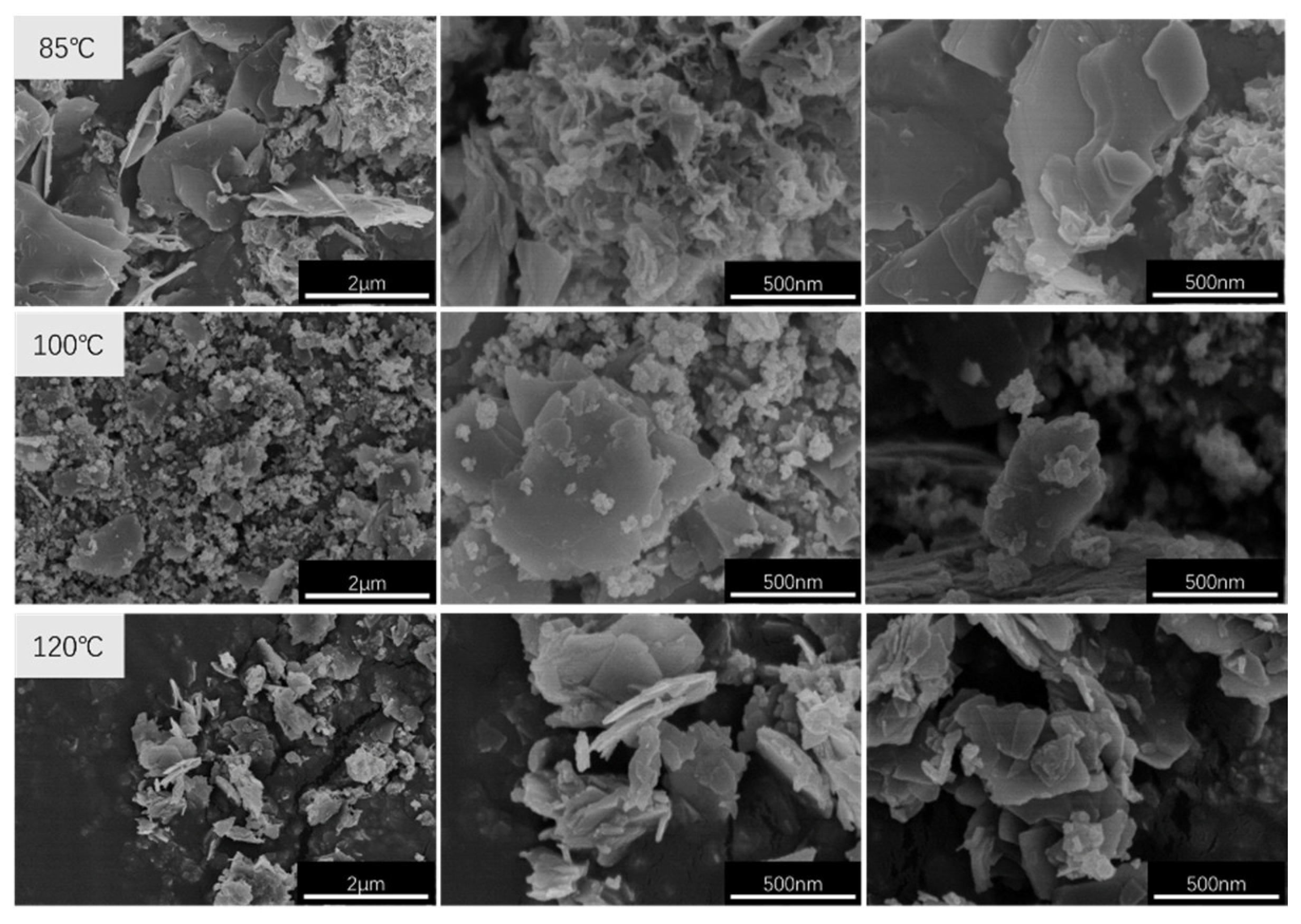
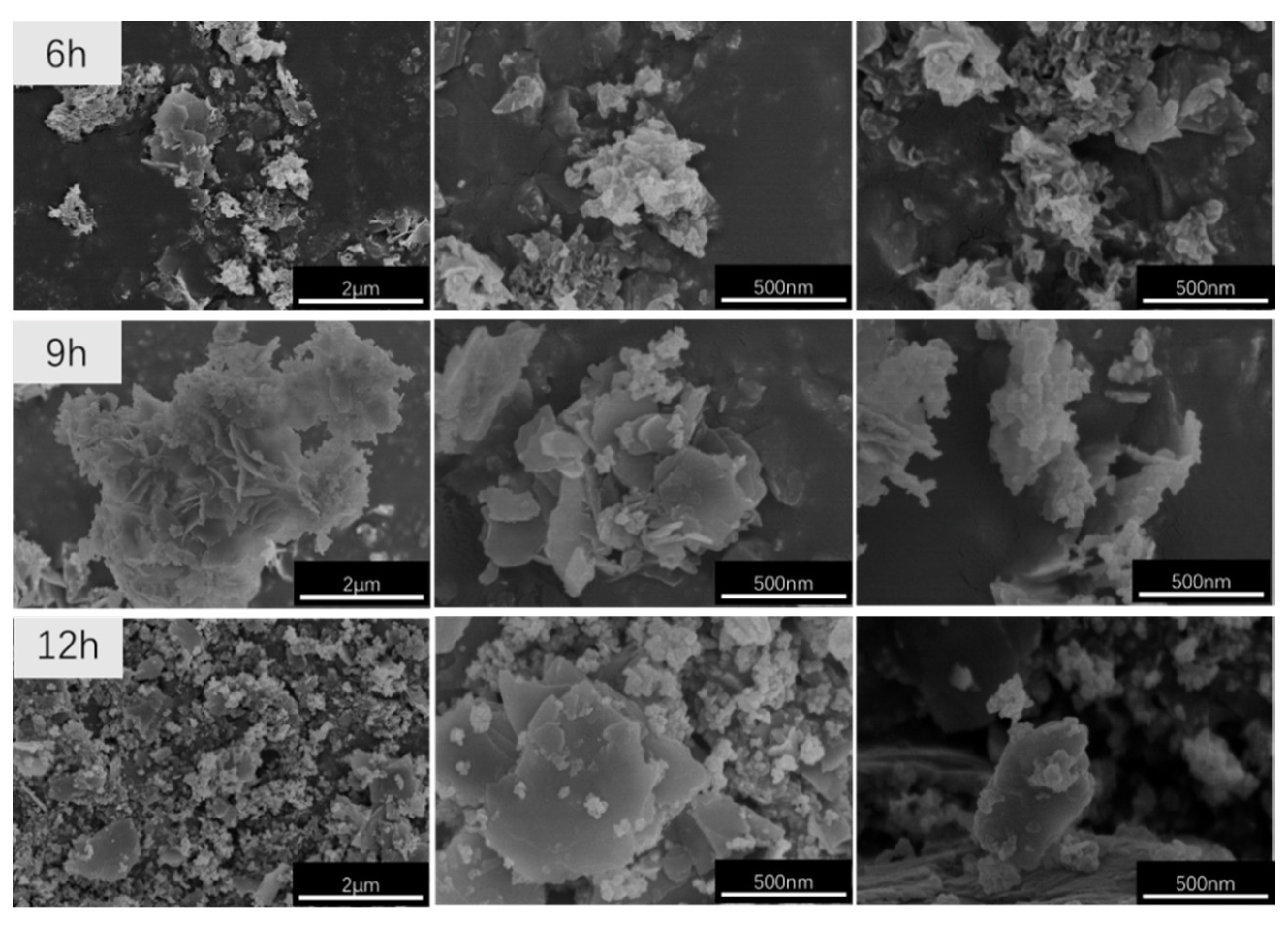
2.4.3. Cold Field Emission Scanning Electron Microscopy (SEM) Analysis
2.4.4. Thermogravimetry (TG–DTG) Analysis
2.4.5. Cone Calorimeter (CONE) Analysis
3. Results
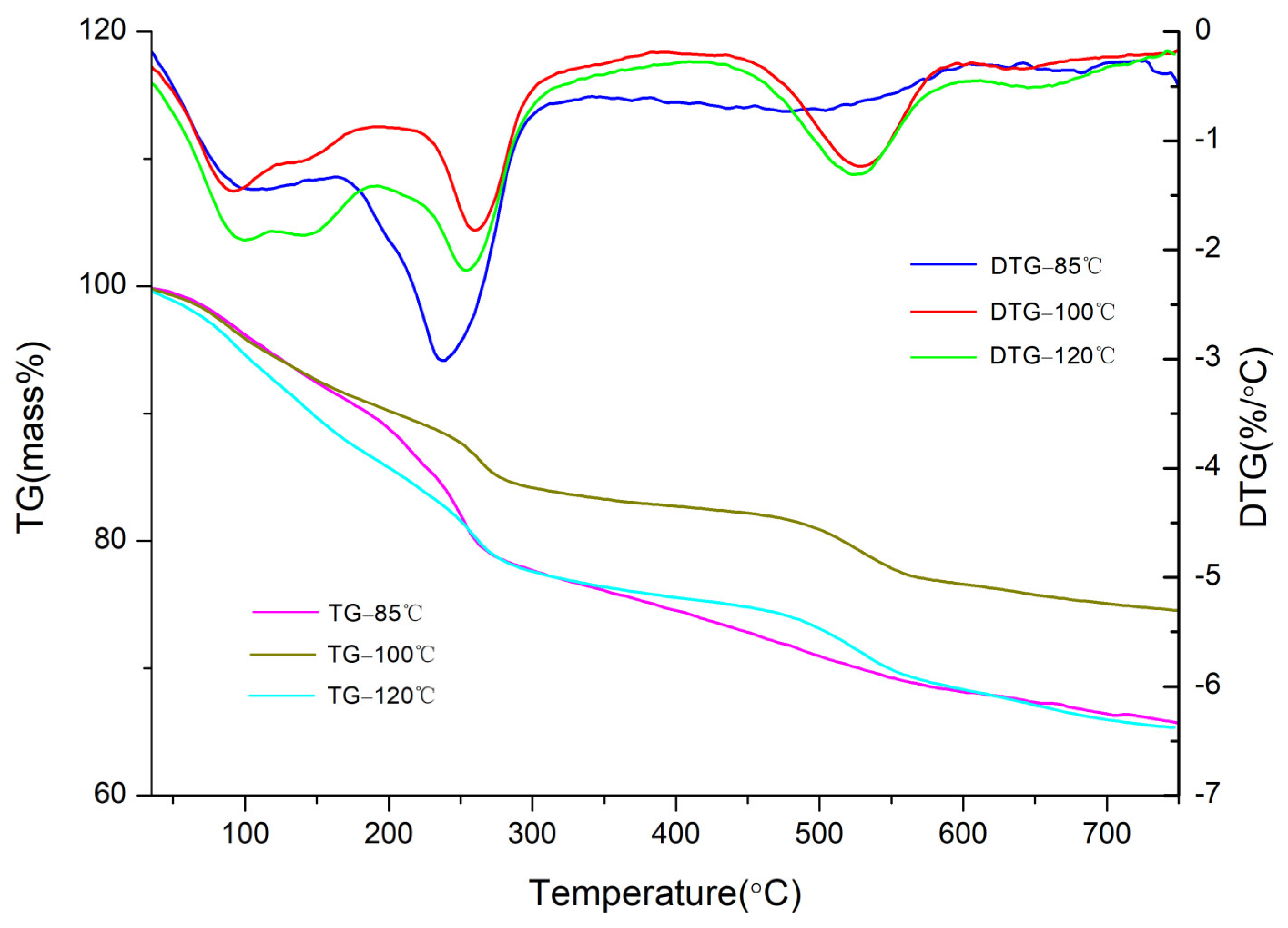
3.1. Effects of Crystallisation Temperature on Structure and Properties of CaAl–SiO3–LDHs
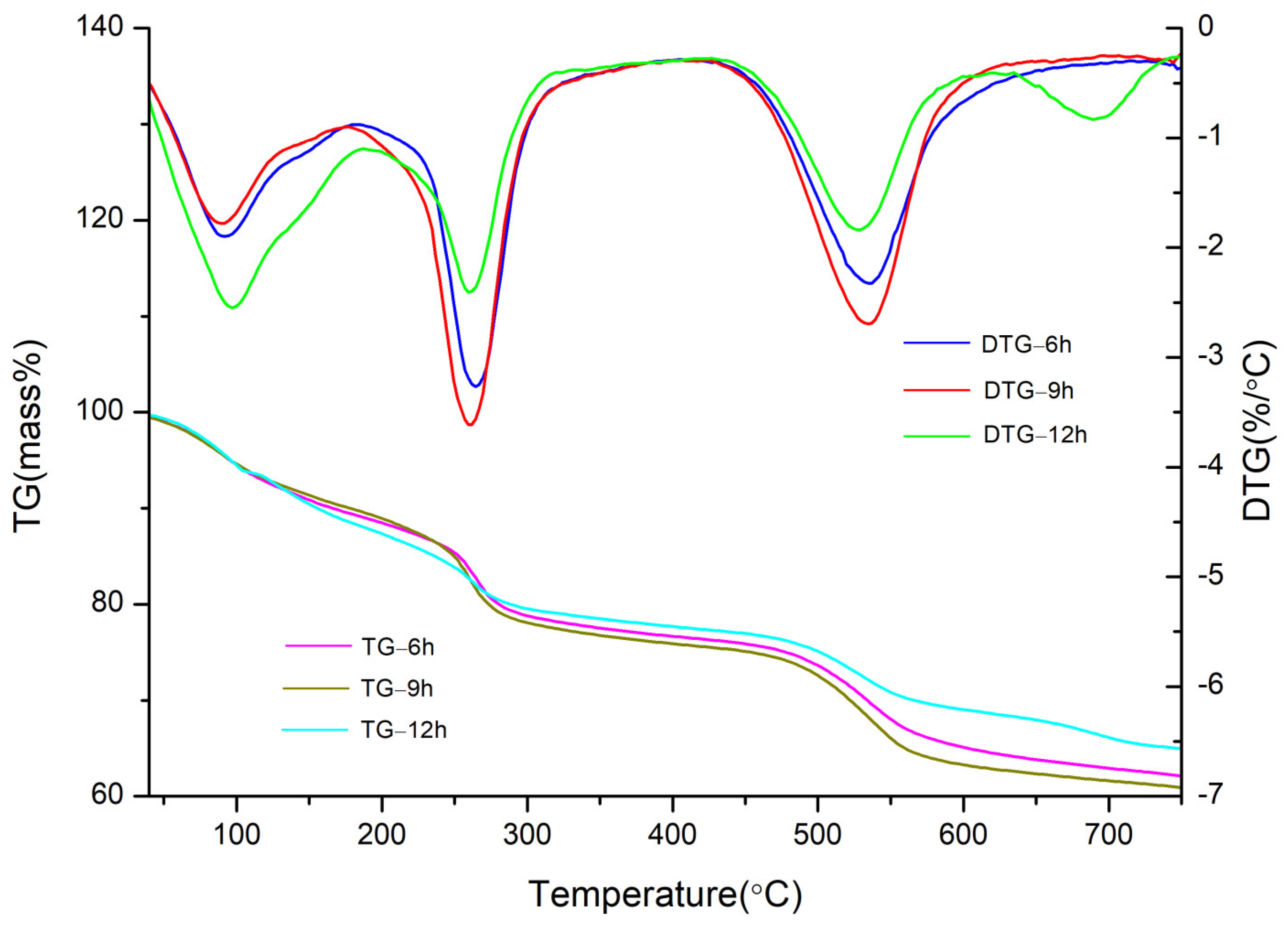
3.2. Effect of the Crystallisation Time on the Structure and Properties of CaAl–SiO3–LDHs
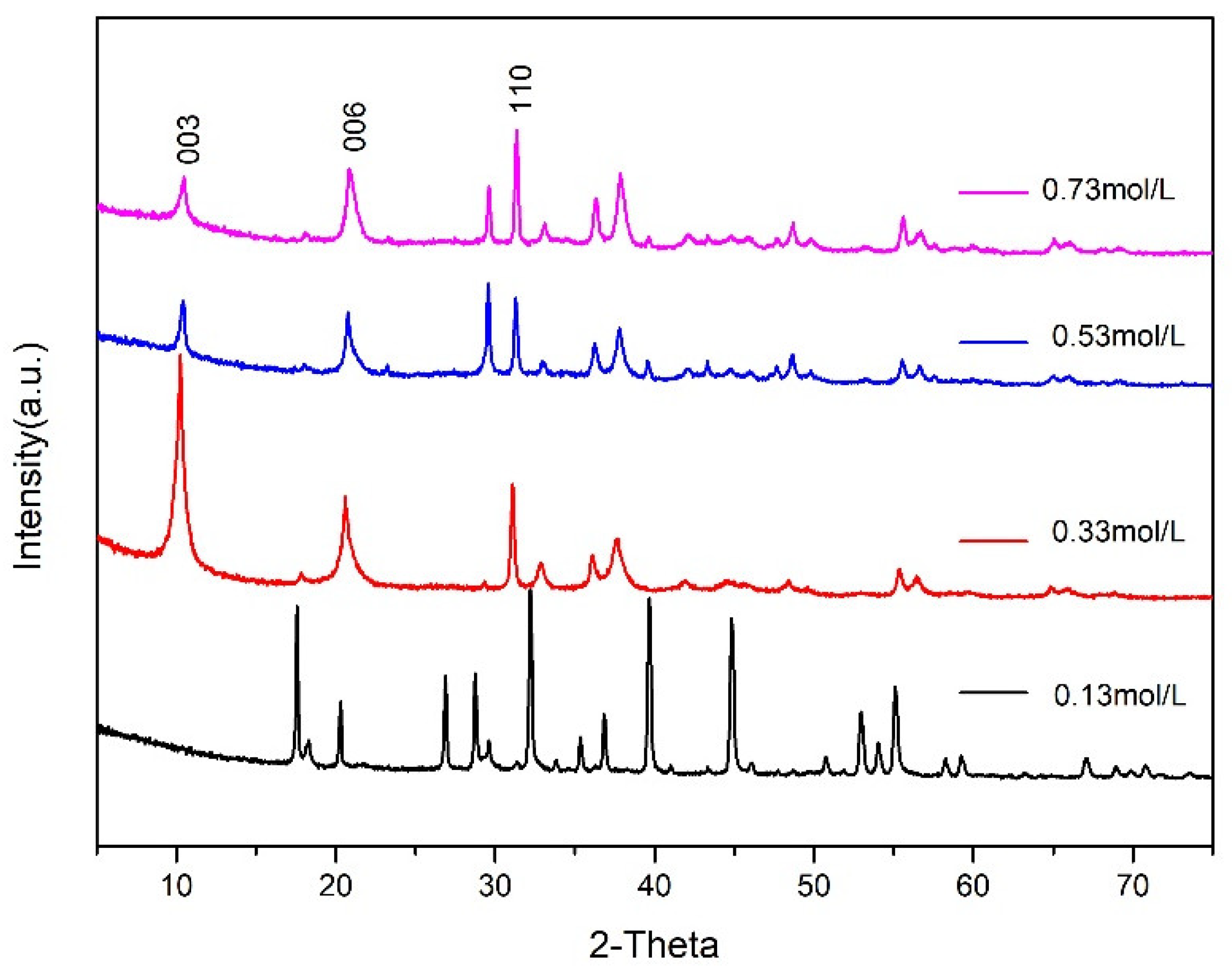
3.3. Effect of the Crystallisation Concentration on the Structure and Properties of CaAl–SiO3–LDHs
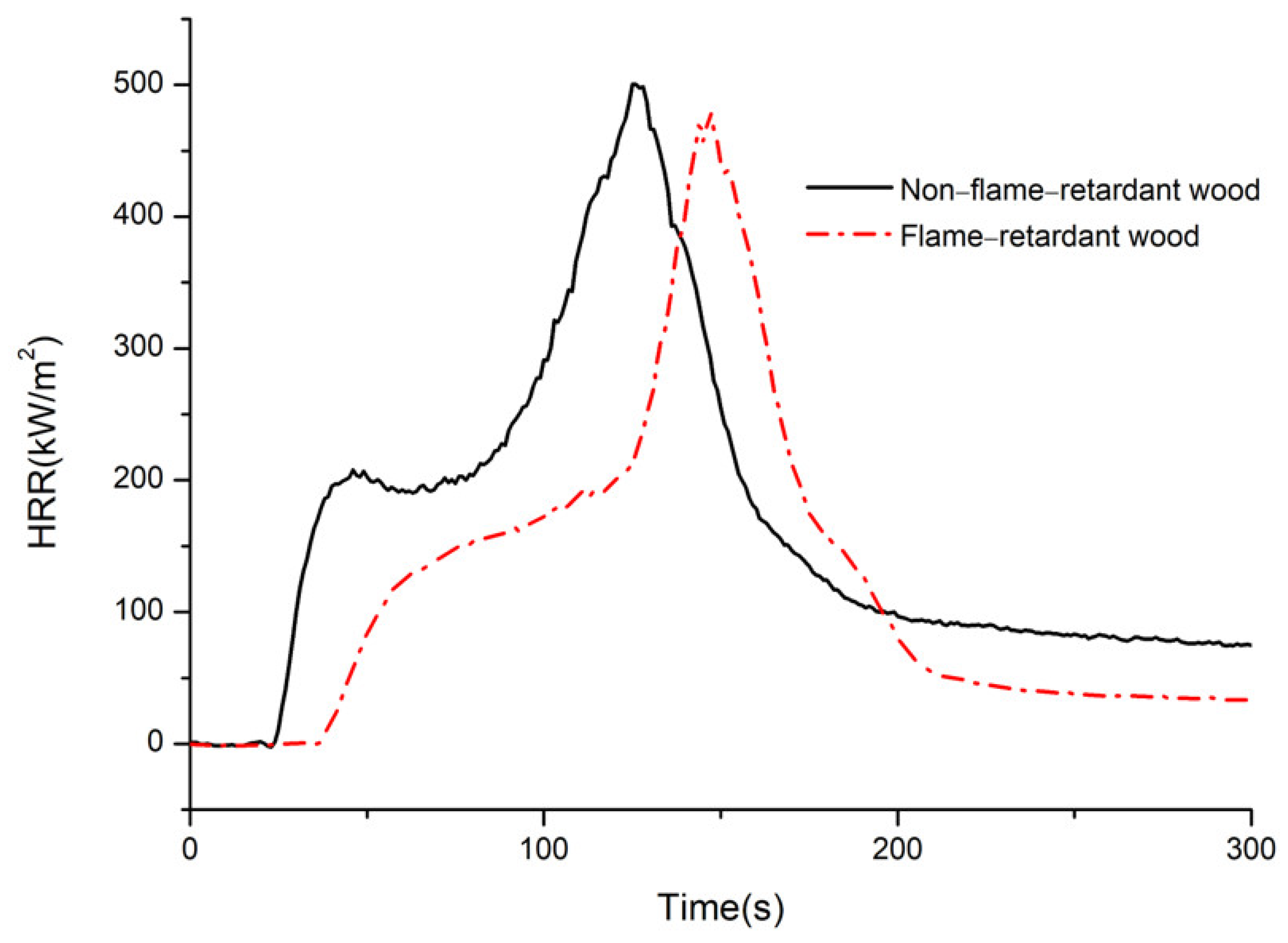
3.4. Heat Release Rate (HRR)
3.5. Time to Ignition (TTI)
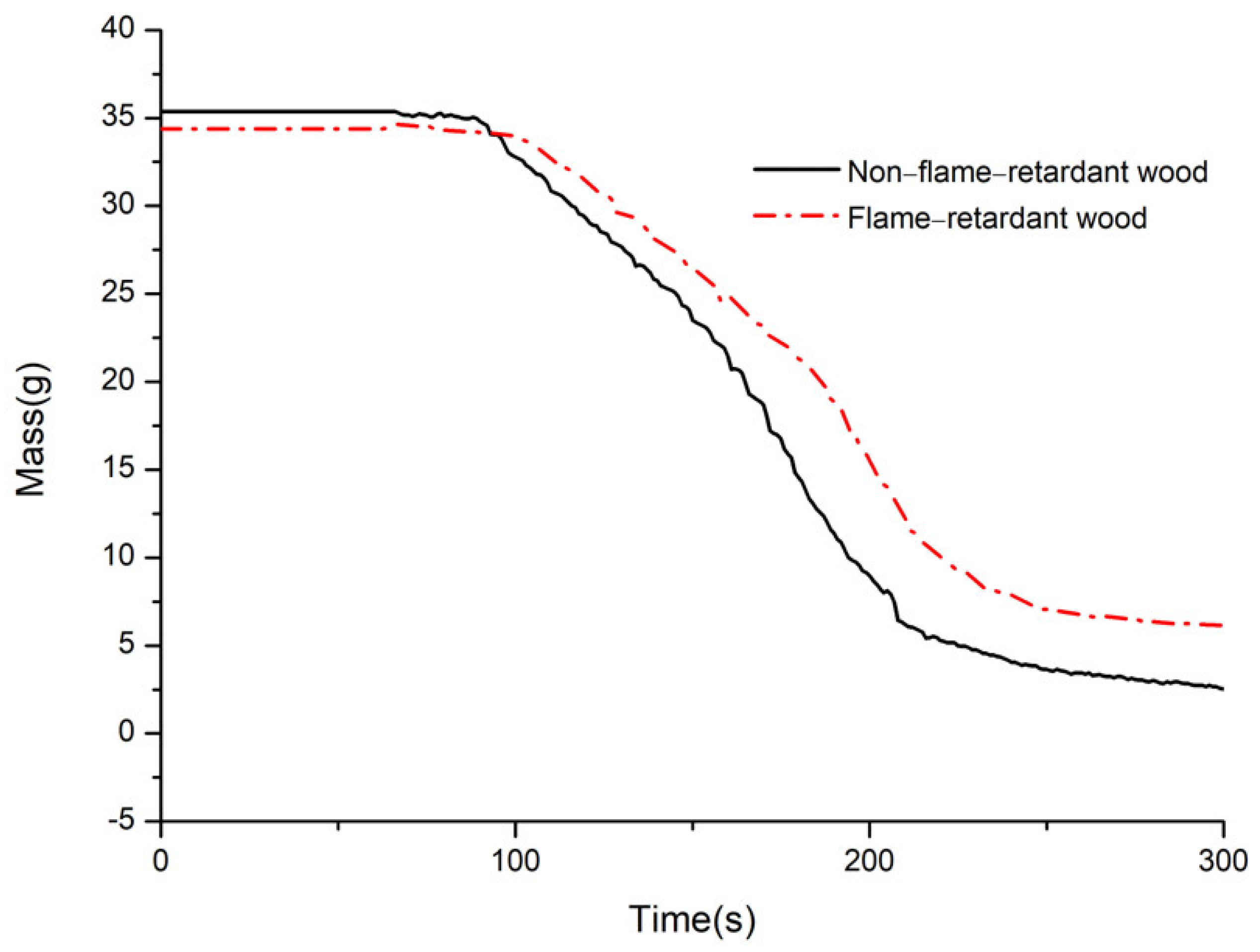
3.6. Mass of Residue(Mass)
4. Conclusions
- (1)
- The crystallisation temperature of CaAl–SiO3–LDHs considerably influenced its structure and properties. The crystallinity and thermal stability of CaAl–SiO3–LDHs prepared at 100 °C was the highest.
- (2)
- The crystallisation time of CaAl–SiO3–LDHs considerably influenced its structure and properties. The sample prepared at 9 h exhibited high crystallinity, obvious layered structure and the best thermal stability.
- (3)
- The molar concentration of solution of CaAl–SiO3–LDHs considerably influenced its structure and properties. When the molar concentration of Ca2+ was 0.33 mol/L, the crystallinity of the sample was the highest, the lamellar was the most uniform and the thermal stability is excellent.
- (4)
- The optimum technological parameters for preparing CaAl–SiO3–LDHs by the coprecipitation method are as follows: crystallisation temperature 100 °C, crystallisation time 9 h and molar concentration of Ca2+ solution 0.33 mol/L. It is not a cheap raw material, but also has high thermal stability.
- (5)
- Compared with the nonflame-retardant bamboo chips, after flame retardant treatment with CaAl–SiO3–LDHs, the peak times of HRR and ignition were delayed by 20 s and 77.78%, respectively, and the residual carbon rate was increased by 9.54%, the results show that the addition of CaAl–SiO3–LDHs greatly improves the flame retardancy of bamboo.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q. Science and innovation should be emphasized in bamboo processing and utilization in China. J. Zhejiang For. Coll. 2003, 20, 1–4. [Google Scholar]
- Zhou, Z.; Du, C.; Wei, J.; Yu, H. General situation and prospect of bamboo flame retardant research. J. Zhejiang For. Sci. Technol. 2016, 36, 71–77. [Google Scholar]
- Yao, X.; Du, C.; Hua, Y.; Zhang, J.; Peng, R.; Huang, Q.; Liu, H. Flame-Retardant and Smoke Suppression Properties of Nano MgAl-LDH Coating on Bamboo Prepared by an In Situ Reaction. J. Nanomater. 2019, 2019, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z. Study of Water-Based Flame Retardant on Main Properties of Bamboo; Zhejiang A&F University: Hangzhou, China, 2018. [Google Scholar]
- Zhao, Z. Study on the Application of Water-based Flame Retardant in Wood. Secur. Technol. 2010, 8, 39–41. [Google Scholar]
- He, W.; Song, P.; Yu, B.; Fang, Z.; Wang, H. Flame retardant polymeric nanocomposites through the combination of nanomaterials and conventional flame retardants. Prog. Mater. Sci. 2020, 114, 100687. [Google Scholar] [CrossRef]
- Dhaliwal, G.S.; Bajwa, D.S.; Bajwa, S. Fabrication and Testing of SoyBased Polyurethane Foam with Flame Retardant Properties. J. Polym. Environ. 2020, 29, 1153–1161. [Google Scholar] [CrossRef]
- Zhang, D.; Meng, D.; Ma, Z.; Zhang, Z.; Ning, H.; Wang, Y. Synthesis of a novel organic–inorganic hybrid flame retardant based on Ca(H2PO4)2 and hexachlorocyclotriphosphazene and its performance in polyvinyl alcohol. J. Appl. Polym. Sci. 2021, 138, 50099. [Google Scholar] [CrossRef]
- Yang, J.; Xie, G. Research Progress in Polymer/Layered Double Hydroxide Nanocomposites. Synth. Fibre 2014, 43, 41–47. [Google Scholar]
- Leão, A.D.; da Silva, L.A.; Ribeiro, F.d.S.; da Silva, D.A.; de França, E.J.; da Silva Aquino, K.A.; Soares-Sobrinho, J.L. Influence of Nonmodified Layered Double Hydroxide (LDH) Metal Constituents in PMMA/LDH Nanocomposites. J. Inorg. Organomet. Polym. Mater. 2021, 31, 836–850. [Google Scholar] [CrossRef]
- Hua, Y.; Du, C.; Yu, H.; Hu, A.; Peng, R.; Zhang, J.; Li, Q.; Ma, Z. Flame-retardancy and smoke suppression characteristics of bamboo impregnated with silicate-intercalated calcium aluminum hydrotalcates. Bioresources 2020, 16, 1311–1324. [Google Scholar] [CrossRef]
- Damindarova, V.N.; Ryl’tsova, I.G.; Tarasenko, E.A.; Wang, X.; Lebedeva, O.E. Tin-Containing Layered Double Hydroxides. Pet. Chem. 2020, 60, 444–450. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, F.; Lu, J.; Zeng, R.; Li, S.; Song, L.; Zeng, J. A comparison of corrosion inhibition of magnesium aluminum and zinc aluminum vanadate intercalated layered double hydroxides on magnesium alloys. Front. Mater. Sci. 2018, 12, 198–206. [Google Scholar] [CrossRef]
- Duan, Z.; Zhang, X.; Cheng, N.; Wu, Y.; Qiu, T. Research Progress of Hydrotalcite Materials for Catalytic Dehydrogenation. Fine Chem. Ind. 2021, 1–13. [Google Scholar] [CrossRef]
- Tsuyumoto, I. High flame retardancy of amorphous sodium silicate on poly(ethylene-co-vinyl acetate) (EVA). Polym. Bull. 2018, 75, 4967–4976. [Google Scholar] [CrossRef]
- Guo, J.; Yin, A.; Chen, J.; Hu, C.; Yi, T.; Shen, X. Different methods to prepare layered hydrotalcite with p-aminobenzenesulfonic acid root cutting. J. Hunan Univ. Humanit. Sci. Technol. 2009, 2, 9–13. [Google Scholar]
- Wang, X. Atlas of geochemical element and the basic necessities of life. Pop. Sci. Territ. Resour. Cult. 2016, 3, 4–9. [Google Scholar]
- Du, C.; Zhou, Z.; Yu, H.; Huang, Q.; Yao, X.; Jin, C. Smoke toxicity and smoke suppression performance of flame-retardant bamboo during burning. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2017, 41, 163–168. [Google Scholar]
- Kovanda, F.; Jindová, E.; Lang, K.; Kubát, P.; Sedláková, Z. Preparation of layered double hydroxides intercalated with organic anions and their application in LDH/poly(butyl methacrylate) nanocomposites. Appl. Clay Sci. 2010, 48, 260–270. [Google Scholar] [CrossRef]
- Yanshan, H.; Linhua, Z.; Jin, Y.; Yanlin, S. Structure, properties, preparation and application in catalysis of hydrotalcite-like anionic intercalation materials. Silic. Bull. 2013, 32, 429–433. [Google Scholar]
- Chang, P.; Lee, T.; Chang, Y.; Chen, S. CO2 Sorbents with Scaffold-like CaAl Layered Double Hydroxides as Precursors for CO2 Capture at High Temperatures. Chemsuschem 2013, 6, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Mora, M.; López, M.I.; Jiménez-Sanchidrián, C.; Ruiz, J.R. Ca/Al Mixed Oxides as Catalysts for the Meerwein–Ponndorf–Verley Reaction. Catal. Lett. 2010, 136, 192–198. [Google Scholar] [CrossRef]
- Yao, X.; Peng, R.; Du, C.; Hua, Y.; Zhang, J.; Huang, Q.; Liu, H. A Two-step Method for Fabricating Bamboo Culm Coated with MgAl-LDHs and its Fire Resistance Properties. Bioresources 2019, 14, 5150–5161. [Google Scholar]
- Li, J.; Wang, Q.; Li, S.; Wu, S. Study on flame retardancy of wood flame retardant FRW by CONE method. For. Sci. 2002, 38, 108–114. [Google Scholar]
- Song, Z. Controllable Preparation, Drug Delivery and Anti-Tumor Properties of Nano-Hydrotalcite Compounds; Shanghai Normal University: Shanghai, China, 2015. [Google Scholar]
- Chang, P.; Chang, Y.; Lai, Y.; Chen, S.; Yu, C.; Chyou, Y. Synthesis, characterization and high temperature CO2 capture capacity of nanoscale Ca-based layered double hydroxides via reverse microemulsion. J. Alloys Compd. 2014, 586, S498–S505. [Google Scholar] [CrossRef]
- Du, Y.; Xie, X.; Wu, X.; Hu, X.; Wang, Z. Application of TG-DTA in thermal decomposition of hydrotalcite compounds. Appl. Chem. Ind. 2005, 34, 12–15. [Google Scholar]
- Kaul, P.K.; Samson, A.J.; Selvan, G.T.; Enoch, I.V.M.V.; Selvakumar, P.M. Synergistic effect of LDH in the presence of organophosphate on thermal and flammable properties of an epoxy nanocomposite. Appl. Clay Sci. 2017, 135, 234–243. [Google Scholar] [CrossRef]
- Du, C.; Yu, H.; Zhou, Z.; Yao, X.; Huang, Q. Flame retardant performance of flame retardant bamboo flooring. For. Prod. Ind. 2017, 44, 7–11. [Google Scholar]
- Zhiwei, H.; Mingjie, G. Cone calorimeter analysis of bamboo floor combustion characteristics. J. Southwest Univ. (Nat. Sci. Ed.) 2017, 39, 187–192. [Google Scholar]




| Sample Number | Crystallisation Temperatures | Crystallisation Times | Ca2+ Molar Concentrations | |
|---|---|---|---|---|
| Samples prepared at different crystallisation temperatures | ① | 85 °C | 12 h | 0.33 mol/L |
| ② | 100 °C | 12 h | 0.33 mol/L | |
| ③ | 120 °C | 12 h | 0.33 mol/L | |
| Samples prepared at different crystallisation times | ④ | 100 °C | 6 h | 0.33 mol/L |
| ⑤ | 100 °C | 9 h | 0.33 mol/L | |
| ⑥ | 100 °C | 12 h | 0.33 mol/L | |
| Samples prepared at different Ca2+ molar concentrations | ⑦ | 100 °C | 9 h | 0.13 mol/L |
| ⑧ | 100 °C | 9 h | 0.33 mol/L | |
| ⑨ | 100 °C | 9 h | 0.53 mol/L | |
| ⑩ | 100 °C | 9 h | 0.73 mol/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, A.; Du, C.; Hua, Y.; Shan, Y.; Liu, C.; Chen, S.; Li, Q.; Yu, H. Preparation and Flame Retardant Properties of Calcium–Aluminium Hydrotalcite with Root Cutting Silicate Layers as Bamboo Flame Retardants. Materials 2021, 14, 7319. https://doi.org/10.3390/ma14237319
Hu A, Du C, Hua Y, Shan Y, Liu C, Chen S, Li Q, Yu H. Preparation and Flame Retardant Properties of Calcium–Aluminium Hydrotalcite with Root Cutting Silicate Layers as Bamboo Flame Retardants. Materials. 2021; 14(23):7319. https://doi.org/10.3390/ma14237319
Chicago/Turabian StyleHu, Ailian, Chungui Du, Yating Hua, Yingying Shan, Chunlin Liu, Shiqin Chen, Qi Li, and Hongwei Yu. 2021. "Preparation and Flame Retardant Properties of Calcium–Aluminium Hydrotalcite with Root Cutting Silicate Layers as Bamboo Flame Retardants" Materials 14, no. 23: 7319. https://doi.org/10.3390/ma14237319






