Recruiting Perovskites to Degrade Toxic Trinitrotoluene
Abstract
:1. Introduction
2. Details of Calculations
3. Results and Discussion
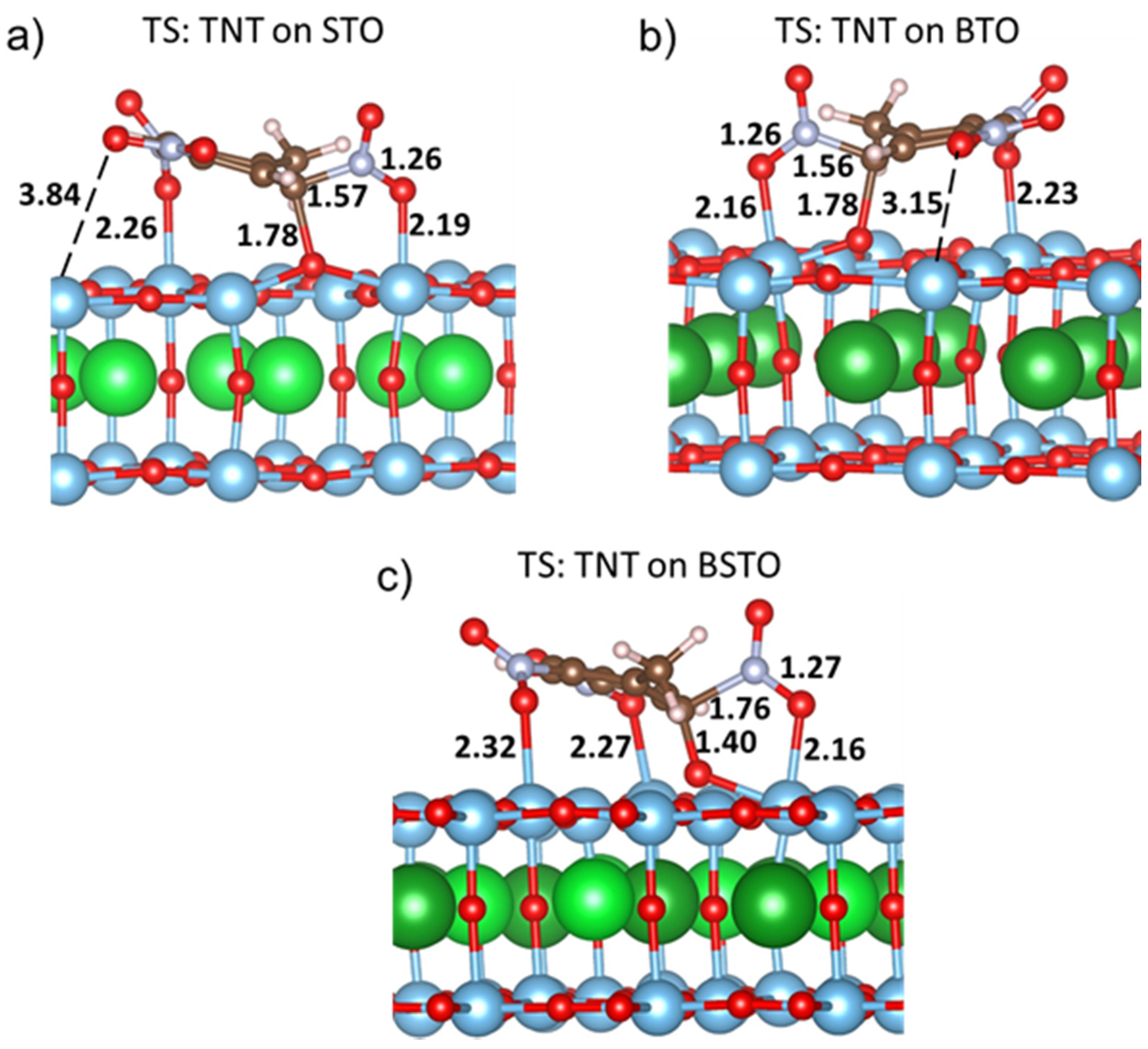
3.1. TNT Adsorption on Perovskites
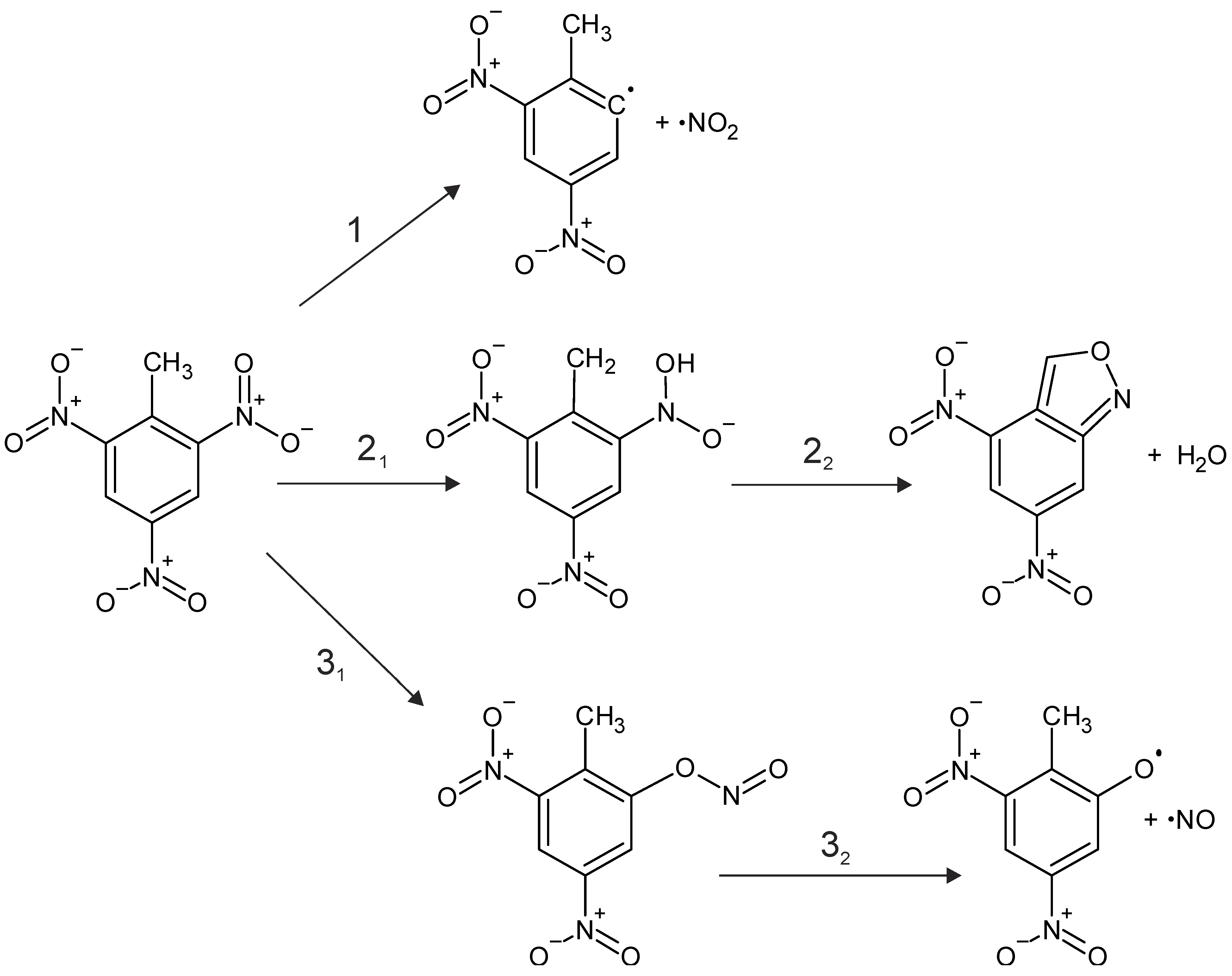
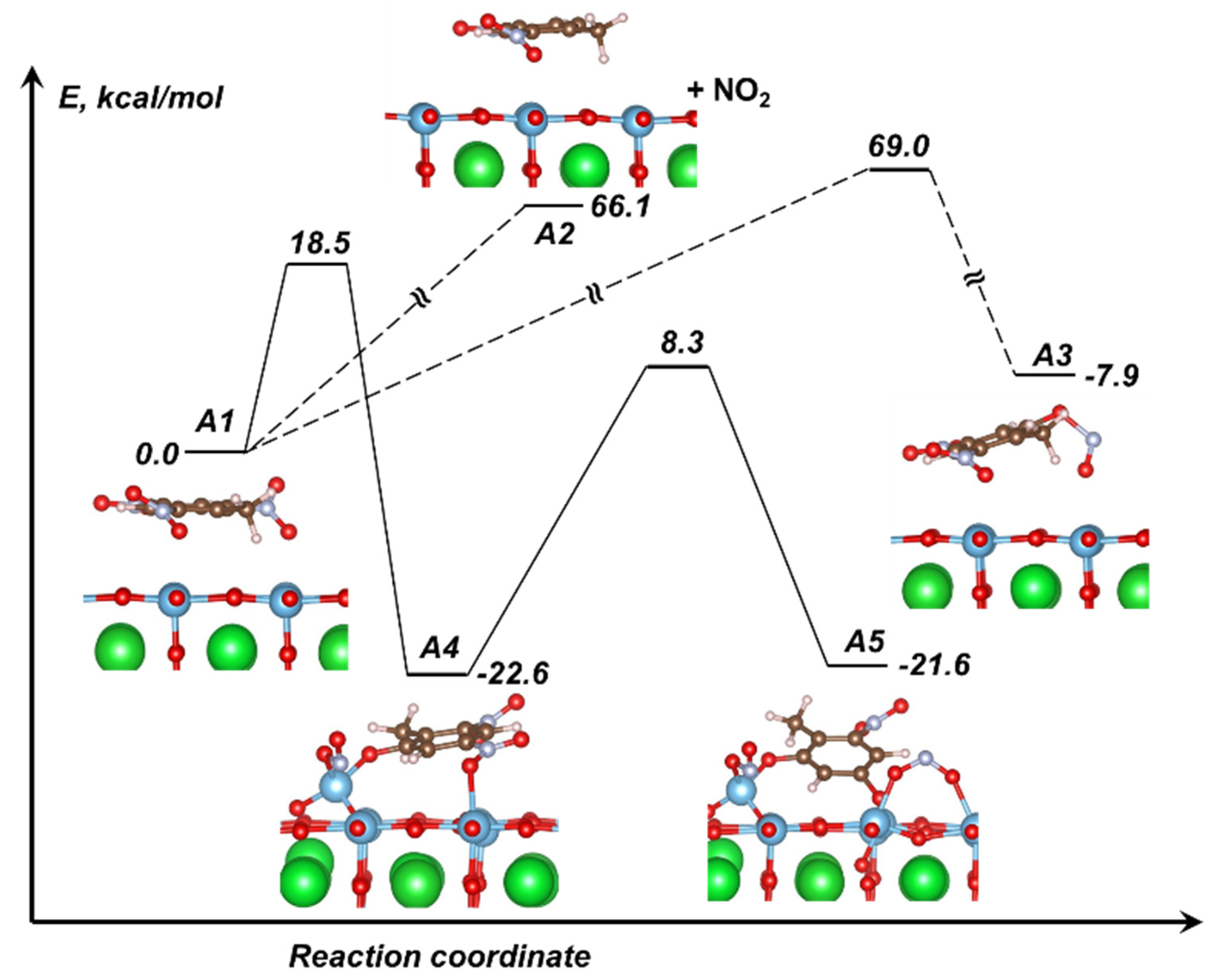
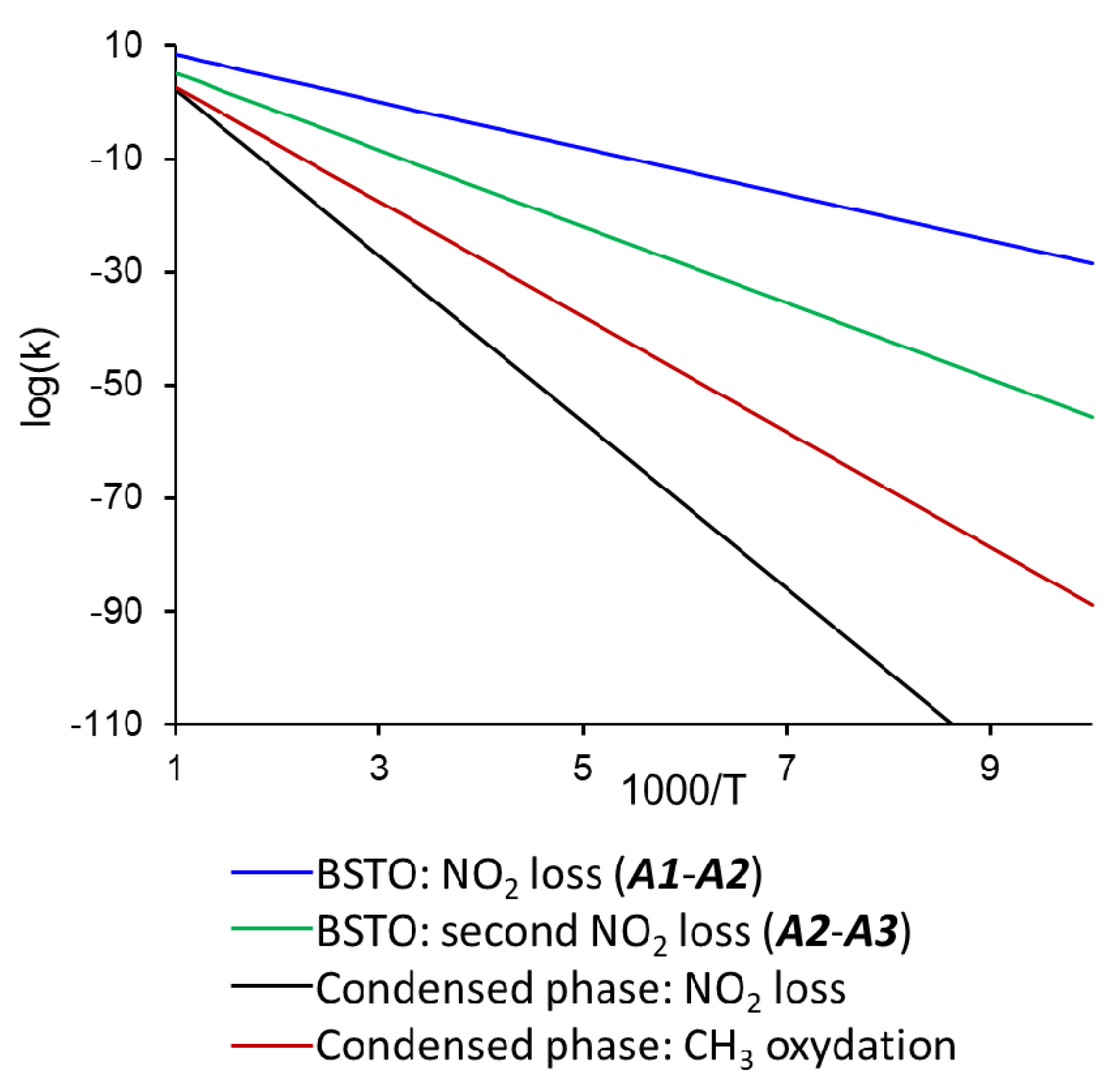
3.2. Perovskite Surface-Enhanced Decomposition of TNT
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cooper, P.W. Explosives Engineering; Wiley-VCH: New York, NY, USA, 1996. [Google Scholar]
- Wilbrand, J. Notiz über Trinitrotoluol. Ann. Chem. Pharm. 1863, 128, 178–179. [Google Scholar] [CrossRef] [Green Version]
- Krehl, P.O.K. History of Shock Waves, Explosions and Impact: A Chronological and Biographical Reference; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Brill, T.B.; James, K.J. Kinetics and mechanisms of thermal decomposition of nitroaromatic explosives. Chem. Rev. 1993, 93, 2667–2692. [Google Scholar] [CrossRef]
- Fedoroff, B.T.; Sheffield, O.E. Encyclopedia of Explosives and Related Items; Dover: Picatinny Arsenal, NJ, USA, 1966. [Google Scholar]
- Urbański, T. Chemistry and Technology of Explosives; Pergamon: Oxford, UK, 1985. [Google Scholar]
- Szala, M.; Sabatini, J.J. 2,4,6-Trinitrotoluene—A Useful Starting Compound in the Synthesis of Modern Energetic Compounds. Z. Anorg. Allg. Chem. 2018, 644, 262–269. [Google Scholar] [CrossRef]
- Landenberger, K.B.; Matzger, A.J. Cocrystal Engineering of a Prototype Energetic Material: Supramolecular Chemistry of 2,4,6-Trinitrotoluene. Cryst. Growth Des. 2010, 10, 5341–5347. [Google Scholar] [CrossRef]
- Bolton, O.; Matzger, A.J. Improved Stability and Smart-Material Functionality Realized in an Energetic Cocrystal. Angew. Chem. 2011, 123, 9122–9125. [Google Scholar] [CrossRef]
- Campbell, J. Naval weapons of World War Two; Conway Maritime Press: London, UK, 1985. [Google Scholar]
- DeCarli, P.S.; Jamieson, J.C. Formation of Diamond by Explosive Shock. Science 1961, 133, 1821–1822. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qian, Y.; Liao, H.; Ding, Y.; Yang, L.; Xu, C.; Li, F.; Zhou, G. A Reduction-Pyrolysis-Catalysis Synthesis of Diamond. Science 1998, 281, 246–247. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Koga, K.; Takeo, H. Structural properties of diamond fine particles and clusters prepared by detonation and decomposition of TNT. Surf. Sci. 1998, 400, 134–139. [Google Scholar] [CrossRef]
- Dolmatov, V.Y. Detonation synthesis ultradispersed diamonds: Properties and applications. Russ. Chem. Rev. 2001, 70, 607–626. [Google Scholar] [CrossRef]
- Brill, T.B.; James, K.J. Thermal decomposition of energetic materials. 62. Reconciliation of the kinetics and mechanisms of TNT on the time scale from microseconds to hours. J. Phys. Chem. 1993, 97, 8759–8763. [Google Scholar] [CrossRef]
- Fried, L.E.; Manaa, M.R.; Pagoria, P.F.; Simpson, R.L. Design and Synthesis of Energetic Materials. Annu. Rev. Mater. Res. 2001, 31, 291–321. [Google Scholar] [CrossRef]
- Kuklja, M.M. Quantum-chemical modeling of energetic materials: Chemical reactions triggered by defects, deformations, and electronic excitations. Adv. Quantum. Chem. 2014, 69, 71–146. [Google Scholar]
- Kuklja, M. On the initiation of chemical reactions by electronic excitations in molecular solids. Appl. Phys. A 2003, 76, 359–366. [Google Scholar] [CrossRef]
- Tsyshevsky, R.V.; Sharia, O.; Kuklja, M.M. Molecular Theory of Detonation Initiation: Insight from First Principles Modeling of the Decomposition Mechanisms of Organic Nitro Energetic Materials. Molecules 2016, 21, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiegand, D.A.; Pinto, J. The mechanical response of tnt and a composite, Composition B, of TNT and RDX to compressive stress: III dependence on processing and composition. J. Energetic Mater. 1991, 9, 349–413. [Google Scholar] [CrossRef]
- Urtiew, P.A.; Vandersall, K.S.; Tarver, C.M.; Garcia, F.; Forbes, J.W. Shock initiation of composition B and C-4 explosives: Experiments and modeling. Russ. J. Phys. Chem. B 2008, 2, 162–171. [Google Scholar] [CrossRef] [Green Version]
- Hussein, A.K.; Elbeih, A.; Zeman, S. Thermo-analytical study of a melt cast composition based on cis-1,3,4,6-tetranitrooctahydroimidazo-[4,5 d]imidazole (BCHMX)/trinitrotoluene (TNT) compared with traditional compositions. Thermochim. Acta 2018, 666, 91–102. [Google Scholar] [CrossRef]
- Gottfried, J.L.; Smith, D.K.; Wu, C.-C.; Pantoya, M.L. Improving the Explosive Performance of Aluminum Nanoparticles with Aluminum Iodate Hexahydrate (AIH). Sci. Rep. 2018, 8, 8036. [Google Scholar] [CrossRef]
- Li, J.-S.; Chen, J.-J.; Hwang, C.-C.; Lu, K.-T.; Yeh, T.-F. Study on Thermal Characteristics of TNT Based Melt-Cast Explosives. Propellants Explos. Pyrotech. 2019, 44, 1270–1281. [Google Scholar] [CrossRef]
- An, C.-W.; Li, F.-S.; Song, X.-L.; Wang, Y.; Guo, X.-D. Surface Coating of RDX with a Composite of TNT and an Energetic-Polymer and its Safety Investigation. Propellants Explos. Pyrotech. 2009, 34, 400–405. [Google Scholar] [CrossRef]
- An, C.; Wang, J.; Xu, W.; Li, F. Preparation and Properties of HMX Coated with a Composite of TNT/Energetic Material. Propellants Explos. Pyrotech. 2010, 35, 365–372. [Google Scholar] [CrossRef]
- Guo, D.; An, Q.; Goddard, W.A., III; Zybin, S.V.; Huang, F. Compressive shear reactive molecular dynamics studies indicating that cocrystals of TNT/CL-20 decrease sensitivity. J. Phys. Chem. C 2014, 118, 30202–30208. [Google Scholar] [CrossRef]
- Bart, J.C.; Judd, L.L.; Hoffman, K.E.; Wilkins, A.M.; Kusterbeck, A.W. Application of a Portable Immunosensor to Detect the Explosives TNT and RDX in Groundwater Samples. Environ. Sci. Technol. 1997, 31, 1505–1511. [Google Scholar] [CrossRef]
- Pichtel, J. Distribution and Fate of Military Explosives and Propellants in Soil: A Review. Appl. Environ. Soil Sci. 2012, 2012, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Fawcett-Hirst, W.; Temple, T.J.; Ladyman, M.K.; Coulon, F. A review of treatment methods for insensitive high explosive contaminated wastewater. Heliyon 2021, 7, e07438. [Google Scholar] [CrossRef]
- Kalderis, D.; Juhasz, A.; Boopathy, R.; Comfort, S. Soils contaminated with explosives: Environmental fate and evaluation of state-of-the-art remediation processes (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 1407–1484. [Google Scholar] [CrossRef]
- Klapec, D.J.; Czarnopys, G.; Pannuto, J. Interpol review of detection and characterization of explosives and explosives residues 2016–2019. Forensic Sci. Int. Synerg. 2020, 2, 670–700. [Google Scholar] [CrossRef] [PubMed]
- Beard, R.R.; Noe, J.T. Aromatic nitro and amino compounds. In Patty’s Industrial Hygiene and Toxicology; Clayton, G.D., Clayton, F.E., Eds.; John Wiley and Sons: New York, NY, USA, 1982; Volume 2A, pp. 2413–2481. [Google Scholar]
- Marshall, A. Explosives: Their History, Manufacture, Properties and Tests, 3rd ed.; Churchill: London, UK, 1932. [Google Scholar]
- Rosenblatt, D.H.; Burrows, E.P.; Mitchell, W.R.; Parmer, D.L. Organic explosives and related compounds. In The Handbook of Environmental Chemistry; Hutzinger, O., Ed.; Springer: Berlin/Heidelberg, Germany, 1991; pp. 195–234. [Google Scholar]
- Wujcik, W.J.; Lowe, W.L.; Marks, P.J.; Sisk, W.E. Granular activated carbon pilot treatment studies for explosives removal from contaminated groundwater. Environ. Prog. 1992, 11, 178–189. [Google Scholar] [CrossRef]
- Sublette, K.L.; Ganapathy, E.V.; Schwartz, S. Degradation of munition wastes by Phanerochaete chrysosporium. Appl. Biochem. Biotechnol. 1992, 34, 709–723. [Google Scholar] [CrossRef]
- Vasilyeva, G.; Kreslavski, V.; Shea, P. Catalytic oxidation of TNT by activated carbon. Chemosphere 2001, 47, 311–317. [Google Scholar] [CrossRef]
- Schmelling, D.C.; Gray, K.A.; Kamat, P.V. Role of Reduction in the Photocatalytic Degradation of TNT. Environ. Sci. Technol. 1996, 30, 2547–2555. [Google Scholar] [CrossRef]
- Carraway, E.R.; Hoffman, A.J.; Hoffman, M.R. Photo-catalytic oxidation of organic acids on quantum-sized semiconductor colloids. Environ. Sci. Technol. 1994, 28, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Son, H.-S.; Lee, S.-J.; Cho, I.-H.; Zoh, K.-D. Kinetics and mechanism of TNT degradation in TiO2 photocatalysis. Chemosphere 2004, 57, 309–317. [Google Scholar] [CrossRef]
- Ayoub, K.; van Hullebusch, E.D.; Cassir, M.; Bermond, A. Application of advanced oxidation processes for TNT removal: A review. J. Hazard. Mater. 2010, 178, 10–28. [Google Scholar] [CrossRef]
- Ayoub, K.; Nélieu, S.; van Hullebusch, E.D.; Labanowski, J.; Schmitz-Afonso, I.; Bermond, A.; Cassir, M. Electro-Fenton removal of TNT: Evidences of the electro-chemical reduction contribution. Appl. Catal. B Environ. 2011, 104, 169–176. [Google Scholar] [CrossRef]
- Lewis, T.; Newcombe, D.; Crawfold, R. Bioremediation of soils contaminated with explosives. J. Environ. Manag. 2004, 70, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.; Chapelle, F.H.; Landmeyer, J.E.; Schumacher, J.G. Microbial Transformation of Nitroaromatics in Surface Soils and Aquifer Materials. Appl. Environ. Microbiol. 1994, 60, 2170–2175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieger, P.-G.; Knackmuss, H.-J. Basic knowledge and perspectives on biodegradation of 2, 4, 6-trinitrotoluene and related nitroaromatic compounds in contaminated soil. In Biodegradation of Nitroaromatic Compounds; Spain, J.C., Ed.; Springer: Boston, MA, USA, 1995; pp. 1–18. [Google Scholar]
- Esteve-Núñez, A.; Caballero, A.; Ramos, J.L. Biological degradation of 2,4,6-trinitrotoluene. Microbiol. Mol. Biol. Rev. 2001, 65, 335–352. [Google Scholar] [CrossRef] [Green Version]
- Lamba, J.; Anand, S.; Dutta, J.; Chatterjee, S.; Nagar, S.; Celin, S.M.; Rai, P.K. Study on aerobic degradation of 2,4,6-trinitrotoluene (TNT) using Pseudarthrobacter chlorophenolicus collected from the contaminated site. Environ. Monit. Assess. 2021, 193, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liu, D.; Sun, P.; Li, Y.; Wu, M.; Liu, W.; Maser, E.; Xiong, G.; Guo, L. Degradation of 2,4,6-Trinitrotoluene (TNT): Involvement of Protocatechuate 3,4-Dioxygenase (P34O) in Buttiauxella sp. S19-1. Toxics 2021, 9, 231. [Google Scholar] [CrossRef] [PubMed]
- Harms, H.; Schlosser, D.; Wick, L.Y. Untapped potential: Exploiting fungi in bioremediation of hazardous chemicals. Nat. Rev. Genet. 2011, 9, 177–192. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Lai, J.-L.; Luo, X.-G.; Han, M.-W.; Zhao, S.-P.; Zhu, Y.-B. Analysis of the biodegradation and phytotoxicity mechanism of TNT, RDX, HMX in alfalfa (Medicago sativa). Chemosphere 2021, 281, 130842. [Google Scholar] [CrossRef]
- Saupe, A.; Garvens, H.J.; Heinze, L. Alkaline hydrolysis of TNT and TNT in soil followed by thermal treatment of the hydrolysates. Chemosphere 1998, 36, 1725–1744. [Google Scholar] [CrossRef]
- Lang, P.S.; Ching, W.-K.; Willberg, D.M.; Hoffmann, M.R. Oxidative Degradation of 2,4,6-Trinitrotoluene by Ozone in an Electrohydraulic Discharge Reactor. Environ. Sci. Technol. 1998, 32, 3142–3148. [Google Scholar] [CrossRef]
- Gilbert-López, B.; Lara-Ortega, F.J.; Robles-Molina, J.; Brandt, S.; Schütz, A.; Moreno-González, D.; García-Reyes, J.F.; Molina-Díaz, A.; Franzke, J. Detection of multiclass explosives and related compounds in soil and water by liquid chromatography-dielectric barrier discharge ionization-mass spectrometry. Anal. Bioanal. Chem. 2019, 411, 4785–4796. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresse, G.; Furthmuller, F. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Woo, S.; Jeong, H.; Lee, S.A.; Seo, H.; Lacotte, M.; David, A.; Kim, H.Y.; Prellier, W.; Kim, Y.; Choi, W.S. Surface properties of atomically flat poly-crystalline SrTiO3. Sci. Rep. 2015, 5, 8822. [Google Scholar] [CrossRef] [Green Version]
- Heifets, E.; Piskunov, S.; Kotomin, E.A.; Zhukovskii, Y.F.; Ellis, D.E. Electronic structure and thermodynamic stability of double-layered SrTiO3 (001) surfaces: Ab initio simulations. Phys. Rev. B 2007, 75, 115417. [Google Scholar] [CrossRef] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Lytle, F.W. X-ray Diffractometry of Low-Temperature Phase Transformations in Strontium Titanate. J. Appl. Phys. 1964, 35, 2212–2215. [Google Scholar] [CrossRef]
- Kwei, G.H.; Lawson, A.C.; Billinge, S.J.L.; Cheong, S.W. Structures of the ferroelectric phases of barium titanate. J. Phys. Chem. 1993, 97, 2368–2377. [Google Scholar] [CrossRef]
- Chorkendorff, I.; Niemantsverdriet, J.W. Concepts of Modern Catalysis and Kinetics, 2nd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Sharia, O.; Kuklja, M.M. Ab Initio Kinetics of Gas Phase Decomposition Reactions. J. Phys. Chem. A 2010, 114, 12656–12661. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Murray, É.D.; Kong, L.; Lundqvist, B.I.; Langreth, D.C. Higher-accuracy van der Waals density functional. Phys. Rev. B 2010, 82, 081101. [Google Scholar] [CrossRef] [Green Version]
- Cohen, R.; Zeiri, Y.; Wurzberg, E.; Kosloff, R. Mechanism of Thermal Unimolecular Decomposition of TNT (2,4,6-Trinitrotoluene): A DFT Study. J. Phys. Chem. A 2007, 111, 11074–11083. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tsyshevsky, R.; Zverev, A.; Mitrofanov, A.; Kuklja, M.M. Can a photosensitive oxide catalyze decomposition of energetic materials? J. Phys. Chem. C 2017, 121, 1153–1161. [Google Scholar] [CrossRef]
- Mishra, S.B.; Marutheeswaran, S.; Roy, S.C.; Natarajan, V.; Rai, P.K.; Nanda, B.R.K. Adsorption and degradation mechanism of 2,4,6-trinitrotoluene on TiO2 (110) surface. Surf. Sci. 2021, 713, 121902. [Google Scholar] [CrossRef]

| Material | Ead, kcal/mol | ΔEb, kcal/mol | log(A, s−1) | Er, kcal/mol |
|---|---|---|---|---|
| SrTiO3 | −42.6 | 24.5 a (22.3) b | 13.1 | −3.7 |
| Ba0.5Sr0.5TiO3 | −42.7 | 18.5 (18.4) | 12.3 | −22.6 |
| BaTiO3 | −43.3 | 23.8 (22.2) | 12.2 | −25.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastrikov, Y.A.; Tsyshevsky, R.; Wang, F.; Kuklja, M.M. Recruiting Perovskites to Degrade Toxic Trinitrotoluene. Materials 2021, 14, 7387. https://doi.org/10.3390/ma14237387
Mastrikov YA, Tsyshevsky R, Wang F, Kuklja MM. Recruiting Perovskites to Degrade Toxic Trinitrotoluene. Materials. 2021; 14(23):7387. https://doi.org/10.3390/ma14237387
Chicago/Turabian StyleMastrikov, Yuri A., Roman Tsyshevsky, Fenggong Wang, and Maija M. Kuklja. 2021. "Recruiting Perovskites to Degrade Toxic Trinitrotoluene" Materials 14, no. 23: 7387. https://doi.org/10.3390/ma14237387
APA StyleMastrikov, Y. A., Tsyshevsky, R., Wang, F., & Kuklja, M. M. (2021). Recruiting Perovskites to Degrade Toxic Trinitrotoluene. Materials, 14(23), 7387. https://doi.org/10.3390/ma14237387






