A Comparative Study on the Mechanical Properties and Microstructure of Cement-Based Materials by Direct Electric Curing and Steam Curing
Abstract
:1. Introduction
2. Materials and Experiments
2.1. Materials and Equipment
2.2. Experimental Methods
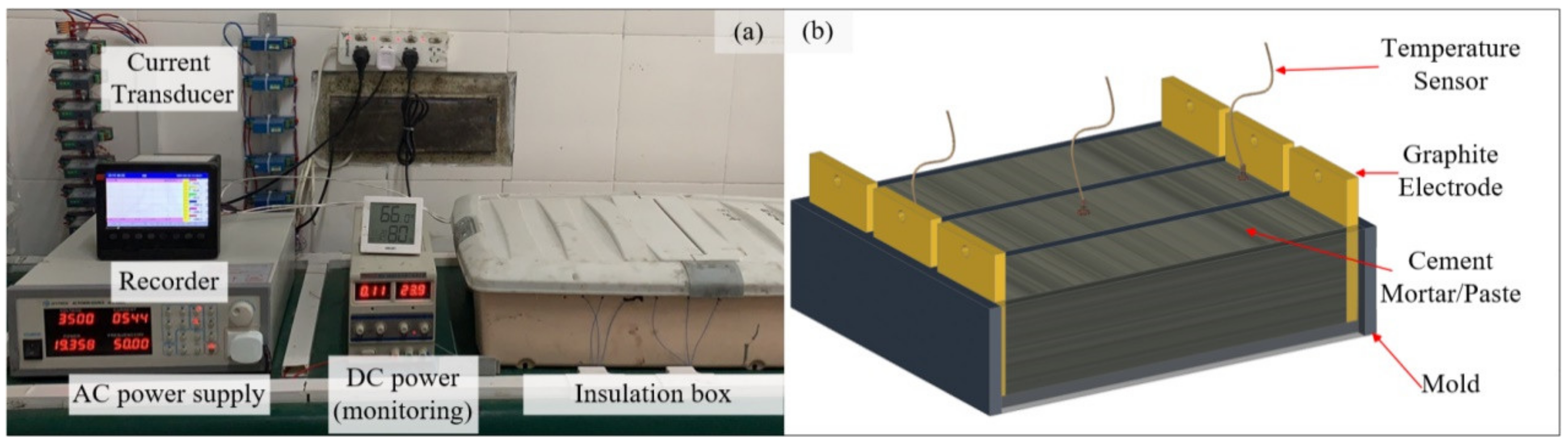
2.2.1. Casting and Curing
2.2.2. Testing
3. Results and Discussion
3.1. Strength Development
3.2. Solid Phase Analysis
3.2.1. TG
3.2.2. XRD
3.3. Pore Structure
3.3.1. Microscopic Pore Structure
3.3.2. Macro/Mesoscopic Pore Image Analysis
3.4. Discussion
3.4.1. Temperature Evolution during Curing Regimes
3.4.2. The Heat Flow Transfer Characteristics of Two Curing Methods
3.4.3. Energy Consumption
4. Conclusions
- 1.
- The compressive strength of both the SC and EC samples increased with the increase of the curing temperature at 1 day, but there was an opposite trend at 28 and 56 days. In addition, the early and late strength of the EC samples were both higher than that of the SC samples. These results indicate that both EC and SC were favorable to the development of early strength but not favorable to the continued growth of later strength, and EC was more favorable to improve the early strength of the samples and alleviate the decrease of later strength.
- 2.
- Based on the solid phase analysis, it was found that the degree of hydration and hydration products of EC was basically the same as that of SC, which presented that the influence of EC on the hydration of cement was similar to that of SC. Moreover, the capillary pore structure and air voids distribution of the EC samples were finer than those of the SC samples. These results suggest that the possible reason for the better strength development of the EC samples is the improved microstructure of the hydration products rather than hydration.
- 3.
- With the increase of the curing temperature from 40 °C to 80 °C, the energy consumption of SC increased from 2.13 times of EC to 8.62 times, which demonstrates that the energy consumption of SC was much higher than that of EC. Overall, EC can achieve better accelerated curing effects of the mortar than SC with lower energy consumption.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statements
Conflicts of Interest
References
- He, J.; Long, G.; Ma, K.; Xie, Y.; Ma, C. Hydration heat evolution of Portland cement paste during unsteady steam curing process: Modelling and optimization. Thermochim. Acta 2020, 694, 178784. [Google Scholar] [CrossRef]
- Zou, C.; Long, G.; Zeng, X.; Ma, C.; Xie, Y.; Sun, Z. Water evolution and hydration kinetics of cement paste under steam-curing condition based on low-field NMR method. Constr. Build. Mater. 2021, 271, 121583. [Google Scholar] [CrossRef]
- Hiremath, P.N.; Yaragal, S.C. Effect of different curing regimes and durations on early strength development of reactive powder concrete. Constr. Build. Mater. 2017, 154, 72–87. [Google Scholar] [CrossRef]
- Liu, B.; Jiang, J.; Shen, S.; Zhou, F.; Shi, J.; He, Z. Effects of curing methods of concrete after steam curing on mechanical strength and permeability. Constr. Build. Mater. 2020, 256, 119441. [Google Scholar] [CrossRef]
- Yang, J.; Hu, H.; He, X.; Su, Y.; Wang, Y.; Tan, H.; Pan, H. Effect of steam curing on compressive strength and microstructure of high volume ultrafine fly ash cement mortar. Constr. Build. Mater. 2021, 266, 120894. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, K.; Xia, T.; Long, G. Mechanical property, sorptivity and microstructure of steam-cured concrete incorporated with the combination of metakaolin-limestone. Case Stud. Constr. Mater. 2019, 11, e00267. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, M. Effect of curing temperature on hydration, microstructure and ionic diffusivity of fly ash blended cement paste: A modelling study. Constr. Build. Mater. 2021, 297, 123834. [Google Scholar] [CrossRef]
- Zou, C.; Long, G.; Zeng, X.; Ma, K.; Xie, Y. Hydration and multiscale pore structure characterization of steam-cured cement paste investigated by X-ray CT. Constr. Build. Mater. 2021, 282, 122629. [Google Scholar] [CrossRef]
- Zou, C.; Long, G.; Xie, Y.; He, J.; Ma, C.; Zeng, X. Evolution of multi-scale pore structure of concrete during steam-curing process. Microporous Mesoporous Mater. 2019, 288, 109566. [Google Scholar] [CrossRef]
- Wang, P.; Fu, H.; Guo, T.; Zuo, W.; Zhao, H.; Tian, L.; Chen, C. Volume deformation of steam-cured concrete with fly ash during and after steam curing. Constr. Build. Mater. 2021, 306, 124854. [Google Scholar] [CrossRef]
- Shi, J.; Liu, B.; Zhou, F.; Shen, S.; Dai, J.; Ji, R.; Tan, J. Heat damage of concrete surfaces under steam curing and improvement measures. Constr. Build. Mater. 2020, 252, 119104. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, Z.; Shao, S.; Zhang, X.; Li, N.; Xiong, C. Evolution of temperature stress and tensile properties of concrete during steam-curing process. Constr. Build. Mater. 2021, 305, 124691. [Google Scholar] [CrossRef]
- Xiang, Y.; Long, G.; Xie, Y.; Zheng, K.; He, Z.; Ma, K.; Zeng, X.; Wang, M. Thermal damage and its controlling methods of high-speed railway steam-cured concrete: A review. Struct. Concr. 2021, 22, 1074. [Google Scholar] [CrossRef]
- Shi, J.; Liu, B.; Zhou, F.; Shen, S.; Guo, A.; Xie, Y. Effect of steam curing regimes on temperature and humidity gradient, permeability and microstructure of concrete. Constr. Build. Mater. 2021, 281, 122562. [Google Scholar] [CrossRef]
- Cecini, D.; Austin, S.A.; Cavalaro, S.; Palmeri, A. Accelerated electric curing of steel-fibre reinforced concrete. Constr. Build. Mater. 2018, 189, 192–204. [Google Scholar] [CrossRef]
- Wilson, J.G.; Gupta, N.K. Assessment of structure formation in fresh concrete by measurement of its electrical resistance: Investigation reports on accelerated curing of concrete using direct electrical conduction with a view to minimizing the temperature rise and so improving durability and reducing energy costs. Build. Res. Inf. 1996, 24, 209–212. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, Y.; He, J.; Zeng, X.; Ma, K.; Long, G. Experimental investigation on mechanical strength and microstructure of cement paste by electric curing with different voltage and frequency. Constr. Build. Mater. 2021, 299, 123615. [Google Scholar] [CrossRef]
- Bredenkamp, S.; Kruger, K.; Bredenkamp, G.L. Direct electric curing of concrete. Mag. Concr. Res. 1993, 162, 71–74. [Google Scholar] [CrossRef]
- Kafry, I.D. Direct Electrical Curing of Concrete: Basic Design; Whittles Publishing: Dunbeath, UK, 1999. [Google Scholar]
- Wadhwa, S.S.; Srivastava, L.K.; Gautam, D.K.; Chandra, D. Direct electric curing of in situ concrete. Build. Res. Inf. 1987, 15, 97–101. [Google Scholar] [CrossRef]
- Wilson, J.; Gupta, N. Analysis of power distribution in reinforced concrete during accelerated curing using electroheat. IEE Proc.-Electr. Power Appl. 1996, 143, 172–176. [Google Scholar] [CrossRef]
- Wilson, J.G.; Gupta, N.K. Equipment for the investigation of the accelerated curing of concrete using direct electrical conduction. Measurement 2004, 35, 243–250. [Google Scholar] [CrossRef]
- Ma, C.; Peng, J.; Zhou, H.; Zhou, R.; Ren, W.; Du, Y. An effective method for preparing high early-strength cement-based materials: The effects of direct electric curing on Portland cement. J. Build. Eng. 2021, 43, 102485. [Google Scholar] [CrossRef]
- Uygunoğlu, T.; Hocaoğlu, I. Effect of electrical curing application on setting time of concrete with different stress intensity. Constr. Build. Mater. 2018, 162, 298–305. [Google Scholar] [CrossRef]
- Susanto, A.; Peng, G.; Koleva, D.A.; Van Breugel, K. Electrical Current Flow and Cement Hydration: Implications on Cement-Based Microstructure. Int. J. Struct. Civ. Eng. Res. 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Kovtun, M.; Ziolkowski, M.; Shekhovtsova, J.; Kearsley, E. Direct electric curing of alkali-activated fly ash concretes: A tool for wider utilization of fly ashes. J. Clean. Prod. 2016, 133, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Tian, W.; Qi, B.; Liu, Y.; Liu, K.; Wang, W. Early frost resistance and permeability properties of carbon fiber/cement-based composite cured by ohmic heating under ultra-low temperature. Constr. Build. Mater. 2021, 282, 122729. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Wang, W. Electric induced curing of graphene/cement-based composites for structural strength formation in deep-freeze low temperature. Mater. Des. 2018, 160, 783–793. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Wang, W. Ohmic heating curing of electrically conductive carbon nanofiber/cement-based composites to avoid frost damage under severely low temperature. Compos. Part A Appl. Sci. Manuf. 2018, 115, 236–246. [Google Scholar] [CrossRef]
- Tian, W.; Wang, M.; Liu, Y.; Wang, W. Ohmic heating curing of high content fly ash blended cement-based composites towards sustainable green construction materials used in severe cold region. J. Clean. Prod. 2020, 276, 123300. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Tian, W.; Qi, B.; Lei, Z.; Wang, W. Ohmic heating curing of carbon fiber/carbon nanofiber synergistically strengthening cement-based composites as repair/reinforcement materials used in ultra-low temperature environment. Compos. Part A Appl. Sci. Manuf. 2019, 125, 105570. [Google Scholar] [CrossRef]
- Tian, W.; Liu, Y.; Wang, W. Multi-structural evolution of conductive reactive powder concrete manufactured by enhanced ohmic heating curing. Cem. Concr. Compos. 2021, 123, 104199. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, W.; Wang, M.; Qi, B.; Wang, W. Rapid strength formation of on-site carbon fiber reinforced high-performance concrete cured by ohmic heating. Constr. Build. Mater. 2020, 244, 118344. [Google Scholar] [CrossRef]
- Jung, M.; Park, J.; Hong, S.-G.; Moon, J. Electrically cured ultra-high performance concrete (UHPC) embedded with carbon nanotubes for field casting and crack sensing. Mater. Des. 2020, 196, 109127. [Google Scholar] [CrossRef]
- Wang, M.; Xie, Y.; Long, G.; Ma, C.; Zeng, X.; Qiang, F. The impact mechanical characteristics of steam-cured concrete under different curing temperature conditions. Constr. Build. Mater. 2020, 241, 118042. [Google Scholar] [CrossRef]
- He, J.; Long, G.; Ma, C.; Ma, K.; Xie, Y.; Shi, Y.; Li, N.; Cheng, Z. Effect of Triethanolamine on Hydration Kinetics of Cement–Fly Ash System at Elevated Curing Temperature. ACS Sustain. Chem. Eng. 2020, 8, 10053–10064. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, J.; Han, S. Fractal analysis of relation between strength and pore structure of hardened mortar. Constr. Build. Mater. 2017, 135, 1–7. [Google Scholar] [CrossRef]
- Fu, Q.; Bu, M.; Li, D.; Xu, W.; He, J.; Niu, D. Resistance to Sulfate Attack and Chemo-Damage-Transport Model of Sulfate Ions for Tunnel Lining Concrete under the Action of Loading and Flowing Groundwater. ACS Sustain. Chem. Eng. 2021, 9, 14307–14326. [Google Scholar] [CrossRef]
- Ren, M.; Wen, X.; Gao, X.; Liu, Y. Thermal and mechanical properties of ultra-high performance concrete incorporated with microencapsulated phase change material. Constr. Build. Mater. 2021, 273, 121714. [Google Scholar] [CrossRef]













| SiO2 | Al2O3 | FexOy | CaO | MgO | SO3 | LOI | C3S | C2S | C3A | C4AF |
|---|---|---|---|---|---|---|---|---|---|---|
| 20.58 | 5.03 | 3.38 | 63.32 | 2.01 | 2.06 | 1.76 | 57.55 | 17.82 | 7.54 | 11.19 |
| Group | Curing Method | Curing Regime (°C) | Cement Pasts |
|---|---|---|---|
| SC40 | Steam | 40 | - |
| SC60 | Steam | 60 | Casted and cured |
| SC80 | Steam | 80 | - |
| EC40 | Electric | 40 | - |
| EC60 | Electric | 60 | Casted and cured |
| EC80 | Electric | 80 | - |
| NC | Normal | 20 | Casted and cured |
| SC60-1d | EC60-1d | SC60-28d | EC60-28d | SC60-56d | EC60-56d | |
|---|---|---|---|---|---|---|
| Chemical bound water | 13.77 | 13.71 | 17.21 | 17.23 | 17.85 | 17.82 |
| Portlandite content | 15.26 | 14.35 | 19.24 | 19.65 | 19.68 | 20.43 |
| Specimen No. | SC60 -1d | EC60 -1d | SC60 -3d | EC60 -3d | SC60 -7d | EC60 -7d | SC60 -28d | EC60 -28d | |
|---|---|---|---|---|---|---|---|---|---|
| Porosity/% | 18.37 | 17.26 | 15.92 | 15.83 | 14.34 | 12.21 | 8.96 | 8.61 | |
| Probable pore size/nm | 26.30 | 23.41 | 26.30 | 21.10 | 13.74 | 11.06 | 11.06 | 9.06 | |
| Pores distribution/% | <10nm | 1.53 | 2.02 | 1.75 | 2.53 | 1.99 | 3.78 | 2.38 | 1.70 |
| 10–100nm | 13.67 | 14.38 | 11.79 | 12.71 | 9.45 | 8.23 | 4.84 | 5.81 | |
| 100–1000nm | 2.77 | 0.52 | 2.11 | 0.39 | 2.46 | 0.50 | 1.54 | 0.88 | |
| >1000nm | 0.39 | 0.35 | 0.27 | 0.21 | 0.06 | 0.30 | 0.20 | 0.22 | |
| Curing Method | Energy Consumption (kWh) | Energy Consumed per Unit Area (kWh/m2) | Energy Density (kWh/kg) |
|---|---|---|---|
| SC80 | 2.35 | 15.28 | 0.162 |
| EC80 | 1.03 | 7.18 | 0.076 |
| SC60 | 1.83 | 11.93 | 0.126 |
| EC60 | 0.59 | 4.10 | 0.043 |
| SC40 | 1.51 | 9.83 | 0.104 |
| EC40 | 0.16 | 1.14 | 0.012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Xie, Y.; He, J.; Wang, F.; Zeng, X.; Ma, K.; Long, G. A Comparative Study on the Mechanical Properties and Microstructure of Cement-Based Materials by Direct Electric Curing and Steam Curing. Materials 2021, 14, 7407. https://doi.org/10.3390/ma14237407
Yang Z, Xie Y, He J, Wang F, Zeng X, Ma K, Long G. A Comparative Study on the Mechanical Properties and Microstructure of Cement-Based Materials by Direct Electric Curing and Steam Curing. Materials. 2021; 14(23):7407. https://doi.org/10.3390/ma14237407
Chicago/Turabian StyleYang, Zhihan, Youjun Xie, Jionghuang He, Fan Wang, Xiaohui Zeng, Kunlin Ma, and Guangcheng Long. 2021. "A Comparative Study on the Mechanical Properties and Microstructure of Cement-Based Materials by Direct Electric Curing and Steam Curing" Materials 14, no. 23: 7407. https://doi.org/10.3390/ma14237407
APA StyleYang, Z., Xie, Y., He, J., Wang, F., Zeng, X., Ma, K., & Long, G. (2021). A Comparative Study on the Mechanical Properties and Microstructure of Cement-Based Materials by Direct Electric Curing and Steam Curing. Materials, 14(23), 7407. https://doi.org/10.3390/ma14237407





