Formation of Polymer-Carbon Nanotube Composites by Two-Step Supercritical Fluid Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
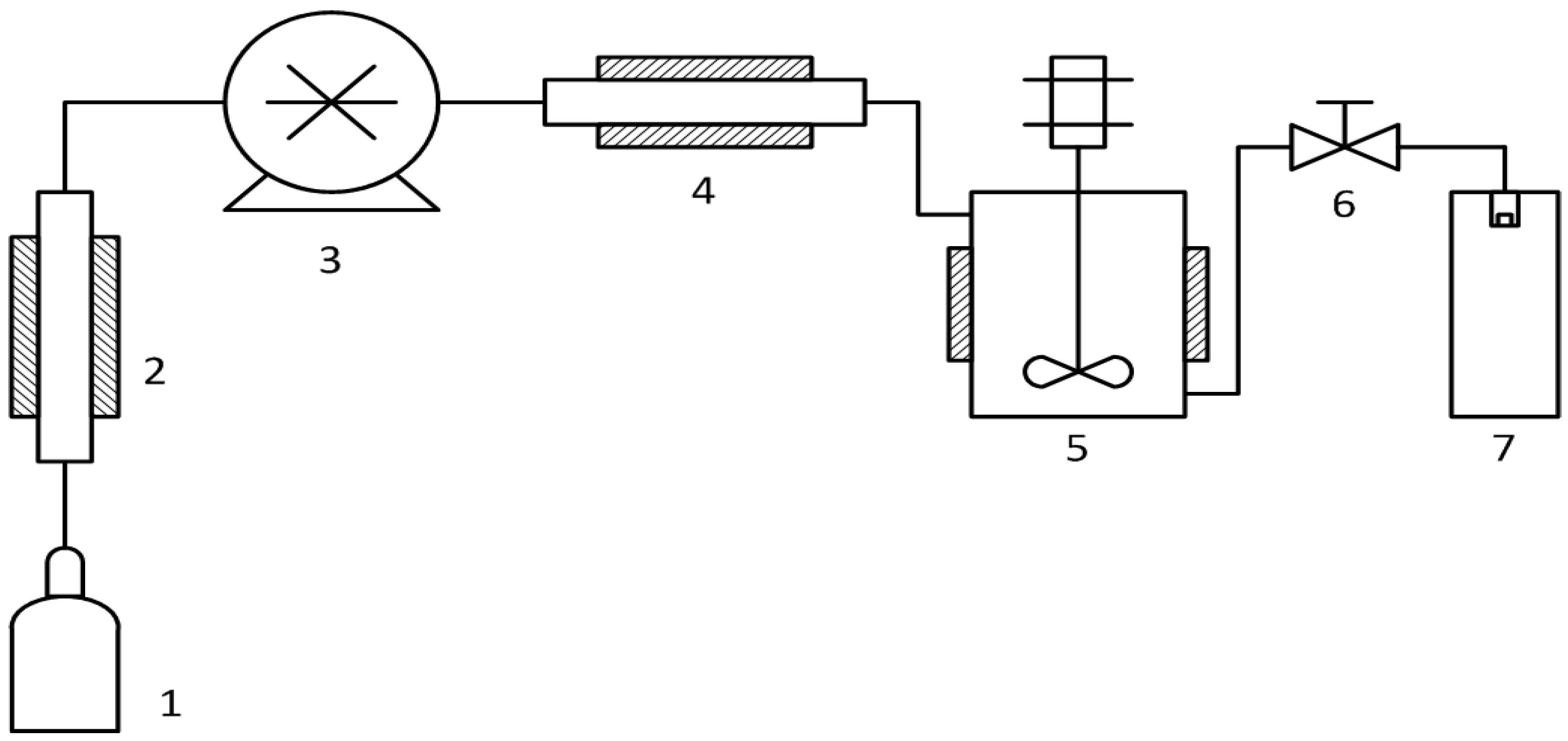
2.2. Rapid Expansion of Supercritical Suspensions (RESS) Carbon Nanotube (CNT) Debundling
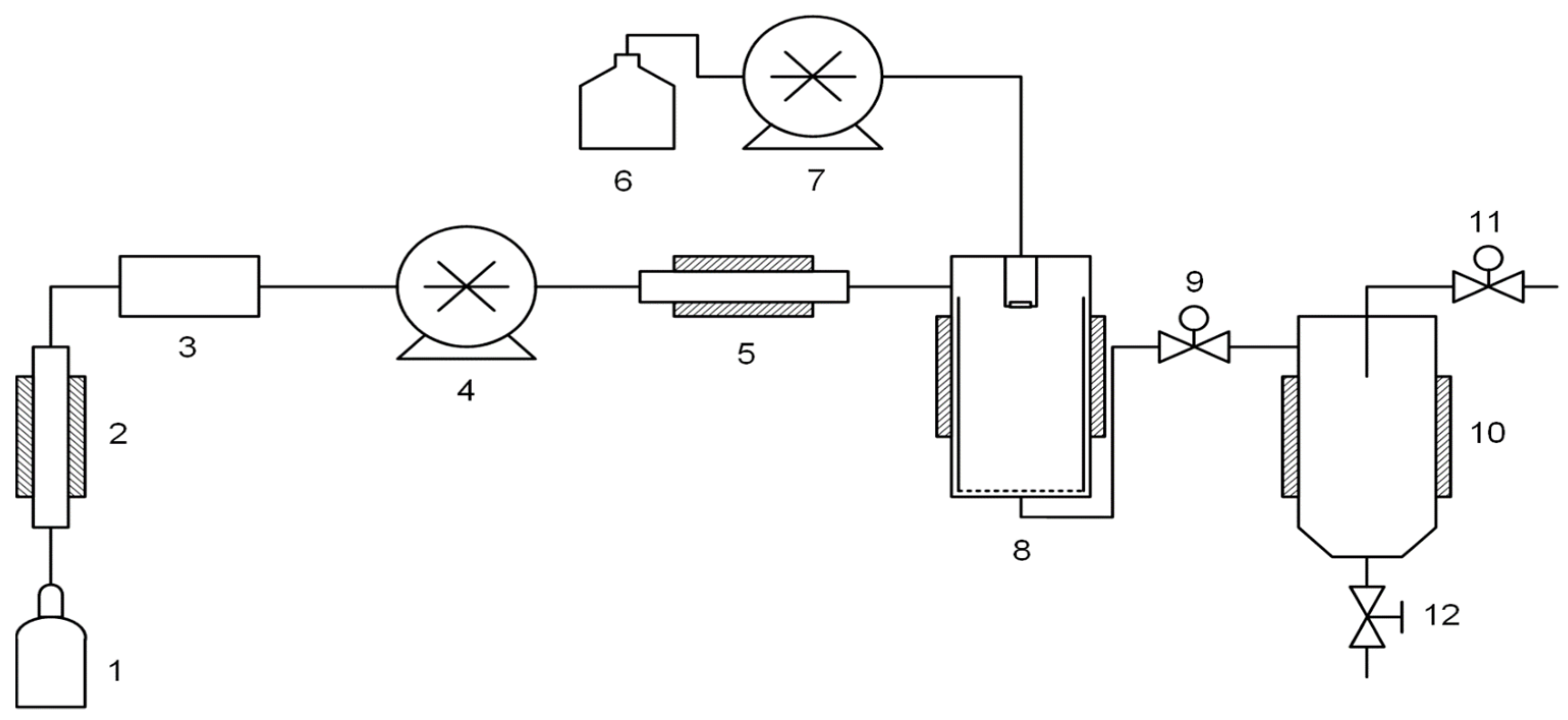
2.3. Preparation of Polymer-CNT Solutions
2.4. Supercritical Antisolvent (SAS) Composite Precipitation
2.5. Preparation of Composites by the Solution Processing Method for Comparison
2.6. Preparation of Samples for Measuring Mechanical Properties by the Hot Pressing Method
2.7. Scanning Electron Microscopy
2.8. X-ray Photoelectron Spectroscopy
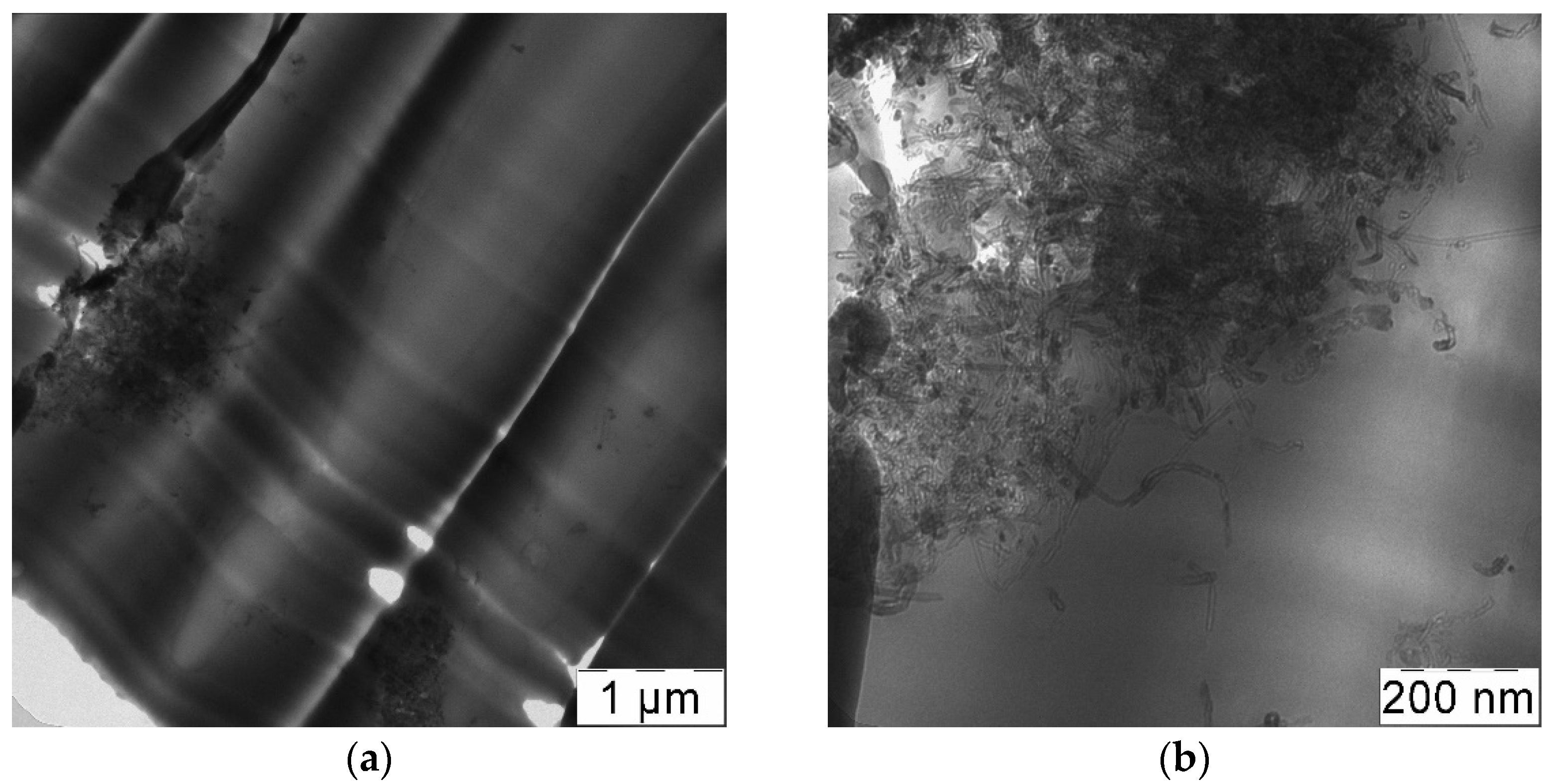
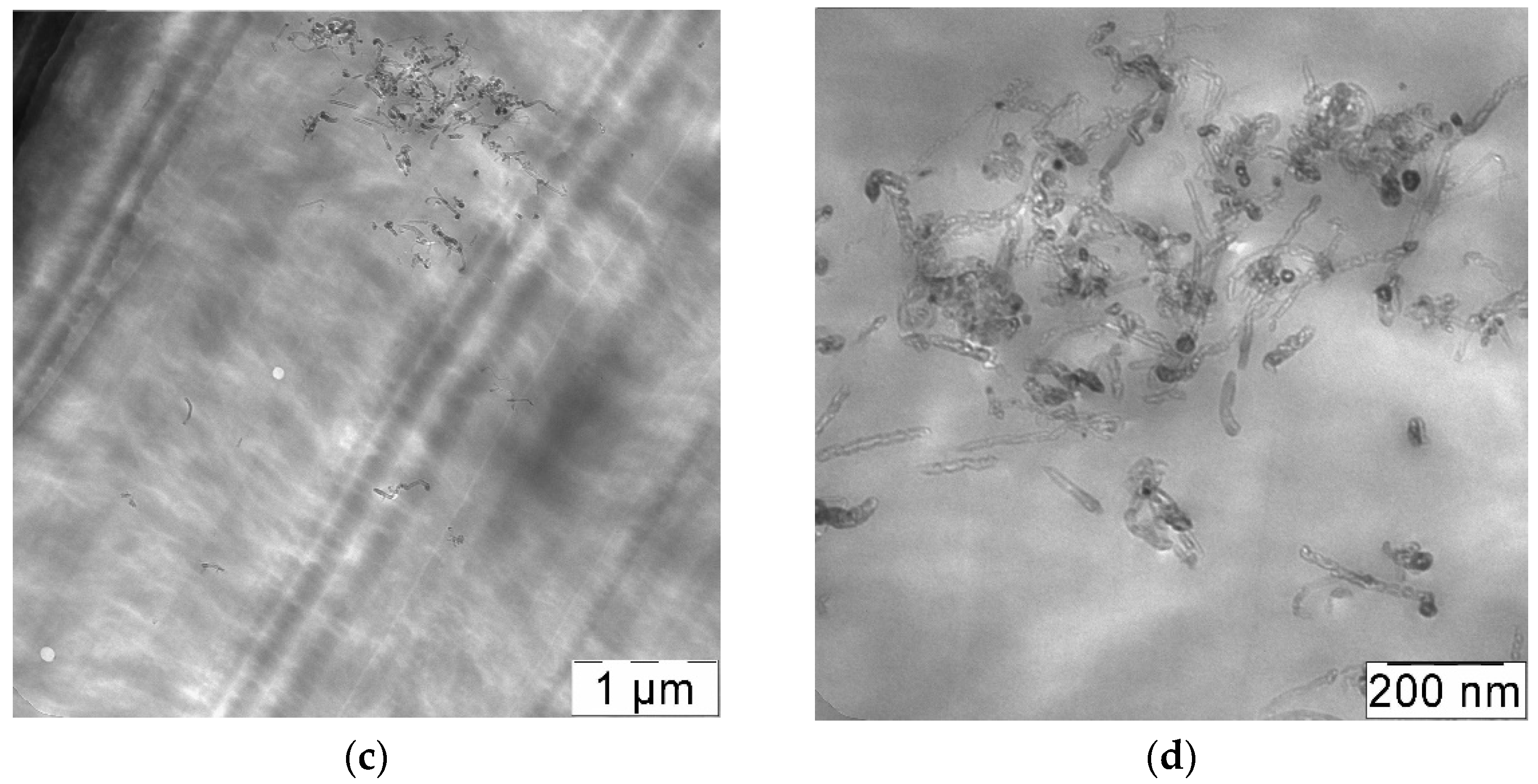
2.9. Transmission Electron Microscopy
2.10. Measurement of Mechanical Characteristics
3. Results and Discussion
3.1. Optimization of the SAS Process for Polycarbonate (PC)
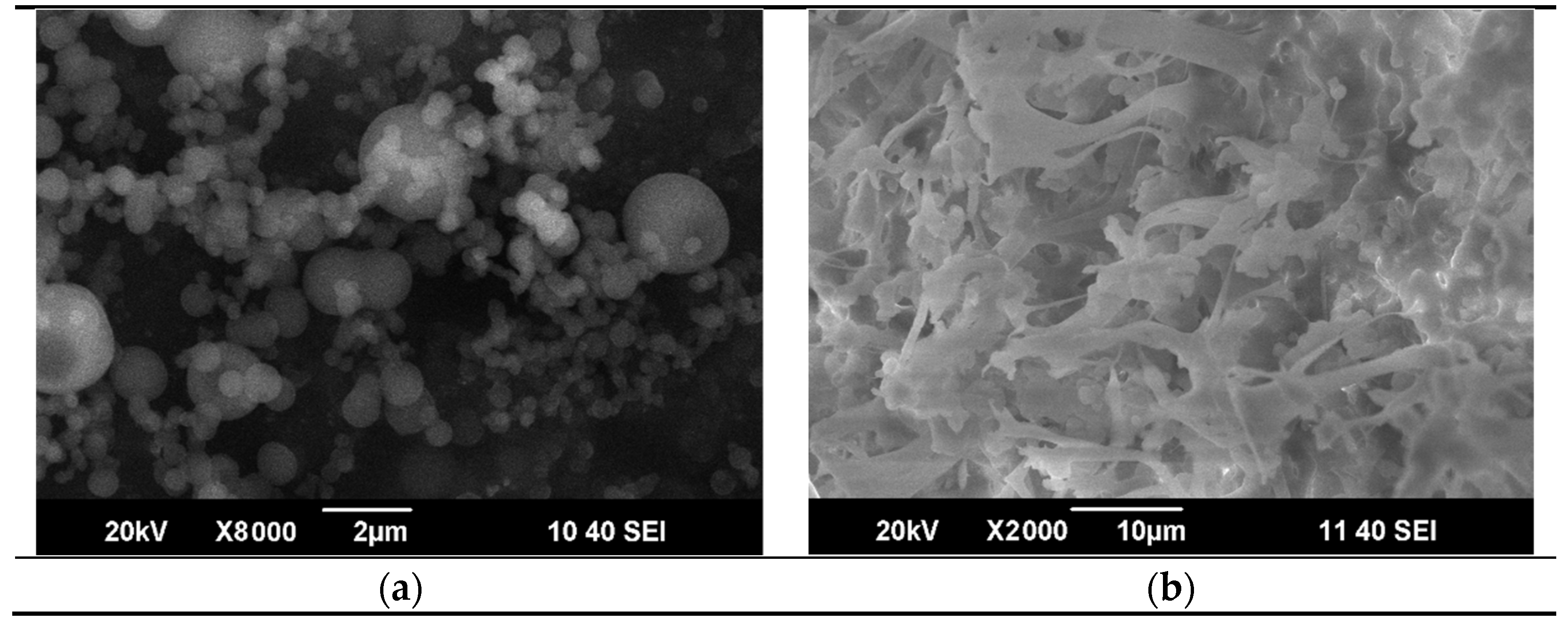
3.2. Preparation of CNT-Polycarbonate Composite Powders by the SAS Method
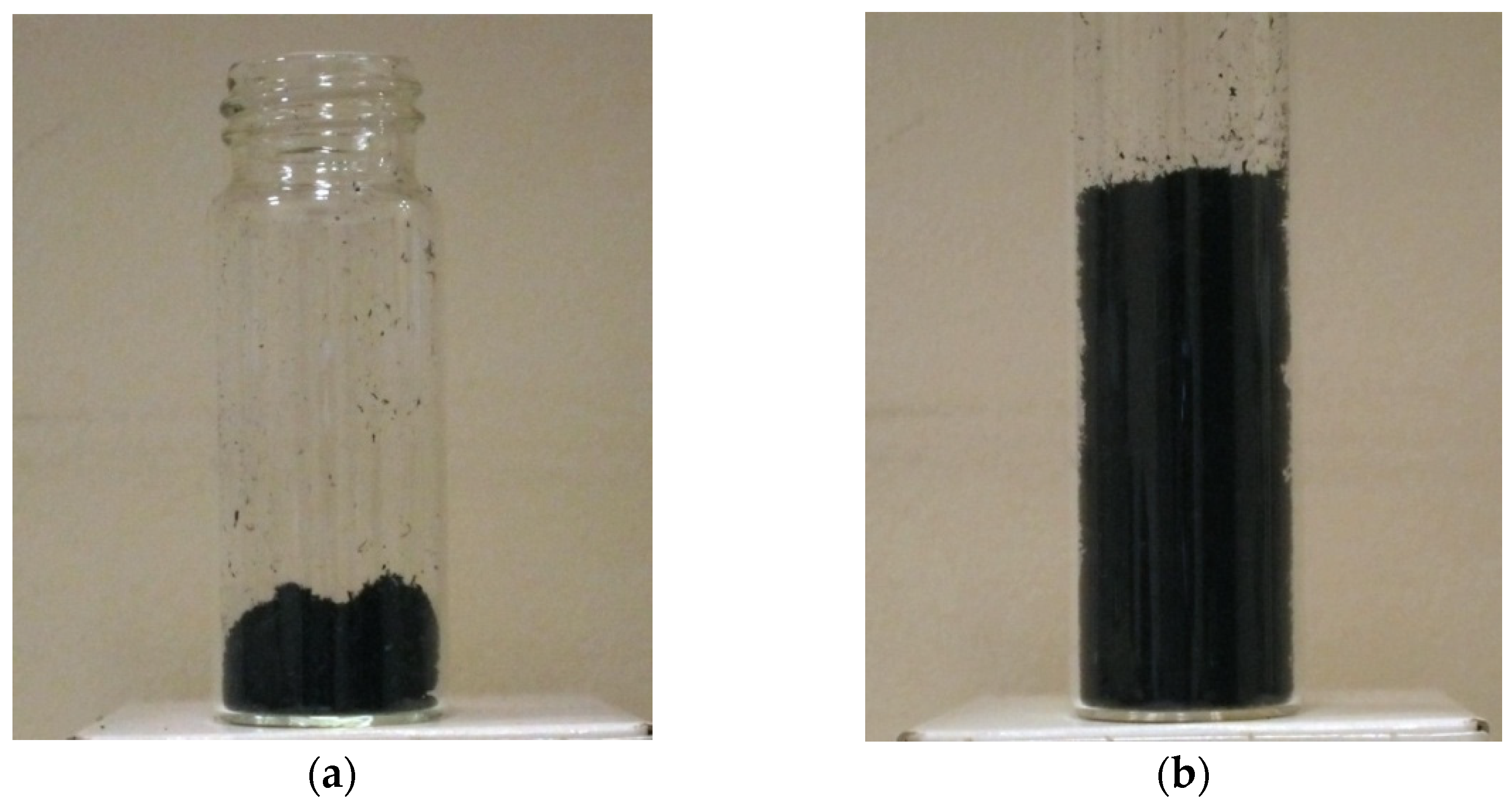
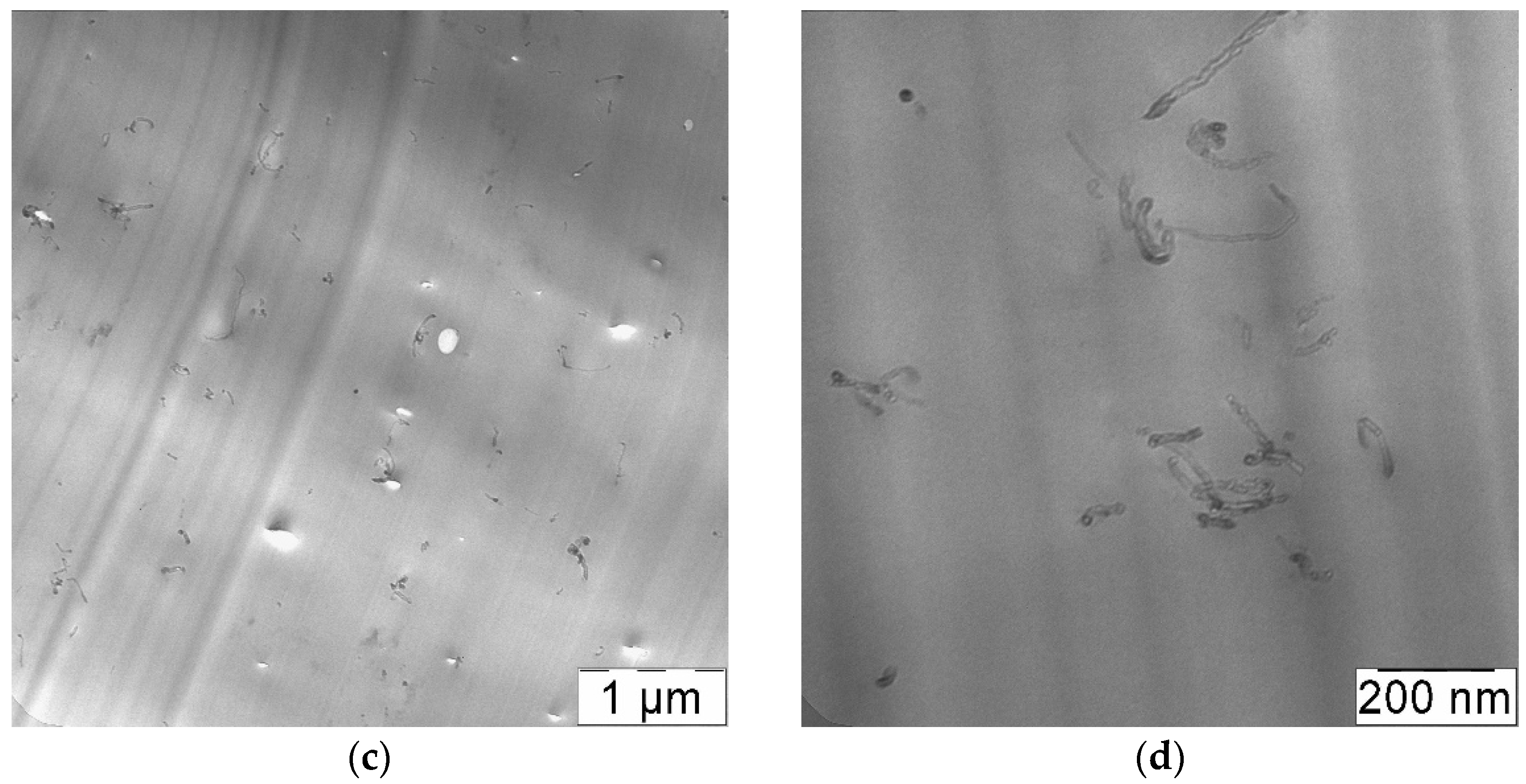
3.3. Rapid Expansion of a CNT Suspension in Supercritical Carbon Dioxide
3.4. Mechanical Properties of Composite Materials
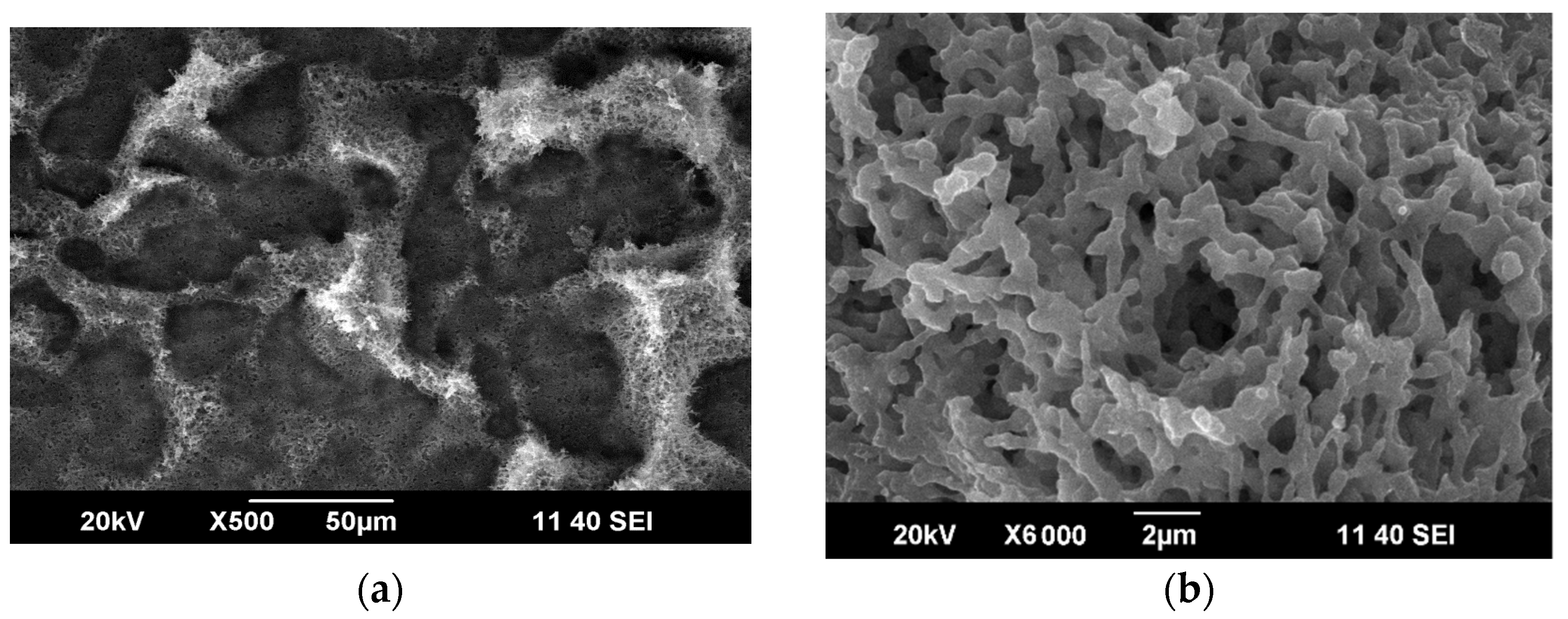
3.4.1. Effect of RESS Treatment
3.4.2. Effect of Powerful Ultrasound Processing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Noor, N.A.M.; Razak, J.A.; Ismail, S.; Mohamad, N.; Tee, L.K.; Munawar, R.F.; Junid, R. Review on carbon nanotube based polymer composites and its applications. J. Adv. Manuf. Technol. 2018, 12, 311–326. [Google Scholar]
- Ates, M.; Eker, A.A.; Eker, B. Carbon nanotube-based nanocomposites and their applications. J. Adhes. Sci. Technol. 2017, 18, 1977–1997. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Source 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Afzal, A.; Abuilaiwi, F.A.; Habib, A.; Awais, M.; Waje, S.B.; Atieh, M.A. Polypyrrole/carbon nanotube supercapacitors: Technological advances and challenges. J. Power Source 2017, 352, 174–186. [Google Scholar] [CrossRef]
- Guo, D.; Pan, X.; He, H. A simple and cost-effective method for improving the sensitivity of flexible strain sensors based on conductive polymer composites. Sens. Actuators A 2019, 298, 111608. [Google Scholar] [CrossRef]
- Pham, G.T.; Park, Y.-B.; Liang, Z.; Zhang, C.; Wang, B. Processing and modeling of conductive thermoplastic/carbon nanotube films for strain sensing. Compos. Part B 2008, 39, 209–216. [Google Scholar] [CrossRef]
- Kang, I.; Schulz, M.J.; Kim, J.H.; Shanov, V.; Shi, D. A carbon nanotube strain sensor for structural health monitoring. Smart Mater. Struct. 2006, 15, 737–748. [Google Scholar] [CrossRef]
- Ferrreira, A.; Rocha, J.G.; Ansón-Casaos, A.; Martínez, M.T.; Vaz, F.; Lanceros-Mendez, S. Electromechanical performance of poly(vinylidene fluoride)/carbon nanotube composites for strain sensor applications. Sens. Actuators A 2012, 178, 10–16. [Google Scholar] [CrossRef]
- Kymakis, E.; Alexandrou, I.; Amaratunga, G.A.J. High open-circuit voltage photovoltaic devices from carbon-nanotube-polymer composites. J. Appl. Phys. 2003, 93, 1764–1768. [Google Scholar] [CrossRef]
- Glanzmann, L.N.; Mowbray, D.J. Theoretical Insight into the Internal Quantum Efficiencies of Polymer/C60 and Polymer/SWNT Photovoltaic Devices. Adv. Mater. J. Phys. Chem. C 2016, 120, 6336–6343. [Google Scholar] [CrossRef] [Green Version]
- Scardaci, V.; Rozhin, A.G.; Hennrich, F.; Milne, W.I.; Ferrari, A.C. Carbon nanotube–polymer composites for photonic devices. Phys. E Low-Dimens. Syst. Nanostruct. 2007, 37, 115–118. [Google Scholar] [CrossRef]
- McEuen, P.; Fuhrer, M.; Park, H. Single-walled carbon nanotube electronics. IEEE Trans. Nanotechnol. 2002, 1, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Avouris, P. Carbon nanotube electronics. Phys. World 2007, 20, 40–45. [Google Scholar] [CrossRef]
- Kumar, S.; Pavelyev, V.; Mishra, P.; Tripathi, N. A review on chemiresistive gas sensors based on carbon nanotubes: Device and technology transformation. Sens. Actuators A 2018, 283, 174–186. [Google Scholar] [CrossRef]
- Kauffman, D.R.; Star, A. Carbon Nanotube Gas and Vapor Sensors. Angew. Chem. Int. Ed. 2008, 47, 6550–6570. [Google Scholar] [CrossRef]
- Shawky, H.A.; Chae, S.R.; Lin, S.; Wiesner, M.R. Synthesis and characterization of a carbon nanotube/polymer nanocomposite membrane for water treatment. Desalination 2011, 272, 46–50. [Google Scholar] [CrossRef]
- Kim, S.; Chen, L.; Johnson, J.K.; Marand, E. Marand, Polysulfone and functionalized carbon nanotube mixed matrix membranes for gas separation: Theory and experiment. J. Membr. Sci. 2007, 294, 147–158. [Google Scholar] [CrossRef]
- Du, J.-H.; Bai, J.; Cheng, H.-M. The present status and key problems of carbon nanotube based polymer composites. Express Polym. Lett. 2007, 1, 253–273. [Google Scholar] [CrossRef]
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon nanotube–polymer composites: Chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci. 2010, 35, 357–401. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Fagan, J.; Douglas, J.F.; Yamamoto, K.; Heckert, A.N.; Leigh, S.D.; Obrzut, J.; Du, F.; Lin-Gibson, S.; Mu, M.; et al. Haggenmueller, Relationship between dispersion metric and properties of PMMA/SWNT nanocomposites. Polymer 2007, 48, 4855–4866. [Google Scholar] [CrossRef] [Green Version]
- Rathi, A.; Kundalwal, S.I. Mechanical and fracture behavior of MWCNT/ZrO2/epoxy nanocomposite systems: Experimental and numerical study. Polym. Compos. 2020, 41, 2491–2507. [Google Scholar] [CrossRef]
- Hennrich, F.; Krupke, R.; Arnold, K.; Rojas Stütz, J.A.; Lebedkin, S.; Koch, T.; Schimmel, T.; Kappes, M.M. The Mechanism of cavitation-induced scission of single-walled carbon nanotubes. J. Phys. Chem. B 2007, 111, 1932–1937. [Google Scholar] [CrossRef]
- Lucas, A.; Zakri, C.; Maugey, M.; Pasquali, M.; Van Der Schoot, P.; Poulin, P. Poulin, Kinetics of nanotube and microfiber scission under sonication. J. Phys. Chem. C 2009, 113, 20599–20605. [Google Scholar] [CrossRef]
- Cheng, Q.; Debnath, S.; Gregan, E.; Byrne, H.J. Ultrasound-assisted SWNTs dispersion: Effects of sonication parameters and solvent properties. J. Phys. Chem. C 2010, 114, 8821–8827. [Google Scholar] [CrossRef] [Green Version]
- Ma, P.-C.; Siddiqui, N.A.; Marom, G.; Kim, J.-K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part B 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Song, S.; Yang, H.; Rao, R.; Liu, H.; Zhang, A. Defects of multi-walled carbon nanotubes as active sites for benzene hydroxylation to phenol in the presence of H2O2. Catal. Commun. 2010, 11, 783–787. [Google Scholar] [CrossRef]
- Shelimov, K.B.; Esenaliev, R.O.; Rinzler, A.G.; Huffman, C.B.; Smalley, R.E. Purification of single-wall carbon nanotubes by ultrasonically assisted filtration. Chem. Phys. Lett. 1998, 282, 429–434. [Google Scholar] [CrossRef]
- Lu, K.; Lago, R.; Chen, Y.; Green, M.; Harris, P.; Tsang, S. Mechanical damage of carbon nanotubes by ultrasound. Carbon 1996, 34, 814–816. [Google Scholar] [CrossRef]
- Arai, Y.; Sako, T.; Takebayashi, Y. Supercritical Fluids: Molecular Interactions, Physical Properties and New Applications; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Reverchon, E.; Adami, R. Nanomaterials and supercritical fluids. J. Supercrit. Fluids 2005, 37, 1–22. [Google Scholar] [CrossRef]
- Tomasko, D.L.; Han, X.; Liu, D.; Gao, W. Supercritical fluid applications in polymer nanocomposites. Curr. Opin. Solid State Mater. Sci. 2003, 7, 407–412. [Google Scholar] [CrossRef]
- Cansell, F.; Aymonier, C. Design of functional nanostructured materials using supercritical fluids. J. Supercrit. Fluids 2008, 47, 508–516. [Google Scholar] [CrossRef] [Green Version]
- Tsutsumi, A.; Ikeda, M.; Chen, W.; Iwatsuki, J. A nano-coating process by the rapid expansion of supercritical suspensions in impinging-stream reactors. Powder Technol. 2003, 138, 211–215. [Google Scholar] [CrossRef]
- Shim, J.-J.; Yates, A.M.Z.; Johnston, K.P. Polymer Coatings by Rapid Expansion of Suspensions in Supercritical Carbon Dioxide. Ind. Eng. Chem. Res. 1999, 38, 3655–3662. [Google Scholar] [CrossRef]
- To, D.; Dave, R.; Yin, X.; Sundaresan, S. Deagglomeration of nanoparticle aggregates via rapid expansion of supercritical or high-pressure suspensions. AIChE J. 2009, 55, 2807–2826. [Google Scholar] [CrossRef]
- Vorobei, A.M.; Pokrovskiy, O.I.; Ustinovich, K.B.; Parenago, O.O.; Savilov, S.V.; Lunin, V.V.; Novotortsev, V.M. Preparation of polymer–multi-walled carbon nanotube composites with enhanced mechanical properties using supercritical antisolvent precipitation. Polymer 2016, 95, 77–81. [Google Scholar] [CrossRef]
- Reverchon, E. Supercritical antisolvent precipitation of micro- and nano-particles. J. Supercrit. Fluids 1999, 15, 1–21. [Google Scholar] [CrossRef]
- Franco, P.; Reverchon, E.; De Marco, I. Production of zein/antibiotic microparticles by supercritical antisolvent coprecipitation. J. Supercrit. Fluids. 2019, 145, 31–38. [Google Scholar] [CrossRef]
- Adami, R.; Reverchon, E.; Järvenpää, E.; Huopalahti, R. Supercritical antisolvent micronization of nalmefene HCl on laboratory and pilot scale. Powder Technol. 2008, 182, 105–112. [Google Scholar] [CrossRef]
- Kalogiannis, C.G.; Pavlidou, E.; Panayiotou, C.G. Production of amoxicillin microparticles by supercritical antisolvent precipitation. Ind. Eng. Chem. Res. 2005, 44, 9339–9346. [Google Scholar] [CrossRef]
- Vorobei, A.M.; Pokrovskiy, O.I.; Ustinovich, K.B.; Parenago, O.O.; Lunin, V.V.; Miroshnichenko, A.G. Micronization of Salbutamol Sulfate by Supercritical Antisolvent Precipitation: The Effect of Process Parameters on the Size and Morphology of Particles. Russ. J. Phys. Chem. B 2018, 12, 1240–1248. [Google Scholar] [CrossRef]
- Aguiar, G.P.S.; Magro, C.D.; Oliveira, J.V.; Lanza, M.; Oliveira, M. Lanza, poly (hydroxybutyrate-co-hydroxyvalerate) micronization by solution enhanced dispersion by supercritical fluids technique. Braz. J. Chem. Eng. 2018, 35, 1275–1282. [Google Scholar] [CrossRef] [Green Version]
- Vorobei, A.M.; Ustinovich, K.B.; Pokrovskiy, O.I.; Parenago, O.O.; Lunin, V.V. Preparation of hydroxypropylmethylcellulose microparticles using supercritical antisolvent precipitation. Russ. J. Phys. Chem. B 2015, 9, 1103–1108. [Google Scholar] [CrossRef]
- Reverchon, E.; Della Porta, G.; De Rosa, I.; Subra, P.; Letourneur, D. Supercritical antisolvent micronization of some biopolymers. J. Supercrit. Fluids. 2000, 18, 239–245. [Google Scholar] [CrossRef]
- Nesterov, N.; Paharukova, V.; Yakovlev, V.; Martyanov, O. The facile synthesis of Ni–Cu catalysts stabilized in SiO2 framework via a supercritical antisolvent approach. J. Supercrit. Fluids 2016, 112, 119–127. [Google Scholar] [CrossRef]
- Hutchings, G.J.; Bartley, J.; Webster, J.M.; Lopez-Sanchez, J.A.; Gilbert, D.J.; Kiely, C.; Carley, A.F.; Howdle, S.M.; Sajipc, S.; Caldarelli, S.; et al. Amorphous Vanadium Phosphate Catalysts from Supercritical Antisolvent Precipitation. J. Catal. 2001, 197, 232–235. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I.; Della Porta, G. Tailoring of nano- and micro- particles of some superconductor precursors by supercritical antisolvent precipitation. J. Supercrit. Fluids. 2002, 23, 81–87. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Hajimirsadeghi, S.S. Application of supercritical carbon dioxide in energetic materials processes: A review. Ind. Eng. Chem. Res. 2005, 44, 6523–6533. [Google Scholar] [CrossRef]
- Chernyak, S.A.; Ivanov, A.S.; Strokova, N.E.; Maslakov, K.I.; Savilov, S.V.; Lunin, V.V. Mechanism of Thermal Defunctionalization of Oxidized Carbon Nanotubes. J. Phys. Chem. C 2016, 120, 17465–17474. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA 300 Database; John Wiley & Sons: Chichester, UK, 1992; 295p. [Google Scholar]
- Zanjanijam, A.R.; Bahrami, M.; Hajian, M. Poly (vinyl chloride)/single wall carbon nanotubes composites: Investigation of mechanical and thermal characteristics. J. Vinyl Addit. Technol. 2016, 22, 128–133. [Google Scholar] [CrossRef]
- Zanjanijam, A.R.; Hajian, M.; Koohmareh, G.A. Improving the thermal and mechanical properties of poly (vinyl butyral) through the incorporation of acid-treated single-walled carbon nanotubes. J. Appl. Polym. Sci. 2014, 131, 40481. [Google Scholar] [CrossRef]
- Jung, W.R.; Choi, J.H.; Lee, N.; Shin, K.; Moon, J.H.; Seo, Y.S. Reduced damage to carbon nanotubes during ultrasound-assisted dispersion as a result of supercritical-fluid treatment. Carbon 2012, 50, 633–636. [Google Scholar] [CrossRef]
- Chen, C.; Bortner, M.; Quigley, J.P.; Baird, D.G. Using Supercritical Carbon Dioxide in Preparing Carbon Nanotube Nanocomposite: Improved Dispersion and Mechanical Properties. Polym. Compos. 2012, 33, 1033–1043. [Google Scholar] [CrossRef]
- Quaresimin, M.; Schulte, K.; Zappalorto, M.; Chandrasekaran, S. Toughening mechanisms in polymer nanocomposites: From experiments to modelling. Compos. Sci. Technol. 2016, 123, 187–204. [Google Scholar] [CrossRef]



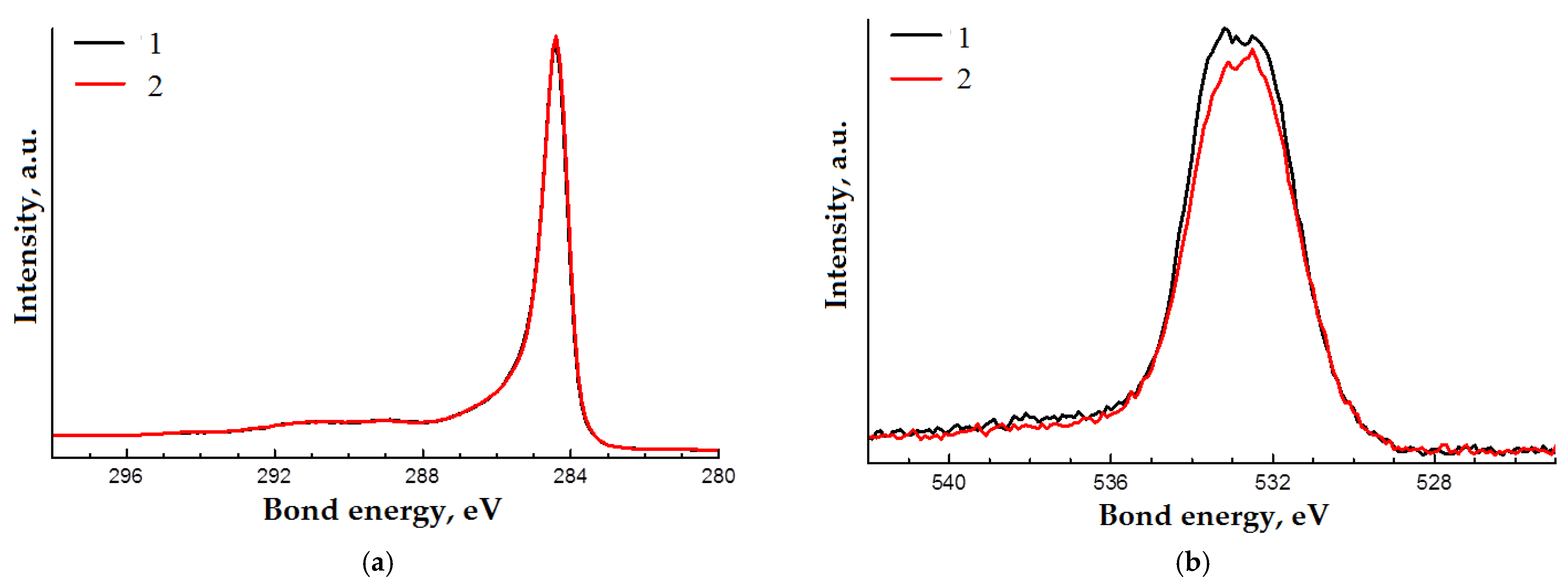
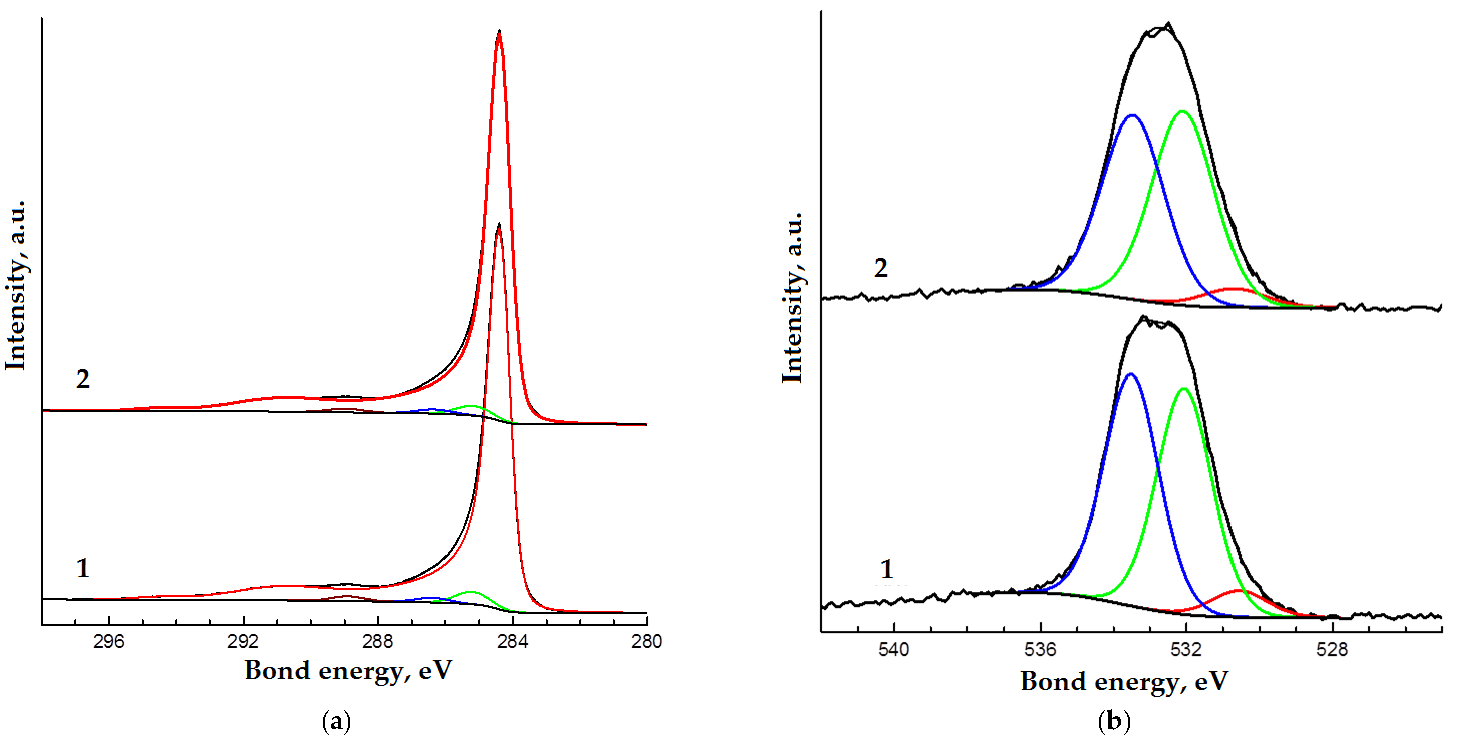


| Spectrum | The Content of the Element, Atomic % | Bond Energy, eV | Atomic % | Type of Bond | ||
|---|---|---|---|---|---|---|
| Initial CNTs | CNTs after RESS | Initial CNTs | CNTs after RESS | |||
| O1s | 3.2 | 3.0 | 530.6 | 0.2 | 0.1 | O− |
| 532.1 | 1.5 | 1.5 | O−C (aliphatic) | |||
| 533.5 | 1.5 | 1.4 | O=C (aliphatic) | |||
| C1s | 96.8 | 97.0 | 284.4 | 91.7 | 92.8 | C−C (sp2) |
| 285.2 | 2.9 | 2.3 | C−C,H (sp3) | |||
| 286.4 | 1.1 | 1.0 | C−O | |||
| 288.9 | 1.1 | 0.9 | O=C−O | |||
| Exp. No. | Composite Preparation Technique | Young’s Modulus, GPa | Tensile Strength, MPa | Total Elongation, % | Elongation in the Elastic Range, % |
|---|---|---|---|---|---|
| 0 | Initial PC for comparison | 1.48 ± 0.06 | 28 ± 2 | 1.3 ± 0.1 | 1.22 ± 0.07 |
| 1 | SAS without additional pre-treatments | 1.49 ± 0.01 | 48 ± 2 | 4 ± 1 | 1.73 ± 0.06 |
| 2 | Solution processing without additional pre-treatment | 1.53 ± 0.09 | 35 ± 4 | 2.0 ± 0.2 | 1.4 ± 0.1 |
| 3 | SAS. The CNTs were pre-processed by RESS | 1.53 ± 0.02 | 58 ± 1 | 5 ± 1 | 1.6 ± 0.1 |
| 4 | Solution processing. The CNTs were pre-processed by RESS | 1.38 ± 0.01 | 40 ± 3 | 2.6 ± 0.3 | 1.3 ± 0.1 |
| 5 | SAS. Powerful ultrasound was used | 1.50 ± 0.04 | 50 ± 5 | 8.3 ± 0.9 | 1.63 ± 0.08 |
| 6 | SAS. The CNTs were pre-processed by RESS and powerful ultrasound was used | 1.4 ± 0.1 | 45 ± 5 | 3.2 ± 0.7 | 1.6 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorobei, A.M.; Ustinovich, K.B.; Chernyak, S.A.; Savilov, S.V.; Parenago, O.O.; Kiselev, M.G. Formation of Polymer-Carbon Nanotube Composites by Two-Step Supercritical Fluid Treatment. Materials 2021, 14, 7428. https://doi.org/10.3390/ma14237428
Vorobei AM, Ustinovich KB, Chernyak SA, Savilov SV, Parenago OO, Kiselev MG. Formation of Polymer-Carbon Nanotube Composites by Two-Step Supercritical Fluid Treatment. Materials. 2021; 14(23):7428. https://doi.org/10.3390/ma14237428
Chicago/Turabian StyleVorobei, Anton M., Konstantin B. Ustinovich, Sergei A. Chernyak, Sergei V. Savilov, Olga O. Parenago, and Mikhail G. Kiselev. 2021. "Formation of Polymer-Carbon Nanotube Composites by Two-Step Supercritical Fluid Treatment" Materials 14, no. 23: 7428. https://doi.org/10.3390/ma14237428
APA StyleVorobei, A. M., Ustinovich, K. B., Chernyak, S. A., Savilov, S. V., Parenago, O. O., & Kiselev, M. G. (2021). Formation of Polymer-Carbon Nanotube Composites by Two-Step Supercritical Fluid Treatment. Materials, 14(23), 7428. https://doi.org/10.3390/ma14237428










