Copper(II) Perfluorinated Carboxylate Complexes with Small Aliphatic Amines as Universal Precursors for Nanomaterial Fabrication
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Instrumentation
2.3. Chemical Vapour Deposition Parameters
2.4. Spin- and Dip-Coating Parameters
2.5. Synthesis of [Cu2(RNH2)2(µ-O2CRf)4]) (1–10)
3. Results and Discussion
3.1. Infrared Spectra Analysis
3.2. Mass Spectra Analysis
3.3. Results of Thermal Analysis
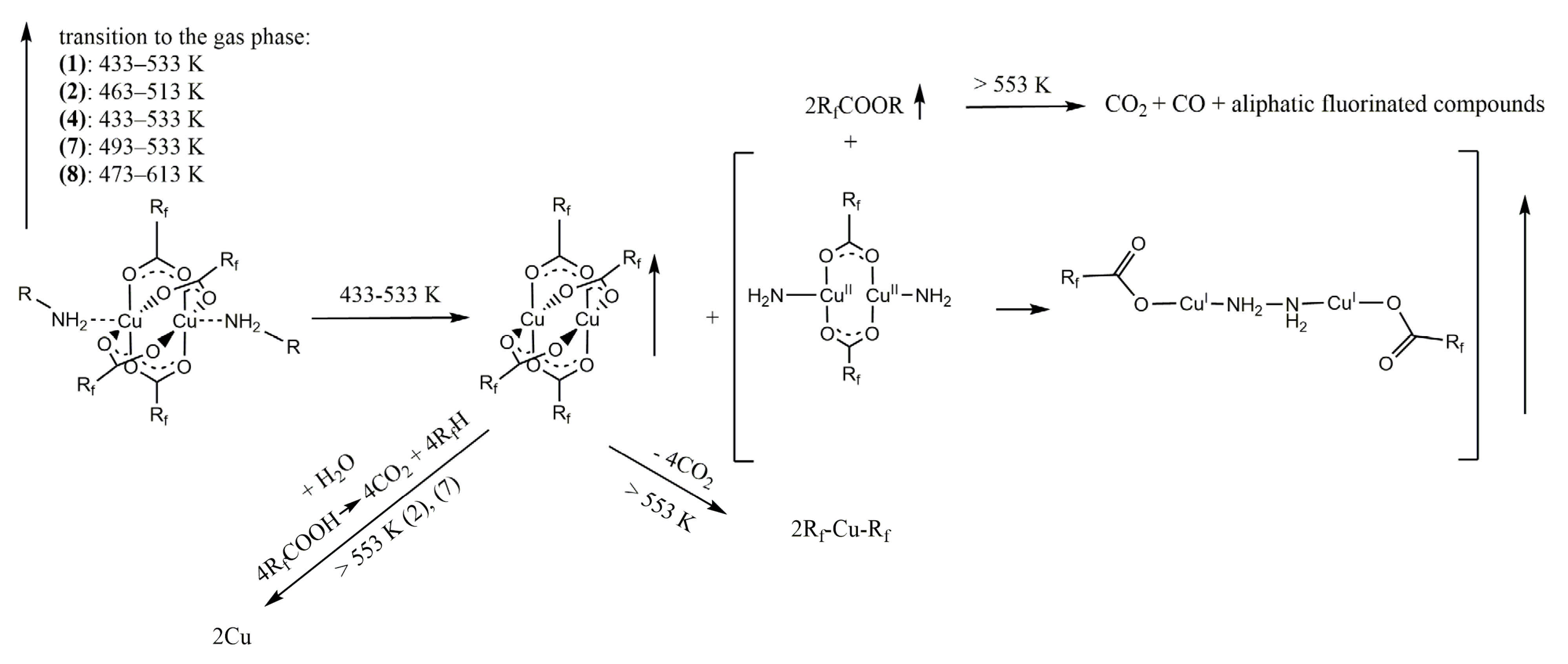
3.4. Temperature Variable Infrared Spectroscopy
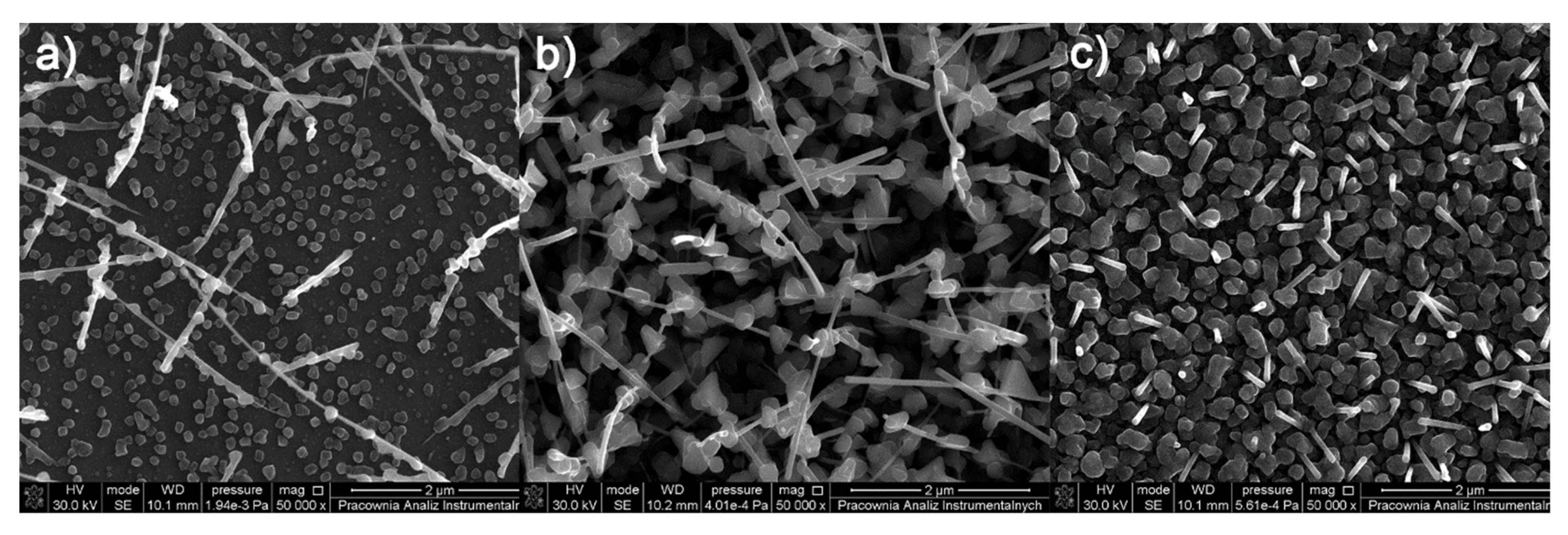
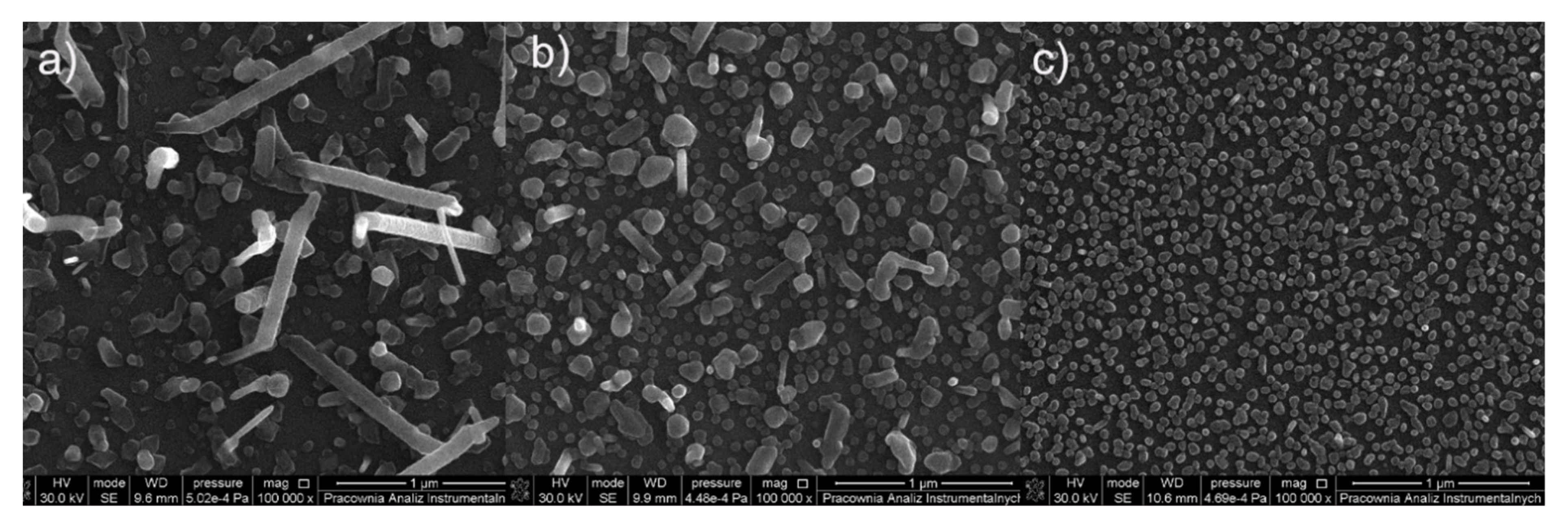
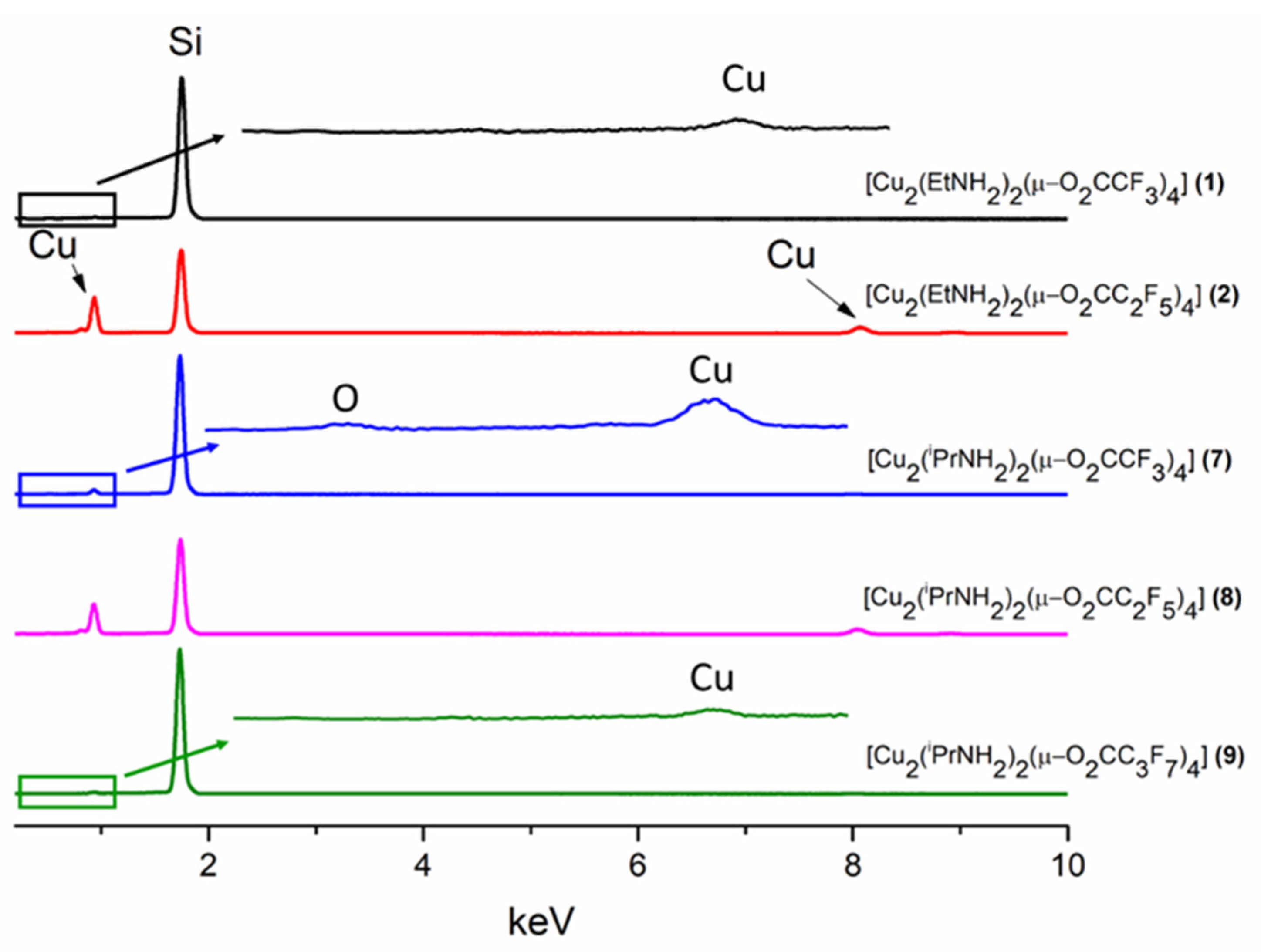
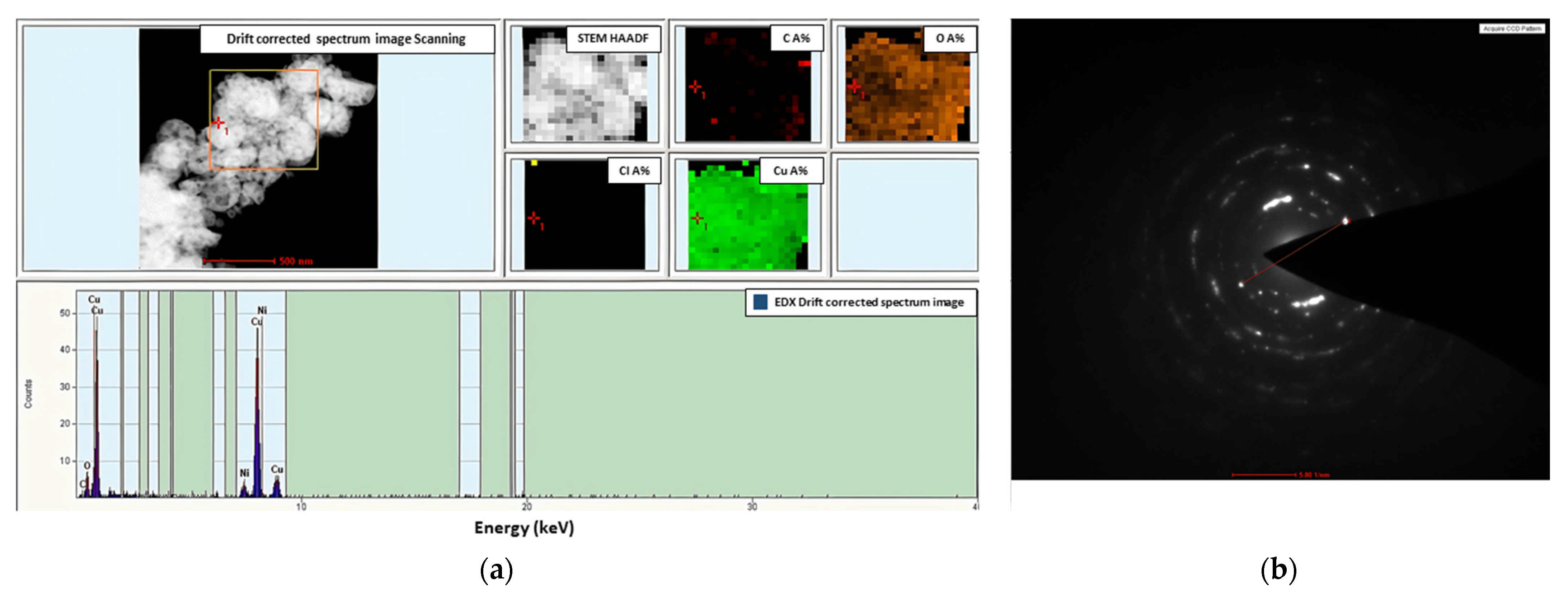
3.5. CVD Experiments
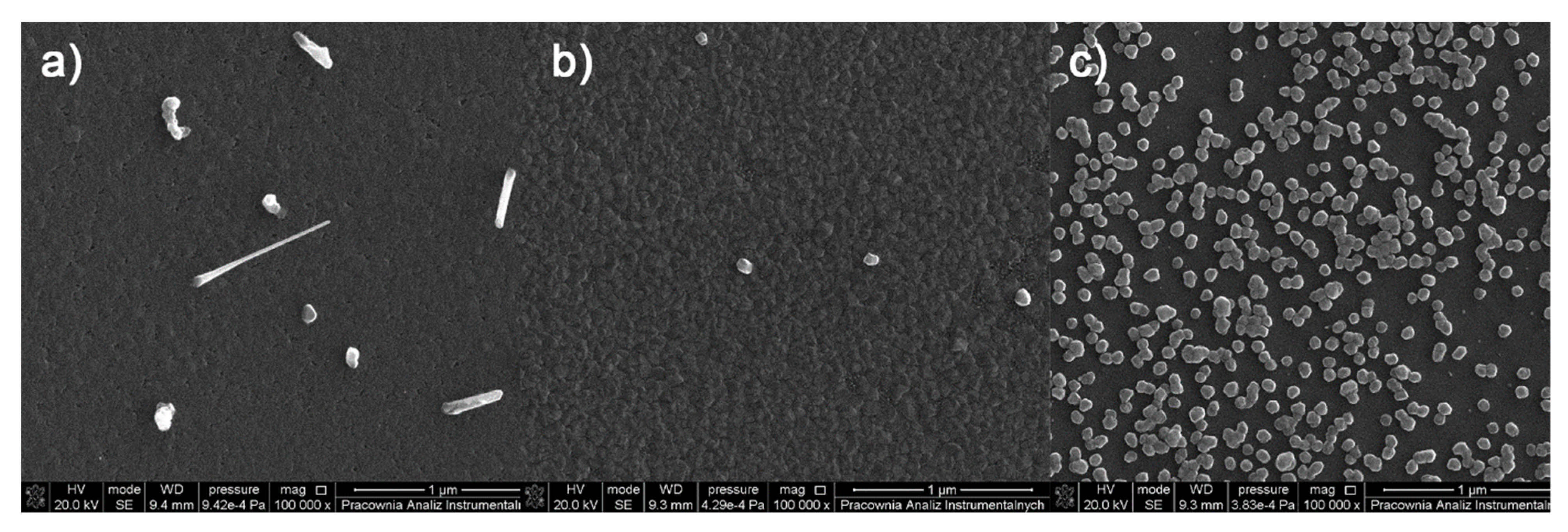
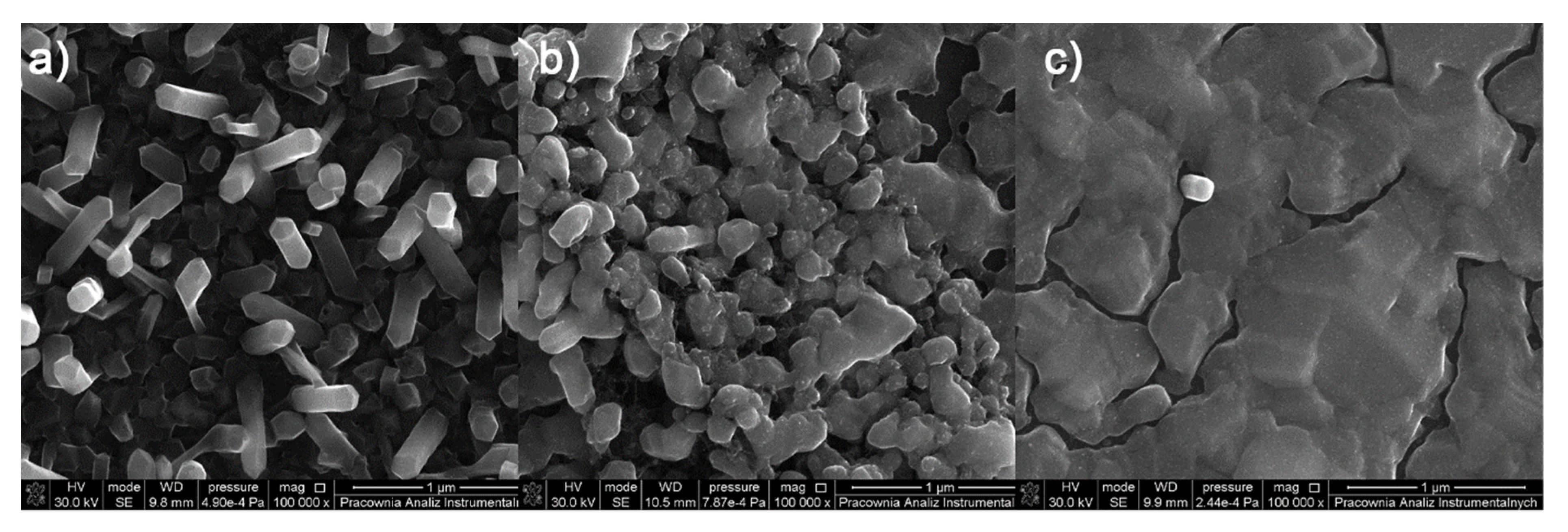
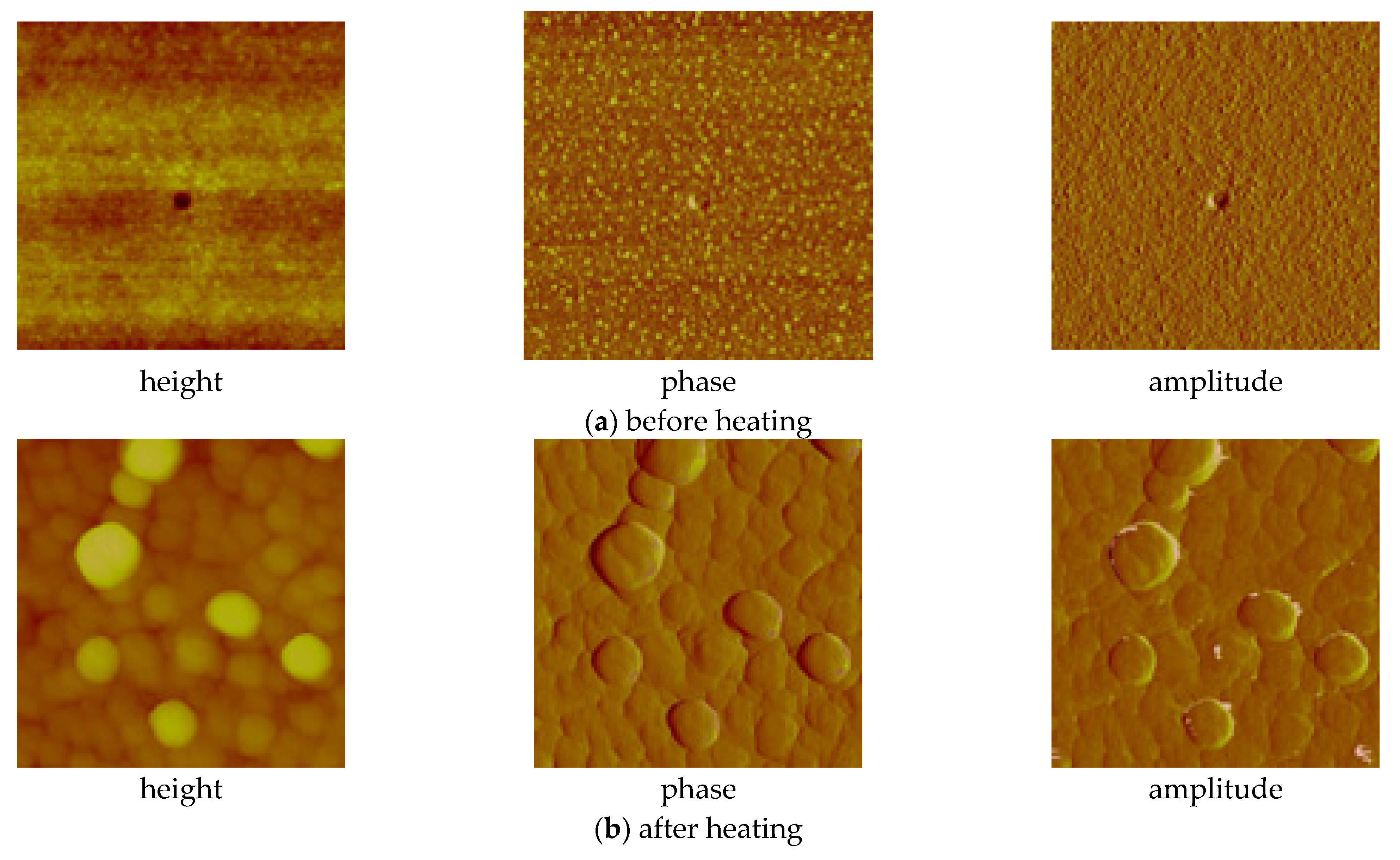
3.6. Spin- and Dip-Coating Deposition
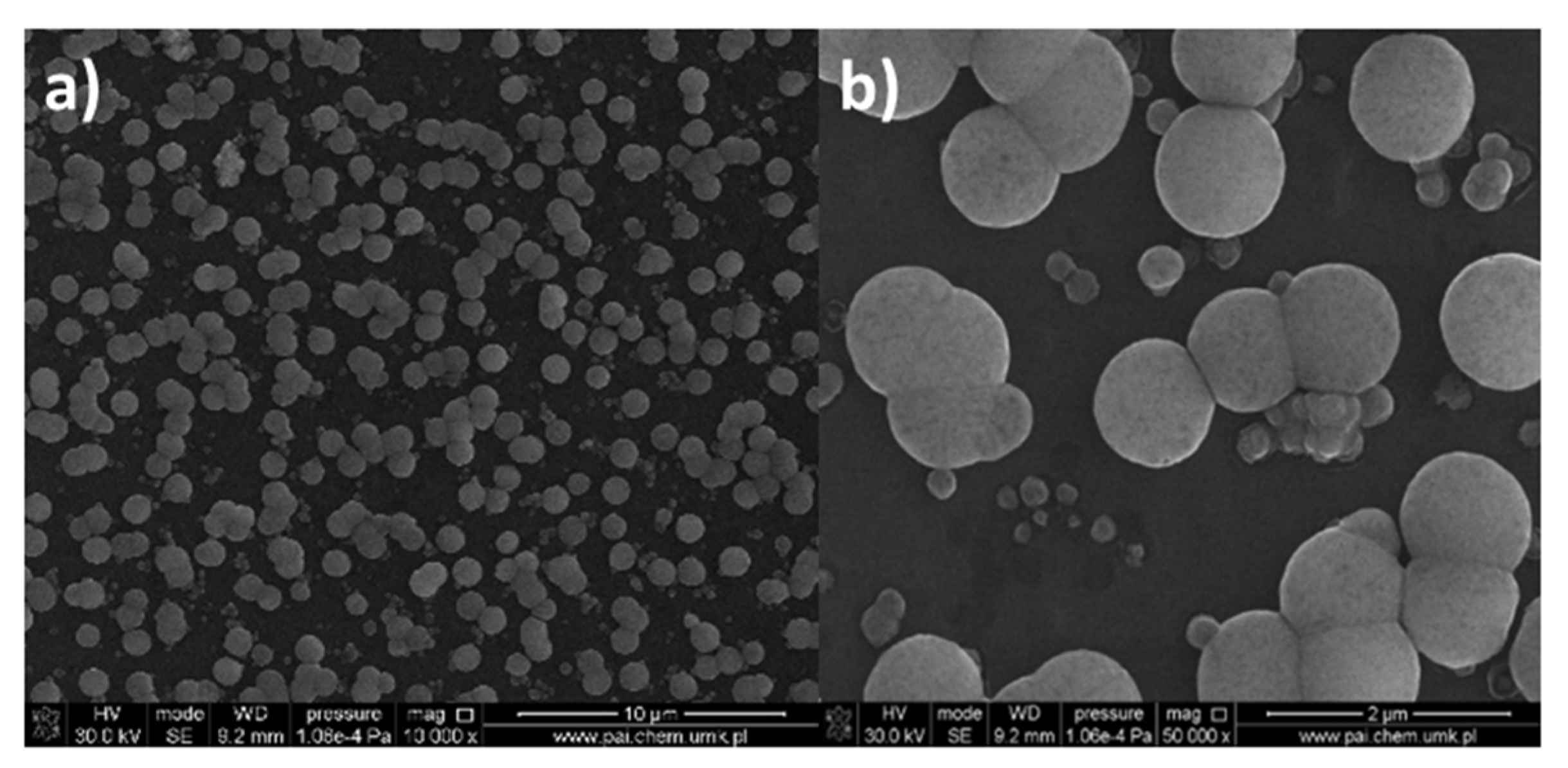
3.6.1. Spin-Coated Materials
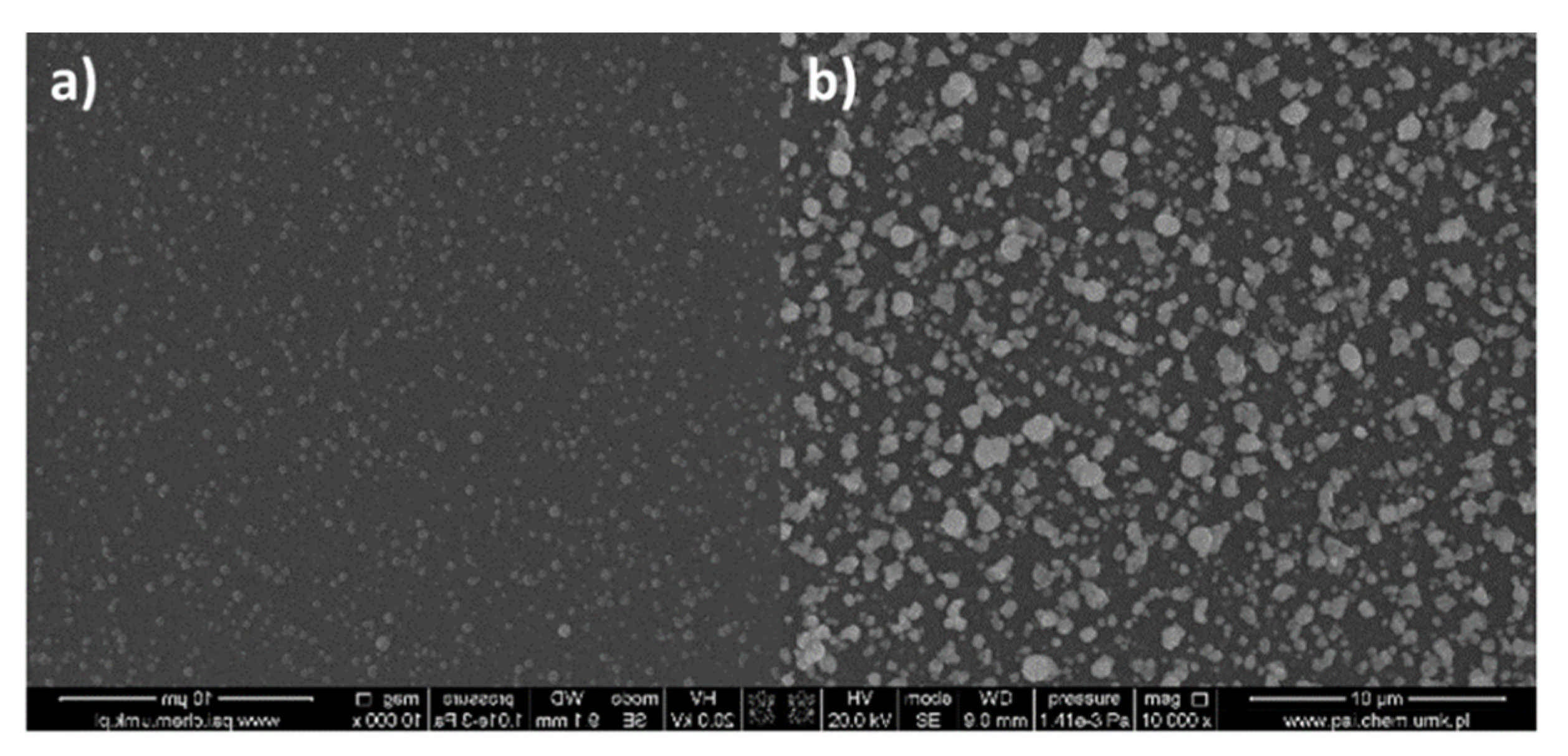
3.6.2. Dip-Coated Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Padnya, P.; Shibaeva, K.; Arsenyev, M.; Baryshnikova, S.; Terenteva, O.; Shiabiev, I.; Khannanov, A.; Boldyrev, A.; Gerasimov, A.; Grishaev, D.; et al. Catechol-Containing Schiff Bases on Thiacalixarene: Synthesis, Copper (II) Recognition, and Formation of Organic-Inorganic Copper-Based Materials. Molecules 2021, 26, 2334. [Google Scholar] [CrossRef]
- Hao, X.-L.; Ma, Y.-Y.; Zhang, C.; Wang, Q.; Cheng, X.; Wang, Y.-H.; Li, Y.-G.; Wang, E.-B. Assembly of new organic-inorganic hybrids based on copper-bis(triazole) complexes and Keggin-type polyoxometalates with different negative charges. CrystEngComm 2012, 14, 6573–6580. [Google Scholar] [CrossRef]
- Wang, L.; Sun, H.; Sun, C.; Xu, D.; Tao, J.; Wei, T.; Zhang, Z.; Zhang, Y.; Wang, Z.; Bi, W. Lead-free, stable orange-red-emitting hybrid copper based organic-inorganic compounds. Dalton Trans. 2021, 50, 2766–2773. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhong, X.; Hu, Y.; Li, B.; Sheng, Y.; Zhang, Y.; Weng, C.; Feng, M.; Han, H.; Wang, J. Organic-Inorganic Copper(II)-Based Material: A Low-Toxic, Highly Stable Light Absorber for Photovoltaic Application. J. Phys. Chem. Lett. 2017, 8, 1804–1809. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.C.; Singh, B.N.; Adhya, S.D.; Banerji, K.C. Thermal behaviour of copper oxalate and its mixed ligand complexes with amines. J. Therm. Anal. 1983, 27, 263–273. [Google Scholar] [CrossRef]
- Perova, E.V.; Yakovleva, M.A.; Baranova, E.O.; Anan’ev, I.V.; Nefedov, S.E. Reactions of aqueous copper acetate with triethylamine and 3,5-dimethylpyrazole. Russ. J. Inorg. Chem. 2010, 55, 714–726. [Google Scholar] [CrossRef]
- Rigamonti, L.; Carlino, S.; Halibi, Y.; Demartin, F.; Castellano, C.; Ponti, A.; Pievo, R.; Pasini, A. Copper 1D coordination polymers and dimers: Role of the carboxylate and the ammonium cation, crystal structures and magnetic studies. Polyhedron 2013, 53, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Kalsotra, B.L. Preparation of Complexes of Copper(II) Aryl Carboxylates with Diethyl Amine and Dipropyl Amine. J. Chin. Chem. Soc. 1974, 21, 267–269. [Google Scholar] [CrossRef]
- Szymańska, I.B. Gaseous phase studies of new copper(II) carboxylate complexes with tert-butylamine as potential precursors for chemical vapor deposition (CVD). Polyhedron 2013, 50, 200–207. [Google Scholar] [CrossRef]
- Piszczek, P.; Szymańska, I.B.; Talik, E.; Heimann, J. Deposition of thin copper layers using copper(II) carboxylate complexes with tert-butylamine as new CVD precursors. Chem. Vap. Depos. 2013, 19, 251–259. [Google Scholar] [CrossRef]
- Lacko, M.; Papp, P.; Szymańska, I.B.; Szłyk, E.; Matejčík, Š. Electron interaction with copper(II) carboxylate compounds. Beilstein J. Nanotechnol. 2018, 9, 384–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sala, L.; Szymańska, I.B.; Dablemont, C.; Lafosse, A.; Amiaud, L. Response under low-energy electron irradiation of a thin film of a potential copper precursor for focused electron beam induced deposition (FEBID). Beilstein J. Nanotechnol. 2018, 9, 57–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakob, A.; Rüffer, T.; Schmidt, H.; Djiele, P.; Körbitz, K.; Ecorchard, P.; Haase, T.; Kohse-Höinghaus, K.; Frühauf, S.; Wächtler, T.; et al. Disilver(I) coordination complexes: Synthesis, reaction chemistry, and their potential use in CVD and spin-coating processes for silver deposition. Eur. J. Inorg. Chem. 2010, 2010, 2975–2986. [Google Scholar] [CrossRef]
- Tuchscherer, A.; Shen, Y.; Jakob, A.; Mothes, R.; Al-Anber, M.; Walfort, B.; Rüffer, T.; Frühauf, S.; Ecke, R.; Schulz, S.E.; et al. Lewis-base copper(I) formates: Synthesis, reaction chemistry, structural characterization and their use as spin-coating precursors for copper deposition. Inorg. Chim. Acta 2011, 365, 10–19. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Q.; Payne, G.F.; Sun, R.; Wang, X. A highly conductive, pliable and foldable Cu/cellulose paper electrode enabled by controlled deposition of copper nanoparticles. Nanoscale 2019, 11, 725–732. [Google Scholar] [CrossRef]
- Kwon, Y.T.; Kim, Y.S.; Lee, Y.; Kwon, S.; Lim, M.; Song, Y.; Choa, Y.H.; Yeo, W.H. Ultrahigh Conductivity and Superior Interfacial Adhesion of a Nanostructured, Photonic-Sintered Copper Membrane for Printed Flexible Hybrid Electronics. ACS Appl. Mater. Interfaces 2018, 10, 44071–44079. [Google Scholar] [CrossRef] [Green Version]
- Chan, G.H.; Zhao, J.; Hicks, E.M.; Schatz, G.C.; van Duyne, R.P. Plasmonic properties of copper nanoparticles fabricated by nanosphere lithography. Nano Lett. 2007, 7, 1947–1952. [Google Scholar] [CrossRef]
- Huang, C.-L.; Kumar, G.; Sharma, G.D.; Chen, F.-C. Plasmonic effects of copper nanoparticles in polymer photovoltaic devices for outdoor and indoor applications. Appl. Phys. Lett. 2020, 116, 253302. [Google Scholar] [CrossRef]
- Gu, Z.; Shen, H.; Shang, L.; Lv, X.; Qian, L.; Zheng, G. Nanostructured Copper-Based Electrocatalysts for CO2 Reduction. Small Methods 2018, 2, 1800121. [Google Scholar] [CrossRef]
- Luc, W.; Fu, X.; Shi, J.; Lv, J.J.; Jouny, M.; Ko, B.H.; Xu, Y.; Tu, Q.; Hu, X.; Wu, J.; et al. Two-dimensional copper nanosheets for electrochemical reduction of carbon monoxide to acetate. Nat. Catal. 2019, 2, 423–430. [Google Scholar] [CrossRef]
- Kuroda, K.; Keller, P.; Kawasaki, H. Mild synthesis of single-nanosized plasmonic copper nanoparticles and their catalytic reduction of methylene blue. Colloids Interface Sci. Commun. 2019, 31, 100187. [Google Scholar] [CrossRef]
- Gao, F.; Pang, H.; Xu, S.; Lu, Q. Copper-based nanostructures: Promising antibacterial agents and photocatalysts. Chem. Commun. 2009, 24, 3571–3573. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, S.; Elmi, A.; Hallaj-Nezhadi, S. Copper Nanoparticles as Antibacterial Agents. J. Mol. Pharm. Org. Process Res. 2018, 6, 1000140. [Google Scholar] [CrossRef]
- Niavol, S.S.; Ghodsi, F.E. Effect of pH on the Properties of Nanocrystalline CuO Thin Films Deposited by Sol-Gel Process. Russ. J. Phys. Chem. A 2013, 87, 84–87. [Google Scholar] [CrossRef]
- Syukri, T.; Ban, Y.; Ohya, Y.; Takahashi. Novel approach to deposition of copper thin films by dip-coating method. J. Mater. Sci. Lett. 2002, 21, 1449–1451. [Google Scholar] [CrossRef]
- Szłyk, E.; Szymańska, I. Studies of new volatile copper(I) complexes with triphenylphosphite and perfluorinated carboxylates. Polyhedron 1999, 18, 2941–2948. [Google Scholar] [CrossRef]
- Furukawa, Y.; Kaneko, A. Investigation of surface formation process of silicon molecular beam epitaxy by atomic force microscopy. CIRP Ann. 1999, 48, 453–457. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; Part A 56–58, 217–220, part B, 276–282. [Google Scholar]
- Sengar, B.R.S.; Narain, G. Magneto-chemistry and Infrared Spectra of Ammonia and Primary Aliphatic Amine Complexes of Copper(II) Chromate. Bull. Chem. Soc. Jpn. 1975, 48, 1087–1088. [Google Scholar] [CrossRef]
- Uznanski, P.; Zakrzewska, J.; Favier, F.; Kazmierski, S.; Bryszewska, E. Synthesis and characterization of silver nanoparticles from (bis)alkylamine silver carboxylate precursors. J. Nanoparticle Res. 2017, 19, 121. [Google Scholar] [CrossRef] [Green Version]
- Zakrzewska, J.; Uznanski, P. Synthesis and characterization of bis(amine)palladium(ii) carboxylate complexes as precursors of palladium nanoparticles. Dalton Trans. 2021, 50, 6933–6948. [Google Scholar] [CrossRef] [PubMed]
- Melník, M. Mono-, bi-, tetra- and polynuclear copper(II) halogenocarboxylates. Coord. Chem. Rev. 1981, 36, 1–44. [Google Scholar] [CrossRef]
- Mikuriya, M.; Yamakawa, C.; Tanabe, K.; Nukita, R.; Amabe, Y.; Yoshioka, D.; Mitsuhashi, R.; Tatehata, R.; Tanaka, H.; Handa, M.; et al. Copper(II) Carboxylates with 2,3,4-Trimethoxybenzoate and 2,4,6-Trimethoxybenzoate: Dinuclear Cu(II) Cluster and µ-Aqua-Bridged Cu(II) Chain Molecule. Magnetochemistry 2021, 7, 35. [Google Scholar] [CrossRef]
- John Wiley & Sons. SpectraBase Compound ID=8A1dEqurmmH SpectraBase Spectrum ID=G4CBuKNSKtQ, (n.d.). Available online: https://spectrabase.com/spectrum/G4CBuKNSKtQ (accessed on 30 October 2021).
- John Wiley & Sons. SpectraBase Compound ID=Cz3wO46i3MG, (n.d.). Available online: https://spectrabase.com/compound/Cz3wO46i3MG (accessed on 30 October 2021).
- John Wiley & Sons. SpectraBase, SpectraBase Compound ID=3IaXq9hN0c6 SpectraBase Spectrum ID=4PAsbe0uoOp, (n.d.). Available online: https://spectrabase.com/spectrum/4PAsbe0uoOp (accessed on 30 October 2021).
- John Wiley & Sons. SpectraBase Compound ID=6i6hO8Z6rbg SpectraBase Spectrum ID=1HL91c6HmtV, SpectraBase, (n.d.). Available online: https://spectrabase.com/spectrum/1HL91c6HmtV (accessed on 30 October 2021).
- John Wiley & Sons. SpectraBase Compound ID=4G3cv2HmDfG SpectraBase Spectrum ID=89U74D3zedG, (n.d.). Available online: https://spectrabase.com/spectrum/89U74D3zedG (accessed on 30 October 2021).
- Fuson, N.; Josien, M.-L.; Jones, E.A.; Lawson, J.R. Infrared and Raman spectroscopy studies of light and heavy trifluoroacetic acids. J. Chem. Phys. 1952, 20, 1627–1634. [Google Scholar] [CrossRef]
- Shipman, S.T.; Douglass, P.C.; Yoo, H.S.; Hinkle, C.E.; Mierzejewski, E.L.; Pate, B.H. Vibrational dynamics of carboxylic acid dimers in gas and dilute solution. Phys. Chem. Chem. Phys. 2007, 9, 4572–4586. [Google Scholar] [CrossRef] [PubMed]
- Höflich, K.; Jurczyk, J.M.; Madajska, K.; Götz, M.; Berger, L.; Guerra-Nuñez, C.; Haverkamp, C.; Szymanska, I.; Utke, I. Towards the third dimension in direct electron beam writing of silver. Beilstein J. Nanotechnol. 2018, 9, 842–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; p. 118. [Google Scholar]
- Gu, N.X.; Oyala, P.H.; Peters, J.C. Hydrazine Formation via Coupling of a Nickel(III)–NH2 Radical. Angew. Chem. Int. Ed. 2021, 60, 4009–4013. [Google Scholar] [CrossRef] [PubMed]
- Jakob, A.; Rüffer, T.; Ecorchard, P.; Walfort, B.; Körbitz, K.; Frühauf, S.; Schulz, S.E.; Gessner, T.; Lang, H. Phosphane Copper(I) Dicarboxylates: Synthesis and Their Potential Use as Precursors for the Spin-coating Process in the Deposition of Copper. Z. Anorg. Allg. Chem. 2010, 636, 1931–1940. [Google Scholar] [CrossRef] [Green Version]
- Bräuer, B.; Zahn, D.R.T.; Rüffer, T.; Salvan, G. Deposition of thin films of a transition metal complex by spin coating. Chem. Phys. Lett. 2006, 432, 226–229. [Google Scholar] [CrossRef]
- Syukri; Terazawa, T.; Ban, T.; Ohya, Y.; Takahashi, Y. Dip Coating of Metal Films from Metal Acetate-Acetol Hydrazone System, Ceram. Trans. In Proceedings of the International Conference on Ceramic Processing Science Meeting, Nagoya, Japan, 15–17 May 2000; 2001; Volume 112, pp. 329–334. [Google Scholar] [CrossRef]
- Theivasanthi, T.; Alagar, M. X-Ray Diffraction Studies of Copper Nanopowder. Arch. Phys. Res. 2010, 1, 112–117. [Google Scholar] [CrossRef]
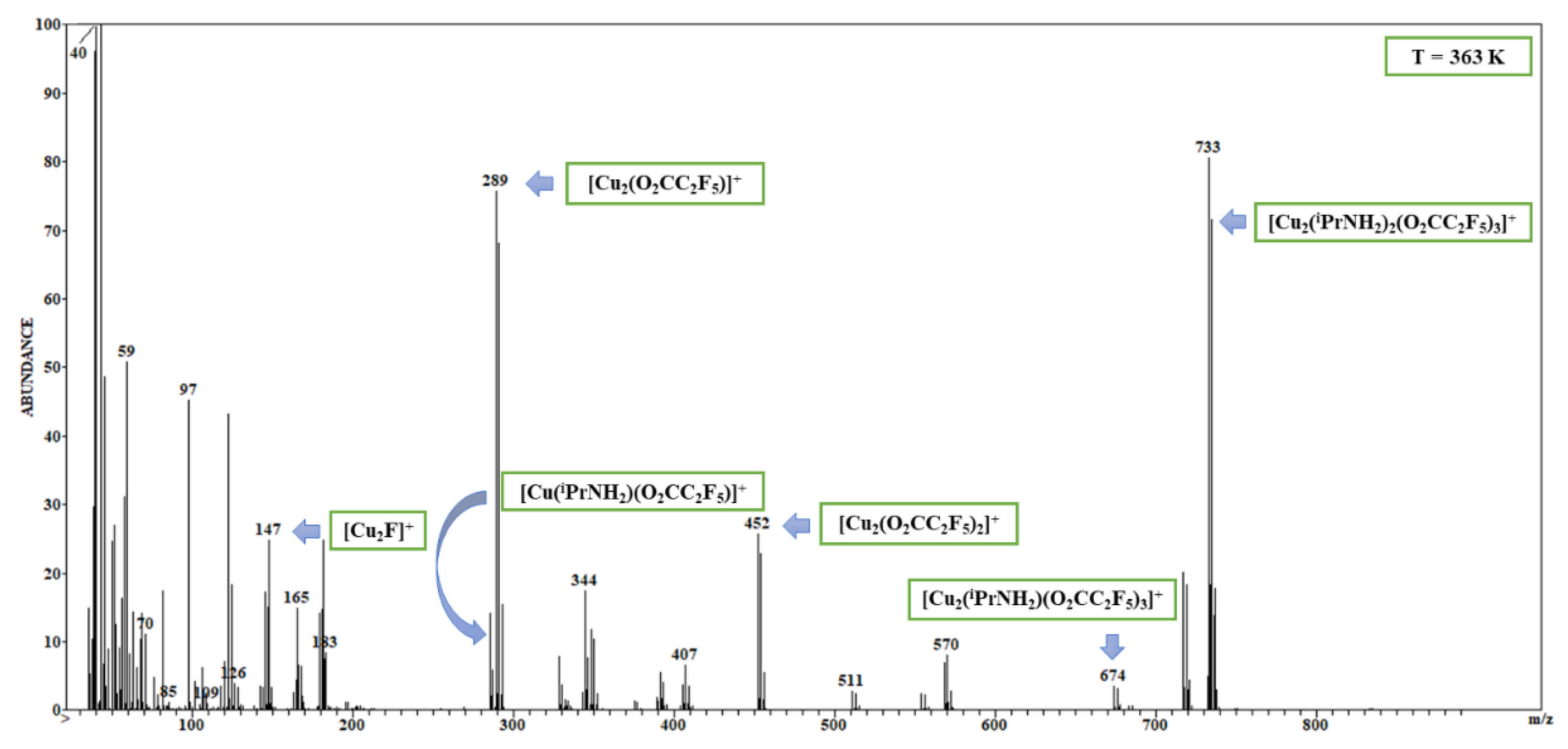
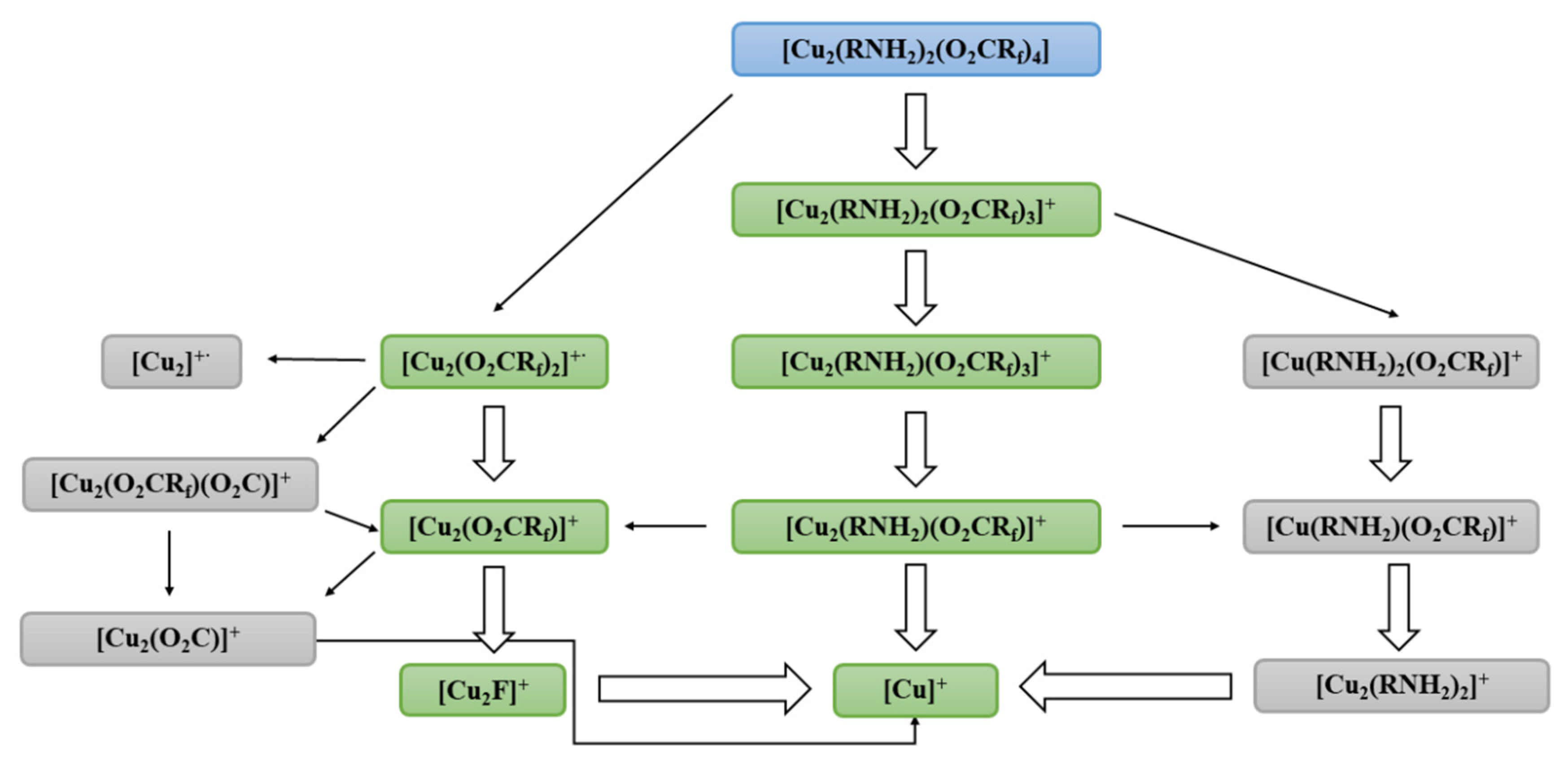
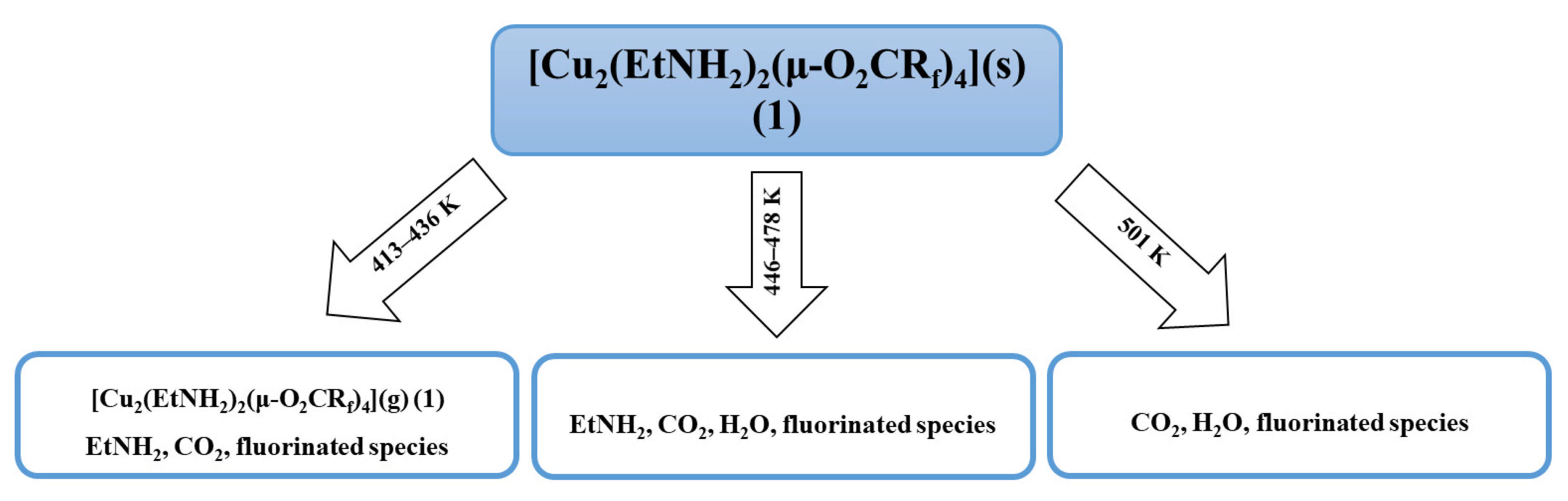
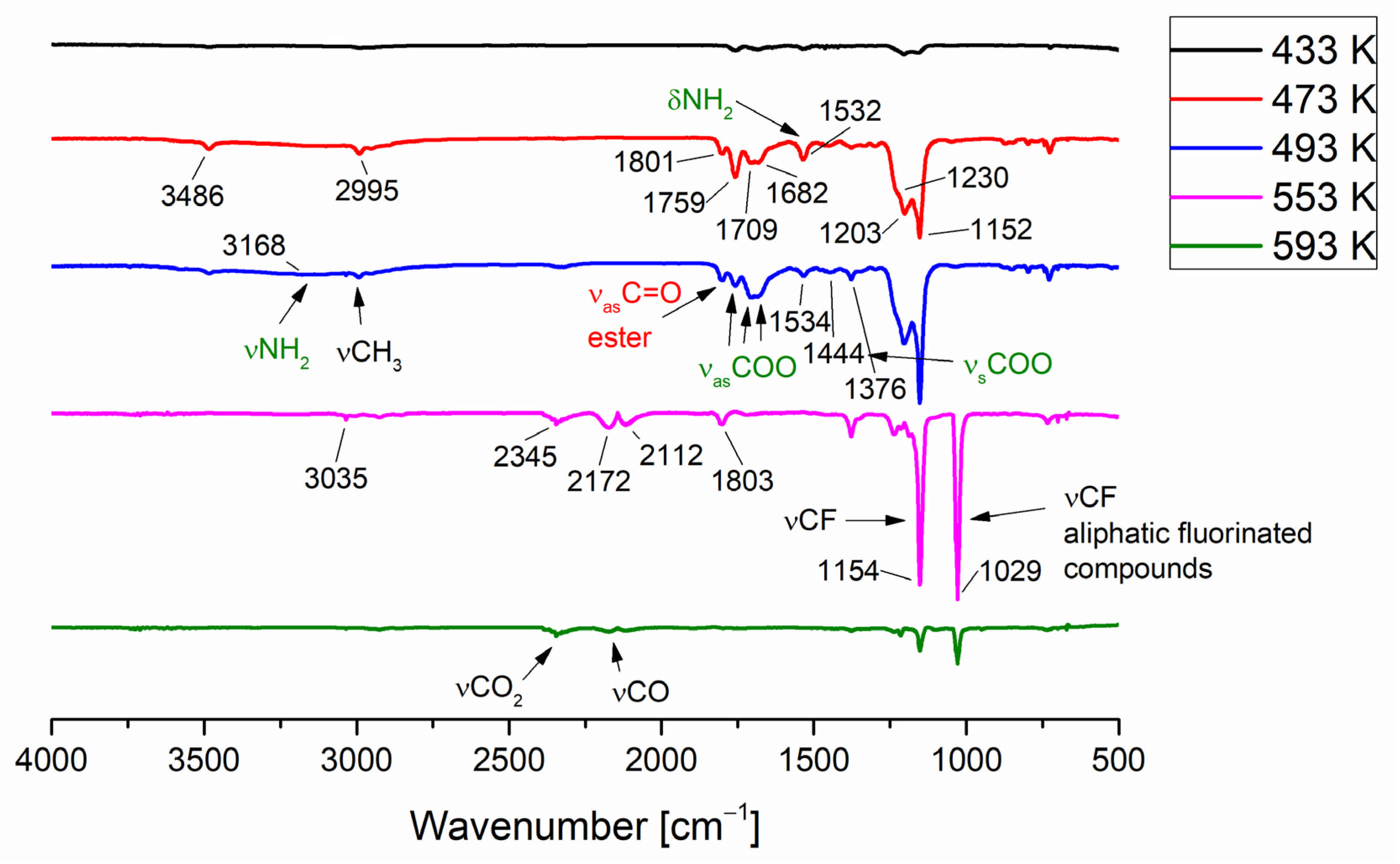
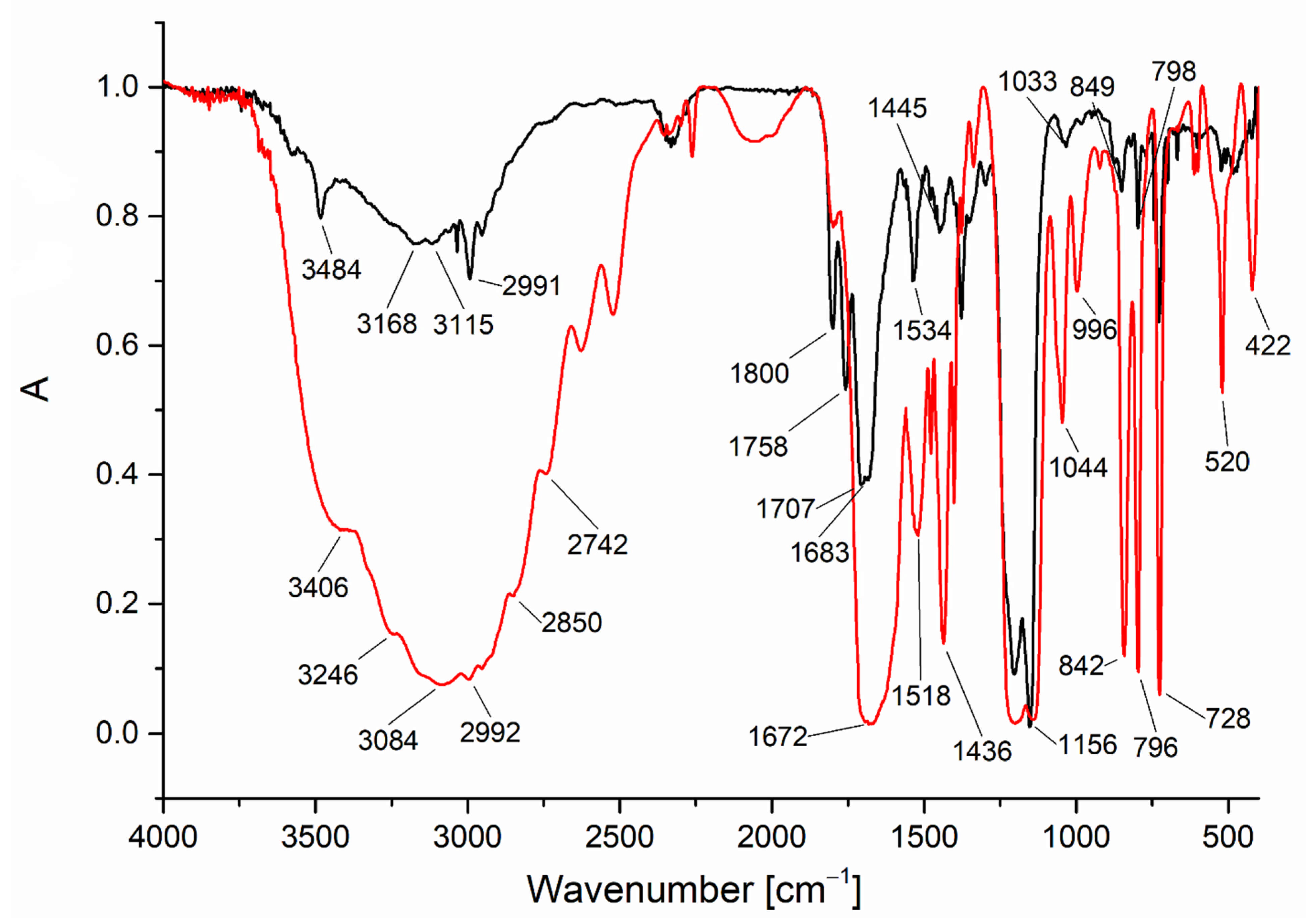



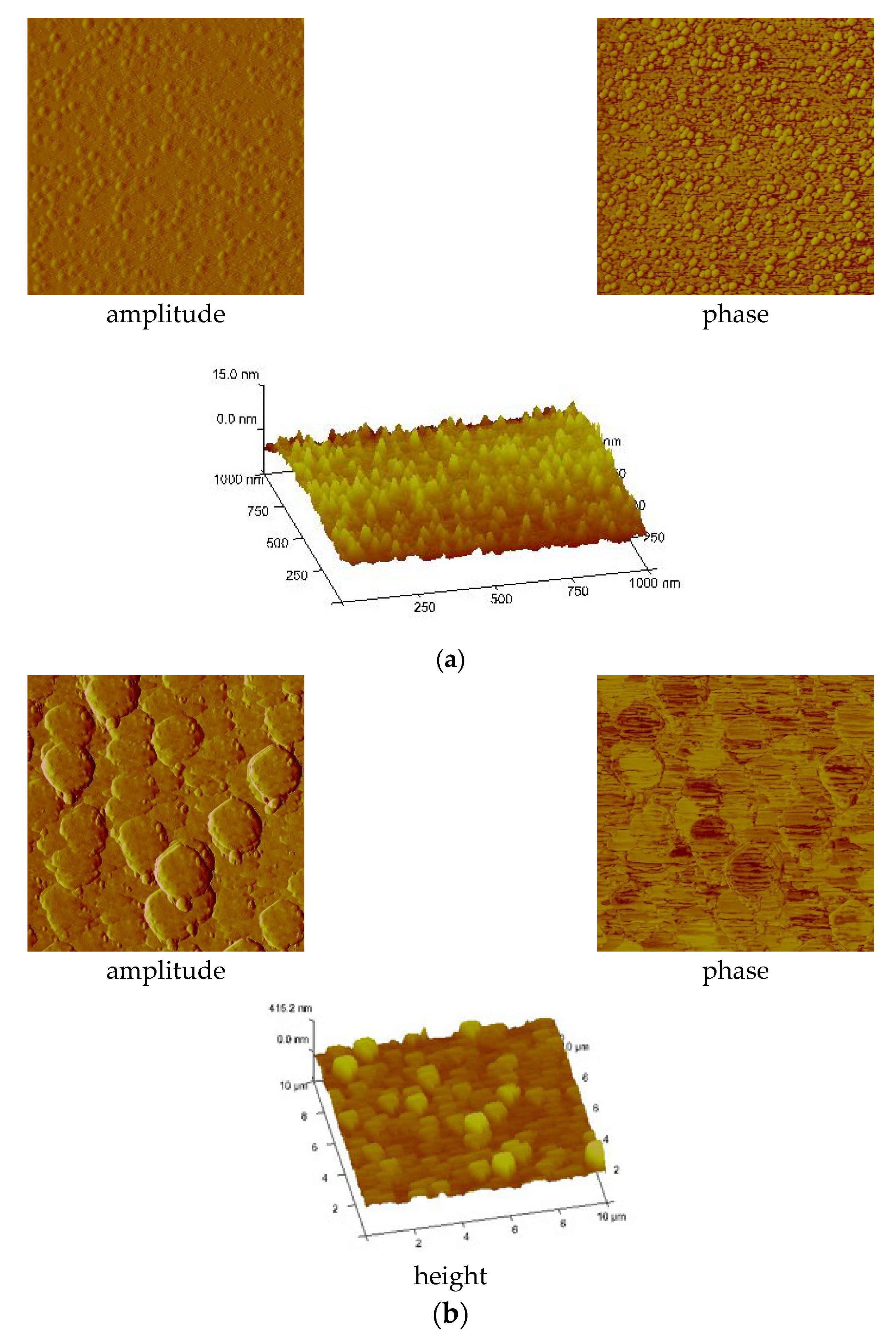


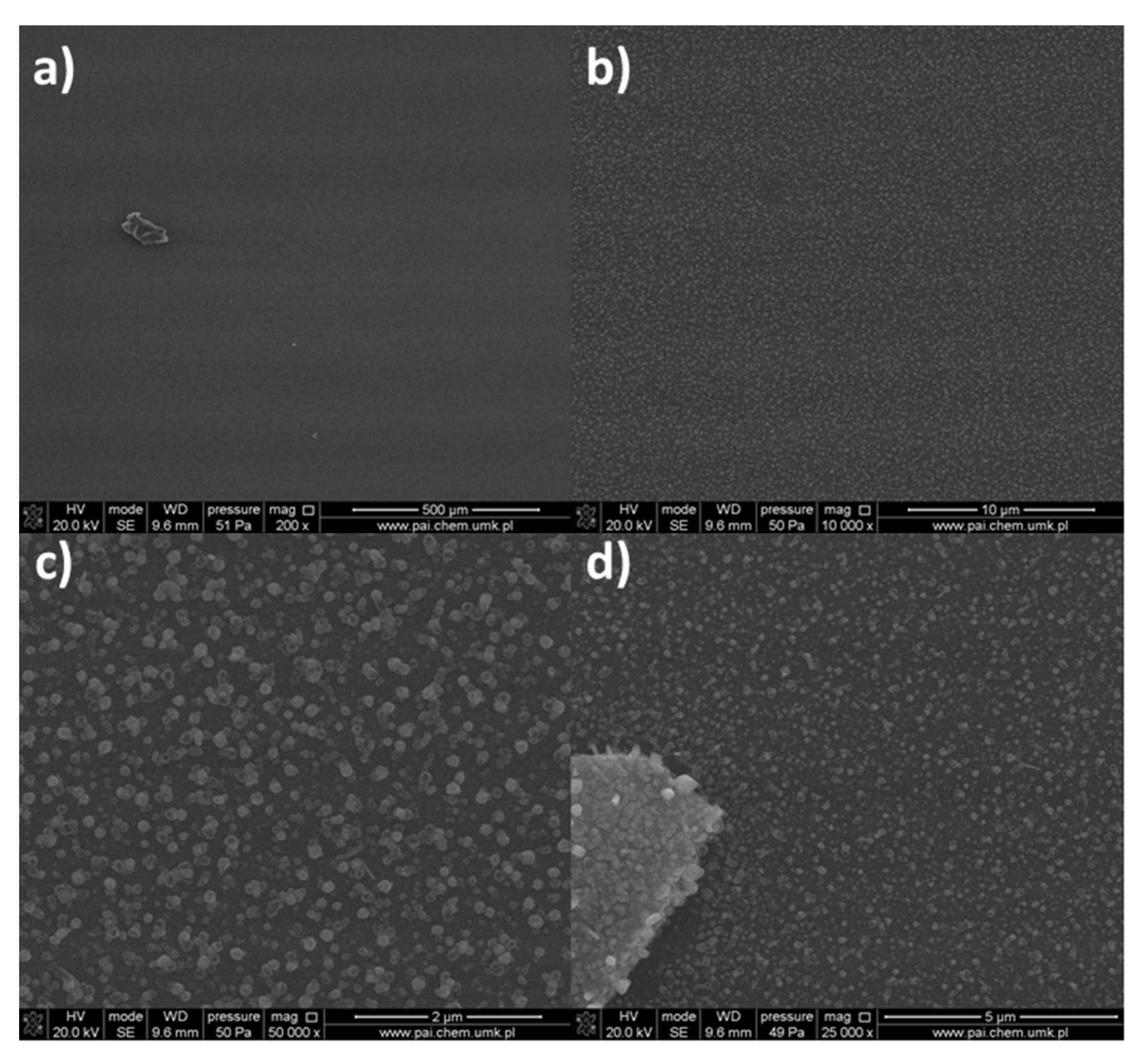
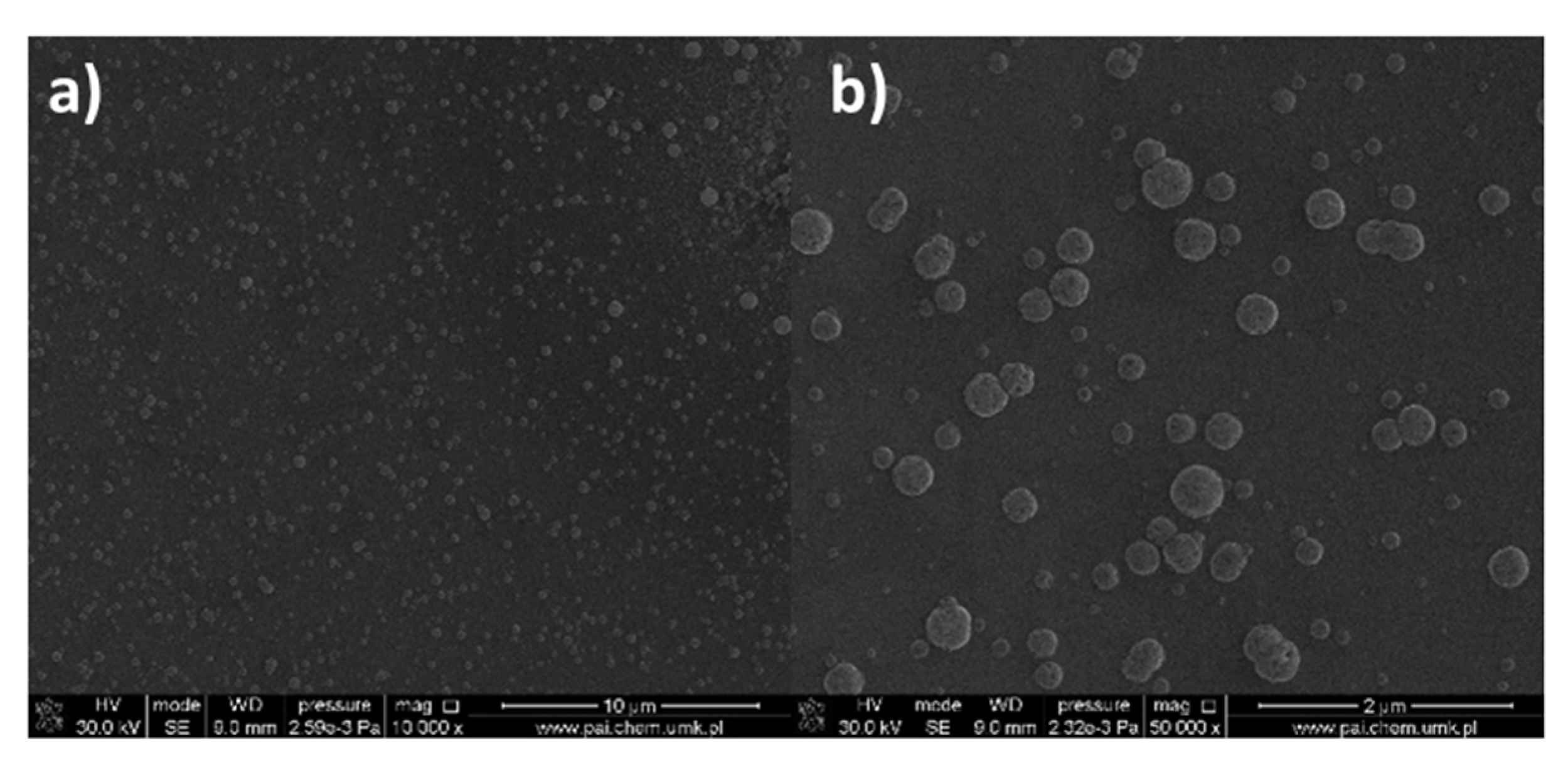
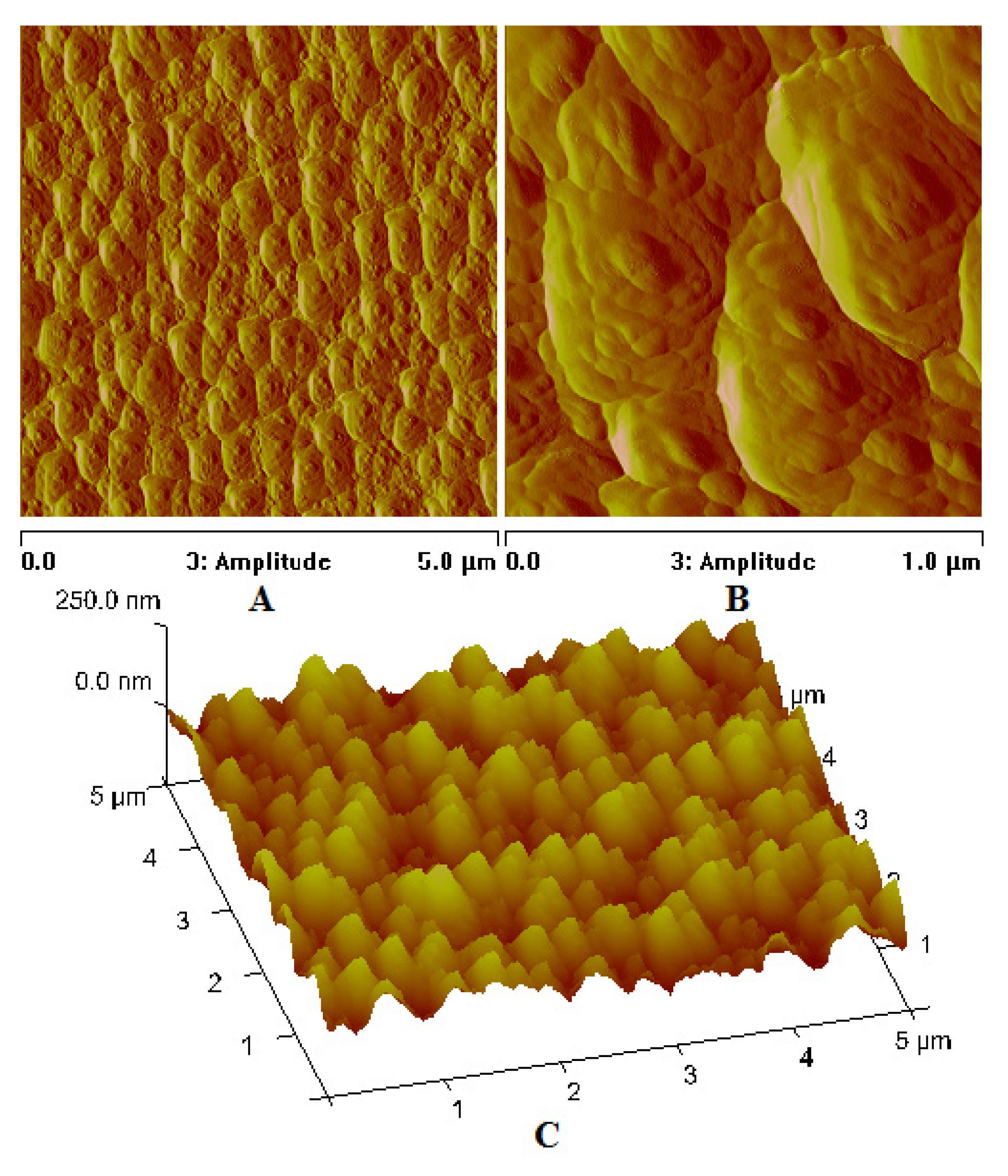

| Compound | νNH2 br | νasCOO | νNH2 scci | νsCOO | ∆νCOO |
|---|---|---|---|---|---|
| 3080 | 1672 | 1518 | 1436 | 236 |
| 3080 | 1675 | 1531 | 1413 | 262 |
| 3060 | 1679 | 1528 | 1405 | 274 |
| 3070 | 1681 | 1531 | 1403 | 278 |
| 3090 | 1679 | 1545 | 1409 | 270 |
| 3070 | 1673 | 1527 | 1404 | 269 |
| 3080 | 1666 | 1520 | 1431 | 235 |
| 3060 | 1675 | 1523 | 1403 | 272 |
| 3090 | 1683 | 1522 | 1406 | 277 |
| 3100 | 1681 | 1522 | 1403 | 278 |
| EtNH2 | 3360 | - | 1625 | - | - |
| iPrNH2 | 3380 | - | 1618 | - | - |
| Fragments | m/z | RI[%] | ||
|---|---|---|---|---|
| 305 K | 355 K | 600 K | ||
| [C2H2N]+ | 40 | 7 | 6 | - |
| [C2H3N]+ | 41 | 11 | 8 | - |
| [C2H4N]+ | 42 | 15 | 13 | - |
| [CO2]+ | 44 | 35 | 33 | 100 |
| [C2H7N]+./[COOH]+. | 45 | 45 | 69 | 3 |
| [CF2]+ | 50 | 20 | 20 | 11 |
| [CF3]+ | 69 | 93 | 100 | 61 |
| [CF3CO]+ | 97 | 9 | 9 | 6 |
| [Cu]+ | 63 | 40 | 26 | 1 |
| [Cu2]+∙ | 126 | 6 | 7 | - |
| [Cu2F]+ | 145 | 25 | 24 | 2 |
| [Cu(EtNH2)(O2CCF3)]+ | 221 | 11 | 3 | - |
| [Cu2(O2CCF3)]+ | 239 | 100 | 98 | 6 |
| [Cu2(EtNH2)(O2CCF3)]+ | 284 | 5 | 2 | - |
| [Cu2(O2CCF3)2]+∙ | 352 | 66 | 61 | 4 |
| [Cu2(O2CCF3)3]+ | 465 | 7 | 1 | - |
| [Cu2(EtNH2)(O2CCF3)3]+ | 510 | 11 | 3 | - |
| [Cu2(EtNH2)2(O2CCF3)3]+ | 555 | 3 | - | - |
| [Cu3(O2CCF3)5]+ | 756 | 2 | - | - |
| Fragments | m/z | RI[%] | ||
|---|---|---|---|---|
| 368 K | 389 K | 548 K | ||
| [C2H2N]+ | 40 | 1 | 50 | 3 |
| [C2H3N]+ | 41 | - | 1 | 8 |
| [C2H4N]+ | 42 | 1 | 1 | 6 |
| [C3H7]+ | 43 | 42 | 80 | 2 |
| [CO2]+ | 44 | 100 | 6 | 4 |
| [C2H7N]+/[COOH]+∙ | 45 | 40 | 37 | 40 |
| [CF2]+ | 50 | 8 | 67 | 52 |
| [C3H7N]+ | 57 | 18 | 19 | - |
| [C3H8N]+ | 58 | 69 | 1 | - |
| [C3H9N]+∙ | 59 | 19 | 41 | 2 |
| [CF3]+ | 69 | 44 | 2 | 1 |
| [CF3CO]+ | 97 | 4 | 55 | 32 |
| [Cu]+ | 63 | - | 20 | 44 |
| [Cu(iPrNH2)]+ | 122 | 2 | 40 | - |
| [Cu2]+∙ | 126 | - | 8 | 11 |
| [Cu2F]+ | 145 | - | 29 | 39 |
| [Cu(iPrNH2)(O2CCF3)]+ | 235 | 1 | 28 | 2 |
| [Cu2(O2CCF3)]+ | 239 | - | - | 99 |
| [Cu(iPrNH2)2(O2CCF3)]+ | 294 | 1 | 22 | - |
| [Cu2(iPrNH2)(O2CCF3)]+ | 298 | 1 | 14 | - |
| [Cu2(O2CCF3)2]+∙ | 352 | - | 63 | 45 |
| [Cu2(O2CCF3)3]+ a) | 465 | - | - | - |
| [Cu2(iPrNH2)(O2CCF3)3]+ | 524 | - | 12 | - |
| [Cu2(iPrNH2)2(O2CCF3)3]+ | 583 | 5 | 77 | - |
| Complex | Temperature (K) | Residue (%) | |||
|---|---|---|---|---|---|
| Ti | Tm | Tf | Found | Calc. | |
| [Cu2(EtNH2)2(μ-O2CCF3)4] (1) | 410 | 486 | 532 | 18.17 | 18.99 (Cu) |
| [Cu2(EtNH2)2(μ-O2CC3F7)4] (3) | 395 | 466 | 505 | 6.03 | 11.88 (Cu) |
| [Cu2(EtNH2)2(μ-O2CC4F9)4] (4) | 388 | 476 | 520 | 6.97 | 10.01 (Cu) |
| [Cu2(iPrNH2)2(μ-O2CCF3)4] (7) | 312 | 474 | 528 | 22.9 | 22.8 (CuO) |
| [Cu2(iPrNH2)2(μ-O2CC2F5)4] (8) | 323 | 449 | 478 | 4.72 | 17.7 (CuO) |
| [Cu2(iPrNH2)2(μ-O2CC3F7)4] (9) | 308 | 464 | 548 | 7.2 | 14.5 (CuO) |
| [Cu2(iPrNH2)2(μ-O2CC4F9)4] (10) | 355 | 463 | 510 | 14.4 | 12.2 (CuO) |
| The Product in the Gas Phase | Temperature Range [K] | ||||
|---|---|---|---|---|---|
| (1) | (2) | (4) | (7) | (8) | |
| [Cu2(RNH2)2(O2CRf)4] | 433–533 | 463–513 | 433–533 | 493–533 | 473–613 |
| [Cu2(O2CRf)4] | 433–533 | 463–513 | 433–513 | 433–533 | 473–613 |
| [Cu2(NH2)2(O2CRf)2] | 433–533 | ≥463 | 473–533 * | 433–533 | ≥473 |
| RfCOOR | 433–573 | ≥463 | ≥433 | ≥453 | 533–613 |
| RfCOOH | – | 473–513 | – | ≥513 | – |
| CO2 | ≥553 | ≥413 | ≥533 | ≥493 | ≥473 |
| CO | ≥553 | – | 533–553 | ≥553 | 553–613 |
| aliphatic fluorinated compounds | ≥553 | – | ≥533 | – | – |
| Vaporization Temperature TV [K] | Deposition Temperature TD [K] | Precursor Mass (m) [mg] | Carrier Gas | Substrate | Deposition Time (t) [min] | |
|---|---|---|---|---|---|---|
| [Cu2(EtNH2)2(µ-O2CCF3)4] (1) | 473 | 573 | 80 | Ar | Si(111) | 60 |
| [Cu2(EtNH2)2(µ-O2CC2F5)4] (2) | 473 | 713 | 100 | |||
| [Cu2(iPrNH2)2(µ-O2CCF3)4] (7) | 453 | 573 | 80 | |||
| [Cu2(iPrNH2)2(µ-O2CC2F5)4] (8) | 473 | 713 | 100 | |||
| [Cu2(iPrNH2)2(µ-O2CC3F7)4] (9) | 473 | 673 | 100 |
| Set Number | Coating Counts | Immersion Rate [mm/min] | Immersion Time[s] |
|---|---|---|---|
| 1 | 10 | 80 | 5 |
| 2 | 10 | 80 | 20 |
| 3 | 10 | 80 | 30 |
| 4 | 30 | 80 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymańska, I.B.; Madajska, K.; Butrymowicz, A.; Barwiołek, M. Copper(II) Perfluorinated Carboxylate Complexes with Small Aliphatic Amines as Universal Precursors for Nanomaterial Fabrication. Materials 2021, 14, 7451. https://doi.org/10.3390/ma14237451
Szymańska IB, Madajska K, Butrymowicz A, Barwiołek M. Copper(II) Perfluorinated Carboxylate Complexes with Small Aliphatic Amines as Universal Precursors for Nanomaterial Fabrication. Materials. 2021; 14(23):7451. https://doi.org/10.3390/ma14237451
Chicago/Turabian StyleSzymańska, Iwona B., Katarzyna Madajska, Aleksandra Butrymowicz, and Magdalena Barwiołek. 2021. "Copper(II) Perfluorinated Carboxylate Complexes with Small Aliphatic Amines as Universal Precursors for Nanomaterial Fabrication" Materials 14, no. 23: 7451. https://doi.org/10.3390/ma14237451








