Characterization of Three Amu-Darya Basin Clays in Ceramic Brick Industry and Their Applications with Brick Waste
Abstract
:1. Introduction
2. Materials and Methods
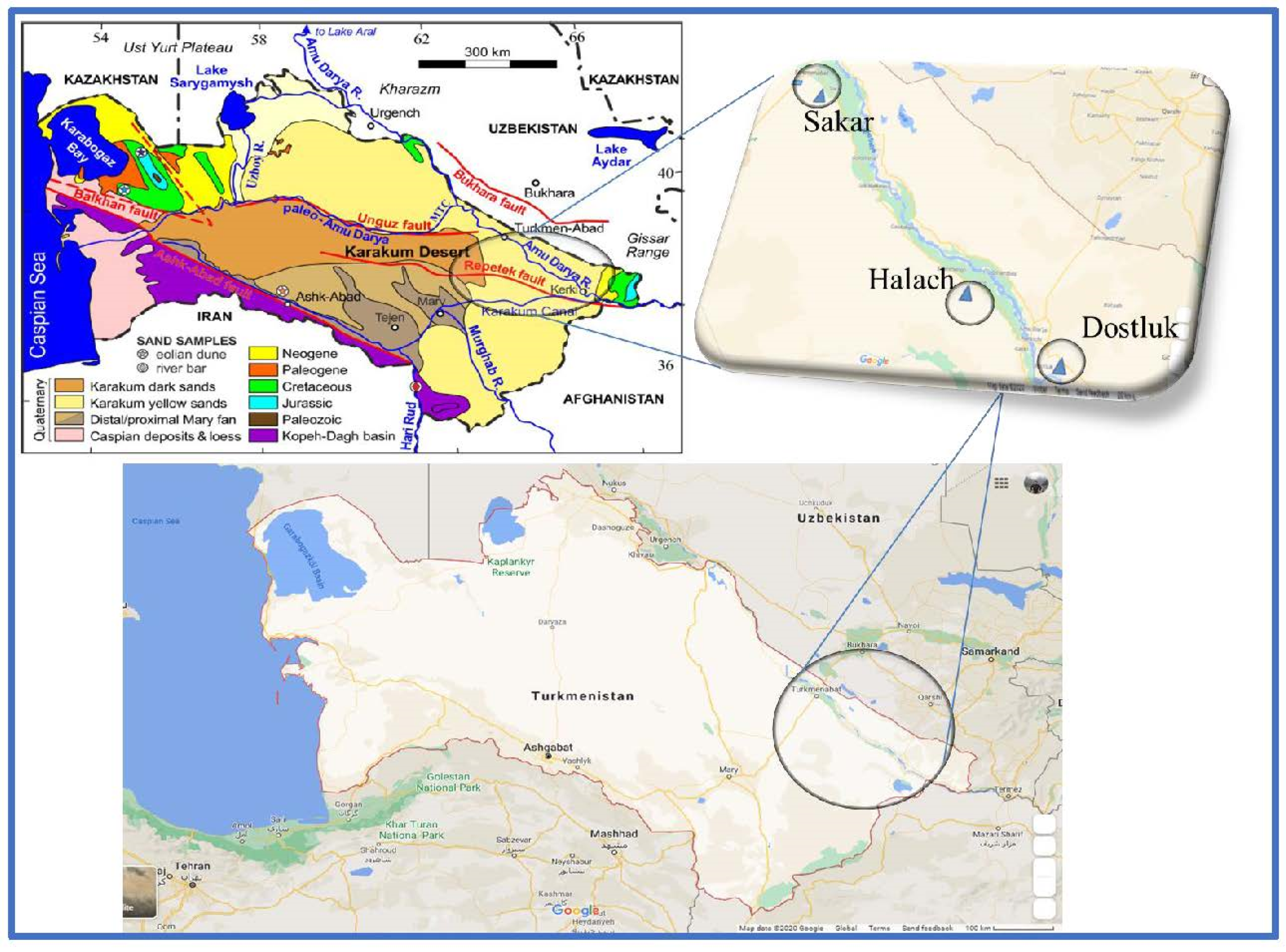
2.1. Sample Materials
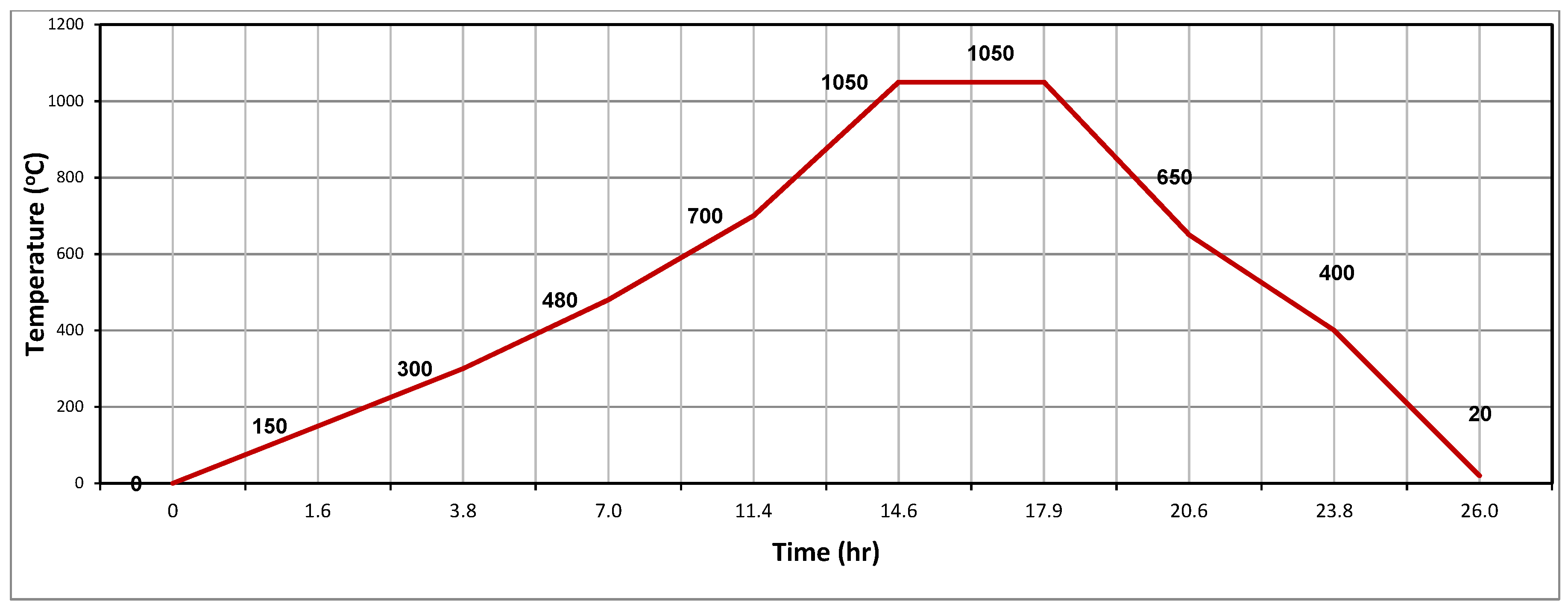
2.2. Specimen Preparation, Extrusion, Drying, and Firing Process of Clay-Based Bricks
2.3. Preparation of Clay and WB Mixtures for Brick Production
2.4. Evaluation of Technological Parameters of the Specimens
2.5. Characterization of Clays and Bricks
2.6. Evaluation of Plasticity
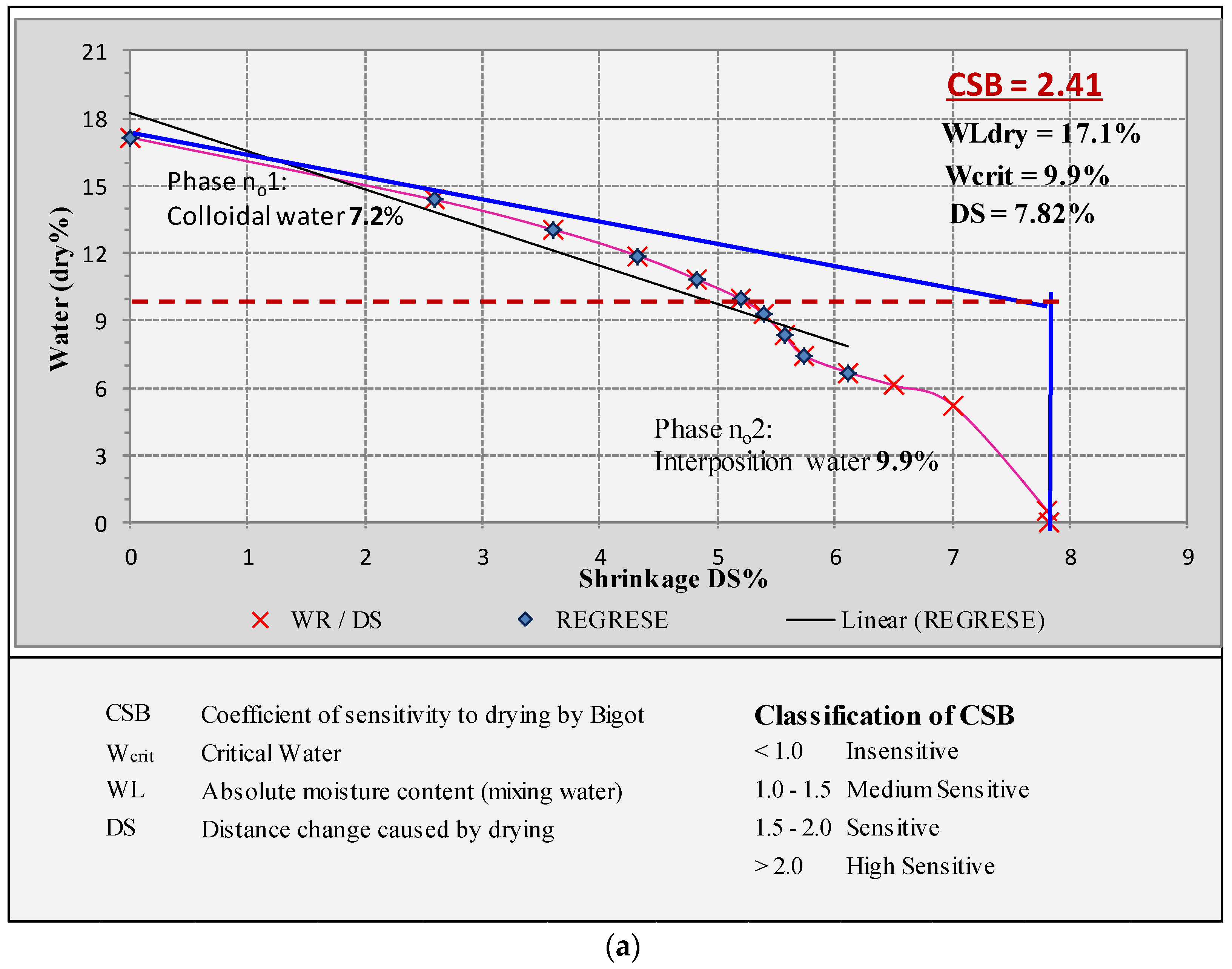
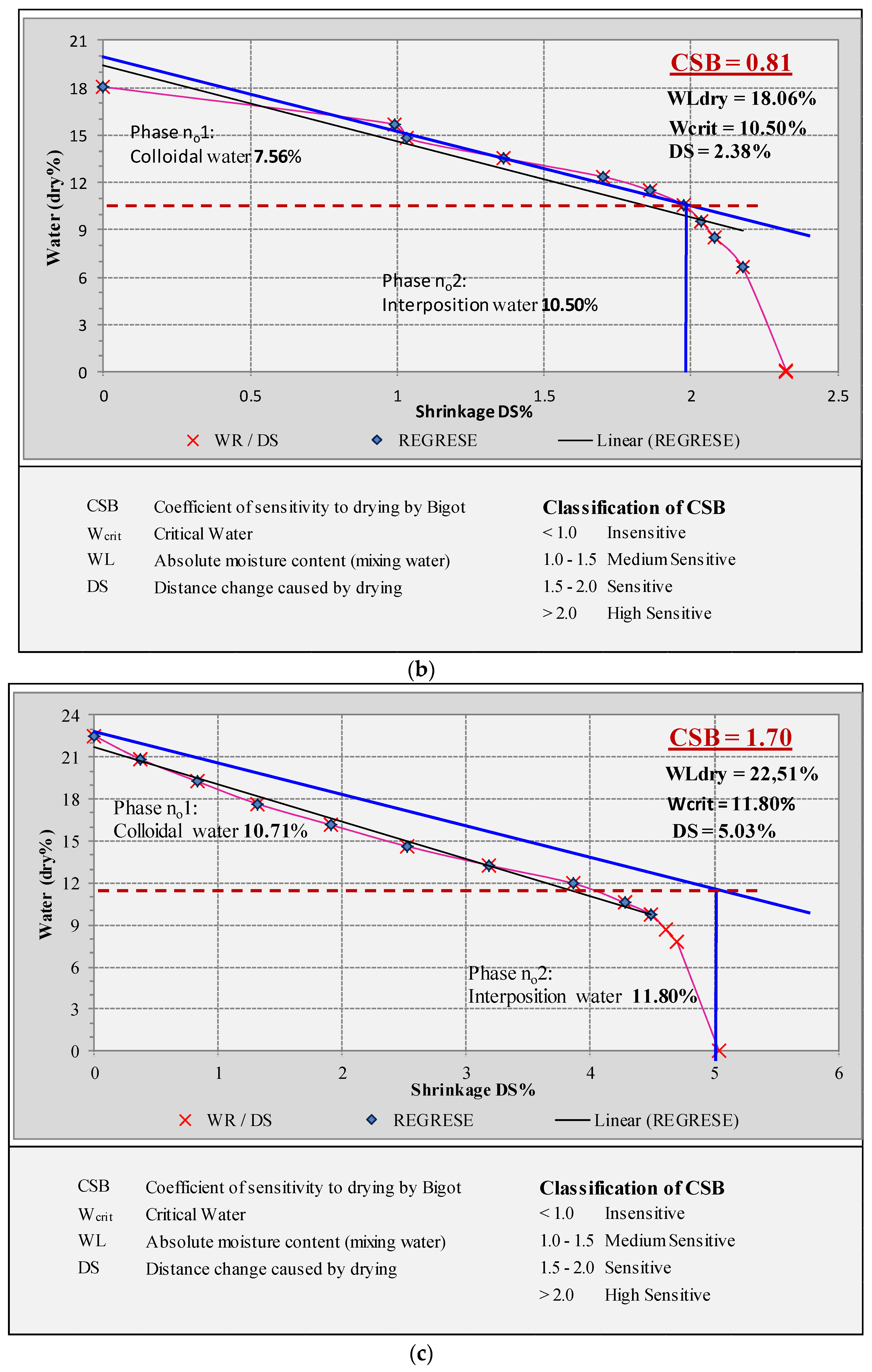
2.7. Bigot’s Curves
2.8. Dilatometer Test
2.9. Frost Resistance of Clay-Based Bricks
3. Results and Discussion
3.1. Sample Material
3.2. Chemical Composition (Oxide Content %) of the Raw Materials
3.3. Mineralogy of the Raw Materials
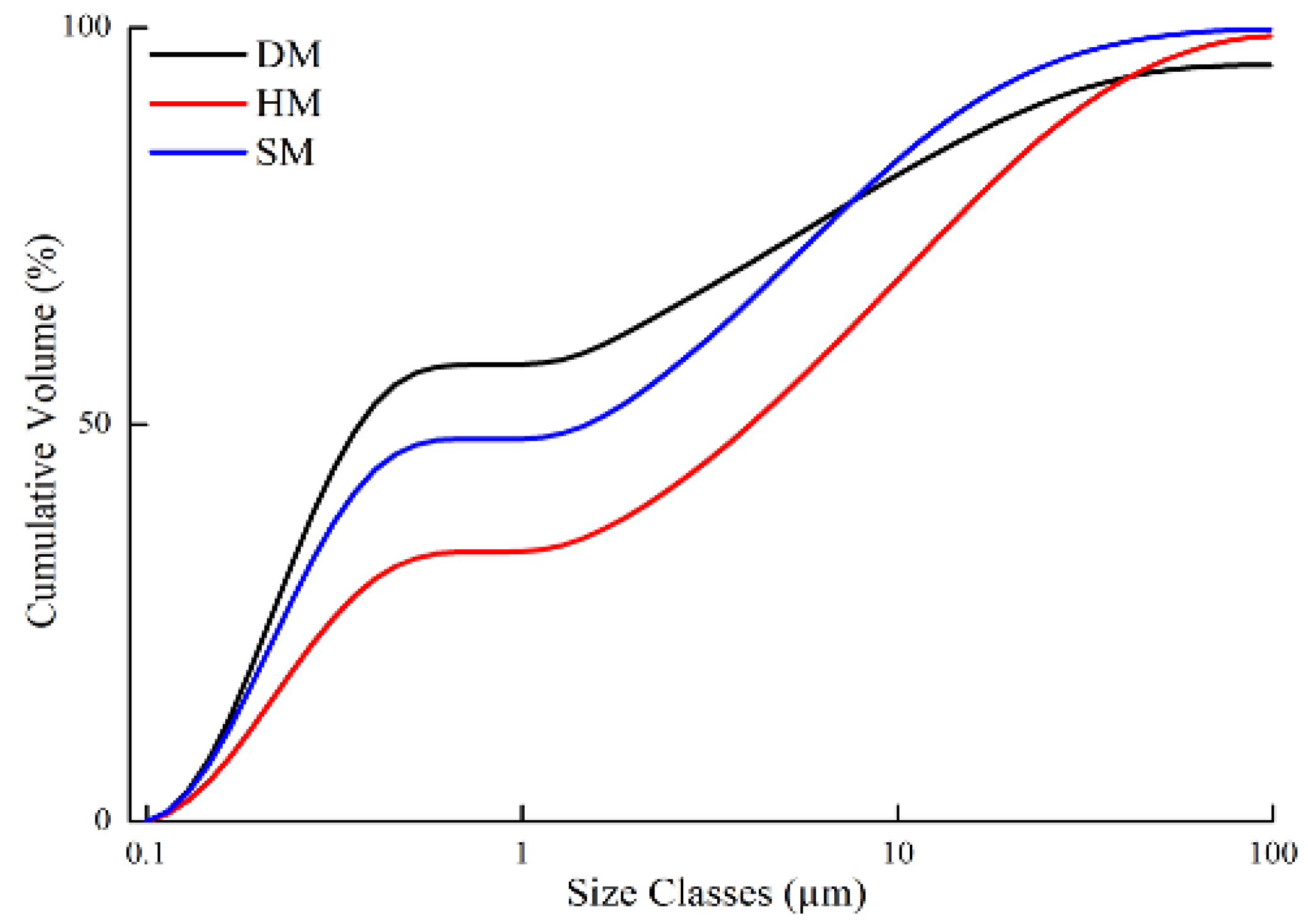
3.4. Microgranulometric and Particle-Size Distribution Analysis of Clays
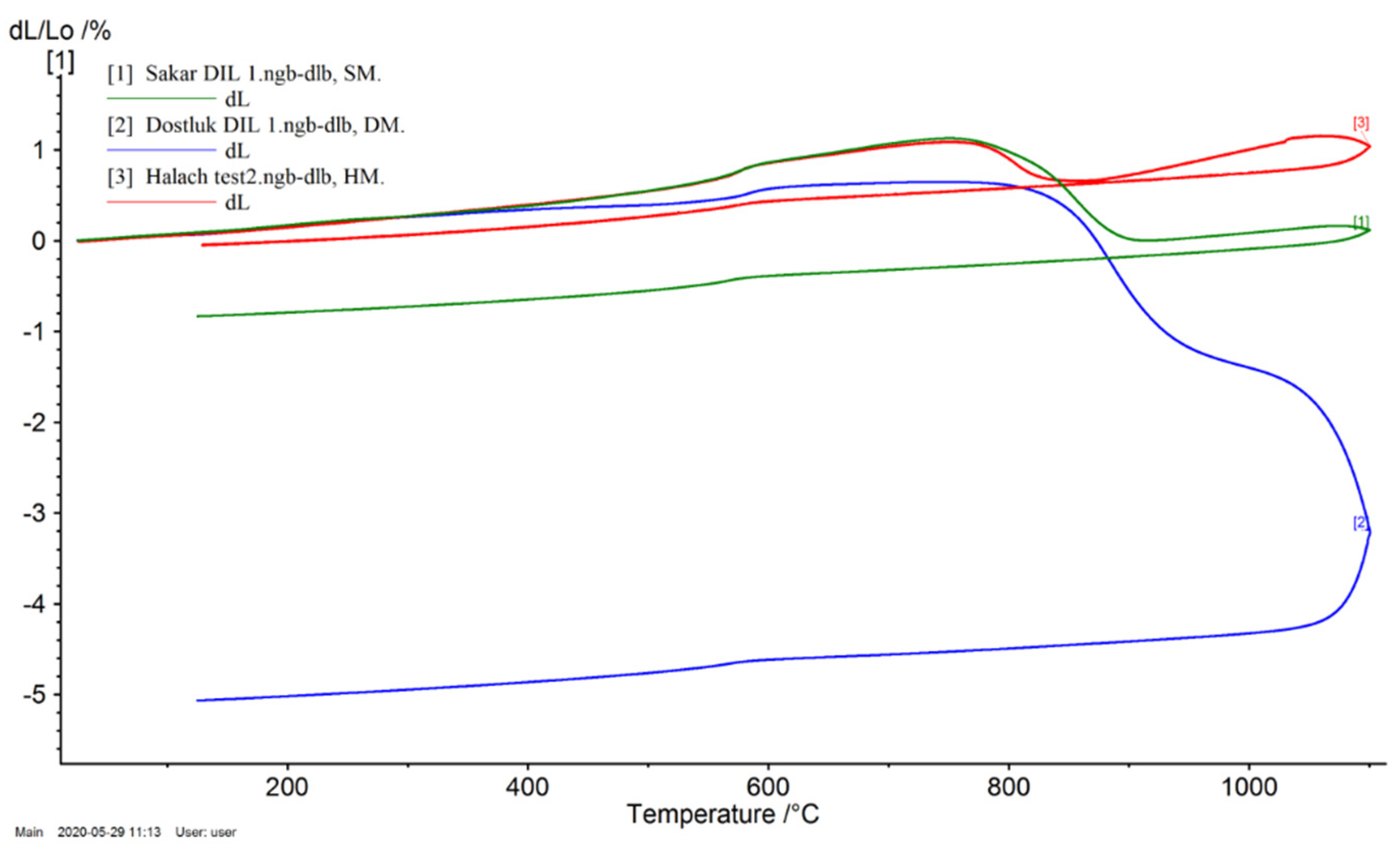
3.5. Dilatometric Curve
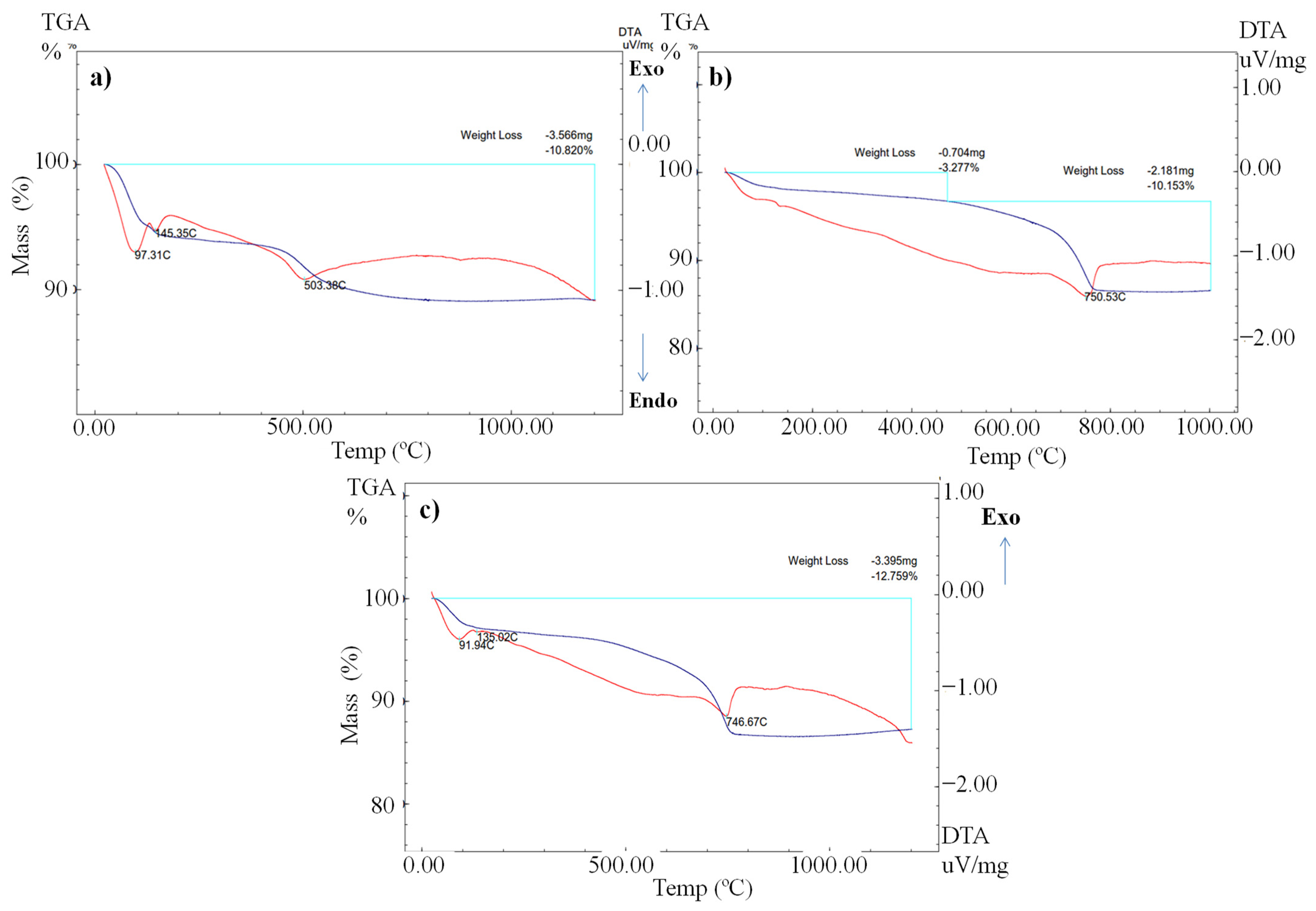
3.6. Thermo-Gravimetric/Differential Thermal Analysis
3.7. Processing of DM, HM, and SM Clays for Brick Production
3.7.1. Moisture Content
3.7.2. Plasticity Evaluation
3.7.3. Extruded Bricks from DM, HM, and SM Clays
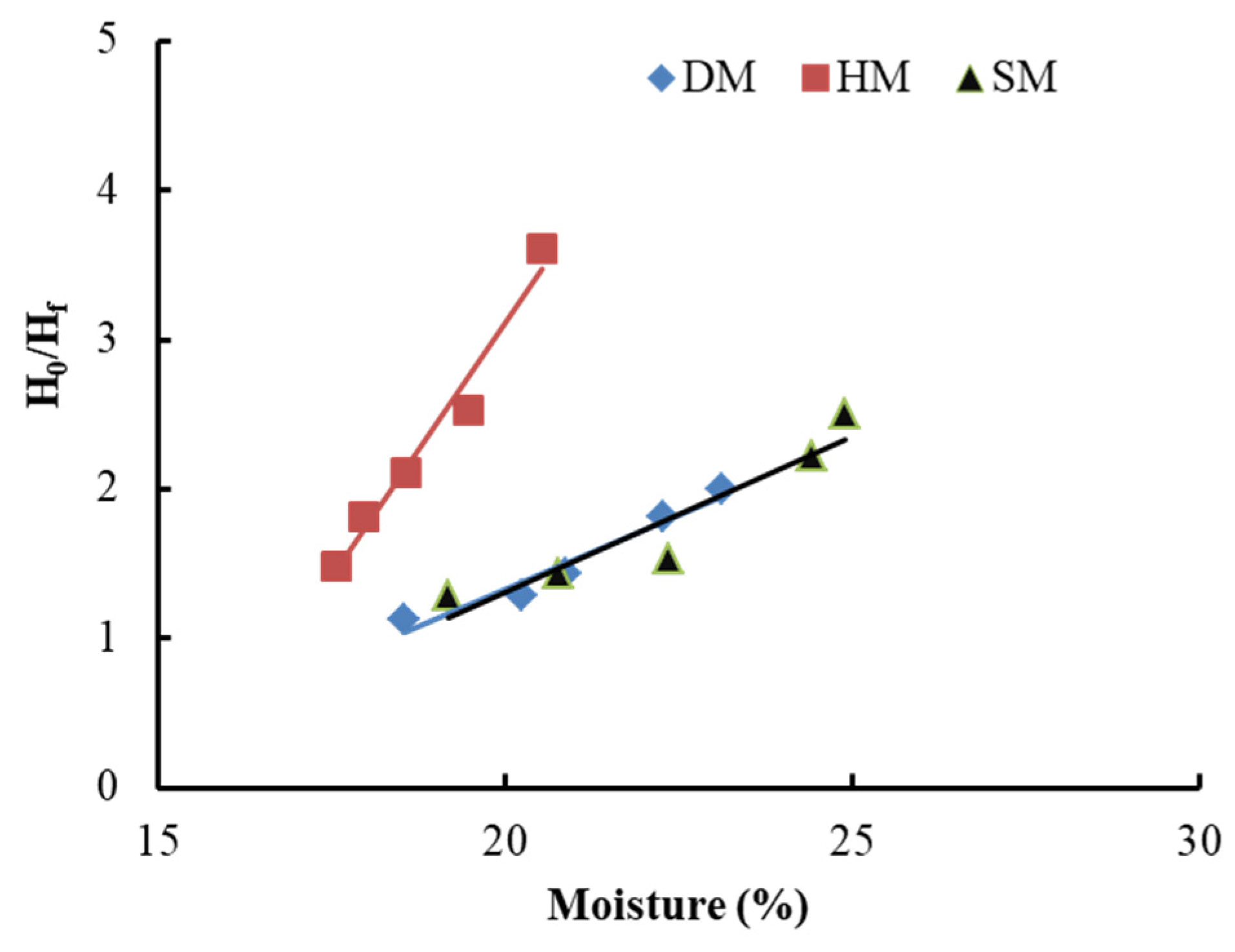
3.7.4. Bigot’s Curves
3.7.5. Reabsorption of Unfired Bricks
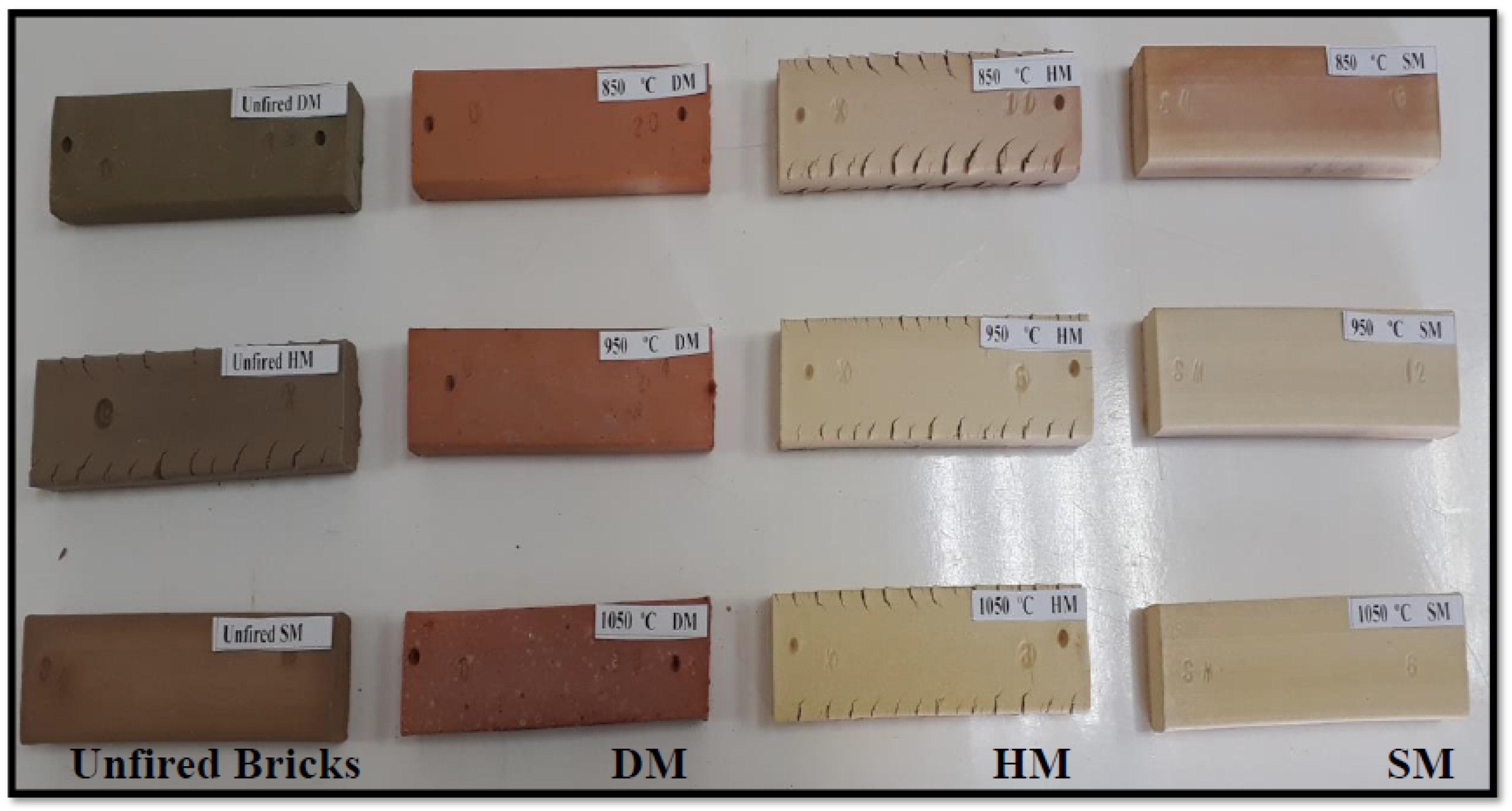
3.7.6. Production of Brick Specimens by Firing
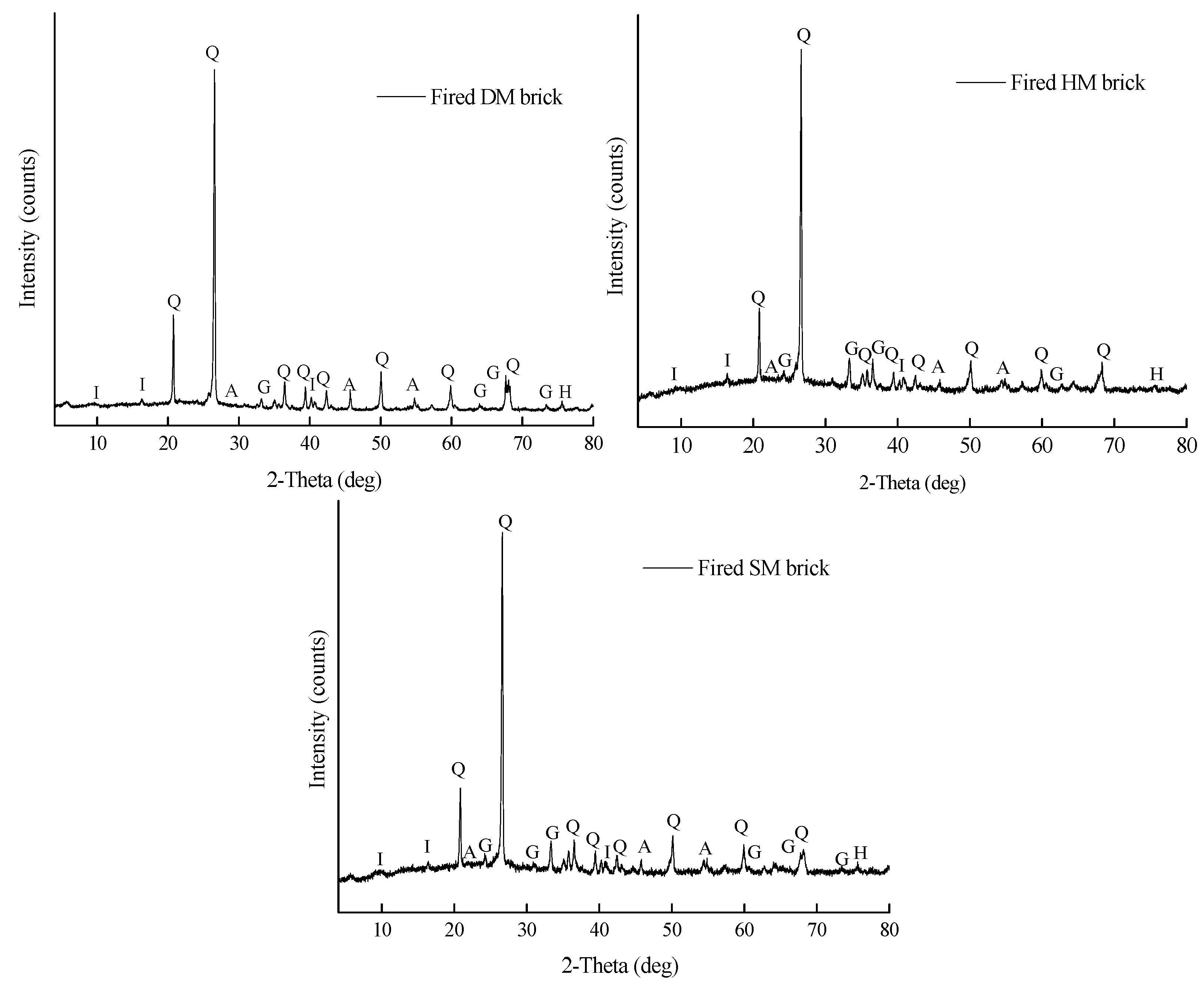
3.7.7. Reactions during Firing and Mineralogy of Fired Brick Specimens
3.7.8. Color and Sound Change of Bricks after Firing
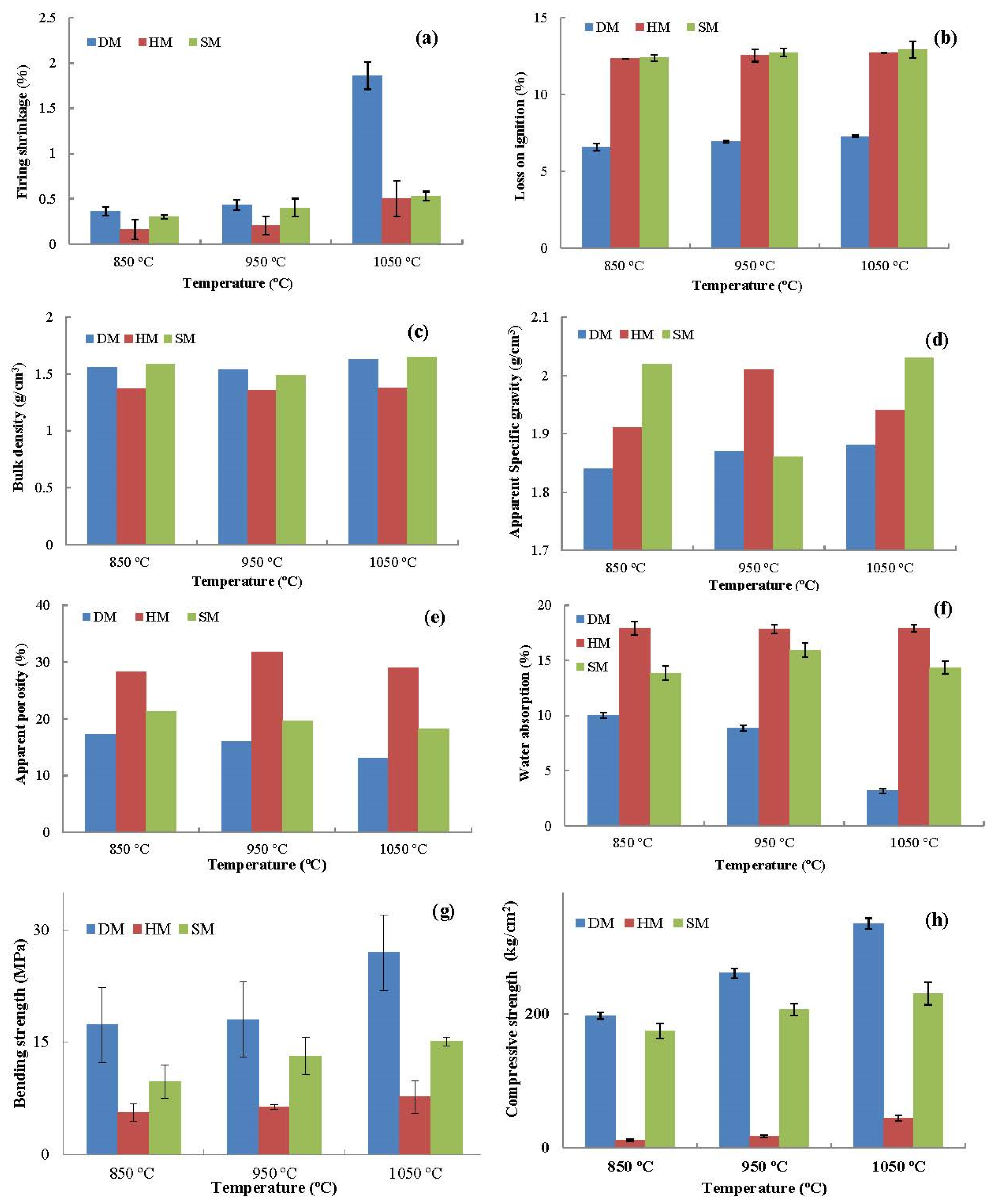
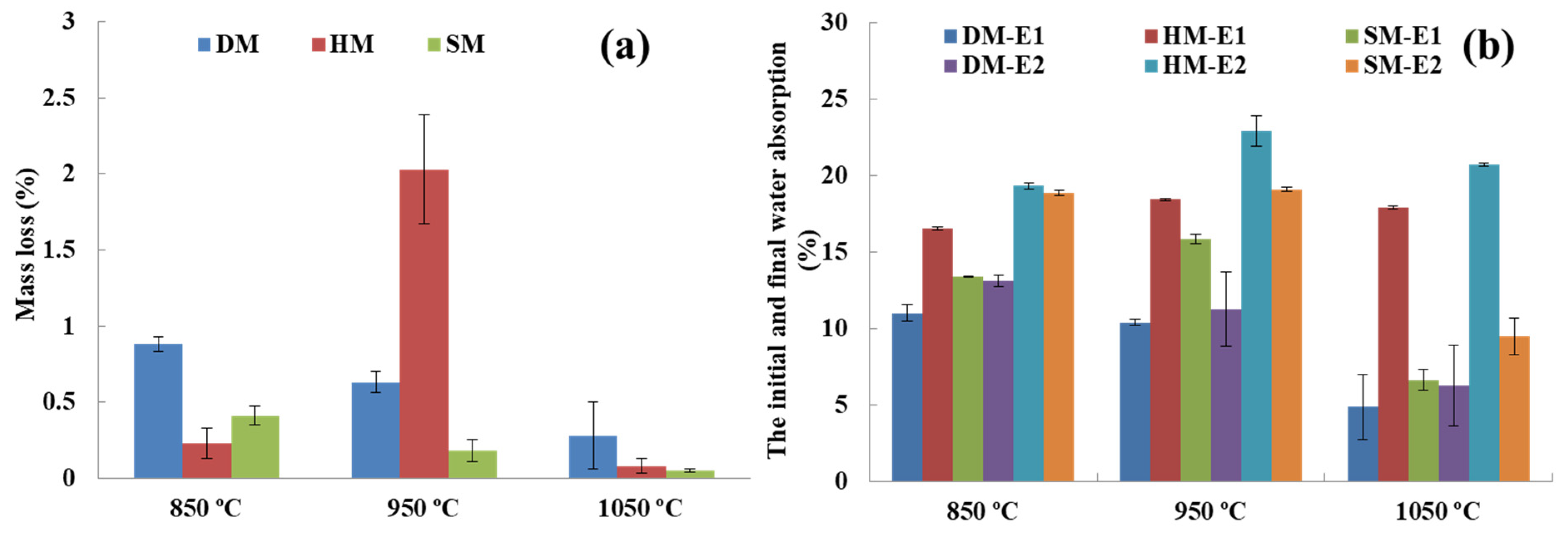
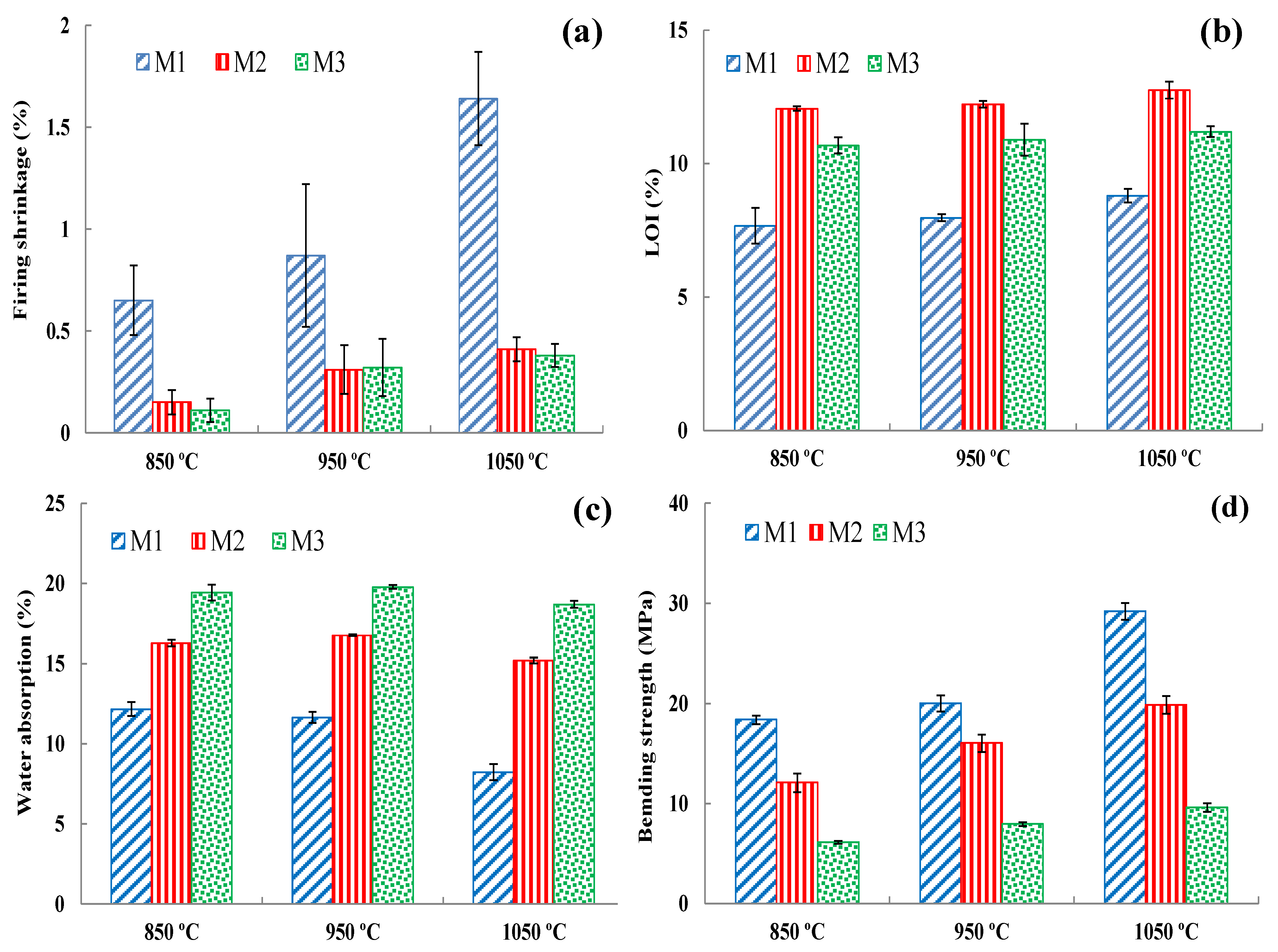
3.7.9. Drying and Firing Shrinkage
3.7.10. Loss on Ignition (LOI)
3.7.11. Apparent Porosity, Apparent Specific Gravity, and Bulk Density
3.7.12. Water Absorption
3.7.13. Bending and Compressive Strength
3.7.14. Optic Analysis
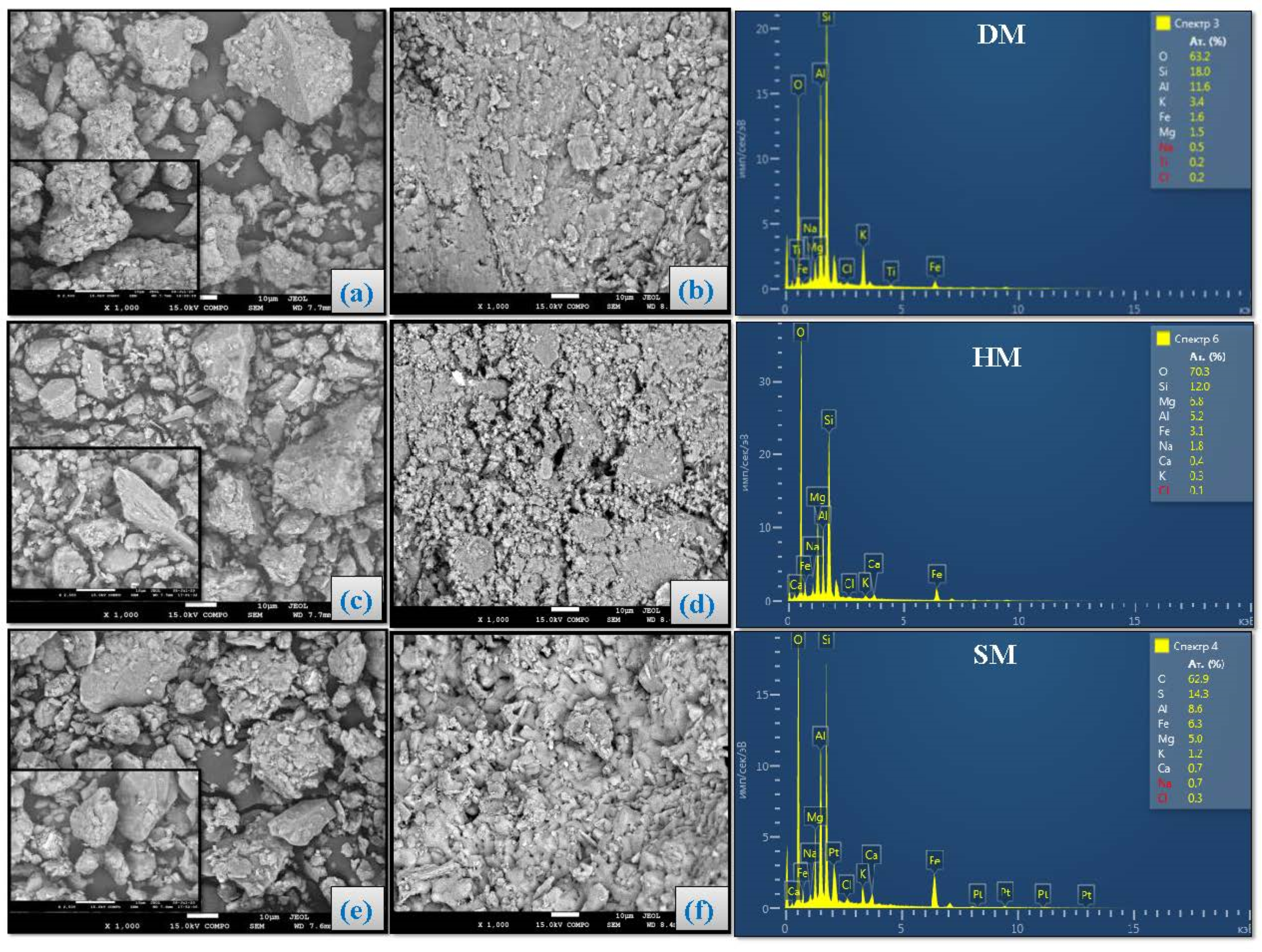
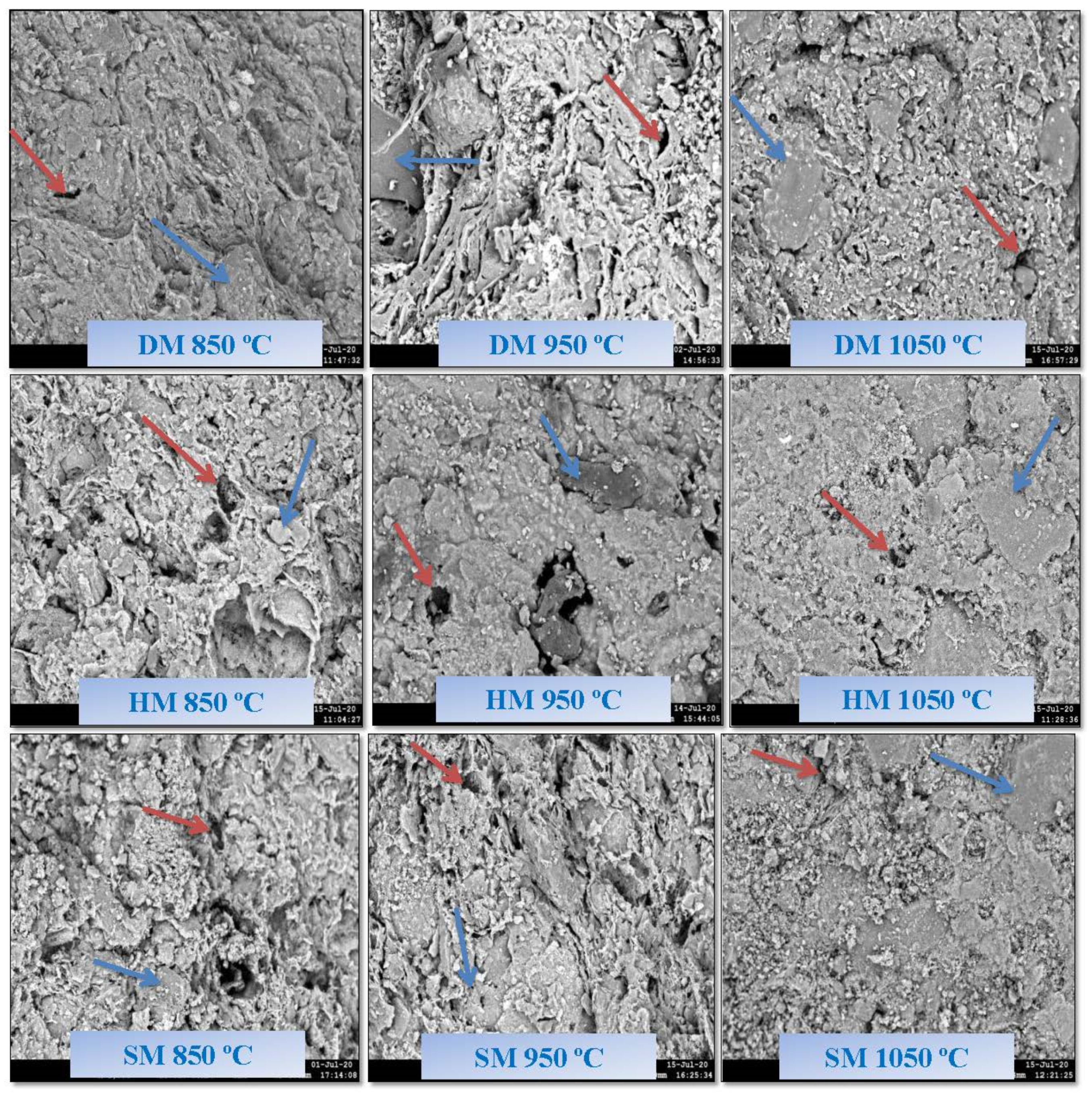
3.8. Scanning Electron Microscopy Analysis of Brick Specimens
3.9. Freeze–Thaw Resistance
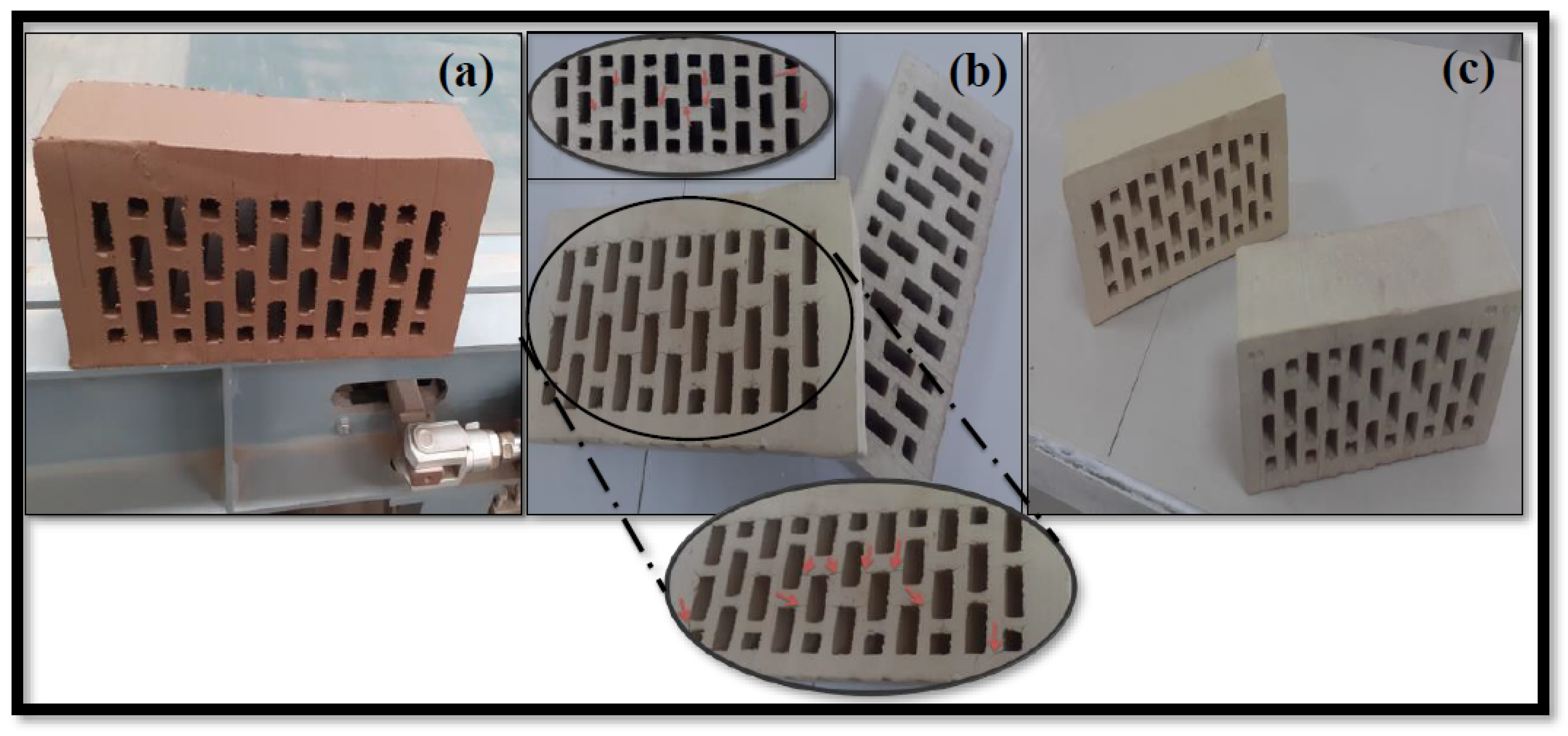
3.10. Semi-industrial Trials of DM, HM, and SM Clay-Based Bricks
3.11. Physical and Mechanical Features of the Mixtures of DM, HM, and SM with Waste Bricks
4. Conclusions
- From the mineralogical analysis, illitic DM, HM, and SM clays were mainly composed of quartz, K-feldspar, and albite, respectively. X-ray spectrum analyses also revealed smectites in all three clays. Other minerals were also detected in DM (bassanite, kaolinite, chlorite, and halite), HM (kaolinite, calcite, smectite, dolomite, and chlorite), and SM (kaolinite, calcite, chlorite, smectite, dolomite, bassanite, and halite). Granulometric and size distribution analysis showed that the DM and SM clays were rich in clay-sized particles and their soil textures were clay and silty clay, respectively. Pfefferkorn analysis showed that these clay types had the highest plasticity values. HM was rich in silt-sized particles, with a prominent amount of total sand (7.34%) and quartz (33.6%). It had lower plasticity than the other clay types (DM and SM).
- The chemical analysis showed that the clays were rich in silica, which was over 50%. Other rich oxides were alumina, ferric oxide (resulting in the reddish color after firing), potassium oxide, and magnesia, respectively. The CaO from calcium carbonate is also observed in SM and HM. The lowest concentration oxides were TiO2, MnO, and P2O3.
- The suitability plot from chemical oxides showed that the illitic DM, HM, and SM clays could be used for producing bricks, but the HM outcome from flux agents (combined effect of Fe2O3, MgO, K2O, and Na2O) for sinterability was out of range, with an 8.90% value. The LOI values resulting from the elimination of organic matter and others for DM, HM, and SM were 7.15%, 12.40%, and 12.31% after firing at 1050 °C, respectively.
- The DM and SM clay-based bricks emerged properly from the extruder and showed high plasticity and dry-bending strength. In contrast, the HM brick specimens displayed cracks (dragon teeth) and lower plasticity and dry-bending strength.
- After firing at different temperatures (850 °C, 950 °C, and 1050 °C), all the brick specimens changed their color and sound (metallic). The bulk density, apparent density, apparent specific gravity, firing shrinkage, mass loss percentage, water absorption, freeze and thaw, bending, and compressive strengths were within the norm (standards) of building brick products. The DM and SM brick specimens had higher bending and compressive strength, while HM demonstrated the lowest strength. The strength values increased with firing temperature in all three clay types. The water absorption and freeze–thaw values of the DM and SM brick specimens were <22% and <3%, indicating that the three clays can be used for brick production. However, HM clay-based bricks displayed cracks on the edges of the bricks after 159 freeze–thaw cycles and the WA% exceeded the 22% threshold value.
- The industrial trials showed positive results for DM and SM, confirming the potential of DM as a raw material in the production of clay-based red bricks and SM as a raw material in the production of clay-based cream and dark beige bricks.
- Based on the study outcomes and observations, it can be concluded that the DM and SM clays can be effectively used for mass scale brick production, leading to energy-efficient, economical, green, and sustainable construction. HM clay could be used by mixing it with clays with high plasticity. If used alone, the brick quality will be inferior.
- Consequently, the WB can be used at optimum ratios, and the usage of WB at optimum ratios by mixing with clays will help to improve the brick features and minimize the negative impacts of their disposal.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Type | CO32− | HCO3− | Cl− | SO42− | Total Anion | Ca2+ | Mg2+ | Na++ K+ | Total Cation | Total Soluble Salt% | pH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mmol/100 g | % | mmol/100 g | % | mmol/100 g | % | mmol/100 g | % | mmol/100 g | % | mmol/100 g | % | mmol/100 g | % | mmol/100 g | % | mmol/100 g | % | |||
| HM | 0.43 | - | 0.55 | 0.034 | 0.55 | 0.02 | 0.69 | 0.033 | 1.79 | 0.086 | 0.55 | 0.011 | 0.30 | 0.004 | 0.940 | 0.022 | 1.79 | 0.036 | 0.13 | 6.8 |
| SM | 2.0 | 0.25 | 0.95 | 0.058 | 0.70 | 0.025 | 0.45 | 0.022 | 4.10 | 0.104 | 0.10 | 0.002 | 0.15 | 0.002 | 1.850 | 0.043 | 2.10 | 0.046 | 0.23 | 7.8 |
| DM | 0.016 | 0.01 | 0.70 | 0.043 | 2.50 | 0.089 | 2.86 | 0.137 | 6.07 | 1.469 | 1.10 | 0.022 | 0.45 | 0.005 | 4.509 | 0.103 | 6.06 | 0.131 | 0.42 | 7.3 |
| DM | HM | SM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T (°C) | 850 | 950 | 1050 | 850 | 950 | 1050 | 850 | 950 | 1050 |
| Brick Colour | Red | Red | Brown | Light coral | Beige | Tan | Reddish | Cream | Dark beige |
References
- Sutcu, M.; Ozturk, S.; Yalamac, E.; Gencel, O. Effect of olive mill waste addition on the properties of porous fi red clay bricks using Taguchi method. J. Environ. Manag. 2016, 181, 185–192. [Google Scholar] [CrossRef]
- Zhang, L. Production of bricks from waste materials—A review. Constr. Build. Mater. 2013, 47, 643–655. [Google Scholar] [CrossRef]
- Vaccari, A. Clays and catalysis: A promising future. Appl. Clay Sci. 1999, 14, 161–198. [Google Scholar] [CrossRef]
- Szewczuk-Karpisz, K.; Tomczyk, A.; Komaniecka, I.; Choma, A.; Adamczuk, A.; Sofińska-Chmiel, W. Impact of sinorhizobium meliloti exopolysaccharide on adsorption and aggregation in the copper(Ii) ions/supporting electrolyte/kaolinite system. Materials 2021, 14, 1950. [Google Scholar] [CrossRef] [PubMed]
- Florian, P.E.; Icriverzi, M.; Ninciuleanu, C.M.; Alexandrescu, E.; Trica, B.; Preda, S.; Ianchis, R.; Roseanu, A. Salecan-clay based polymer nanocomposites for chemotherapeutic drug delivery systems; characterization and in vitro biocompatibility studies. Materials 2020, 13, 5389. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite nanotubes as nanoreactors for heterogeneous micellar catalysis. J. Colloid Interface Sci. 2022, 608, 424–434. [Google Scholar] [CrossRef]
- Nzeugang Nzeukou, A.; Fagel, N.; Njoya, A.; Beyala Kamgang, V.; Eko Medjo, R.; Chinje Melo, U. Mineralogy and physico-chemical properties of alluvial clays from Sanaga valley (Center, Cameroon): Suitability for ceramic application. Appl. Clay Sci. 2013, 83–84, 238–243. [Google Scholar] [CrossRef]
- Valaskova, M. Clays, clay minerals and cordierite ceramics—A review. Ceram. Silik. 2015, 50, 331–340. [Google Scholar]
- Andrade, F.A.; Al-Qureshi, H.A.; Hotza, D. Measuring the plasticity of clays: A review. Appl. Clay Sci. 2011, 51, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, P.; Mendívil, M.A.; Letelier, V.; Morales, M.P. Thermal and mechanical properties of fired clay bricks made by using grapevine shoots as pore forming agent. Influence of particle size and percentage of replacement. Constr. Build. Mater. 2019, 224, 639–658. [Google Scholar] [CrossRef]
- García Ten, J.; Orts, M.J.; Saburit, A.; Silva, G. Thermal conductivity of traditional ceramics. Part I: Influence of bulk density and firing temperature. Ceram. Int. 2010, 36, 1951–1959. [Google Scholar] [CrossRef]
- Sutcu, M.; Erdogmus, E.; Gencel, O.; Gholampour, A.; Atan, E.; Ozbakkaloglu, T. Recycling of bottom ash and fly ash wastes in eco-friendly clay brick production. J. Clean. Prod. 2019, 233, 753–764. [Google Scholar] [CrossRef]
- Madurwar, M.V.; Mandavgane, S.A.; Ralegaonkar, R.V.; Asce, A.M. Development and Feasibility Analysis of Bagasse Ash Bricks. J. Energy Eng. 2013, 141, 04014022. [Google Scholar] [CrossRef]
- El-Mahllawy, M.S.; Kandeel, A.M. Engineering and mineralogical characteristics of stabilized unfired montmorillonitic clay bricks. HBRC J. 2014, 10, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, P.; Morales, M.P.; Mendívil, M.A.; Juárez, M.C.; Muñoz, L. Using of waste pomace from winery industry to improve thermal insulation of fired clay bricks. Eco-friendly way of building construction. Constr. Build. Mater. 2014, 71, 181–187. [Google Scholar] [CrossRef]
- Kadir, A.A.; Sarani, N.A. An Overview of Wastes Recycling in Fired Clay Bricks. Int. J. Integr. Eng. 2012, 4, 53–69. [Google Scholar]
- Yusoff, S.; Abd Rashid, A.F. Adopting the Life Cycle Assessment Approach. In Greening Affordable Housing: An Interactive Approach; CRC Press: Boca Raton, FL, USA, 2019; p. 71. [Google Scholar]
- El Fgaier, F.; Lafhaj, Z.; Brachelet, F.; Antczak, E.; Chapiseau, C. Thermal performance of unfired clay bricks used in construction in the north of France: Case study. Case Stud. Constr. Mater. 2015, 3, 102–111. [Google Scholar] [CrossRef]
- Brunet, M.F.; Ershov, A.V.; Korotaev, M.V.; Melikhov, V.N.; Barrier, E.; Mordvintsev, D.O.; Sidorova, I.P. Late Palaeozoic and Mesozoic evolution of the Amu Darya Basin (Turkmenistan, Uzbekistan). Geol. Soc. Lond. Spec. Publ. 2017, 427, 89–144. [Google Scholar] [CrossRef]
- Cheng, H. Reuse Research Progress on Waste Clay Brick. Procedia Environ. Sci. 2016, 31, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Akinyele, J.O.; Igba, U.T.; Ayorinde, T.O.; Jimoh, P.O. Structural efficiency of burnt clay bricks containing waste crushed glass and polypropylene granules. Case Stud. Constr. Mater. 2020, 13, e00404. [Google Scholar] [CrossRef]
- Khitab, A.; Riaz, M.S.; Jalil, A.; Bilal, R.; Khan, N.; Anwar, W.; Khan, R.A.; Arshad, M.T.; Kirgiz, M.S.; Tariq, Z.; et al. Manufacturing of Clayey Bricks by Synergistic Use of Waste Brick and Ceramic Powders as Partial Replacement of Clay. Sustainability 2021, 15, 10214. [Google Scholar] [CrossRef]
- ASTM. C373-88 Standard Test Method for Water Absorption, Bulk Density, Apparent Porosity, and Apparent Specific Gravity of Fired Whiteware Products; ASTM: West Conshohocken, PA, USA, 2006. [Google Scholar]
- Aouba, L.; Bories, C.; Coutand, M.; Perrin, B.; Lemercier, H. Properties of fired clay bricks with incorporated biomasses: Cases of Olive Stone Flour and Wheat Straw residues. Constr. Build. Mater. 2016, 102, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Moore, D.M.; Reynolds, R. X-ray Diffraction and İdentification and Analysis of Clay Minerals; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- ASTM. Standard Test Methods for Sampling and Testing Brick and Structural Clay Tile; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Pfefferkorn, K. Ein Beitrag zur Bestimmung der Plastizität in Tonen und Kaolinen. Sprechsaal 1924, 57, 297–299. [Google Scholar]
- Amorós, J.L.; Sánchez, E.; García-Ten, J.S.V. Manual para el Control de Calidad de Materias Primas Arcillosas; Instituto de Tecnología Cerámica: Castellón de la Plana, Spain, 1998. [Google Scholar]
- Hamza, A.; Kocserha, I.; Géber, R.; Buzimov, A. Plasticity of Red Mud and Clay Mixtures. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; p. 012051. [Google Scholar]
- De, J.A.; Lorite, M.; Jiménez, J.; Castro, E. Valorisation of wastewater from two-phase olive oil extraction in fired clay brick production. J. Hazard. Mater. 2009, 169, 271–278. [Google Scholar] [CrossRef]
- Klosek-Wawrzyn, E.; Malolepszy, J.; Murzyn, P. Sintering behavior of kaolin with calcite. Procedia Eng. 2013, 57, 572–582. [Google Scholar] [CrossRef] [Green Version]
- Abu Bakar, B.H.; Wan Ibrahim, M.H.; Megat Johari, M.A. A review: Durability of fired clay brick mansory wall due to salt attack. Int. J. Integr. Enginnering 2011, 1, 111–127. [Google Scholar]
- Dondi, M.; Ercolani, G.; Fabbri, B. Concentration of pollutant elements (F, S, Cl) into the clays used by the Italian brick industry. Clays: Controlling the Environment. In Proceedings of the 10th International Clay Conference, Adelaide, Australia, 18–23 July 1993; pp. 79–83. [Google Scholar]
- Hu, H.; Zhou, K.; Meng, K.; Song, L.; Lin, Q. Effects of SiO2/Al2O3 ratios on sintering characteristics of synthetic coal ash. Energies 2017, 10, 242. [Google Scholar] [CrossRef] [Green Version]
- Loutou, M.; Taha, Y.; Benzaazoua, M.; Daa, Y.; Hakkou, R. Valorization of clay by-product from moroccan phosphate mines for the production of fi red bricks. J. Clean. Prod. 2019, 229, 169–179. [Google Scholar] [CrossRef]
- Kazmi, S.M.S.; Abbas, S.; Nehdi, M.L.; Saleem, M.A.; Munir, M.J. Feasibility of using waste glass sludge in production of ecofriendly clay bricks. J. Mater. Civ. Eng. 2017, 29, 04017056. [Google Scholar] [CrossRef]
- Munir, M.J.; Kazmi, S.M.S.; Wu, Y.F.; Hanif, A.; Khan, M.U.A. Thermally efficient fired clay bricks incorporating waste marble sludge: An industrial-scale study. J. Clean. Prod. 2018, 174, 1122–1135. [Google Scholar] [CrossRef]
- Lahcen, D.; Hicham, E.E.; Latifa, S.; Abderrahmane, A.; Jamal, B.; Mohamed, W.; Meriam, E.; Nathalie, F. Characteristics and ceramic properties of clayey materials from Amezmiz region (Western High Atlas, Morocco). Appl. Clay Sci. 2014, 102, 139–147. [Google Scholar] [CrossRef]
- Ferrari, S.; Gualtieri, A.F. The use of illitic clays in the production of stoneware tile ceramics. Appl. Clay Sci. 2006, 32, 73–81. [Google Scholar] [CrossRef]
- Moreno-Maroto, J.M.; Uceda-Rodríguez, M.; Cobo-Ceacero, C.J.; Cotes-Palomino, T.; Martínez-García, C.; Alonso-Azcárate, J. Studying the feasibility of a selection of Southern European ceramic clays for the production of lightweight aggregates. Constr. Build. Mater. 2020, 237, 117583. [Google Scholar] [CrossRef]
- Abdelmalek, B.; Rekia, B.; Youcef, B.; Lakhdar, B.; Nathalie, F. Mineralogical characterization of Neogene clay areas from the Jijel basin for ceramic purposes (NE Algeria-Africa). Appl. Clay Sci. 2017, 136, 176–183. [Google Scholar] [CrossRef]
- Temga, J.P.; Mache, J.R.; Madi, A.B.; Nguetnkam, J.P.; Bitom, D.L. Ceramics applications of clay in Lake Chad Basin, Central Africa. Appl. Clay Sci. 2019, 171, 118–132. [Google Scholar] [CrossRef]
- Diko, M.L.; Ekosse, G.E.; Ayonghe, S.N.; Ntasin, E.B. Physical characterization of clayey materials from tertiary volcanic cones in Limbe (Cameroon) for ceramic applications. Appl. Clay Sci. 2011, 51, 380–384. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Felipe-Sesé, M.A.; López-Pérez, J.A.; Infantes-Molina, A. Characterization and evaluation of rice husk ash and wood ash in sustainable clay matrix bricks. Ceram. Int. 2017, 43, 463–475. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; Hamdan, S.; Van Deventer, J.S.J. Microstructural changes in alkali activated fly ash/slag geopolymers with sulfate exposure. Mater. Struct. Mater. Constr. 2013, 46, 361–373. [Google Scholar] [CrossRef]
- Tarhan, M.; Tarhan, B.; Aydin, T. The effects of fine fire clay sanitaryware wastes on ceramic wall tiles. Ceram. Int. 2016, 42, 17110–17115. [Google Scholar] [CrossRef]
- Escalera, E.; Tegman, R.; Antti, M.L.; Odén, M. High temperature phase evolution of Bolivian kaolinitic-illitic clays heated to 1250 °C. Appl. Clay Sci. 2014, 101, 100–105. [Google Scholar] [CrossRef]
- De Andrade, F.A.; Al-Qureshi, H.A.; Hotza, D. Measuring and modeling the plasticity of clays. Mater. Res. 2010, 13, 395–399. [Google Scholar] [CrossRef]
- Feng, T.W. Using a small ring and a fall-cone to determine the plastic limit. J. Geotech. Geoenvironmental Eng. 2004, 130, 630–635. [Google Scholar] [CrossRef]
- Akinshipe, O.; Kornelius, G. Chemical and Thermodynamic Processes in Clay Brick Firing Technologies and Associated Atmospheric Emissions Metrics—A Review. J. Pollut. Eff. Control 2017, 5, 1–12. [Google Scholar] [CrossRef]
- González-García, F.; Romero-Acosta, V.; García-Ramos, G.; González-Rodríguez, M. Firing transformations of mixtures of clays containing illite, kaolinite and calcium carbonate used by ornamental tile industries. Appl. Clay Sci. 1990, 5, 361–375. [Google Scholar] [CrossRef]
- Sutcu, M.; Alptekin, H.; Erdogmus, E.; Er, Y.; Gencel, O. Characteristics of fired clay bricks with waste marble powder addition as building materials. Constr. Build. Materıals 2015, 82, 1–8. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Leite-Costa, J. Use of bottom ash from olive pomace combustion in the production of eco-friendly fired clay bricks. Waste Manag. 2016, 48, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Emrullahoglu Abi, C.B. Effect of borogypsum on brick properties. Constr. Build. Mater. 2014, 59, 195–203. [Google Scholar] [CrossRef]
- Baccour, H.; Medhioub, M.; Jamoussi, F.; Mhiri, T.; Daoud, A. Mineralogical evaluation and industrial applications of the Triassic clay deposits, Southern Tunisia. Mater. Charact. 2008, 59, 1613–1622. [Google Scholar] [CrossRef]
- Artigas, R.; Rodas, M.; Sánchez, C.J.; Mas, R.; Dondi, M.; Arribas, J. Clayey materials from the Sierra de la Demanda Range (Spain): Their potential as raw materials for the building ceramics industry. Clay Miner. 2005, 40, 25–41. [Google Scholar] [CrossRef] [Green Version]
- Semiz, B. Characteristics of clay-rich raw materials for ceramic applications in Denizli region (Western Anatolia). Appl. Clay Sci. 2017, 137, 83–93. [Google Scholar] [CrossRef]
- Demir, I. Reuse of waste glass in building brick production. Waste Manag. Res. 2009, 27, 572–577. [Google Scholar] [CrossRef]
- Vieira, C.M.F.; Amaral, L.F.; Monteiro, S.N. Recycling of Steelmaking Plant Wastes in Clay Bricks. In Current Topics in the Utilization of Clay in Industrial and Medical Applications; InTech Open: Rijeka, Croatia, 2018; pp. 25–43. [Google Scholar]
- Kazmi, S.M.S.; Abbas, S.; Saleem, M.A.; Munir, M.J.; Khitab, A. Manufacturing of sustainable clay bricks: Utilization of waste sugarcane bagasse and rice husk ashes. Constr. Build. Mater. 2016, 120, 29–41. [Google Scholar] [CrossRef]
- Terrones-Saeta, J.M.; Suárez-Macías, J.; Iglesias-Godino, F.J.; Corpas-Iglesias, F.A. Study of the incorporation of biomass bottom ashes in ceramic materials for the manufacture of bricks and evaluation of their leachates. Materials 2020, 13, 2099. [Google Scholar] [CrossRef] [PubMed]
- Javed, U.; Arsalan, R.; Ali, S.; Jalal, F.E.; Saeed, M. Sustainable incorporation of lime-bentonite clay composite for production of ecofriendly bricks. J. Clean. Prod. 2020, 263, 121469. [Google Scholar] [CrossRef]
- Khalfaoui, A.; Hajjaji, M. A Chloritic-illitic clay from Morocco: Temperature-time-transformation and neoformation. Appl. Clay Sci. 2009, 45, 83–89. [Google Scholar] [CrossRef]
- Cultrone, G.; Sebastián, E.; Elert, K.; de la Torre, M.J.; Cazalla, O.; Rodriguez-Navarro, C. Influence of mineralogy and firing temperature on the porosity of bricks. J. Eur. Ceram. Soc. 2004, 24, 547–564. [Google Scholar] [CrossRef]
- Cultrone, G.; Sebastián, E.; De La Torre, M.J. Mineralogical and physical behaviour of solid bricks with additives. Constr. Build. Mater. 2005, 19, 39–48. [Google Scholar] [CrossRef]
- Mallidi, S.R. Application of mercury intrusion porosimetry on clay bricks to assess freeze-thaw durability—A bibliography with abstracts. Constr. Build. Mater. 1996, 10, 461–465. [Google Scholar] [CrossRef]
- Seaverson, E.J.; Brosnan, D.A.; Frederic, J.C.; Sanders, J.P. Predicting the freeze-thaw durability of bricks based on residual expansion. In Masonry: Opportunities for the 21st Century; ASTM International: West Conshohocken, PA, USA, 2002. [Google Scholar]
- Chidiac, S.E.; Federico, L.M. Effects of waste glass additions on the properties and durability of fired clay brick. Can. J. Civ. Eng. 2007, 34, 1458–1466. [Google Scholar] [CrossRef]
- Wiemes, L.; Pawlowsky, U.; Mymrin, V. Incorporation of industrial wastes as raw materials in brick’s formulation. J. Clean. Prod. 2017, 142, 69–77. [Google Scholar] [CrossRef]
- Okunade, E.A. The effect of wood ash and sawdust admixtures on the engineering properties of a burnt laterite-clay brick. J. Appl. Sci. 2008, 8, 1042–1048. [Google Scholar] [CrossRef] [Green Version]
- Zouaoui, H.; Bouaziz, J. Physical and mechanical properties improvement of a porous clay ceramic. Appl. Clay Sci. 2017, 150, 131–137. [Google Scholar] [CrossRef]
- Garzanti, E.; Ghassemi, M.R.; Limonta, M.; Resentini, A. Provenance of karakum desert sand (Turkmenistan): Lithic-rich orogenic signature of central Asian dune fields. Riv. Ital. Paleontol. Stratigr. 2019, 125, 77–89. [Google Scholar] [CrossRef]





| Sample | <80 µm | >80 µm | Total | CaCO3 | |||
|---|---|---|---|---|---|---|---|
| in gr | in % | in gr | in % | in gr | in % | in % | |
| DM | 133.98 | 94.43 | 7.91 | 5.57 | 141.89 | 100 | 1–1.5 |
| HM | 138.29 | 93.8 | 9.14 | 6.2 | 147.43 | 100 | 10.5 |
| SM | 128.29 | 86.71 | 19.66 | 13.29 | 147.95 | 100 | 12.90 |
| DM (%) | HM (%) | SM (%) | |
|---|---|---|---|
| Physical properties | |||
| Clay-sized particles (<2 µm) | 69 | 40 | 49 |
| Silt-sized particles: (2–50 µm) | 29 | 39 | 39 |
| Sand-sized particles: (>50 µm) | 2 | 21 | 12 |
| Chemical composition (%) | |||
| SiO2 | 57.39 | 50.20 | 53.09 |
| Al2O3 | 16.90 | 14.70 | 12.30 |
| Fe2O3 | 6.28 | 2.69 | 5.55 |
| K2O | 3.52 | 1.76 | 2.91 |
| Na2O | 1.87 | 1.98 | 2.21 |
| MgO | 2.23 | 2.47 | 2.35 |
| TiO2 | 0.78 | – | – |
| P2O5 | 0.23 | 0.19 | 0.12 |
| CaO | 2.15 | 12.70 | 8.55 |
| MnO | 0.23 | 0.19 | 0.21 |
| LOI (1050 °C) | 7.15 | 12.40 | 12.31 |
| Total (oxides) | 99.47 | 99.53 | 99.58 |
| Total C (%) | 0.12 | 1.73 | 2.08 |
| Total S | 0.3 | – | 0.07 |
| Mineralogical properties | |||
| Illite | +++++ | +++ | ++++ |
| Quartz | +++ | ++++ | +++ |
| K-Feldspar | +++ | +++ | +++ |
| Albite | – | + | – |
| Calcite | + | ++ | ++ |
| Chlorite | + | + | + |
| Dolomite | – | + | + |
| Smectite | + | + | + |
| Kaolinite | ++ | ++ | + |
| Bassanite | + | – | + |
| Halite | + | – | + |
| Type | DM | HM | SM |
|---|---|---|---|
| D10 (µm) | 0.154 | 4.05 | 0.158 |
| D50 (µm) | 0.369 | 30.7 | 1.48 |
| D90 (µm) | 22.9 | - | 15.3 |
| Specific surface area (m²/kg) | 12,840 | 7896 | 11,150 |
| Clay-sized (<2 μm) | 62.17 | 39.06 | 53.6 |
| Silt-sized (2–50 μm) | 32.35 | 53.6 | 44.34 |
| Very fine sand (50–100 μm) | 0.78 | 3.32 | 0.81 |
| Fine sand (100–250 μm) | 0.03 | 0.08 | 0.13 |
| Medium sand (250–500 μm) | 1.59 | 1.05 | 0.12 |
| Coarse sand (500–1000 μm) | 2.92 | 2.8 | 0 |
| Very coarse sand (1000–2000 μm) | 0.16 | 0.09 | 0 |
| Total sand (50–2000 μm) | 5.49 | 7.34 | 2.07 |
| Soil texture | Clay | Silty clay loam | Silty clay |
| Samples | Thermal Expansion Coefficient (α) 10−6 K−1 | |
|---|---|---|
| 300 °C | 600 °C | |
| DM | 8.34 | 18.72 |
| HM | 22.92 | 31.27 |
| SM | 9.71 | 24.34 |
| DM | HM | SM | |
|---|---|---|---|
| Color of clay | Greenish | Dark beige | Red |
| Moisture content% | 19 | 17 | 18 |
| PPI | 29.45 | 19.04 | 28.74 |
| Penetrometer consistency | 2.2 | 2.1 | 2.3 |
| Plasticity (Ps) | 19.12 ± 0.23 | 16.30 ± 0.15 | 16.42 ± 0.41 |
| Readsorption (%) | 5.47 ± 0.2 | 2.86 ± 0.2 | 5.46 ± 0.78 |
| Unfired specimen bending strength (MPa) | 6.16 ± 0.77 | 2.04 ± 0.21 | 3.99 ± 0.2 |
| Compressive strength unfired (kg/cm2) | 44.2 | 7.5 | 41 |
| Drying Shrinkage (%) | 6.66 ± 0.45 | 2.04 ± 0.21 | 3.99 ± 0.2 |
| LOI (%) by TGA | 10.82% | 10.15% | 12.75% |
| Samples | Drying Shrinkage (%) | Temperature (°C) | Firing Shrinkage (%) | LOI % | Water Absorption (%) | Bending Strength (MPa) |
|---|---|---|---|---|---|---|
| DM | 3.95 ± 0.2 | 950 | 1.3 ± 0.05 | 5.4 ± 0.3 | 8.5 ± 0.3 | 16.43 ± 0.2 |
| HM | 2.35 ± 0.05 | 950 | 0.15 ± 0.01 | 16.4 ± 1.2 | 17.75 ± 1.0 | 6.07 ± 0.6 |
| SM | 3.28 ± 0.1 | 950 | 0.39 ± 0.05 | 13.8 ± 0.5 | 13.76 ± 0.7 | 12.9 ± 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korpayev, S.; Bayramov, M.; Durdyev, S.; Hamrayev, H. Characterization of Three Amu-Darya Basin Clays in Ceramic Brick Industry and Their Applications with Brick Waste. Materials 2021, 14, 7471. https://doi.org/10.3390/ma14237471
Korpayev S, Bayramov M, Durdyev S, Hamrayev H. Characterization of Three Amu-Darya Basin Clays in Ceramic Brick Industry and Their Applications with Brick Waste. Materials. 2021; 14(23):7471. https://doi.org/10.3390/ma14237471
Chicago/Turabian StyleKorpayev, Serdar, Meretdurdy Bayramov, Serdar Durdyev, and Hemra Hamrayev. 2021. "Characterization of Three Amu-Darya Basin Clays in Ceramic Brick Industry and Their Applications with Brick Waste" Materials 14, no. 23: 7471. https://doi.org/10.3390/ma14237471
APA StyleKorpayev, S., Bayramov, M., Durdyev, S., & Hamrayev, H. (2021). Characterization of Three Amu-Darya Basin Clays in Ceramic Brick Industry and Their Applications with Brick Waste. Materials, 14(23), 7471. https://doi.org/10.3390/ma14237471







