Influence of Natural Polysaccharides on Properties of the Biomicroconcrete-Type Bioceramics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
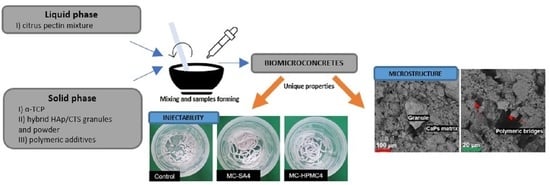
2.2.1. Injectability and Setting Times
2.2.2. Structural Studies
2.2.3. Microstructure
2.2.4. Mechanical Strength
2.2.5. Chemical Stability and Bioactivity In Vitro
2.2.6. Statistics
3. Results and Discussion
3.1. Injectability and Setting Times
3.2. Structural Studies
3.3. Microstructure
3.4. Mechanical Strength
3.5. Chemical Stability and Bioactivity In Vitro
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kokubo, T. Bioceramics and Their Clinical Applications; Woodhead Publishing: Cambridge, UK, 2008; ISBN 9781845692049. [Google Scholar]
- Phillips, M.; Stonoga, Z.J.K. Bone disease. In Orthopaedic Bone Cements; Woodhead Publishing: Cambridge, UK, 2008; pp. 3–40. ISBN 9781845693763. [Google Scholar]
- Ambard, A.J.; Mueninghoff, L. Calcium phosphate cement: Review of mechanical and biological properties. J. Prosthodont. 2006, 15, 321–328. [Google Scholar] [CrossRef]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Puscaselu, R.G.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From food industry to biomedical applications and management of metabolic disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 1–22, Erratum in 2020, 14, 8. [Google Scholar] [CrossRef]
- Mazur, K.; Buchner, R.; Bonn, M.; Hunger, J. Hydration of sodium alginate in aqueous solution. Macromolecules 2014, 47, 771–776. [Google Scholar] [CrossRef]
- Lee, G.S.; Park, J.H.; Won, J.E.; Shin, U.S.; Kim, H.W. Alginate combined calcium phosphate cements: Mechanical properties and in vitro rat bone marrow stromal cell responses. J. Mater. Sci. Mater. Med. 2011, 22, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Štulajterová, R.; Medvecký, L.; Giretová, M.; Sopčák, T.; Briančin, J. Influence of Sodium Alginate on Properties of Tetracalcium Phosphate/Nanomonetite Biocement. Powder Metall. Prog. 2020, 19, 1–11. [Google Scholar] [CrossRef]
- Devjak Novak, S.; Šporar, E.; Baumgartner, S.; Vrečer, F. Characterization of physicochemical properties of hydroxypropyl methylcellulose (HPMC) type 2208 and their influence on prolonged drug release from matrix tablets. J. Pharm. Biomed. Anal. 2012, 66, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Burguera, E.F.; Xu, H.H.K.; Weir, M.D. Injectable and rapid-setting calcium phosphate bone cement with dicalcium phosphate dihydrate. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 77, 126–134. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Rethore, G.; Khairoun, K.; Pilet, P.; Tancret, F.; Bouler, J.M.; Weiss, P. A novel injectable, cohesive and toughened Si-HPMC (silanized-hydroxypropyl methylcellulose) composite calcium phosphate cement for bone substitution. Acta Biomater. 2014, 10, 3335–3345. [Google Scholar] [CrossRef]
- Adrian, G.; Mihai, M.; Vodnar, D.C. The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector- Biocompatibility, Bioadheviveness, and Biodegradability. Polymers 2019, 11, 1837. [Google Scholar]
- Vunain, E.; Mishra, A.K.; Mamba, B.B. Fundamentals of Chitosan for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1, ISBN 9780081002575. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Du, S.; Lu, Y.; Li, Y.; Wang, D. The application of biomedical polymer material hydroxy propyl methyl cellulose(HPMC) in pharmaceutical preparations. J. Chem. Pharm. Res. 2014, 6, 155–160. [Google Scholar]
- Zima, A.; Czechowska, J.; Szponder, T.; Ślósarczyk, A. In vivo behavior of biomicroconcretes based on α-tricalcium phosphate and hybrid hydroxyapatite/chitosan granules and sodium alginate. J. Biomed. Mater. Res. Part A 2020, 108, 1243–1255. [Google Scholar] [CrossRef]
- Czechowska, J.; Zima, A.; Ślósarczyk, A. Comparative study on physicochemical properties of alpha-TCP / calcium sulphate dihydrate biomicroconcretes containing chitosan, sodium alginate or methylcellulose. Acta Bioeng. Biomech. 2020, 22. [Google Scholar] [CrossRef]
- Hasan, M.L.; Kim, B.; Padalhin, A.R.; Faruq, O.; Sultana, T.; Lee, B.T. In vitro and in vivo evaluation of bioglass microspheres incorporated brushite cement for bone regeneration. Mater. Sci. Eng. C 2019, 103, 109775. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Dong, L.; Yuan, Y.; Jiang, Q. In vitro and in vivo analysis of the biocompatibility of two novel and injectable calcium phosphate cements. Regen. Biomater. 2019, 6, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Shi, H.; Ye, J. Effect of PLGA/lecithin hybrid microspheres and β-tricalcium phosphate granules on the physicochemical properties, in vitro degradation and biocompatibility of calcium phosphate cement. RSC Adv. 2015, 5, 47749–47756. [Google Scholar] [CrossRef]
- Nezafati, N.; Farokhi, M.; Heydari, M.; Hesaraki, S.; Nasab, N.A. In vitro bioactivity and cytocompatablity of an injectable calcium phosphate cement/silanated gelatin microsphere composite bone cement. Compos. Part B Eng. 2019, 175, 107146. [Google Scholar] [CrossRef]
- Marudova, M.; MacDougall, A.J.; Ring, S.G. Pectin-chitosan interactions and gel formation. Carbohydr. Res. 2004, 339, 1933–1939. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, J.; Liang, X.; Cao, Y.; Wang, S.; Zhang, L. High strength chitosan hydrogels with biocompatibility via new avenue based on constructing nanofibrous architecture. Macromolecules 2015, 48, 2706–2714. [Google Scholar] [CrossRef]
- Neufeld, L.; Bianco-Peled, H. Pectin–chitosan physical hydrogels as potential drug delivery vehicles. Int. J. Biol. Macromol. 2017, 101, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Dziadek, M.; Zima, A.; Cichoń, E.; Czechowska, J.; Ślósarczyk, A. Biomicroconcretes based on the hybrid HAp/CTS granules, α-TCP and pectins as a novel injectable bone substitutes. Mater. Lett. 2020, 265, 127457. [Google Scholar] [CrossRef]
- Chacón-Cerdas, R.; Medaglia-Mata, A.; Flores-Mora, D.; Starbird-Pérez, R. Synthesis of chitosan, pectin, and chitosan/pectin microspheres by two water-in-oil emulsion crosslinking methods. Chem. Pap. 2020, 74, 509–520. [Google Scholar] [CrossRef]
- Andriani, Y. Retracted-Glutaraldehyde-Crosslinked Chitosan-Pectin Nanoparticles as a Potential Carrier for Curcumin Delivery and Its In Vitro Release Study. Int. J. Drug Deliv. 2015, 7, 167–173. [Google Scholar] [CrossRef]
- Kolmas, J.; Kaflak, A.; Zima, A.; Ślósarczyk, A. Alpha-tricalcium phosphate synthesized by two different routes: Structural and spectroscopic characterization. Ceram. Int. 2015, 41, 5727–5733. [Google Scholar] [CrossRef]
- Czechowska, J.; Zima, A.; Paszkiewicz, Z.; Lis, J.; Ślósarczyk, A. Physicochemical properties and biomimetic behaviour of α-TCP-chitosan based materials. Ceram. Int. 2014, 40, 5523–5532. [Google Scholar] [CrossRef]
- Zima, A. Hydroxyapatite-chitosan based bioactive hybrid biomaterials with improved mechanical strength. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 193, 175–184. [Google Scholar] [CrossRef]
- Standard Test Method for Time of Setting of Hydraulic-Cement Paste by Gillmore Needles. Available online: https://standards.globalspec.com/std/14347806/astm-c266-20 (accessed on 6 December 2021).
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Douglas, T.E.L.; Dziadek, M.; Schietse, J.; Boone, M.; Declercq, H.A.; Coenye, T.; Vanhoorne, V.; Vervaet, C.; Balcaen, L.; Buchweitz, M.; et al. Pectin-bioactive glass self-gelling, injectable composites with high antibacterial activity. Carbohydr. Polym. 2019, 205, 427–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Z.; Zhai, Q.; Hu, M.; Cao, C.; Wang, J.; Yang, H.; Li, B. Bone cements for percutaneous vertebroplasty and balloon kyphoplasty: Current status and future developments. J. Orthop. Transl. 2015, 3, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durucan, C.; Brown, P.W. Reactivity of α-tricalcium phosphate. J. Mater. Sci. 2002, 37, 963–969. [Google Scholar] [CrossRef]
- Rabiee, S.M.; Baseri, H. Prediction of the setting properties of calcium phosphate bone cement. Comput. Intell. Neurosci. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Calcium Phosphate Bone Cements. Available online: https://www.intechopen.com/chapters/61543 (accessed on 6 December 2021).
- Ewald, A.; Hösel, D.; Patel, S.; Grover, L.M.; Barralet, J.E.; Gbureck, U. Silver-doped calcium phosphate cements with antimicrobial activity. Acta Biomater. 2011, 7, 4064–4070. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.P.; Hollinger, J.O.; Milam, S.B. Reconstruction of bone using calcium phosphate bone cements: A critical review. J. Oral Maxillofac. Surg. 1999, 57, 1122–1126. [Google Scholar] [CrossRef]
- Komath, M.; Varma, H.K.; Sivakumar, R. On the development of an apatitic calcium phosphate bone cement. Bull. Mater. Sci. 2000, 23, 135–140. [Google Scholar] [CrossRef]
- Christel, T.; Kuhlmann, M.; Vorndran, E.; Groll, J.; Gbureck, U. Dual setting α-tricalcium phosphate cements. J. Mater. Sci. Mater. Med. 2013, 24, 573–581. [Google Scholar] [CrossRef]
- Thibault, J.-F.; Ralet, M.-C. Pectins, their Origin, Structure and Functions. Adv. Diet. Fibre Technol. 2008, 32, 367–378. [Google Scholar] [CrossRef]
- Taha, M.O.; Aiedeh, K.M.; Al-Hiari, Y.; Al-Khatib, H. Synthesis of zinc-crosslinked thiolated alginic acid beads and their in vitro evaluation as potential enteric delivery system with folic acid as model drug. Pharmazie 2005, 60, 736–742. [Google Scholar] [PubMed]
- Bashir, S.; Zafar, N.; Lebaz, N.; Mahmood, A.; Elaissari, A. Hydroxypropyl methylcellulose-based hydrogel copolymeric for controlled delivery of galantamine hydrobromide in Dementia. Processes 2020, 8, 1350. [Google Scholar] [CrossRef]
- Mickiewicz, R.A.; Mayes, A.M.; Knaack, D. Polymer-calcium phosphate cement composites for bone substitutes. J. Biomed. Mater. Res. 2002, 61, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Belalia, F.; Djelali, N.E. Rheological properties of sodium alginate solutions. Rev. Roum. Chim. 2014, 59, 135–145. [Google Scholar]
- Silva, S.M.C.; Pinto, F.V.; Antunes, F.E.; Miguel, M.G.; Sousa, J.J.S.; Pais, A.A.C.C. Aggregation and gelation in hydroxypropylmethyl cellulose aqueous solutions. J. Colloid Interface Sci. 2008, 327, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Perez, R.A.; Kim, H.W.; Ginebra, M.P. Polymeric additives to enhance the functional properties of calcium phosphate cements. J. Tissue Eng. 2012, 3, 2041731412439555. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, Y.; Xu, D.; Lin, X.; Feng, Y.; Hong, Y. Hydroxypropyl Methylcellulose Reduces Particle Adhesion and Improves Recovery of Herbal Extracts During Spray Drying of Chinese Herbal Medicines. Dry. Technol. 2014, 32, 557–566. [Google Scholar] [CrossRef]
- Chatzistavrou, X.; Newby, P.; Boccaccini, A.R. Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering; Woodhead Publishing Limited: Cambridge, UK, 2011; ISBN 9781845697686. [Google Scholar]
- Noshadi, I.; Walker, B.W.; Portillo-Lara, R.; Sani, E.S.; Gomes, N.; Aziziyan, M.R.; Annabi, N. Engineering Biodegradable and Biocompatible Bio-ionic Liquid Conjugated Hydrogels with Tunable Conductivity and Mechanical Properties. Sci. Rep. 2017, 7, 4345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czechowska, J.; Zima, A.; Siek, D.; Ślósarczyk, A. Influence of sodium alginate and methylcellulose on hydrolysis and physicochemical properties of α-TCP based materials. Ceram. Int. 2018, 44, 6533–6540. [Google Scholar] [CrossRef]
- Guarino, V.; Caputo, T.; Altobelli, R.; Ambrosio, L. Degradation properties and metabolic activity of alginate and chitosan polyelectrolytes for drug delivery and tissue engineering applications. AIMS Mater. Sci. 2015, 2, 497–502. [Google Scholar] [CrossRef]
- Joshi, S.C. Sol-gel behavior of hydroxypropyl methylcellulose (HPMC) in ionic media including drug release. Materials 2011, 4, 1861–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Material Label | Solid (Powder) Phase (P) | Liquid Phase (L) | L/P (mL/g) |
|---|---|---|---|
| Control | 25 wt% α-TCP: 35 wt% HAp/CTS granules: 40 wt% HAp/CTS powder | 5 wt% CUL in distilled water (gel) | 0.8 |
| MC-SA2 | Control + 2 wt% SA powder | ||
| MC-SA4 | Control + 4 wt% SA powder | ||
| MC-HPMC2 | Control + 2 wt% HPMC powder | ||
| MC-HPMC4 | Control + 4 wt% HPMC powder |
| Material | Initial, ti [Min] | Final, tf [Min] |
|---|---|---|
| Control | 28 ± 2 | >60 |
| MC-SA2 | 33 ± 2 | |
| MC-SA4 | 36 ± 3 | |
| MC-HPMC2 | 34 ± 1 | |
| MC-HPMC4 | 37 ± 2 |
| Material Label | Phase Composition [wt%] | |||
|---|---|---|---|---|
| 7 Days after Setting and Hardening | After 7 Days of Incubation in SBF (37 °C) | |||
| α-TCP | HAp | α-TCP | HAp | |
| Control | 25.0 | 75.0 | 2.0 | 98.0 |
| MC-SA2 | 26.0 | 74.0 | 2.0 | 98.0 |
| MC-SA4 | 29.0 | 71.0 | 3.0 | 97.0 |
| MC-HPMC2 | 26.0 | 74.0 | 2.0 | 98.0 |
| MC-HPMC4 | 28.0 | 72.0 | 1.0 | 99.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pańtak, P.; Cichoń, E.; Czechowska, J.; Zima, A. Influence of Natural Polysaccharides on Properties of the Biomicroconcrete-Type Bioceramics. Materials 2021, 14, 7496. https://doi.org/10.3390/ma14247496
Pańtak P, Cichoń E, Czechowska J, Zima A. Influence of Natural Polysaccharides on Properties of the Biomicroconcrete-Type Bioceramics. Materials. 2021; 14(24):7496. https://doi.org/10.3390/ma14247496
Chicago/Turabian StylePańtak, Piotr, Ewelina Cichoń, Joanna Czechowska, and Aneta Zima. 2021. "Influence of Natural Polysaccharides on Properties of the Biomicroconcrete-Type Bioceramics" Materials 14, no. 24: 7496. https://doi.org/10.3390/ma14247496