Laser Processing of Diffusion Boronized Layer Produced on Monel® Alloy 400—Microstructure, Microhardness, Corrosion and Wear Resistance Tests
Abstract
:1. Introduction
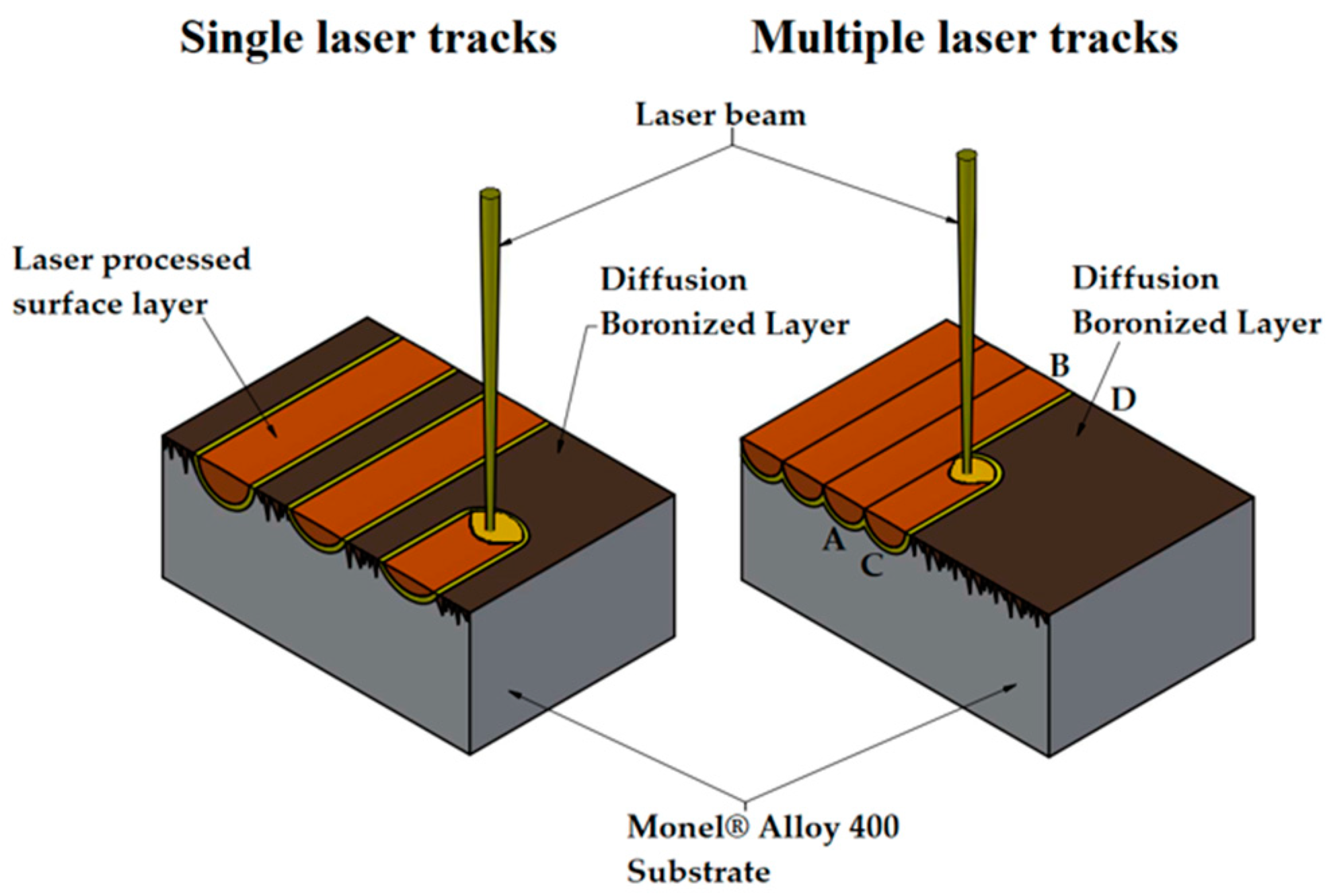
2. Materials and Methods
3. Results and Discussion
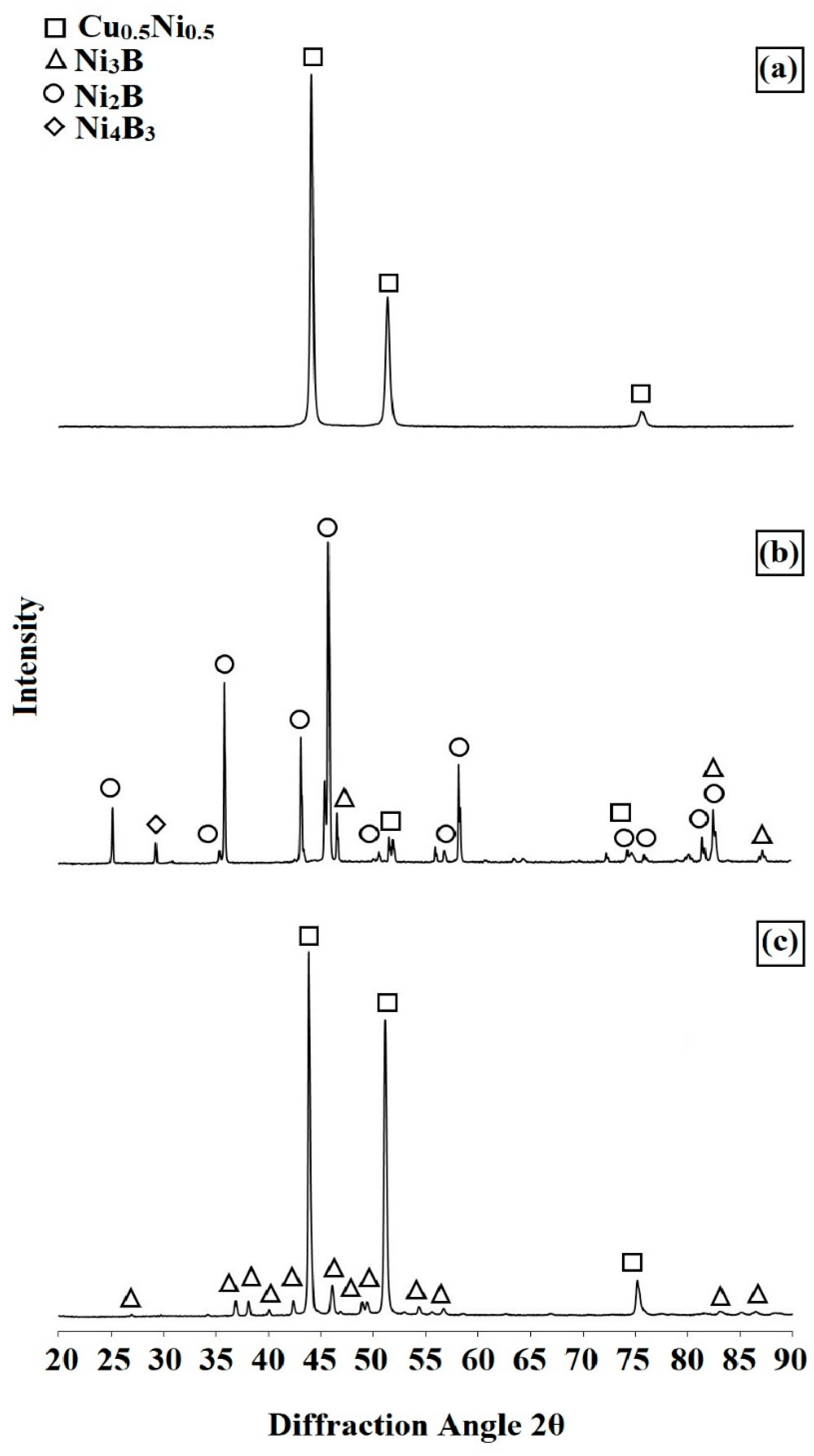
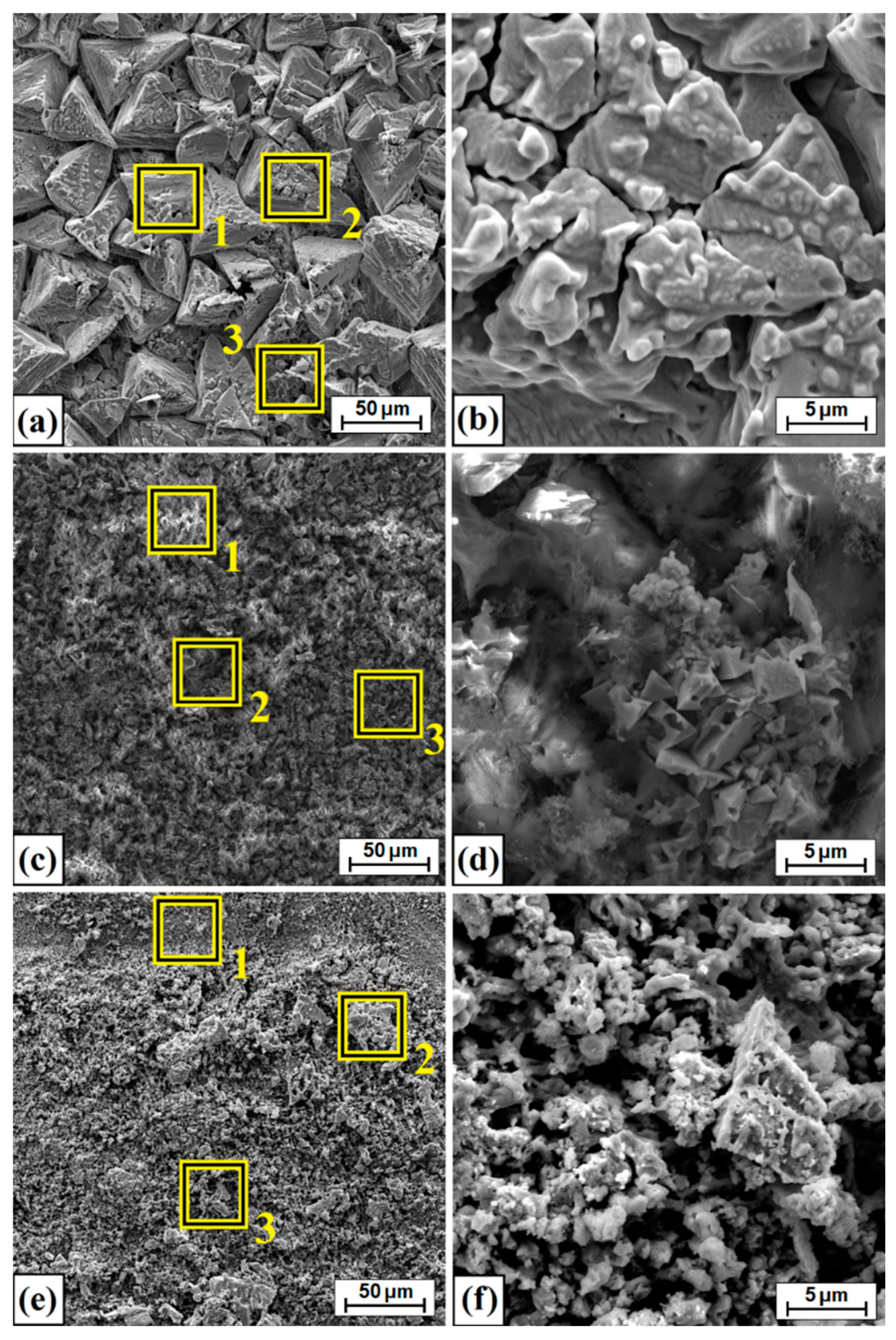
3.1. Microstructure, Chemical and Phase Analysis
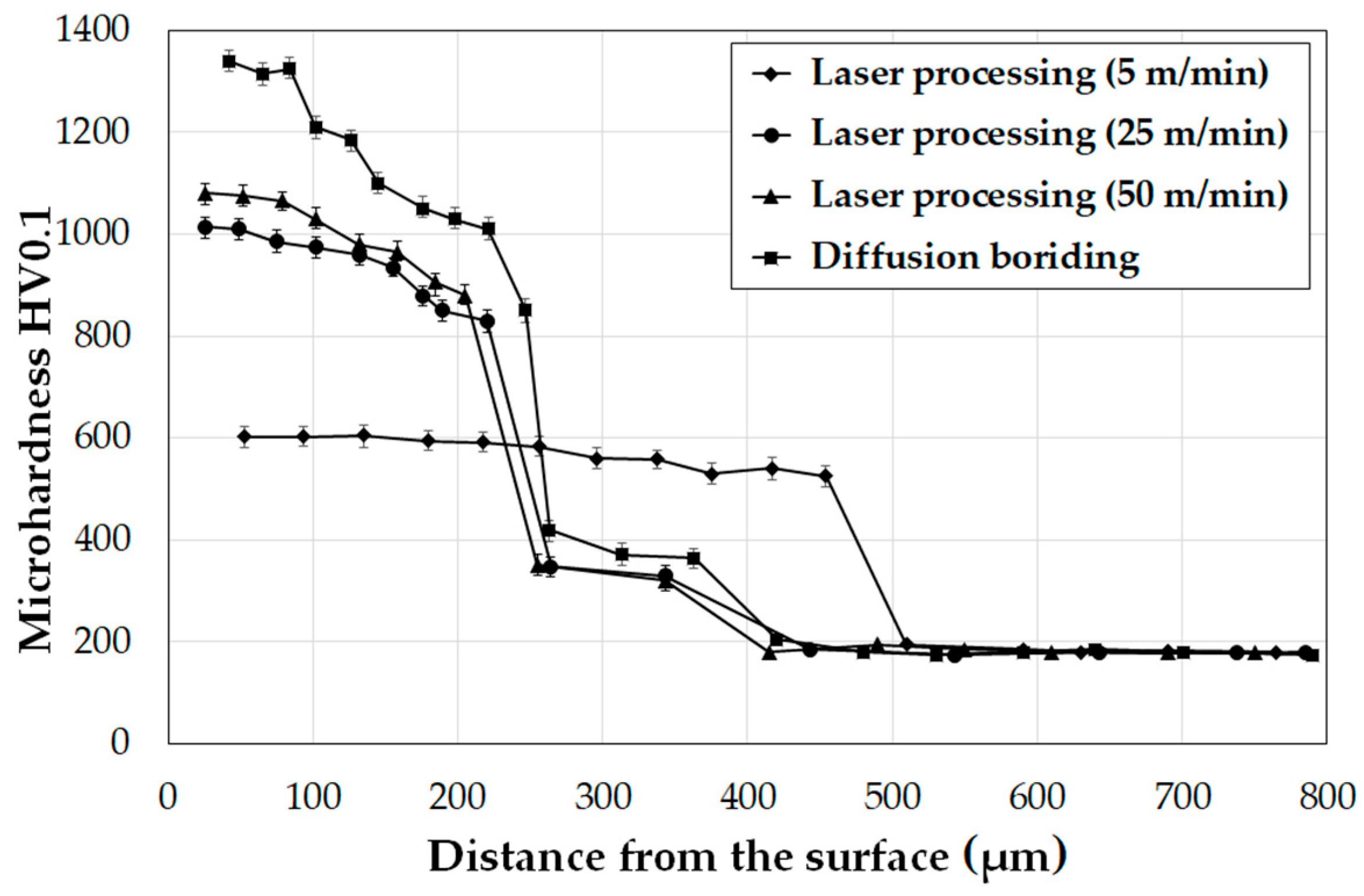
3.2. Microhardness
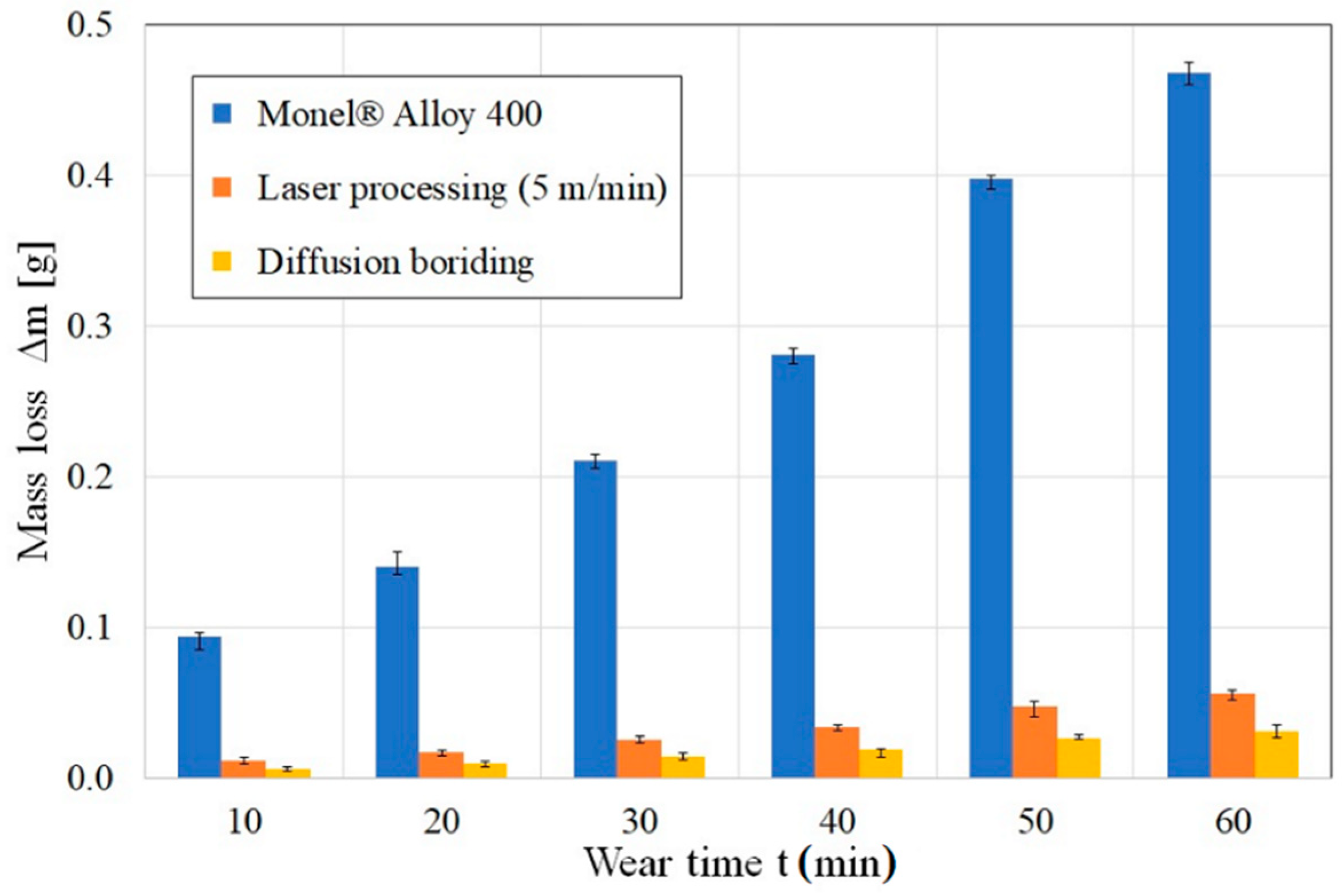
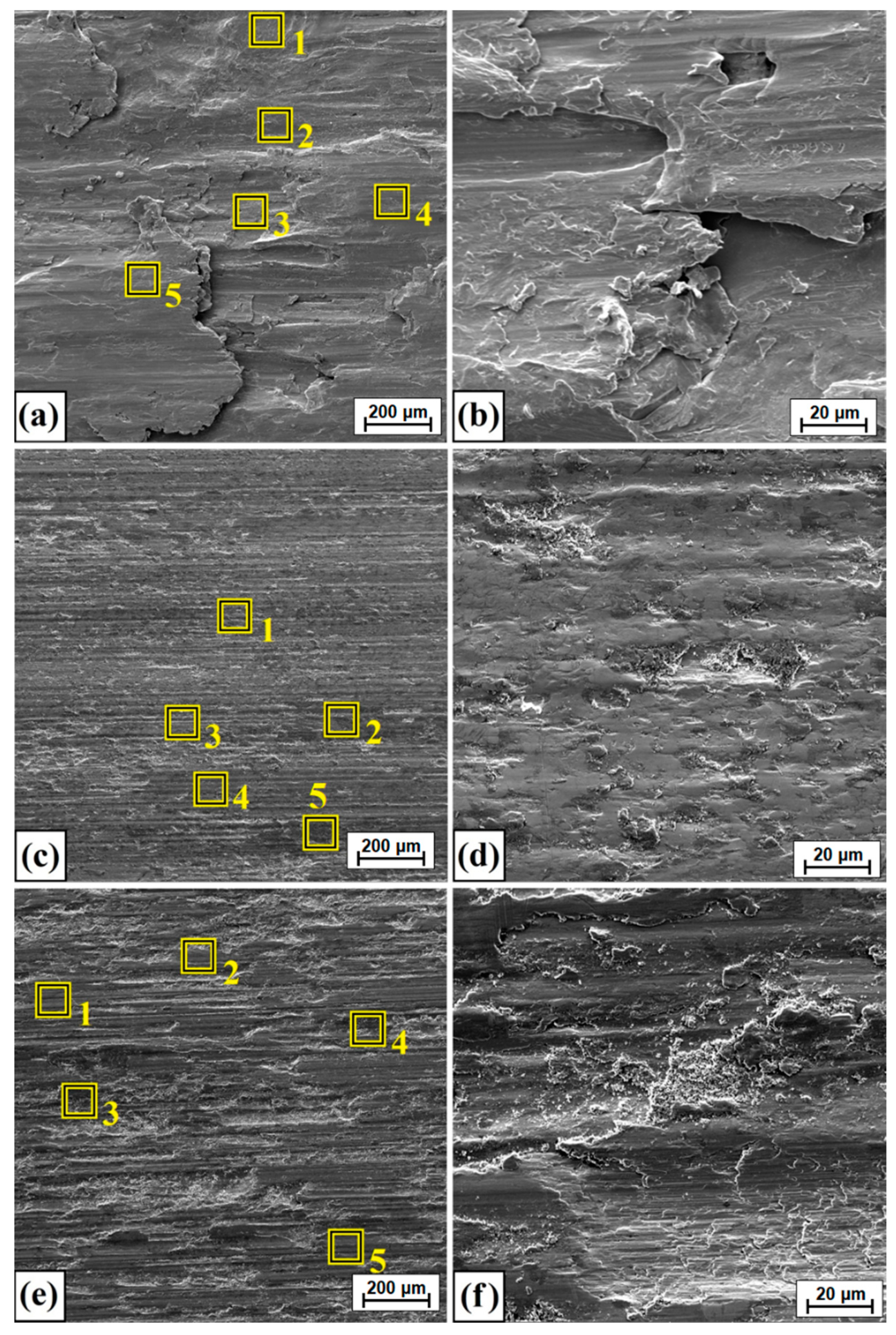
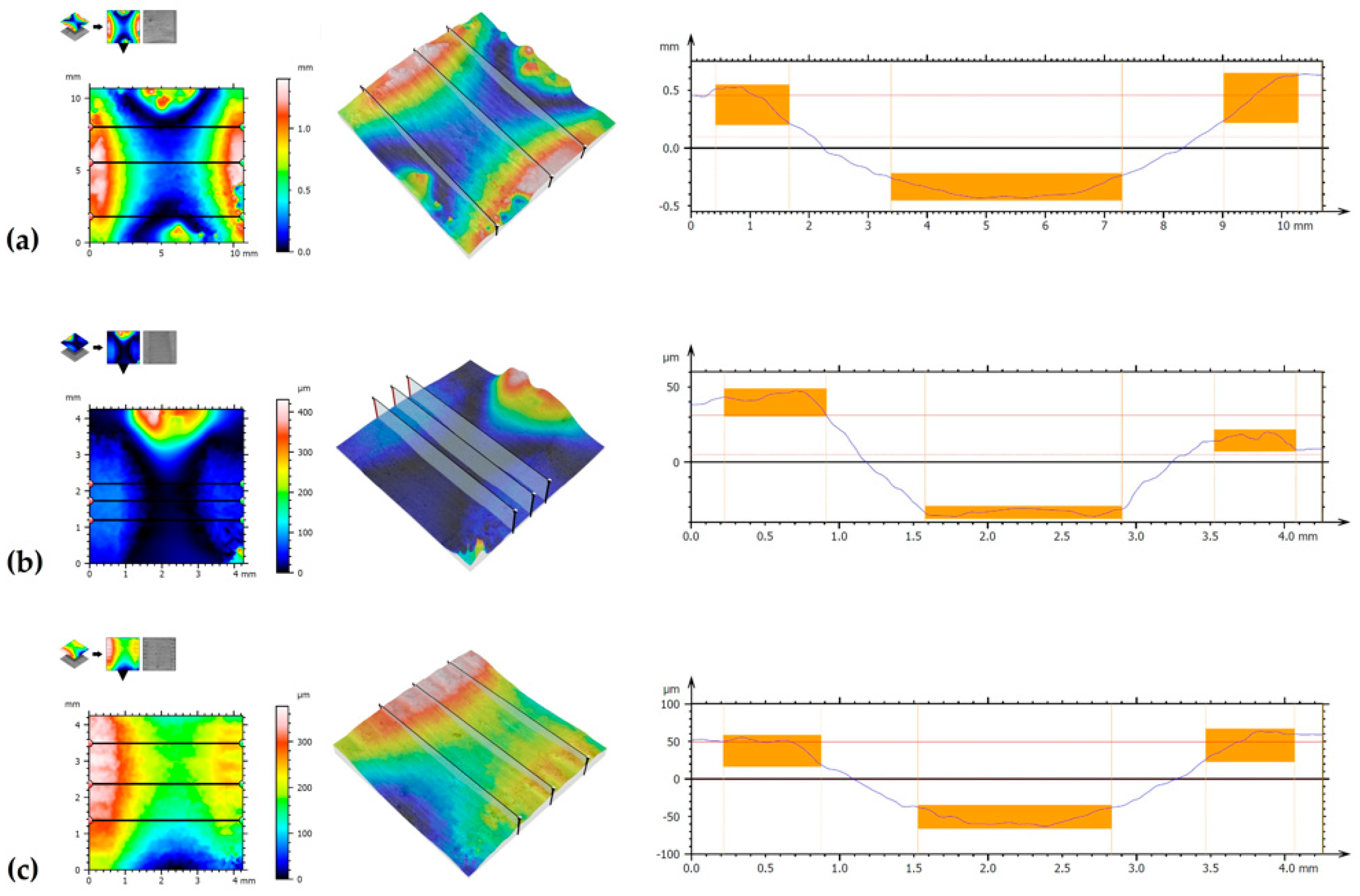
3.3. Wear Resistance
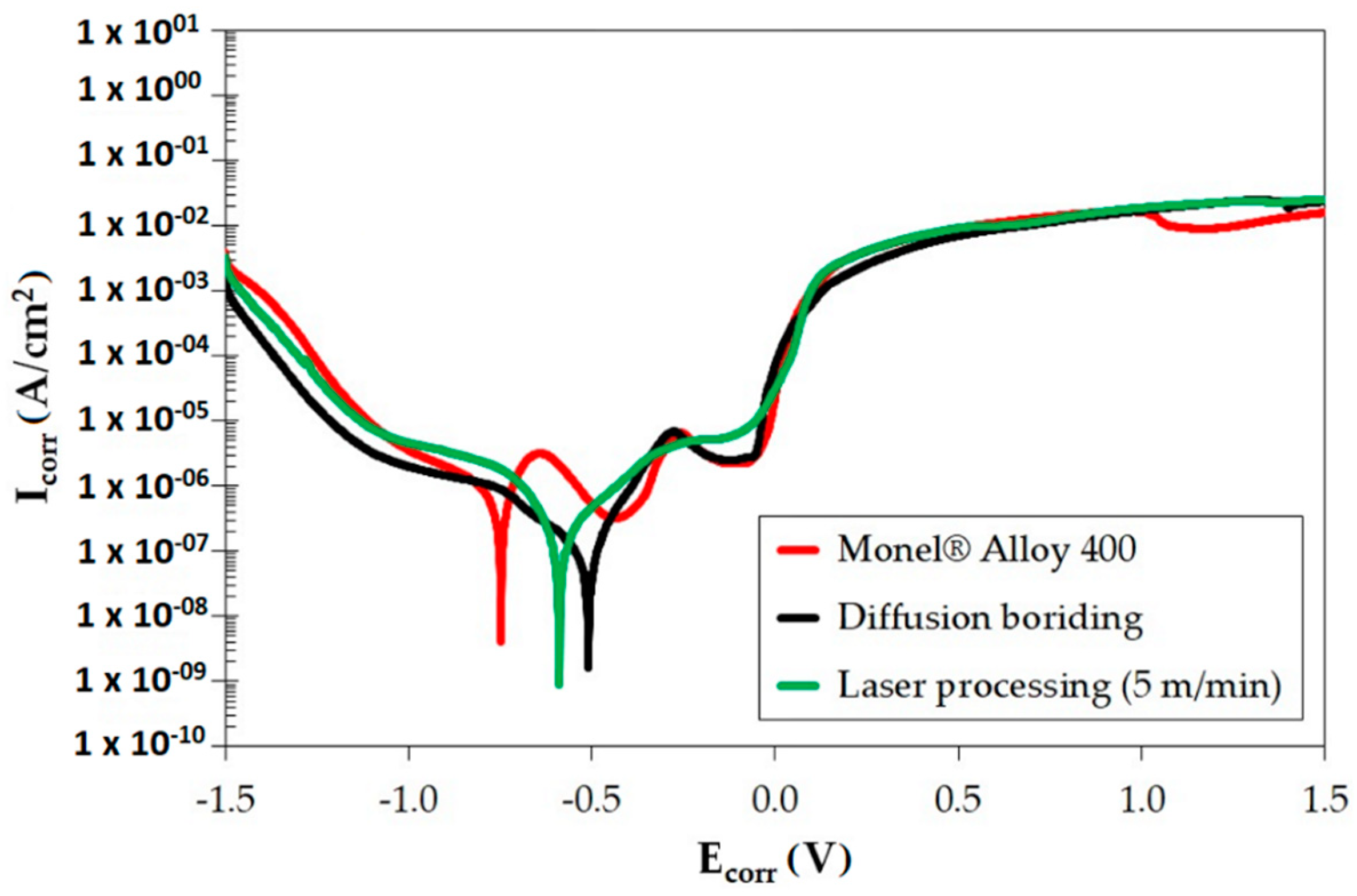
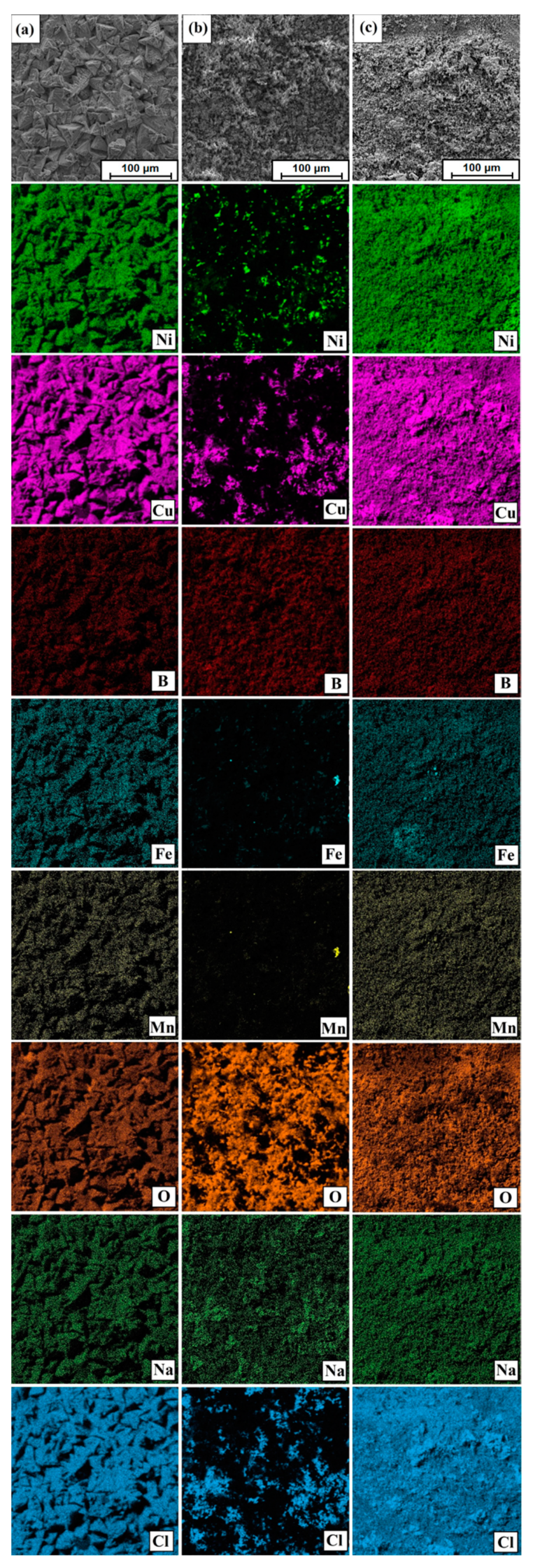
3.4. Corrosion Resistance
4. Conclusions
- A diffusion borided layer produced on Monel® Alloy 400 was characterized by fine needle-like microstructure of nickel borides at the surface, under which there was a solution zone enriched with boron.
- Microhardness of the diffusion boron layer ranged from 1400 HV0.05 in the sub-surface zone to 1100 HV0.05 in the zone near the substrate. Laser processing contributed to microhardness reduction. It was found that microhardness decreased with a decrease in scanning speed. Using the highest scanning speed, a microhardness of around 1050 HV0.05 was achieved, while at the lowest scanning speed of 600 it was HV0.05.
- As a result of remelting the borided layer with a laser beam, the microstructure of the remelted zone was composed of boron-nickel eutectic with nickel boride phases Ni3B, Ni2B and Ni4B3.
- Both diffusion boriding and laser modification contributed to increased corrosion resistance and friction wear resistance compared to the properties of untreated Monel® Alloy 400 material.
- Laser processing of the diffusion borided layer on Monel® Alloy 400 leads to a reduction in mechanical and physicochemical properties (corrosion resistance, wear resistance) compared to the diffusion borided layer. It should be emphasized, however, that the boron layer after laser processing was much thicker than the diffusion layer. At the same time, such a layer is less prone to cracking due to lower brittleness, which means that it can be used in selected applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esgin, U.; Özyürek, D.; Kaya, H. An investigation of wear behaviors of different Monel alloys produced by powder metallurgy. AIP Conf. Proc. 2016, 1727, 020008. [Google Scholar]
- Ma, F.; Zeng, Z.; Gao, Y. Tribocorrosion and the surface repassivation behavior of Monel 400 alloy in artificial seawater. Ind. Lubr. Tribol. 2018, 70, 1331–1340. [Google Scholar] [CrossRef]
- Petrova, R.S.; Suwattananont, N.; Samardzic, V. The effect of boronizing on metallic alloys for automotive applications. J. Mater. Eng. Perform. 2008, 17, 340–345. [Google Scholar] [CrossRef]
- Czerwinski, F. Heat Treatment: Conventional and Novel Applications; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Krukovich, M.G.; Prusakov, B.A.; Sizov, I.G. Plasticity of Boronized Layers; Springer Series in Materials Science; Spinger: Berlin/Heidelberg, Germany, 2016; p. 237. ISBN 978-3-319-40012-9. [Google Scholar]
- Pertek, A.; Kulka, M. Characterization of single tracks after laser surface modification of borided 41Cr4 steel. Appl. Surf. Sci. 2003, 205, 137–142. [Google Scholar] [CrossRef]
- Bartkowska, A.; Jurči, P.; Hudáková, M.; Bartkowski, D.; Kusý, M.; Przestacki, D. Effect of diode laser beam fluence on change in microstructure, microhardness and phase composition of FeB-Fe2B layers produced on Vanadis-6 steel. Arch. Metall. Mater. 2018, 63, 791–800. [Google Scholar]
- Bartkowska, A.; Bartkowski, D.; Swadźba, R.; Przestacki, D.; Miklaszewski, A. Microstructure, chemical composition, wear, and corrosion resistance of FeB-Fe2B-Fe3B surface layers produced on Vanadis-6 steel using CO2 laser. Int. J. Adv. Manuf. Technol. 2018, 95, 1763–1776. [Google Scholar] [CrossRef] [Green Version]
- Kulka, M. Current Trends in Boriding; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 978-3-030-06782-3. [Google Scholar]
- Campos-Silva, I.; Contla-Pacheco, A.D.; Ruiz-Rios, A.; Martínez-Trinidad, J.; Rodríguez-Castro, G.; Meneses-Amador, A.; Wong-Angel, W.D. Effects of scratch tests on the adhesive and cohesive properties of borided Inconel 718 superalloy. Surf. Coat. Technol. 2018, 349, 917–927. [Google Scholar] [CrossRef]
- Günen, A.; Kanca, E.; Çakir, H.; Karakaş, M.S.; Gök, M.S.; Küçük, Y.; Demir, M. Effect of borotitanizing on microstructure and wear behavior of Inconel 625. Surf. Coat. Technol. 2017, 311, 374–382. [Google Scholar] [CrossRef]
- Deng, D.W.; Wang, C.G.; Liu, Q.Q.; Niu, T.T. Effect of standard heat treatment on microstructure and properties of borided Inconel 718, Trans. Nonferr. Met. Soc. China 2015, 25, 437–443. [Google Scholar] [CrossRef]
- Sista, V.; Kahvecioglu, O.; Kartal, G.; Zeng, Q.Z.; Kim, J.H.; Eryilmaz, O.L.; Erdemir, A. Evaluation of electrochemical boriding of Inconel 600. Surf. Coat. Technol. 2013, 215, 452–459. [Google Scholar] [CrossRef]
- Gunen, A.; Kanca, E. Microstructure and Mechanical Properties of Borided Inconel 625 Superalloy. Materia 2017, 22, 11829. [Google Scholar] [CrossRef]
- Lou, D.C.; Solberg, J.K.; Akselsen, O.M.; Dahl, N. Microstructure and property investigation of paste boronized pure nickel and Nimonic 90 superalloy. Mater. Chem. Phys. 2009, 115, 239–244. [Google Scholar] [CrossRef]
- Özbek, I.; Akbulut, H.; Zeytin, S.; Bindal, C.; Ucisik, A.H. The characterization of borided 99.5% purity nickel. Surf. Coat. Technol. 2000, 126, 166–170. [Google Scholar] [CrossRef]
- Gunes, I.; Kayali, Y. Investigation of mechanical properties of borided Nickel 201 alloy. Mater. Des. 2014, 53, 577–580. [Google Scholar] [CrossRef]
- Ueda, N.; Mizukoshi, T.; Demizu, K.; Sone, T.; Ikenaga, A.; Kawamoto, M. Boriding of nickel by the powder-pack method. Surf. Coat. Technol. 2000, 126, 25–30. [Google Scholar] [CrossRef]
- Mu, D.; Shen, B.; Yang, C.; Zhao, X. Microstructure analysis of boronized pure nickel using boronizing powders with SiC as diluent. Vacuum 2009, 83, 1481–1484. [Google Scholar] [CrossRef]
- Anthymidis, K.G.; Zinoviadis, P.; Roussos, D.; Tsipas, D.N. Boriding of nickel in a fluidized bed reactor. Mater. Res. Bull. 2002, 37, 515–522. [Google Scholar] [CrossRef]
- Krellinga, A.P.; Melo, F.S.; Almeida, E.A.S.; da Costa, C.E.; Milan, J.C.G. Microstructure and properties of borided Monel 400 alloy. Mater. Res. Express 2019, 6, 106410. [Google Scholar] [CrossRef]
- Nakata, K.; Tomoto, K.; Matsuda, F. Laser Boronizing of Copper Alloy. Trans. JWRI 1996, 25, 37–41. [Google Scholar]
- Kukliński, M.; Bartkowska, A.; Przestacki, D. Microstructure and selected properties of Monel 400 alloy after laser heat treatment and laser boriding using diode laser. Int. J. Adv. Manuf. Technol. 2018, 98, 3005–3017. [Google Scholar] [CrossRef] [Green Version]
- Kukliński, M.; Bartkowska, A.; Przestacki, D. Laser Alloying Monel 400 with Amorphous Boron to Obtain Hard Coatings. Materials 2019, 12, 3494. [Google Scholar] [CrossRef] [Green Version]
- Kukliński, M.; Bartkowska, A.; Przestacki, D.; Kinal, G. Influence of Microstructure and Chemical Composition on Microhardness and Wear Properties of Laser Borided Monel 400. Materials 2020, 13, 5757. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.S. Corrosion aspects of Ni-Cr-Fe based and Ni-Cu based steam generator tube materials. J. Nucl. Mater. 2009, 393, 343–349. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, C.; Zhang, S.; Jia, Y.; Guan, M.; Tan, J.; Lin, B. Laser cladding of NiCrSiB on Monel 400 to enhance cavitation erosion and corrosion resistance. Rare Met. 2016, 1–9. [Google Scholar] [CrossRef]







| Cu | Si | Fe | Mn | C | S | Ni |
|---|---|---|---|---|---|---|
| 31 | 0.5 | 2.5 | 2.0 | 0.3 | 0.024 | bal. |
| Scanning Speed of Laser Beam | Single Track | Multiple Tracks | |
|---|---|---|---|
| In the Axes of the Laser Tracks | On Border of the Laser Tracks | ||
| v = 5 m/min | 600 | 459 | 370 |
| v = 25 m/min | 212 | no tested | |
| v = 50 m/min | 150 | no tested | |
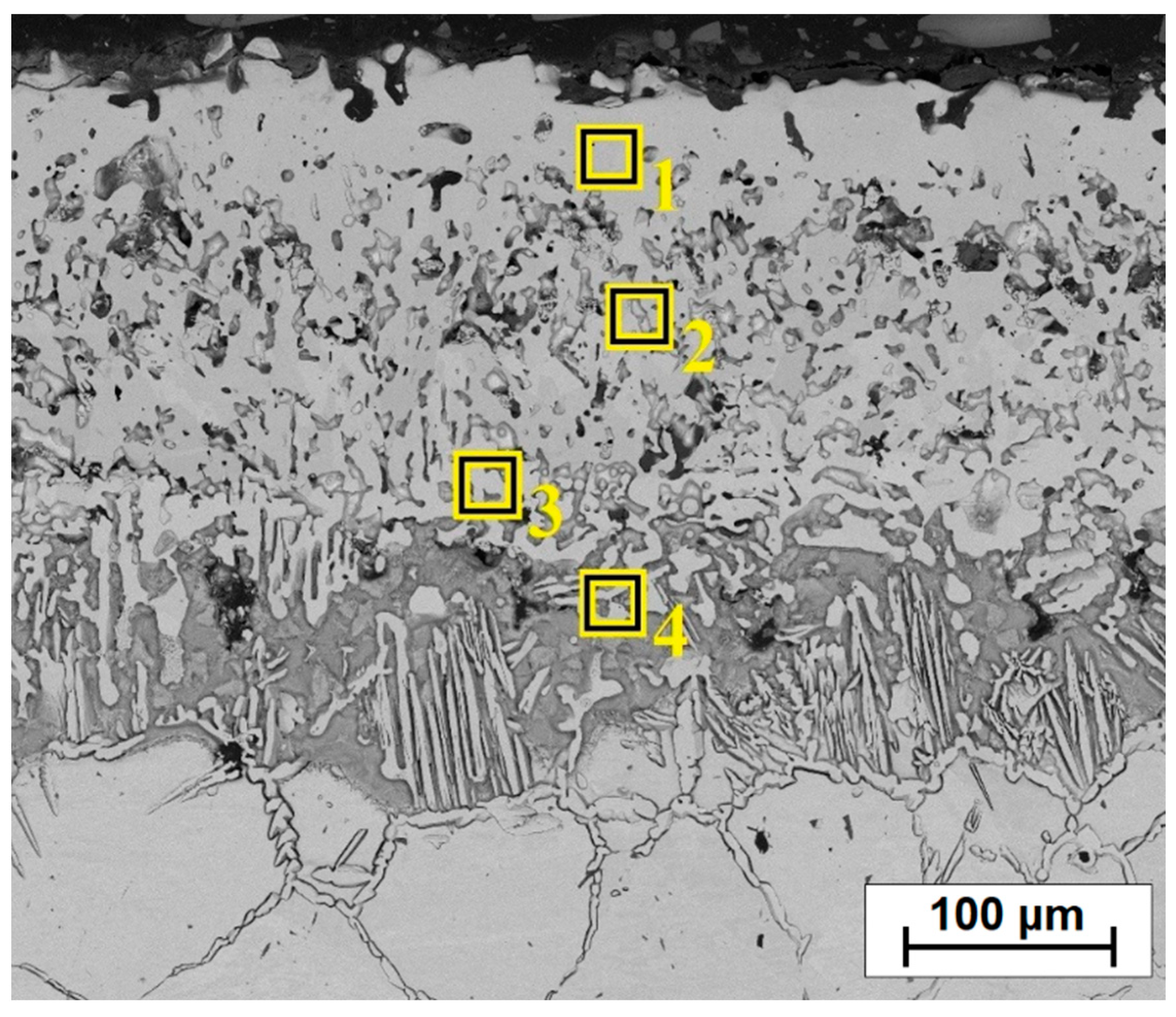
| Type of Layer | No | Ni | Cu | B | Fe | Mn |
|---|---|---|---|---|---|---|
| Diffusion boronized | 1 | 86.8 | 2.1 | 7.7 | 2.7 | 0.6 |
| 2 | 86.0 | 2.9 | 7.1 | 3.6 | 0.4 | |
| 3 | 86.1 | 4.5 | 6.2 | 2.7 | 0.5 | |
| 4 | 86.4 | 5.0 | 5.3 | 3.0 | 0.3 | |
| Laser processing of diffusion boronizing layer v = 5 m/min | 1 | 82.0 | 9.8 | 5.2 | 2.6 | 0.4 |
| 2 | 81.5 | 10.8 | 4.7 | 2.4 | 0.6 | |
| 3 | 85.8 | 6.7 | 5.2 | 2.1 | 0.2 | |
| 4 | 85.6 | 6.3 | 4.7 | 3.0 | 0.4 | |
| 5 | 67.3 | 28.2 | 1.2 | 2.5 | 0.8 | |
| 6 | 65.4 | 30.8 | 0.5 | 2.2 | 1.1 | |
| Laser processing of diffusion boronizing layer v = 25 m/min | 1 | 80.2 | 10.0 | 6.6 | 2.7 | 0.6 |
| 2 | 81.2 | 9.6 | 6.4 | 2.4 | 0.3 | |
| 3 | 80.1 | 11.7 | 5.5 | 2.4 | 0.3 | |
| 4 | 87.8 | 5.4 | 3.8 | 2.5 | 0.5 | |
| 5 | 89.3 | 4.4 | 3.7 | 2.4 | 0.3 | |
| 6 | 64.8 | 33.1 | 0.0 | 1.4 | 0.7 | |
| Laser processing of diffusion boronizing layer v = 50 m/min | 1 | 81.4 | 9.7 | 6.8 | 2.1 | 0.1 |
| 2 | 82.3 | 8.7 | 6.5 | 2.3 | 0.2 | |
| 3 | 87.3 | 2.9 | 7.3 | 2.4 | 0.2 | |
| 4 | 82.7 | 8.8 | 5.9 | 2.4 | 0.2 | |
| 5 | 87.5 | 4.8 | 4.9 | 2.4 | 0.4 | |
| 6 | 66.7 | 30.1 | 0.0 | 2.0 | 1.2 |
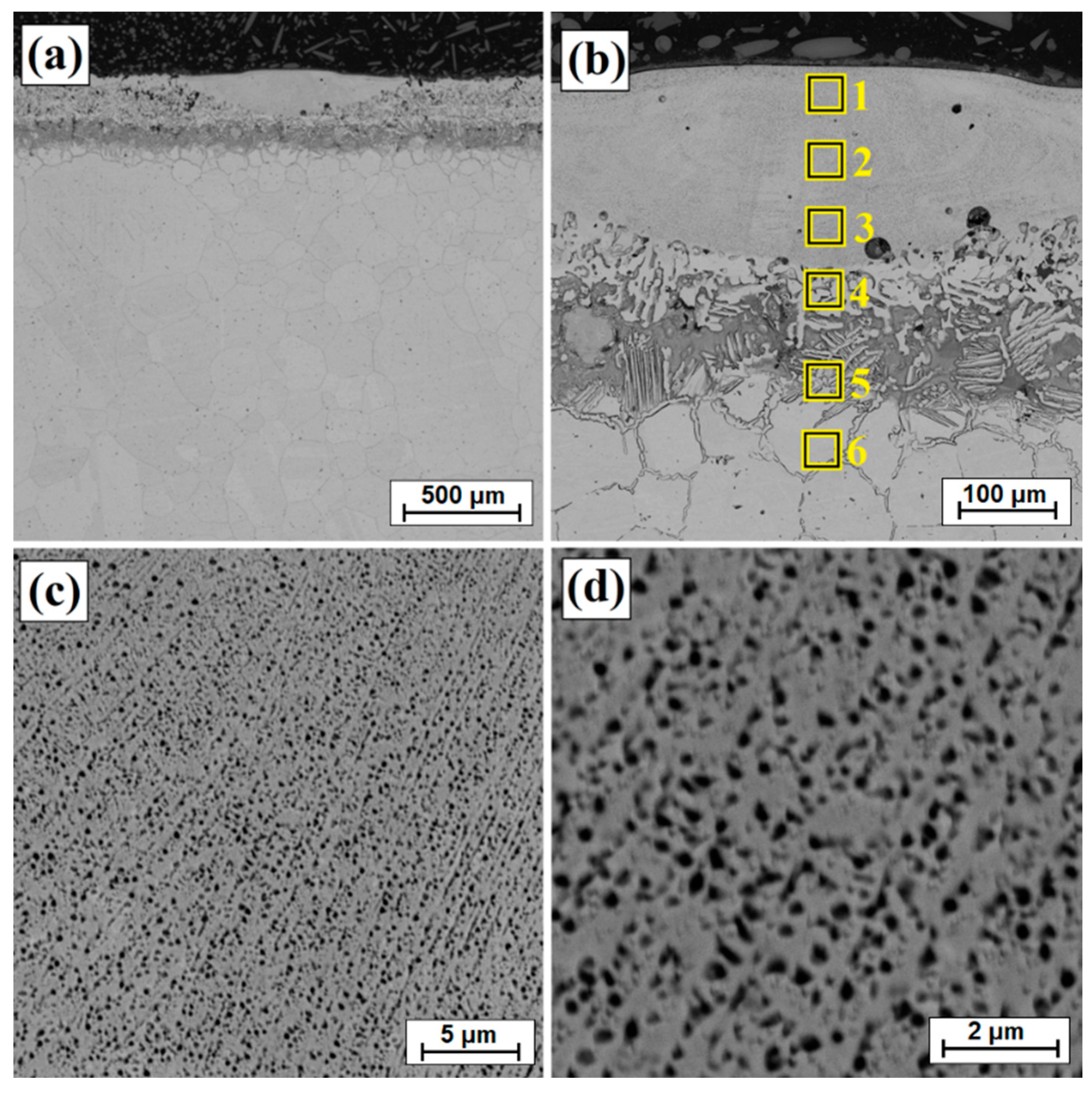
| Scanning Speed of Laser Beam | No | Ni | Cu | B | Fe | Mn |
|---|---|---|---|---|---|---|
| v = 5 m/min | 1 | 84.6 | 7.8 | 4.1 | 2.8 | 0.7 |
| 2 | 85.5 | 7.6 | 3.8 | 2.4 | 0.8 | |
| 3 | 87.9 | 5.4 | 3.9 | 2.2 | 0.5 | |
| 4 | 85.5 | 7.5 | 4.4 | 2.1 | 0.6 | |
| v = 25 m/min | 1 | 77.6 | 13.8 | 6.1 | 2.1 | 0.4 |
| 2 | 80.9 | 10.8 | 6.1 | 2.1 | 0.2 | |
| 3 | 78.0 | 13.3 | 5.7 | 2.4 | 0.6 | |
| 4 | 81.2 | 10.7 | 5.6 | 2.4 | 0.1 | |
| v = 50 m/min | 1 | 80.5 | 9.0 | 7.5 | 2.7 | 0.2 |
| 2 | 79.4 | 11.2 | 6.9 | 2.3 | 0.2 | |
| 3 | 85.2 | 5.4 | 7.2 | 2.2 | 0.2 | |
| 4 | 81.7 | 9.1 | 6.8 | 2.0 | 0.3 |
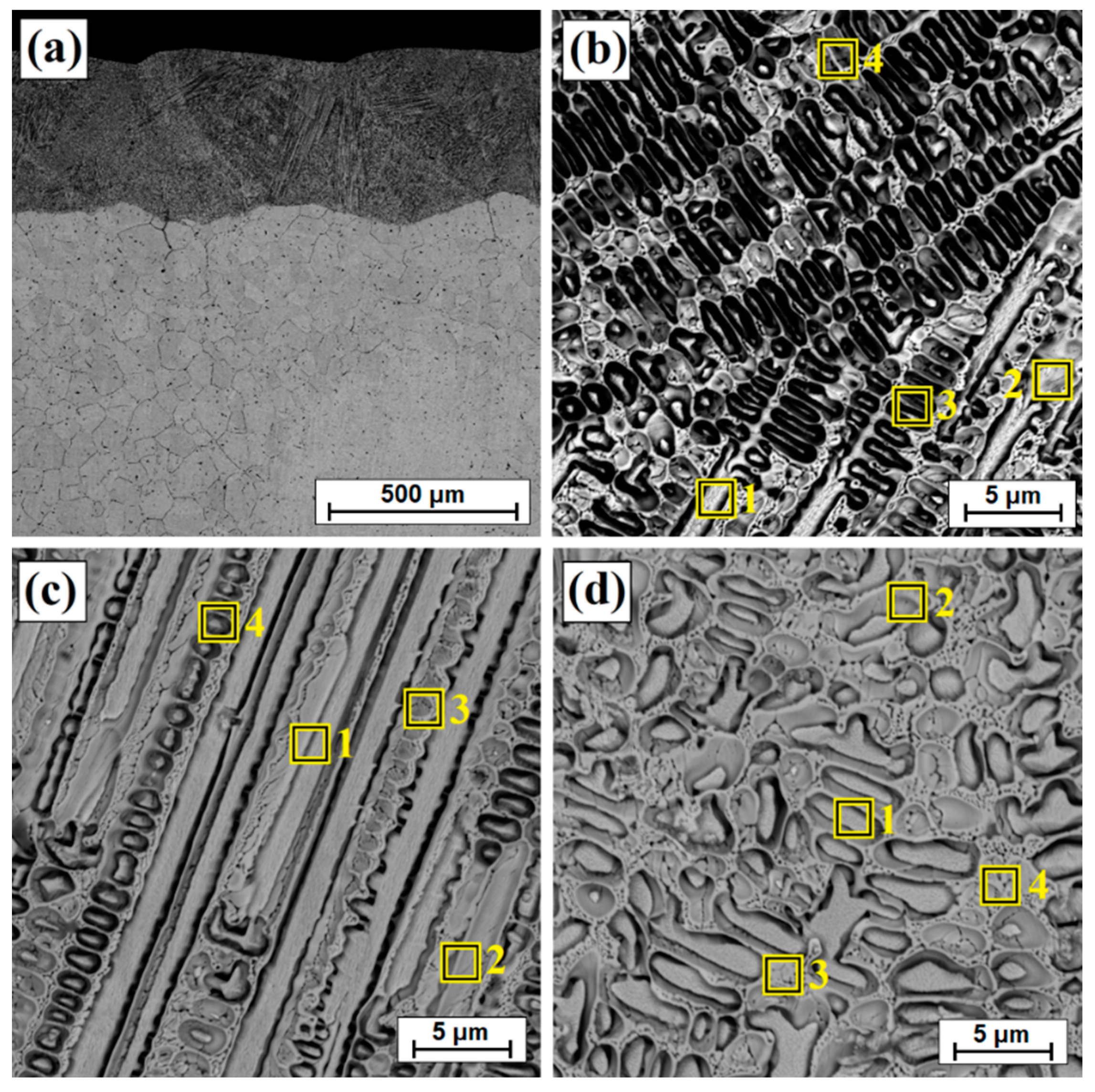
| Zone of Layer | No | Ni | Cu | B | Fe | Mn |
|---|---|---|---|---|---|---|
| Subsurface zone of laser track | 1 | 81.8 | 10.7 | 5.3 | 1.5 | 0.6 |
| 2 | 86.6 | 7.0 | 4.2 | 1.7 | 0.5 | |
| 3 | 88.0 | 6.8 | 3.5 | 1.1 | 0.5 | |
| 4 | 87.1 | 7.3 | 3.5 | 1.5 | 0.6 | |
| Middle zone of laser track | 1 | 84.2 | 8.2 | 5.9 | 1.3 | 0.3 |
| 2 | 79.0 | 13.1 | 5.5 | 1.7 | 0.7 | |
| 3 | 84.3 | 8.1 | 4.7 | 2.3 | 0.7 | |
| 4 | 88.2 | 5.7 | 4.2 | 1.5 | 0.5 | |
| Sub-substrate zone of laser track | 1 | 89.3 | 6.1 | 2.1 | 1.8 | 0.8 |
| 2 | 87.0 | 7.6 | 3.0 | 2.0 | 0.4 | |
| 3 | 89.6 | 4.9 | 3.8 | 1.2 | 0.5 | |
| 4 | 89.4 | 4.9 | 3.9 | 1.4 | 0.3 |
| Type of Layer | No | Ni | Cu | B | Fe | Mn | O |
|---|---|---|---|---|---|---|---|
| Monel® Alloy 400 (substrate material) | 1 | 61.6 | 32.4 | - | 4.2 | 1.2 | 0.6 |
| 2 | 58.7 | 32.6 | - | 7.3 | 0.7 | 0.7 | |
| 3 | 62.0 | 34.0 | - | 2.5 | 1.0 | 0.4 | |
| 4 | 66.4 | 29.4 | - | 2.9 | 0.9 | 0.5 | |
| 5 | 60.8 | 32.1 | - | 5.9 | 0.7 | 0.6 | |
| Diffusion boronzing | 1 | 71.8 | 20.1 | 1.4 | 4.1 | 1.0 | 1.6 |
| 2 | 55.1 | 25.5 | 0.1 | 13.5 | 0.6 | 5.3 | |
| 3 | 21.2 | 12.8 | 0.0 | 44.4 | 0.6 | 20.9 | |
| 4 | 25.1 | 15.5 | 0.0 | 39.8 | 0.9 | 18.7 | |
| 5 | 22.4 | 13.7 | 0.0 | 44.1 | 0.6 | 19.1 | |
| Laser processing of diffusion boronizing layer | 1 | 32.0 | 16.4 | 0.0 | 38.5 | 0.7 | 12.5 |
| 2 | 64.5 | 29.7 | 0.0 | 3.6 | 0.9 | 1.4 | |
| 3 | 35.1 | 17.9 | 0.0 | 30.4 | 0.6 | 15.9 | |
| 4 | 63.9 | 30.0 | 0.0 | 3.7 | 1.1 | 1.3 | |
| 5 | 65.4 | 28.7 | 0.4 | 4.0 | 0.5 | 1.0 |
| Parameters | Monel® Alloy 400 (Substrate Material) | Diffusion Boronizing | Laser Processing of Diffusion Boronizing Layer |
|---|---|---|---|
| Ra | 4.746 | 1.697 | 2.011 |
| Rz | 18.23 | 7.713 | 8.015 |
| Sa | 309.9 | 45.69 | 45.77 |
| Sq | 363.9 | 66.11 | 53.82 |
| Sz | 1422 | 444.5 | 234.9 |
| wear track width | 3908.0 | 1327 | 1306 |
| max. wear track depth | 889.0 | 67.48 | 112.3 |
| average wear track depth | 828.3 | 64.46 | 102.8 |
| Specimen | Current Icorr [A·cm2] | Potential Ecorr [V] |
|---|---|---|
| Monel® Alloy 400 | 3.54 × 10−7 | −7.48 × 10−1 |
| Diffusion boronizing | 4.07 × 10−8 | −5.09 × 10−1 |
| Laser processing of diffusion boronizing layer | 5.54 × 10−6 | −5.97 × 10−1 |
| Process | No | Ni | Cu | O | Cl |
|---|---|---|---|---|---|
| Monel® Alloy 400 (substrate material without modification process) | 1 | 14.7 | 45.9 | 17.6 | 21.8 |
| 2 | 25.1 | 39.2 | 13.4 | 22.3 | |
| 3 | 11.1 | 39.6 | 7.7 | 41.7 | |
| Diffusion boronzing | 1 | 87.4 | 9.8 | 2.4 | 0.5 |
| 2 | 9.8 | 20.6 | 62.3 | 7.2 | |
| 3 | 38.9 | 8.1 | 51.8 | 1.2 | |
| Laser processing of diffusion boronizing layer | 1 | 2.2 | 51.2 | 15.9 | 30.9 |
| 2 | 2.7 | 52.6 | 13.7 | 31.1 | |
| 3 | 1.4 | 57.9 | 5.9 | 34.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartkowska, A.; Bartkowski, D.; Przestacki, D.; Kukliński, M.; Miklaszewski, A.; Kieruj, P. Laser Processing of Diffusion Boronized Layer Produced on Monel® Alloy 400—Microstructure, Microhardness, Corrosion and Wear Resistance Tests. Materials 2021, 14, 7529. https://doi.org/10.3390/ma14247529
Bartkowska A, Bartkowski D, Przestacki D, Kukliński M, Miklaszewski A, Kieruj P. Laser Processing of Diffusion Boronized Layer Produced on Monel® Alloy 400—Microstructure, Microhardness, Corrosion and Wear Resistance Tests. Materials. 2021; 14(24):7529. https://doi.org/10.3390/ma14247529
Chicago/Turabian StyleBartkowska, Aneta, Dariusz Bartkowski, Damian Przestacki, Mateusz Kukliński, Andrzej Miklaszewski, and Piotr Kieruj. 2021. "Laser Processing of Diffusion Boronized Layer Produced on Monel® Alloy 400—Microstructure, Microhardness, Corrosion and Wear Resistance Tests" Materials 14, no. 24: 7529. https://doi.org/10.3390/ma14247529
APA StyleBartkowska, A., Bartkowski, D., Przestacki, D., Kukliński, M., Miklaszewski, A., & Kieruj, P. (2021). Laser Processing of Diffusion Boronized Layer Produced on Monel® Alloy 400—Microstructure, Microhardness, Corrosion and Wear Resistance Tests. Materials, 14(24), 7529. https://doi.org/10.3390/ma14247529









