Synthesis Method and Thermodynamic Characteristics of Anode Material Li3FeN2 for Application in Lithium-Ion Batteries
Abstract
:1. Introduction
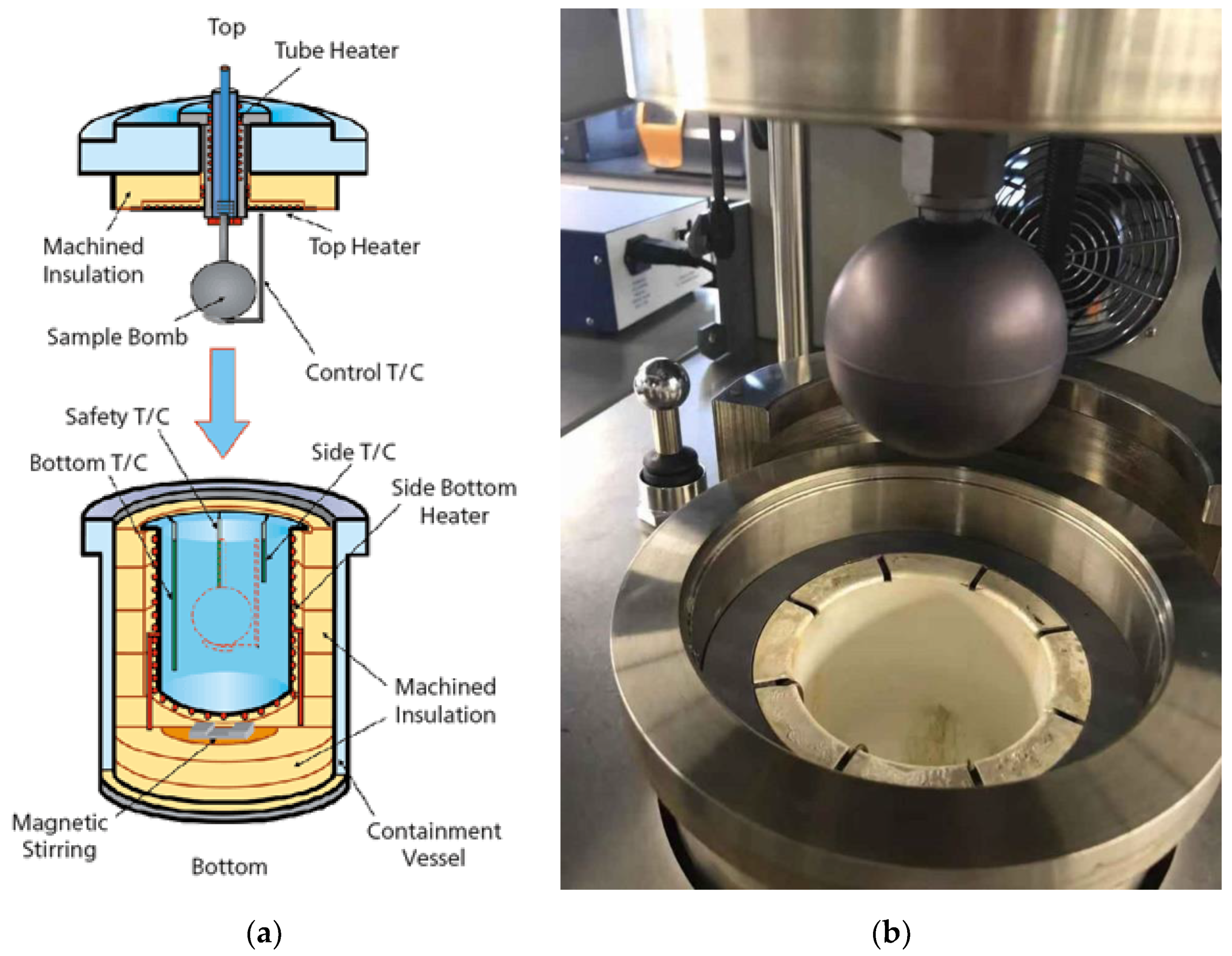
2. Materials and Methods
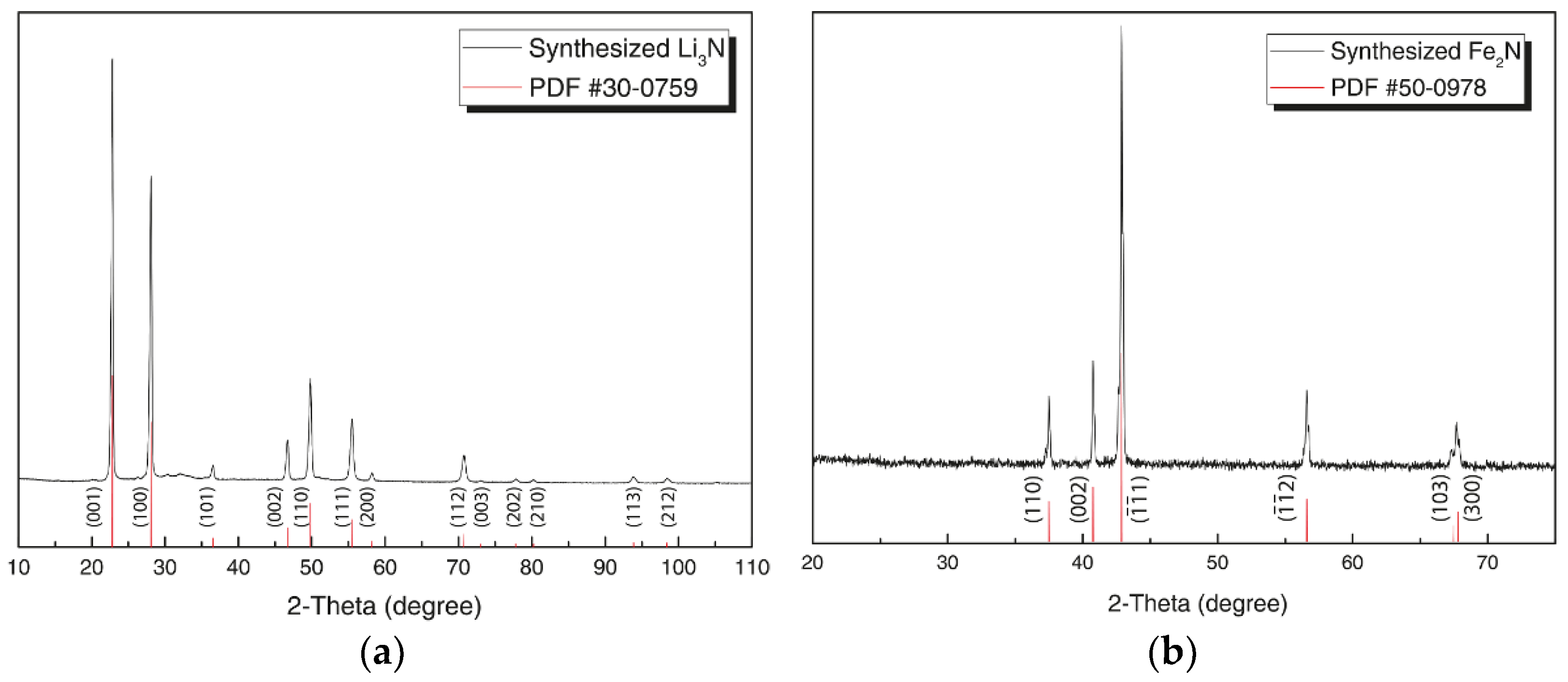
3. Results
4. Discussion
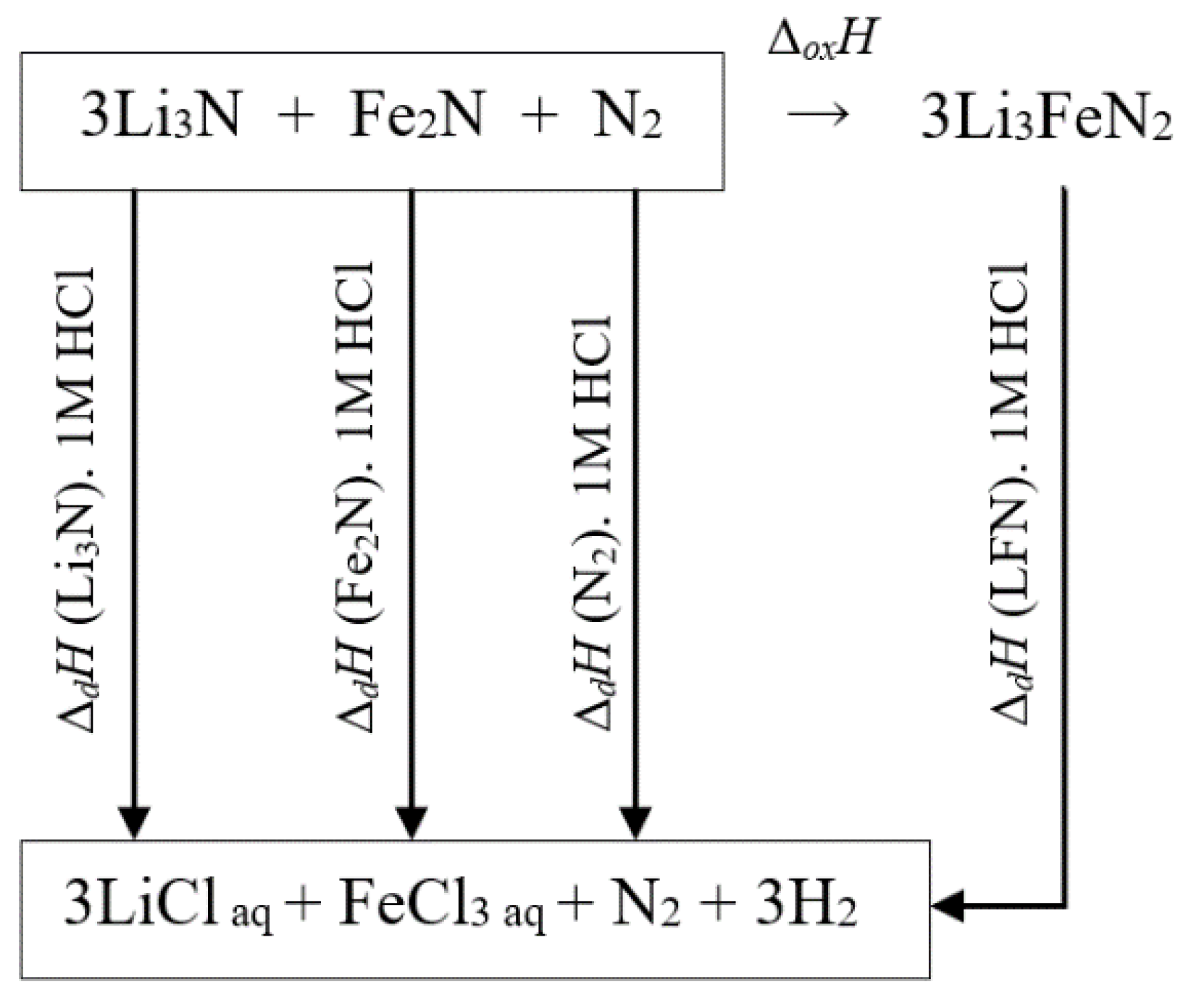
4.1. The Standard Enthalpy of Formation
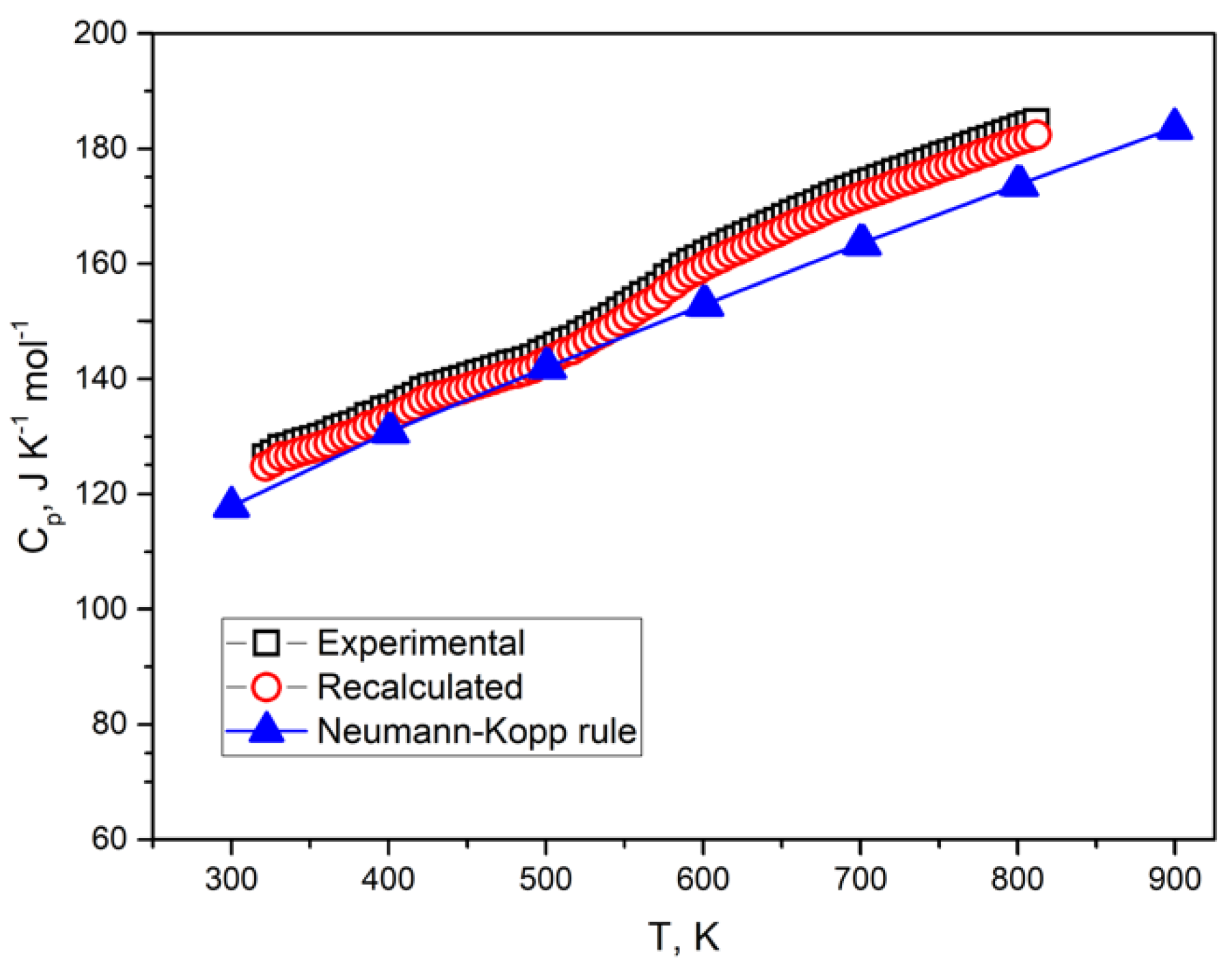
4.2. The Isobaric Heat Capacity
4.3. Entropy
4.4. The Standard Gibbs Free Energy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bruce, P.G.; Scrosati, B.; Tarascon, J.M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef]
- Du, F.H.; Li, S.Q.; Yan, Y.; Lu, X.M.; Guo, C.; Ji, Z.; Hu, P.; Shen, X. Facile fabrication of Fe0.8Mn1.2O3 with various nanostructures for high-performance lithium-ion batteries. Chem. Eng. J. 2022, 427, 131697. [Google Scholar] [CrossRef]
- Faizan, M.; Hussain, S.; Vikraman, D.; Ali, B.; Kim, H.S.; Jung, J.; Nam, K.W. MoS2@ Mo2C hybrid nanostructures formation as an efficient anode material for lithium-ion batteries. J. Mater. Res. Technol. 2021, 14, 2382–2393. [Google Scholar] [CrossRef]
- Yoon, J.; Choi, W.; Kim, H.; Choi, Y.S.; Kim, J.M.; Yoon, W.S. The effects of nanostructures on lithium storage behavior in Mn2O3 anodes for next-generation lithium-ion batteries. J. Power Sources 2021, 493, 229682. [Google Scholar] [CrossRef]
- Devi, V.S.; Athika, M.; Duraisamy, E.; Prasath, A.; Sharma, A.S.; Elumalai, P. Facile sol-gel derived nanostructured spinel Co3O4 as electrode material for high-performance supercapattery and lithium-ion storage. J. Energy Storage 2019, 25, 100815. [Google Scholar] [CrossRef]
- Kosova, N.V. Mechanochemical reactions and processing of nanostructured electrode materials for lithium-ion batteries. Mater. Today Proc. 2016, 3, 391–395. [Google Scholar] [CrossRef]
- Duan, W.; Yan, W.; Yan, X.; Munakata, H.; Jin, Y.; Kanamura, K. Synthesis of nanostructured Ni3S2 with different morphologies as negative electrode materials for lithium-ion batteries. J. Power Sources 2015, 293, 706–711. [Google Scholar] [CrossRef]
- Wang, G.; Shen, X.; Yao, J. One-dimensional nanostructures as electrode materials for lithium-ion batteries with improved electrochemical performance. J. Power Sources 2009, 189, 543–546. [Google Scholar] [CrossRef]
- Zhang, M.; Li, L.; Jian, X.; Zhang, S.; Shang, Y.; Xu, T.; Dai, S.; Xu, J.; Kong, D.; Wang, Y.; et al. Free-standing and flexible CNT/(Fe@ Si@ SiO2) composite anodes with kernel-pulp-skin nanostructure for high-performance lithium-ion batteries. J. Alloys Compd. 2021, 878, 160396. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, G.; Cai, X.; Yang, F.; Ma, C.; Xue, M.; Tao, X. Nanostructured strategies towards boosting organic lithium-ion batteries. J. Energy Chem. 2021, 54, 179–193. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Phung, V.D.; Mittova, V.O.; Ngo, H.D.; Vo, T.N.; Le Thi, M.L.; Mittova, I.Y.; Le, M.L.P.; Ahn, Y.N.; Kim, I.T.; et al. Fabricating nanostructured HoFeO3 perovskite for lithium-ion battery anodes via co-precipitation. Scr. Mater. 2022, 207, 114259. [Google Scholar] [CrossRef]
- Lan, X.; Cui, J.; Yu, H.; Xiong, X.; Tan, L.; Hu, R. Nanostructured Sn–Mo multilayer film anode with stable electrode-interfaces for long-cycle lithium storage. J. Power Sources 2021, 509, 230391. [Google Scholar] [CrossRef]
- Kakarla, A.K.; Narsimulu, D.; Yu, J.S. Two-dimensional porous NiCo2O4 nanostructures for use as advanced high-performance anode material in lithium-ion batteries. J. Alloys Compd. 2021, 886, 161224. [Google Scholar] [CrossRef]
- Woo, S.; Chung, K.; Bae, J.; Lee, Y.W.; Lee, S. Microwave-assisted hydrothermal synthesis of a high-voltage microcube LiMn1.5Ni0.5O4 spinel cathode material. J. Electroanal. Chem. 2021, 902, 115798. [Google Scholar] [CrossRef]
- Kozawa, T.; Fukuyama, K.; Kondo, A.; Naito, M. Wet milling synthesis of NH4CoPO4·H2O platelets: Formation reaction, growth mechanism, and conversion into high-voltage LiCoPO4 cathode for Li-ion batteries. Mater. Res. Bull. 2021, 135, 111149. [Google Scholar] [CrossRef]
- Tolganbek, N.; Yerkinbekova, Y.; Kalybekkyzy, S.; Bakenov, Z.; Mentbayeva, A. Current state of high voltage olivine structured LiMPO4 cathode materials for energy storage applications: A review. J. Alloys Compd. 2021, 882, 160774. [Google Scholar] [CrossRef]
- Hou, X.; Li, W.; Wang, Y.; Li, S.; Meng, Y.; Yu, H.; Chen, B.; Wu, X. Sodium-based dual-ion batteries via coupling high-capacity selenium/graphene anode with high-voltage graphite cathode. Chin. Chem. Lett. 2020, 31, 2314–2318. [Google Scholar] [CrossRef]
- Pang, P.; Tan, X.; Wang, Z.; Cai, Z.; Nan, J.; Xing, Z.; Li, H. Crack-free single-crystal LiNi0.83Co0.10Mn0.07O2 as cycling/thermal stable cathode materials for high-voltage lithium-ion batteries. Electrochim. Acta 2021, 365, 137380. [Google Scholar] [CrossRef]
- Papp, J.K.; Li, N.; Kaufman, L.A.; Naylor, A.J.; Younesi, R.; Tong, W.; McCloskey, B.D. A comparison of high voltage outgassing of LiCoO2, LiNiO2, and Li2MnO3 layered Li-ion cathode materials. Electrochim. Acta 2021, 368, 137505. [Google Scholar] [CrossRef]
- Peng, Q.; Wang, Y.; Yang, G. High crystallinity, preferred orientation and superior reversible capacity P2–Na0.67Ni0.25Mn0.75O2 thin film as cathode material for wide voltage sodium-ion battery. Electrochim. Acta 2020, 337, 135761. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, L.; Cui, Z.; Jia, H.; Engelhard, M.H.; Matthews, B.E.; Cao, X.; Xie, Q.; Wang, C.; Manthiram, A.; et al. Stabilizing ultrahigh-nickel layered oxide cathodes for high-voltage lithium metal batteries. Mater. Today 2021, 44, 15–24. [Google Scholar] [CrossRef]
- Kasim, M.F.; Azizan, W.A.H.W.; Elong, K.A.; Kamarudin, N.; Yaakob, M.K.; Badar, N. Enhancing the structural stability and capacity retention of Ni-rich LiNi0.7Co0.3O2 cathode materials via Ti doping for rechargeable Li-ion batteries: Experimental and computational approaches. J. Alloys Compd. 2021, 888, 161559. [Google Scholar] [CrossRef]
- Yang, M.; Hu, B.; Geng, F.; Li, C.; Lou, X.; Hu, B. Mitigating voltage decay in high-capacity Li1.2Ni0.2Mn0.6O2 cathode material by surface K+ doping. Electrochim. Acta 2018, 291, 278–286. [Google Scholar] [CrossRef]
- Kocak, T.; Wu, L.; Wang, J.; Savaci, U.; Turan, S.; Zhang, X. The effect of vanadium doping on the cycling performance of LiNi0.5Mn1.5O4 spinel cathode for high voltage lithium-ion batteries. J. Electroanal. Chem. 2021, 881, 114926. [Google Scholar] [CrossRef]
- Xu, Y.S.; Zhou, Y.N.; Zhang, Q.H.; Qi, M.Y.; Guo, S.J.; Luo, J.M.; Sun, Y.G.; Gu, L.; Cao, A.M.; Wan, L.J. Layered oxides with solid-solution reaction for high voltage potassium-ion batteries cathode. Chem. Eng. J. 2021, 412, 128735. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, S.; Liu, X.; Zhong, S.; Zou, Z. Facile Synthesis of 3D Urchin-like V6O13 Microflowers as Cathode Materials for High-Capacity and High-Rate Lithium-Ion Batteries. J. Electroanal. Chem. 2021, 900, 115742. [Google Scholar] [CrossRef]
- Deng, W.; Shi, W.; Liu, Q.; Jiang, J.; Wang, Q.; Guo, C. Conductive nonconjugated radical polymer as high capacity organic cathode material for high-energy Li/Na ion batteries. J. Power Sources 2020, 479, 228796. [Google Scholar] [CrossRef]
- Zhang, S.; Zou, Z.; Zhang, H.; Liu, J.; Zhong, S. Al/Ga co-doped V6O13 nanorods with high discharge specific capacity as cathode materials for lithium-ion batteries. J. Electroanal. Chem. 2021, 890, 115220. [Google Scholar] [CrossRef]
- Lin, J.; Chen, S.; Zhu, L.; Yuan, Z.; Liu, J. Soft-template fabrication of hierarchical nanoparticle iron fluoride as high-capacity cathode materials for Li-ion batteries. Electrochim. Acta 2020, 364, 137293. [Google Scholar] [CrossRef]
- Wu, Z.L.; Xie, H.; Li, Y.; Zhang, F.; Wang, Z.; Zheng, W.; Yang, M.; Xu, Z.; Lu, Z. 2Ni0.25Mn0.55O2: A high-capacity cathode material with a homogeneous monoclinic Li2MnO3-like superstructure. J. Alloys Compd. 2020, 827, 154202. [Google Scholar] [CrossRef]
- Park, J.S.; Cho, J.S.; Kim, J.H.; Choi, Y.J.; Kang, Y.C. Electrochemical properties of micron-sized Co3O4 hollow powders consisting of size controlled hollow nanospheres. J. Alloys Compd. 2016, 689, 554–563. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, L.; Wu, M.; Wang, Y.; Lou, X.W. Construction of complex Co3O4@ Co3V2O8 hollow structures from metal–organic frameworks with enhanced lithium storage properties. Adv. Mater. 2018, 30, 1702875. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xiang, K.; Zhu, Y.; Xiao, L.; Chen, W.; Liao, H.; Chen, X.; Chen, H. Porous, nitrogen-doped Li3V2 (PO4) 3/C cathode materials derived from oroxylum and their exceptional electrochemical properties in lithium-ion batteries. Ceram. Int. 2019, 45, 4980–4989. [Google Scholar] [CrossRef]
- Kim, N.Y.; Yim, T.; Lee, Z. A novel specimen preparation of porous cathode materials in lithium-ion batteries for high-resolution transmission electron microscopy. Mater. Charact. 2019, 155, 109804. [Google Scholar] [CrossRef]
- Qiu, S.; Fang, T.; Zhu, Y.; Hua, J.; Chu, H.; Zou, Y.; Zeng, J.L.; Xu, F.; Sun, L. 2Mn0.6Ni0.2O2 with 3D porous rod-like hierarchical micro/nanostructure for high-performance cathode material. J. Alloys Compd. 2019, 790, 863–870. [Google Scholar] [CrossRef]
- Gajraj, V.; Azmi, R.; Darma, M.S.D.; Indris, S.; Ehrenberg, H.; Mariappan, C.R. Correlation between structural, electrical and electrochemical performance of Zn doped high voltage spinel LiNi0.5−xZnxMn1.5O4 porous microspheres as a cathode material for Li-Ion batteries. Ceram. Int. 2021, 47, 35275–35286. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Luo, L.; Zhao, J.; He, J.; Zhao, X.; Liu, H.; Qin, Y.; Wang, F.; Song, J. Hierarchically porous MXene decorated carbon coated LiFePO4 as cathode material for high-performance lithium-ion batteries. J. Alloys Compd. 2021, 876, 160210. [Google Scholar] [CrossRef]
- Zhou, Z.; Luo, Z.; He, Z.; Zheng, J.; Li, Y. A novel hollow porous structure designed for Na0.44Mn2/3Co1/6Ni1/6O2 cathode material of sodium-ion batteries. J. Power Sources 2020, 479, 228788. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, W.; Ding, G.; Yan, F.; Jing, X.; Zhu, Z.; Gao, Y.; Wu, L.; Xu, G.; Sun, F. Hierarchically porous LiMn0.1Fe0.9PO4@ C microspherical cathode materials prepared by a facile template-free hydrothermal method for high-performance lithium-ion batteries. J. Alloys Compd. 2021, 859, 157825. [Google Scholar] [CrossRef]
- Karami, M.; Masoudpanah, S.M.; Rezaie, H.R. Solution combustion synthesis of hierarchical porous LiFePO4 powders as cathode materials for lithium-ion batteries. Adv. Powder Technol. 2021, 32, 1935–1942. [Google Scholar] [CrossRef]
- Gu, H.; Wang, J.; Wang, Z.; Tong, J.; Qi, N.; Han, G.; Zhang, M. Self-assembled porous LiNi0.8Co0.1Mn0.1O2 cathode materials with micro/nano-layered hollow morphologies for high-power lithium-ion batteries. Appl. Surf. Sci. 2021, 539, 148034. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Shen, S.; Yan, X.; Wu, A.; Yin, J.; Zhang, J. Hollow porous bowl-shaped lithium-rich cathode material for lithium-ion batteries with exceptional rate capability and stability. J. Power Sources 2018, 380, 164–173. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, M.; Tamirate, A.G.; Zhu, H.; Wang, C.; Xia, Y. Carbon coated nano-sized LiMn0.8Fe0.2PO4 porous microsphere cathode material for Li-ion batteries. J. Power Sources 2020, 448, 227438. [Google Scholar] [CrossRef]
- Deng, B.; Chen, Y.; Wu, P.; Han, J.; Li, Y.; Zheng, H.; Xie, Q.; Wang, L.; Peng, D.L. Lithium-rich layered oxide nanowires bearing porous structures and spinel domains as cathode materials for lithium-ion batteries. J. Power Sources 2019, 418, 122–129. [Google Scholar] [CrossRef]
- Balakrishnan, P.G.; Ramesh, R.; Kumar, T.P. Safety mechanisms in lithium-ion batteries. J. Power Sources 2006, 155, 401–414. [Google Scholar] [CrossRef]
- Huang, W.; Feng, X.; Han, X.; Zhang, W.; Jiang, F. Questions and Answers Relating to Lithium-Ion Battery Safety Issues. Cell Rep. Phys. Sci. 2021, 2, 100285. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Yang, M.; Chen, W. High-safety separators for lithium-ion batteries and sodium-ion batteries: Advances and perspective. Energy Storage Mater. 2021, 41, 522–545. [Google Scholar] [CrossRef]
- Hu, G.; Huang, P.; Bai, Z.; Wang, Q.; Qi, K. Comprehensively analysis the failure evolution and safety evaluation of automotive lithium ion battery. eTransportation 2021, 10, 100140. [Google Scholar] [CrossRef]
- Zhou, M.; Hu, L.; Chen, S.; Zhao, X. Different mechanical-electrochemical coupled failure mechanism and safety evaluation of lithium-ion pouch cells under dynamic and quasi-static mechanical abuse. J. Power Sources 2021, 497, 229897. [Google Scholar] [CrossRef]
- Lyu, P.; Liu, X.; Qu, J.; Zhao, J.; Huo, Y.; Qu, Z.; Rao, Z. Recent advances of thermal safety of lithium ion battery for energy storage. Energy Storage Mater. 2020, 31, 195–220. [Google Scholar] [CrossRef]
- Liang, L.; Yuan, W.; Chen, X.; Liao, H. Flexible, nonflammable, highly conductive and high-safety double cross-linked poly (ionic liquid) as quasi-solid electrolyte for high performance lithium-ion batteries. Chem. Eng. J. 2021, 421, 130000. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, Y.; Zhou, Q. Effect of low-temperature aging on the safety performance of lithium-ion pouch cells under mechanical abuse condition: A comprehensive experimental investigation. Energy Storage Mater. 2021, 40, 268–281. [Google Scholar] [CrossRef]
- Aikhuele, D.O. Development of a fixable model for the reliability and safety evaluation of the components of a commercial lithium-ion battery. J. Energy Storage 2020, 32, 101819. [Google Scholar] [CrossRef]
- Mo, L.; Zheng, H. Solid coated Li4Ti5O12 (LTO) using polyaniline (PANI) as anode materials for improving thermal safety for lithium ion battery. Energy Rep. 2020, 6, 2913–2918. [Google Scholar] [CrossRef]
- Münster, P.; Diehl, M.; Frerichs, J.E.; Börner, M.; Hansen, M.R.; Winter, M.; Niehoff, P. Effect of Li plating during formation of lithium ion batteries on their cycling performance and thermal safety. J. Power Sources 2021, 484, 229306. [Google Scholar] [CrossRef]
- Yiding, L.; Wenwei, W.; Cheng, L.; Xiaoguang, Y.; Fenghao, Z. A safety performance estimation model of lithium-ion batteries for electric vehicles under dynamic compression. Energy 2021, 215, 119050. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, D.; Duo, X.; Sheng, X. A review of spinel lithium titanate (Li4Ti5O12) as electrode material for advanced energy storage devices. Ceram. Int. 2021, 47, 5870–5895. [Google Scholar] [CrossRef]
- Ferg, E.; Gummow, R.D.; De Kock, A.; Thackeray, M.M. Spinel anodes for lithium-ion batteries. J. Electrochem. Soc. 1994, 141, L147. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, B.; Lu, Z.; Liu, N.; Zhu, J. Challenges and recent progress in the development of Si anodes for lithium-ion battery. Adv. Energy Mater. 2017, 7, 1700715. [Google Scholar] [CrossRef] [Green Version]
- Wilkening, M.; Amade, R.; Iwaniak, W.; Heitjans, P. Ultraslow Li diffusion in spinel-type structured Li4Ti5O12—A comparison of results from solid state NMR and impedance spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1239–1246. [Google Scholar] [CrossRef]
- Yuan, T.; Tan, Z.; Ma, C.; Yang, J.; Ma, Z.F.; Zheng, S. Challenges of spinel Li4Ti5O12 for lithium-ion battery industrial applications. Adv. Energy Mater. 2017, 7, 1601625. [Google Scholar] [CrossRef]
- Aravindan, V.; Lee, Y.S.; Madhavi, S. Research progress on negative electrodes for practical Li-ion batteries: Beyond carbonaceous anodes. Adv. Energy Mater. 2015, 5, 1402225. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Schön, J.C.; Wevers, M.A.C.; Jansen, M. Investigation of the possible ternary nitrides in the system Li3N/Na3N. Solid State Sci. 2000, 2, 449–456. [Google Scholar] [CrossRef]
- Somer, M.; Carrillo-Cabrera, W.; Peters, E.M.; Peters, K.; Von Schnering, H.G. Crystal structure of lithium beryllium nitride, LiBeN. Z. Krist.-Cryst. Mater. 1996, 211, 635. [Google Scholar] [CrossRef]
- Yamane, H.; Okabe, T.H.; Ishiyama, O.; Waseda, Y.; Shimada, M. Ternary nitrides prepared in the Li3N–Mg3N2 system at 900–1000 K. J. Alloys Compd. 2001, 319, 124–130. [Google Scholar] [CrossRef]
- Nishijima, M.; Takeda, Y.; Imanishi, N.; Yamamoto, O.; Takano, M. Li deintercalation and structural change in the lithium transition metal nitride Li3FeN2. J. Solid State Chem. 1994, 113, 205–210. [Google Scholar] [CrossRef]
- Emery, N.; Sougrati, M.T.; Panabière, E.; Bach, S.; Fraisse, B.; Jumas, J.C.; Pereira-Ramos, J.P.; Willmann, P. Unidimensional unit cell variation and Fe+3/Fe+4 redox activity of Li3FeN2 in Li-ion batteries. J. Alloys Compd. 2017, 696, 971–979. [Google Scholar] [CrossRef]
- Panabière, E.; Emery, N.; Lorthioir, C.; Sougrati, M.T.; Jumas, J.C.; Bach, S.; Pereira-Ramos, J.P.; Smith, R.I.; Willmann, P. Structural reinvestigation of Li3FeN2: Evidence of cationic disorder through XRD, solid-state NMR and Mössbauer spectroscopy. J. Phys. Chem. Solids 2016, 95, 37–42. [Google Scholar] [CrossRef]
- Frankenburger, W.; Andrussow, L.; Dürr, F. Eine neue Komplexverbindung von Lithium, Eisen und Stickstoff. Ein Beitrag zur Frage der Stickstoffbindung an Eisen. Z. Elektrochem. Angew. Phys. Chem. 1928, 34, 632–637. [Google Scholar]
- Fromont, M. Preparation and Study of Double Nitride Li3FeN2. Rev. Chim. Miner. 1967, 4, 447. [Google Scholar]
- Langmi, H.W.; Culligan, S.D.; McGrady, G.S. Mixed-metal Li3N-based systems for hydrogen storage: Li3AlN2 and Li3FeN2. Int. J. Hydrogen Energy 2009, 34, 8108–8114. [Google Scholar] [CrossRef]
- Wang, P.; Guo, J.; Xiong, Z.; Wu, G.; Wang, J.; Chen, P. The interactions of Li3FeN2 with H2 and NH3. Int. J. Hydrogen Energy 2016, 41, 14171–14177. [Google Scholar] [CrossRef]
- Cabana, J.; Ling, C.D.; Oró-Solé, J.; Gautier, D.; Tobias, G.; Adams, S.; Canadell, E.; Palacin, M.R. Antifluorite-type lithium chromium oxide nitrides: Synthesis, structure, order, and electrochemical properties. Inorg. Chem. 2004, 43, 7050–7060. [Google Scholar] [CrossRef]
- Lei, X.; Wang, J.; Peng, R.; Wang, W. The controllable magnetic properties of Fe3N nanoparticles synthesized by a simple urea route. Mater. Res. Bull. 2020, 122, 110662. [Google Scholar] [CrossRef]
- Veryatin Ud Mashirev, V.P.; Ryabtsev, N.G.; Tarasov, V.I.; Rogozkin, B.D.; Korobov, I.V. Thermodinamicheskie Svoitsva Neorganicheskikh Veshchestv; Atomizdat: Moscow, Russia, 1965. [Google Scholar]
- Glushko, V.P.; Gurvich, L.V.; Bergman, G.A.; Veits, I.V.; Medvedev, V.A.; Khachkuruzov, G.A.; Yungman, V.S. Thermodinamicheskie Svoitsva Individual’nykh Veshchestv; Nauka: Moscow, Russia, 1978. [Google Scholar]
- McHale, J.M.; Navrotsky, A.; Kowach, G.R.; Balbarin, V.E.; DiSalvo, F.J. Energetics of Ternary Nitrides: Li−Ca−Zn−N and Ca−Ta−N Systems. Chem. Mater. 1997, 9, 1538–1546. [Google Scholar] [CrossRef]
- McHale, J.M.; Navrotsky, A.; DiSalvo, F.J. Energetics of Ternary Nitride Formation in the (Li, Ca)−(B, Al)−N System. Chem. Mater. 1999, 11, 1148–1152. [Google Scholar] [CrossRef]
- Elder, S.H.; DiSalvo, F.J.; Topor, L.; Navrotsky, A. Thermodynamics of ternary nitride formation by ammonolysis: Application to lithium molybdenum nitride (LiMoN2), sodium tungsten nitride (Na3WN3), and sodium tungsten oxide nitride (Na3WO3N). Chem. Mater. 1993, 5, 1545–1553. [Google Scholar] [CrossRef]
- He, G.; Herbst, J.F.; Ramesh, T.N.; Pinkerton, F.E.; Meyer, M.S.; Nazar, L. Investigation of hydrogen absorption in Li7VN4 and Li7MnN4. Phys. Chem. Chem. Phys. 2011, 13, 8889–8893. [Google Scholar] [CrossRef]
- Pankratz, L.B. Thermodynamic Properties of Carbides, Nitrides, and Other Selected Substances; U.S. Department of the Interior: Washington, DC, USA, 1995.
- Knunyants, I.L. Khimicheskaya Entsiklopediya; Sovetskaya Entsiklopediya: Moscow, Russia, 1988. [Google Scholar]
- Gudat, A.; Kniep, R.; Rabenau, A.; Bronger, W.; Ruschewitz, U. Li3FeN2, a ternary nitride with 1∞[FeN423−] chains: Crystal structure and magnetic properties. J. Less Common Met. 1990, 161, 31–36. [Google Scholar] [CrossRef]
- Morachevskiy, A.G.; Sladkov, I.B.; Firsova Ye, G. Termodinamicheskiye Raschety v Khimii i Metallurgii; Lan’: St. Petersburg, Russia, 2018. [Google Scholar]

| Name | Formula | Source | Purity, % |
|---|---|---|---|
| Iron nanopowder | Fe | Changsha Easchem Co., Ltd. (Changsha, China) | 99.9 |
| Lithium | Li | Xiamen Tmax Battery Equipments Ltd. (Xiamen, China) | 99.9 |
| Nitrogen | N2 | Qingdao Guida Special Gas Co., Ltd. (Qingdao, China) | 99.9–99.999 |
| Ammonia | NH3 | Wuhan Newradar Trade Company Ltd. (Wuhan, China) | 99.9 |
| Lithium nitride | Li3N | Prepared here | 98.9 1 |
| Iron nitride | Fe2N | Prepared here | 98.4 1 |
| Lithium iron nitride | Li3FeN2 | Prepared here | 99.1 1 |
| Atom/Void | Site | g | x | y | z |
|---|---|---|---|---|---|
| Li1 | 8g | 0.91 | 0.0 | 0.25745 | 0.25 |
| Li2 | 4b | 1 | 0.0 | 0.5 | 0.25 |
| Fe | 4a | 1 | 0.0 | 0.0 | 0.25 |
| N | 8j | 0.98 | 0.219979 | 0.113757 | 0.5 |
| Compound | Specific Enthalpy, J g−1 | Molar Mass, g mol−1 | Molar Enthalpy of Dissolution, kJ mol−1 | Ref. |
|---|---|---|---|---|
| Li3N | −3163.853 ± 30 | 34.83 | −110.197 ± 1.7 | this work |
| Fe2N | −13.79 ± 1.5 | 125.701 | −1.734 ± 0.04 | this work |
| N2 | −71.716 ± 10 | 28.014 | −2.56 ± 0.12 | this work |
| Li3FeN2 | −1972.96 ± 25 | 104.684 | −206.537 ± 2.8 | this work |
| Li3Na3N2 | −2285.96 ± 13.4 | 117.807 | −269.3018 | [66] |
| Compound | ΔfH0298.15, kJ mol−1 | Reference |
|---|---|---|
| Li3N(cryst) | −196.78 ± 0.3 | [76] |
| Fe2N(cryst) | −3.77 ± 0.1 | [76] |
| N2(gas) | 8.67 ± 0.1 | [77] |
| Li3FeN2(cryst) | −291.331 ± 5.7 | this work |
| LiCaN(cryst) | −216.8 ± 10.8 | [78] |
| Li3BN2(cryst) | −534.5 ± 16.7 | [79] |
| Li3AlN2(cryst) | −567.8 ± 12.4 | [79] |
| LiMoN2(cryst) | −386.0 ± 6.4 | [80] |
| Li7MnN4 | −661 | [81] |
| T, K | Cp(exp.), J K−1 mol−1 | Cp(rec.), J K−1 mol−1 | Cp(N-K), J K−1 mol−1 |
|---|---|---|---|
| 300 | 126.9 | 124.1 | 117.8 |
| 400 | 134.1 | 132.6 | 130.7 |
| 500 | 146.3 | 144.3 | 141.9 |
| 600 | 160.5 | 158.3 | 152.8 |
| 700 | 173.8 | 171.9 | 163.4 |
| 800 | 183.3 | 180.7 | 173.6 |
| 900 | 186.1 | 178.8 | 183.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popovich, A.; Novikov, P.; Wang, Q.; Pushnitsa, K.; Aleksandrov, D. Synthesis Method and Thermodynamic Characteristics of Anode Material Li3FeN2 for Application in Lithium-Ion Batteries. Materials 2021, 14, 7562. https://doi.org/10.3390/ma14247562
Popovich A, Novikov P, Wang Q, Pushnitsa K, Aleksandrov D. Synthesis Method and Thermodynamic Characteristics of Anode Material Li3FeN2 for Application in Lithium-Ion Batteries. Materials. 2021; 14(24):7562. https://doi.org/10.3390/ma14247562
Chicago/Turabian StylePopovich, Anatoliy, Pavel Novikov, Qingsheng Wang, Konstantin Pushnitsa, and Daniil Aleksandrov. 2021. "Synthesis Method and Thermodynamic Characteristics of Anode Material Li3FeN2 for Application in Lithium-Ion Batteries" Materials 14, no. 24: 7562. https://doi.org/10.3390/ma14247562
APA StylePopovich, A., Novikov, P., Wang, Q., Pushnitsa, K., & Aleksandrov, D. (2021). Synthesis Method and Thermodynamic Characteristics of Anode Material Li3FeN2 for Application in Lithium-Ion Batteries. Materials, 14(24), 7562. https://doi.org/10.3390/ma14247562





