Epitaxial Growth of Diamond-Shaped Au1/2Ag1/2CN Nanocrystals on Graphene
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Formation of Diamond-Shaped Au1/2Ag1/2CN Nanocrystals
3.2. Epitaxial Alignment between Au1/2Ag1/2CN Nanocrystals and Graphene
3.3. Geometrical Characteristics of the Au1/2Ag1/2CN Nanocrystals
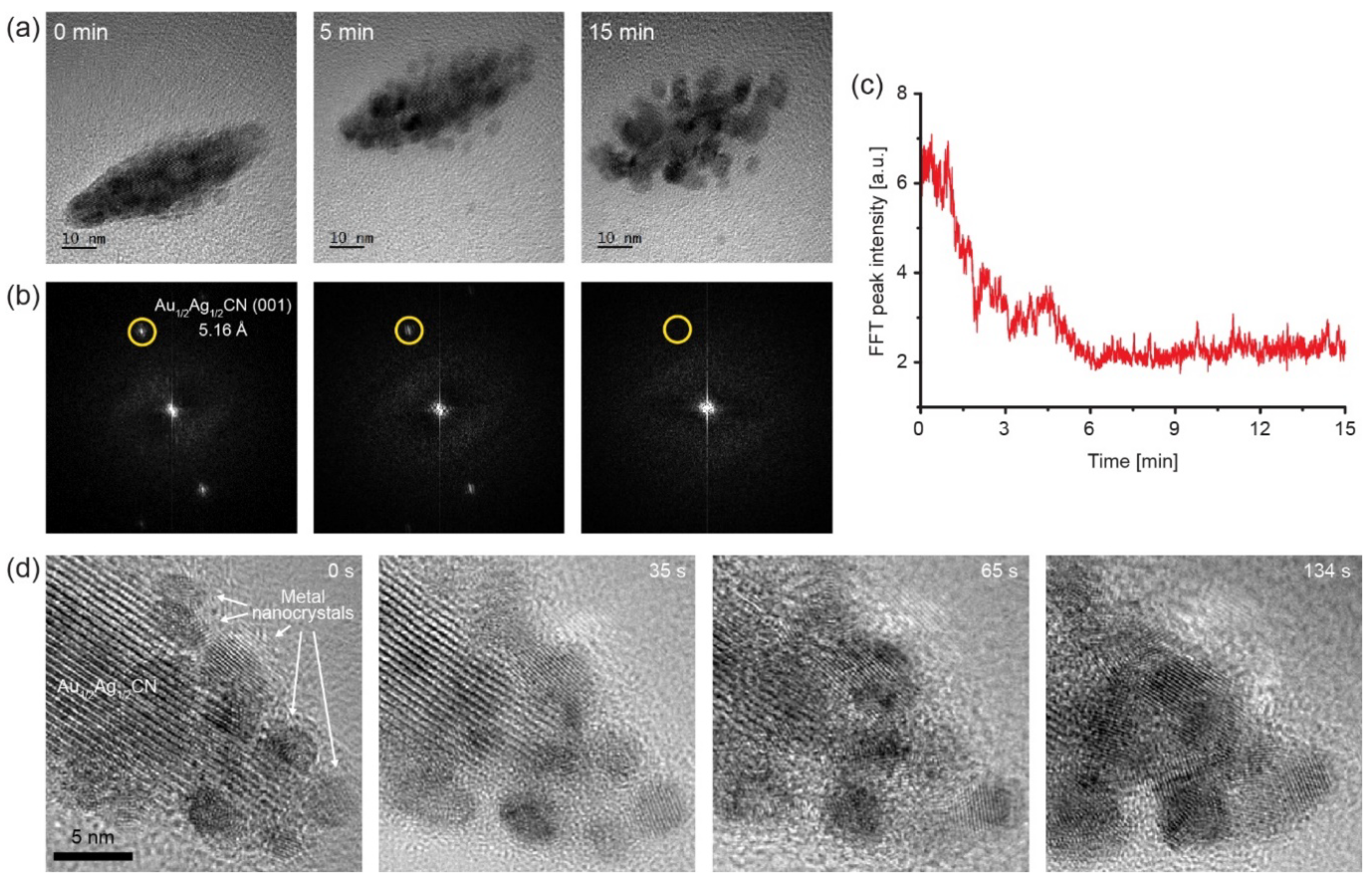
3.4. In Situ TEM Observation of e-Beam-Induced Decomposition of Au1/2Ag1/2CN
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Mishchenko, A.; Carvalho, A.; Neto, A.H.C. 2D materials and van der Waals heterostructures. Science 2016, 353, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geim, A.K.; Grigorieva, I.V. Van der Waals heterostructures. Nature 2013, 499, 419–425. [Google Scholar] [CrossRef]
- Blackstone, C.; Ignaszak, A. Van der Waals Heterostructures—Recent Progress in Electrode Materials for Clean Energy Applications. Materials 2021, 14, 3754. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kim, K.; Lee, Y.; Kim, K.; Lee, W.C.; Park, J. Self-organized growth and self-assembly of nanostructures on 2D materials. FlatChem 2017, 5, 50–68. [Google Scholar] [CrossRef]
- Chung, K.; Lee, C.H.; Yi, G.C. Transferable GaN Layers Grown on ZnO-Coated Graphene Layers for Optoelectronic Devices. Science 2010, 330, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.J.; Lee, W.H.; Wu, Y.P.; Ruoff, R.S.; Fukui, T. van der Waals Epitaxy of InAs Nanowires Vertically Aligned on Single-Layer Graphene. Nano Lett. 2012, 12, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, H.B.R.; Johnson, R.W.; Tanskanen, J.T.; Liu, N.; Kim, M.G.; Pang, C.; Ahn, C.; Bent, S.F.; Bao, Z.N. Selective metal deposition at graphene line defects by atomic layer deposition. Nat. Commun. 2014, 5, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.; Liao, L.; Gong, Y.J.; Li, Y.B.; Shi, F.F.; Pei, A.; Sun, J.; Zhang, R.F.; Kong, B.; Subbaraman, R.; et al. Stitching h-BN by atomic layer deposition of LiF as a stable interface for lithium metal anode. Sci. Adv. 2017, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Khalil, I.; Julkapli, N.M.; Yehye, W.A.; Basirun, W.J.; Bhargava, S.K. Graphene–Gold Nanoparticles Hybrid—Synthesis, Functionalization, and Application in a Electrochemical and Surface-Enhanced Raman Scattering Biosensor. Materials 2016, 9, 406. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.R.; Tabakman, S.M.; Dai, H.J. Atomic layer deposition of metal oxides on pristine and functionalized graphene. J. Am. Chem. Soc. 2008, 130, 8152–8153. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Zhou, X.Z.; Wu, S.X.; Wei, Y.Y.; Qi, X.Y.; Zhang, J.; Boey, F.; Zhang, H. Reduced Graphene Oxide-Templated Photochemical Synthesis and in situ Assembly of Au Nanodots to Orderly Patterned Au Nanodot Chains. Small 2010, 6, 513–516. [Google Scholar] [CrossRef]
- Lin, Y.C.; Ghosh, R.K.; Addou, R.; Lu, N.; Eichfeld, S.M.; Zhu, H.; Li, M.Y.; Peng, X.; Kim, M.J.; Li, L.J.; et al. Atomically thin resonant tunnel diodes built from synthetic van der Waals heterostructures. Nat. Commun. 2015, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Ago, H.; Fukamachi, S.; Endo, H.; Solis-Fernandez, P.; Yunus, R.M.; Uchida, Y.; Panchal, V.; Kazakova, O.; Tsuji, M. Visualization of Grain Structure and Boundaries of Polycrystalline Graphene and Two-Dimensional Materials by Epitaxial Growth of Transition Metal Dichalcogenides. ACS Nano 2016, 10, 3233–3240. [Google Scholar] [CrossRef]
- Zhou, H.Q.; Yu, F.; Chen, M.J.; Qiu, C.Y.; Yang, H.C.; Wang, G.; Yu, T.; Sun, L.F. The transformation of a gold film on few-layer graphene to produce either hexagonal or triangular nanoparticles during annealing. Carbon 2013, 52, 379–387. [Google Scholar] [CrossRef]
- Gong, Y.; Lin, J.; Wang, X.; Shi, G.; Lei, S.; Lin, Z.; Zou, X.; Ye, G.; Vajtai, R.; Yakobson, B.I.; et al. Vertical and in-plane heterostructures from WS2/MoS2 monolayers. Nat. Mater. 2014, 13, 1135–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.C.; Kim, K.; Park, J.; Koo, J.; Jeong, H.Y.; Lee, H.; Weitz, D.A.; Zettl, A.; Takeuchi, S. Graphene-templated directional growth of an inorganic nanowire. Nat. Nanotechnol. 2015, 10, 423–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Lim, K.; Lee, Y.; Kim, J.; Kim, K.; Park, J.; Kim, K.; Lee, W.C. Precise Identification of Graphene’s Crystal Structures by Removable Nanowire Epitaxy. J. Phys. Chem. Lett. 2017, 8, 1302–1309. [Google Scholar] [CrossRef]
- Jang, J.; Lee, Y.; Yoon, J.Y.; Yoon, H.H.; Koo, J.; Choe, J.; Jeon, S.; Sung, J.; Park, J.; Lee, W.C.; et al. One-Dimensional Assembly on Two-Dimensions: AuCN Nanowire Epitaxy on Graphene for Hybrid Phototransistors. Nano Lett. 2018, 18, 6214–6221. [Google Scholar] [CrossRef]
- Jang, M.; Bae, H.; Lee, Y.; Na, W.; Yu, B.; Choi, S.; Cheong, H.; Lee, H.; Kim, K. Unidirectional Alignment of AgCN Microwires on Distorted Transition Metal Dichalcogenide Crystals. ACS Appl. Mater. Interfaces 2021, 13, 8727–8735. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Koo, J.; Lee, S.; Yoon, J.Y.; Kim, K.; Jang, M.; Jang, J.; Choe, J.; Li, B.W.; Le, C.T.; et al. Universal Oriented van der Waals Epitaxy of 1D Cyanide Chains on Hexagonal 2D Crystals. Adv. Sci. 2020, 7, 9. [Google Scholar] [CrossRef]
- Koma, A. Van der Waals epitaxy—a new epitaxial growth method for a highly lattice-mismatched system. Thin Solid Films 1992, 216, 72–76. [Google Scholar] [CrossRef]
- Koma, A. Van der Waals epitaxy for highly lattice-mismatched systems. J. Cryst. Growth 1999, 201-202, 236–241. [Google Scholar] [CrossRef]
- Chippindale, A.M.; Hibble, S.J.; Bilbe, E.J.; Marelli, E.; Hannon, A.C.; Allain, C.; Pansu, R.; Hartl, F. Mixed Copper, Silver, and Gold Cyanides, (MxM ’(1−x))CN: Tailoring Chain Structures To Influence Physical Properties. J. Am. Chem. Soc. 2012, 134, 16387–16400. [Google Scholar] [CrossRef] [PubMed]
- Toth, Z.; Zsoter, Z.; Beck, M.T. Testing the photocatalytic activity of cyanogen-and thiocyanogen-based inorganic polymers. React. Kinet. Catal. Lett. 1992, 47, 29–35. [Google Scholar] [CrossRef]
- Daroczi, L.; Beck, M.T.; Beke, D.L.; Kis-Varga, M.; Harasztosi, L.; Takacs, N. Production of Fe and Cu nanocrystalline particles by thermal decomposition of ferro- and copper-cyanides. In Mechanically Alloyed, Metastable and Nanocrystalline Materials, Part 1; Materials Science Forum; Baro, M.D., Surinach, S., Eds.; Transtec Publications Ltd.: Zurich-Uetikon, Switzerland, 1998; Volume 269-2, pp. 319–324. [Google Scholar]
- Bowmaker, G.A.; Kennedy, B.J.; Reid, J.C. Crystal structures of AuCN and AgCN and vibrational spectroscopic studies of AuCN, AgCN, and CuCN. Inorg. Chem. 1998, 37, 3968–3974. [Google Scholar] [CrossRef] [PubMed]
- Regan, W.; Alem, N.; Aleman, B.; Geng, B.S.; Girit, C.; Maserati, L.; Wang, F.; Crommie, M.; Zettl, A. A direct transfer of layer-area graphene. Appl. Phys. Lett. 2010, 96, 3. [Google Scholar] [CrossRef] [Green Version]
- Jeon, S.; Heo, T.; Hwang, S.Y.; Ciston, J.; Bustillo, K.C.; Reed, B.W.; Ham, J.M.; Kang, S.S.; Kim, S.G.; Lim, J.; et al. Reversible disorder-order transitions in atomic crystal nucleation. Science 2021, 371, 498. [Google Scholar] [CrossRef]
- Liao, H.G.; Zherebetskyy, D.; Xin, H.L.; Czarnik, C.; Ercius, P.; Elmlund, H.; Pan, M.; Wang, L.W.; Zheng, H.M. Facet development during platinum nanocube growth. Science 2014, 345, 916–919. [Google Scholar] [CrossRef]
- Beck, M.T.; Bertoti, I.; Mohai, M.; Nemeth, P.; Jakab, E.; Szabo, L.; Szepvolgyi, J. Gold nano-particle formation from crystalline AuCN: Comparison of thermal, plasma- and ion-beam activated decomposition. J. Solid State Chem. 2017, 246, 65–74. [Google Scholar] [CrossRef]
- Akhtar, M.N.; Isab, A.A.; Hassan, A. Thermal decomposition of trialkyl/arylphosphine gold(I) cyanide complexes. J. Therm. Anal. 2000, 61, 119–125. [Google Scholar] [CrossRef]
- Lim, J.; Ham, J.; Lee, W.; Hwang, E.; Lee, W.C.; Hong, S. A Transformative Gold Patterning through Selective Laser Refining of Cyanide. Nanomaterials 2021, 11, 1921. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.; Ham, J.; Heo, Y.J.; Lee, W.C. Epitaxial Growth of Diamond-Shaped Au1/2Ag1/2CN Nanocrystals on Graphene. Materials 2021, 14, 7569. https://doi.org/10.3390/ma14247569
Park C, Ham J, Heo YJ, Lee WC. Epitaxial Growth of Diamond-Shaped Au1/2Ag1/2CN Nanocrystals on Graphene. Materials. 2021; 14(24):7569. https://doi.org/10.3390/ma14247569
Chicago/Turabian StylePark, Chunggeun, Jimin Ham, Yun Jung Heo, and Won Chul Lee. 2021. "Epitaxial Growth of Diamond-Shaped Au1/2Ag1/2CN Nanocrystals on Graphene" Materials 14, no. 24: 7569. https://doi.org/10.3390/ma14247569
APA StylePark, C., Ham, J., Heo, Y. J., & Lee, W. C. (2021). Epitaxial Growth of Diamond-Shaped Au1/2Ag1/2CN Nanocrystals on Graphene. Materials, 14(24), 7569. https://doi.org/10.3390/ma14247569





