Rehydration Driven Na-Activation of Bentonite—Evolution of the Clay Structure and Composition
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
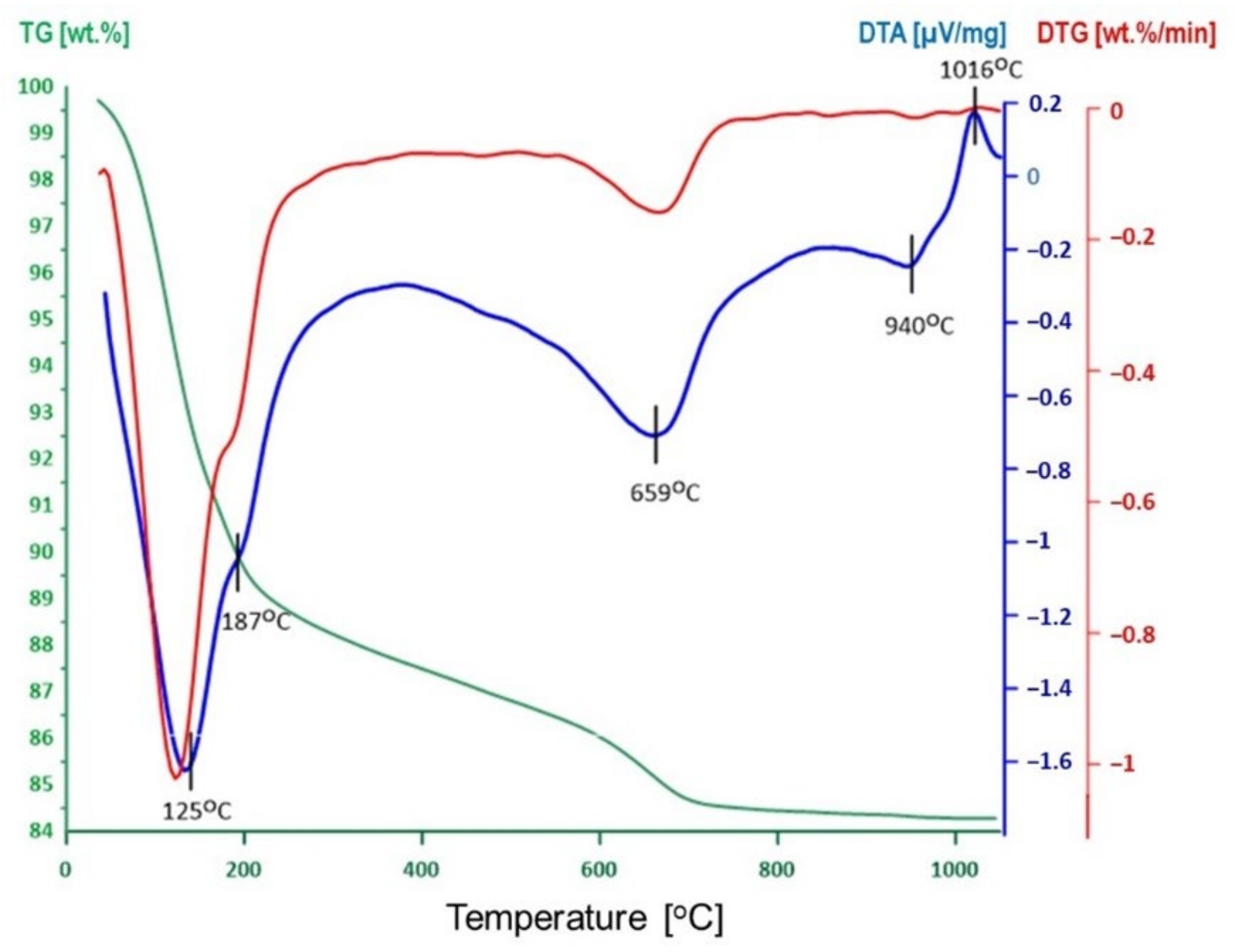
3.1. Thermal Activation
3.2. Sodium Activation
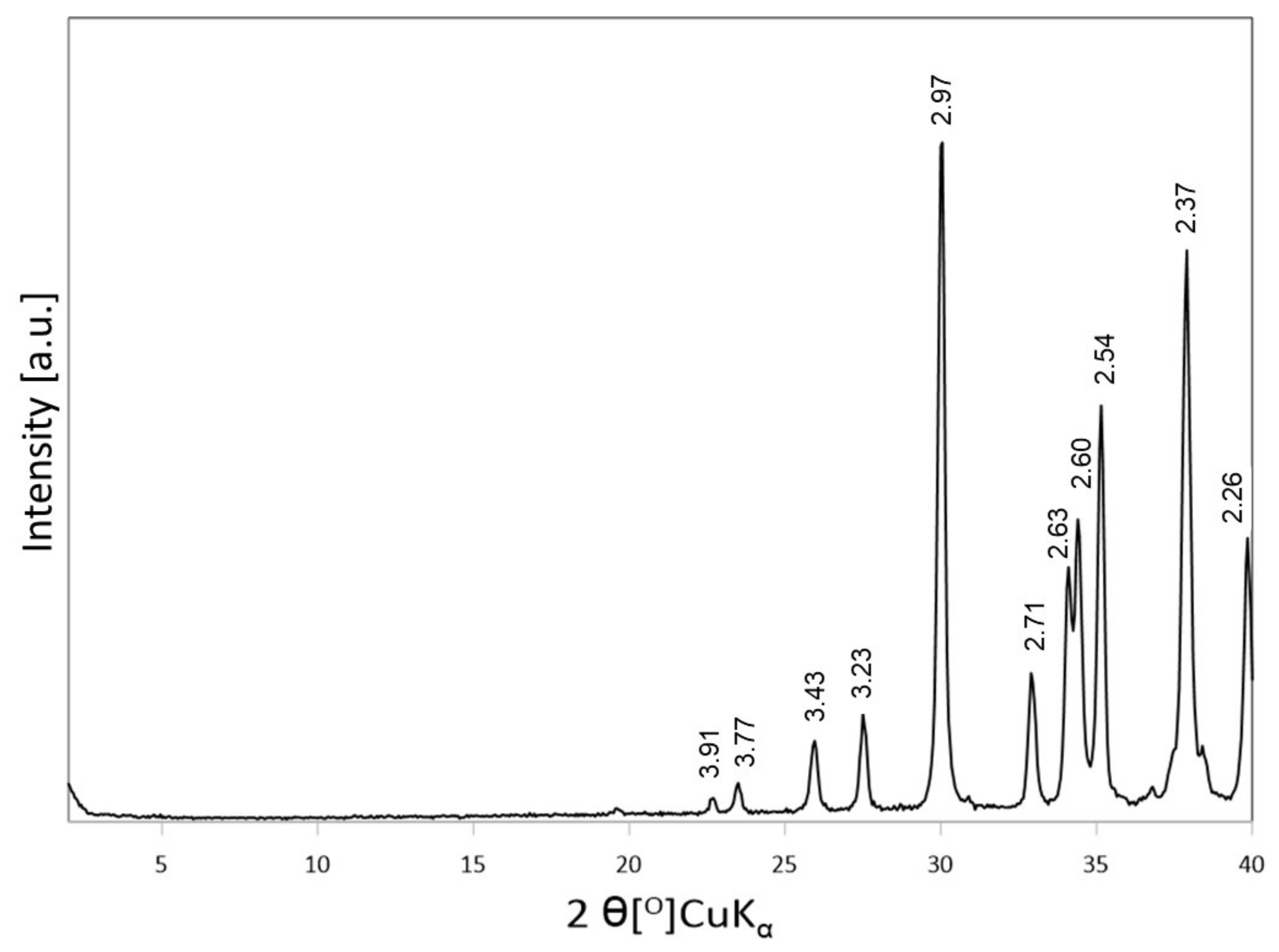
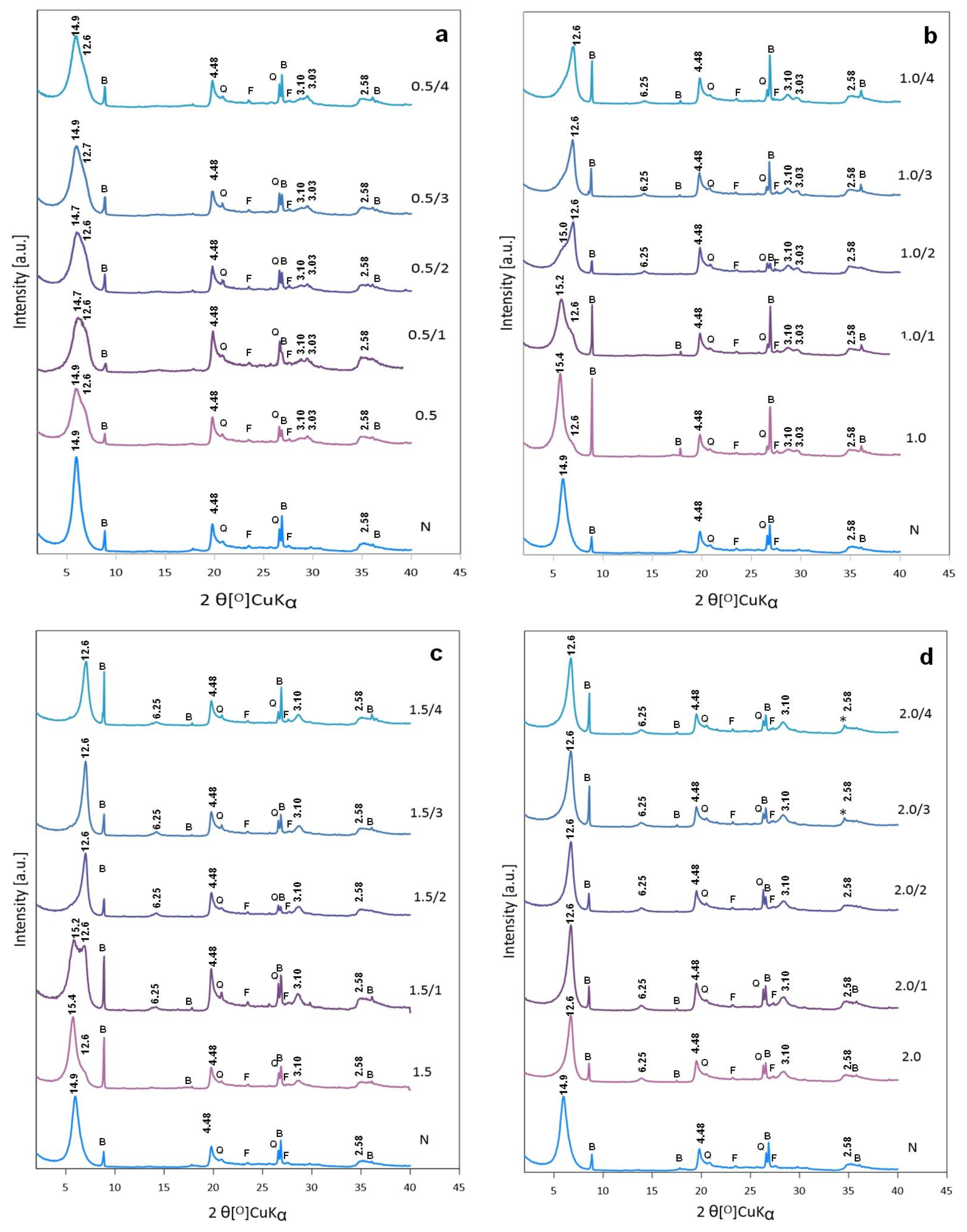
3.2.1. XRD Analysis of Activated Bentonites
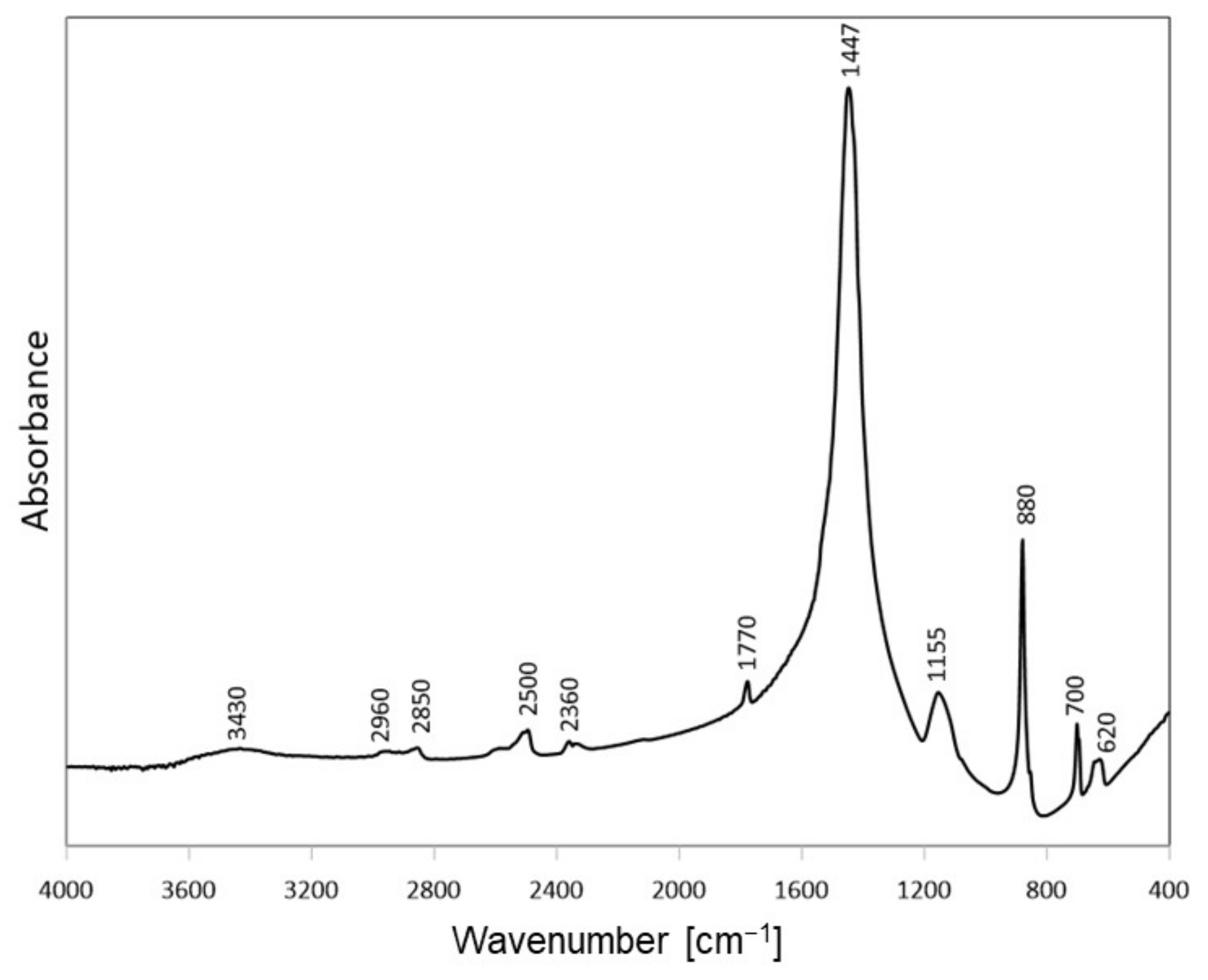
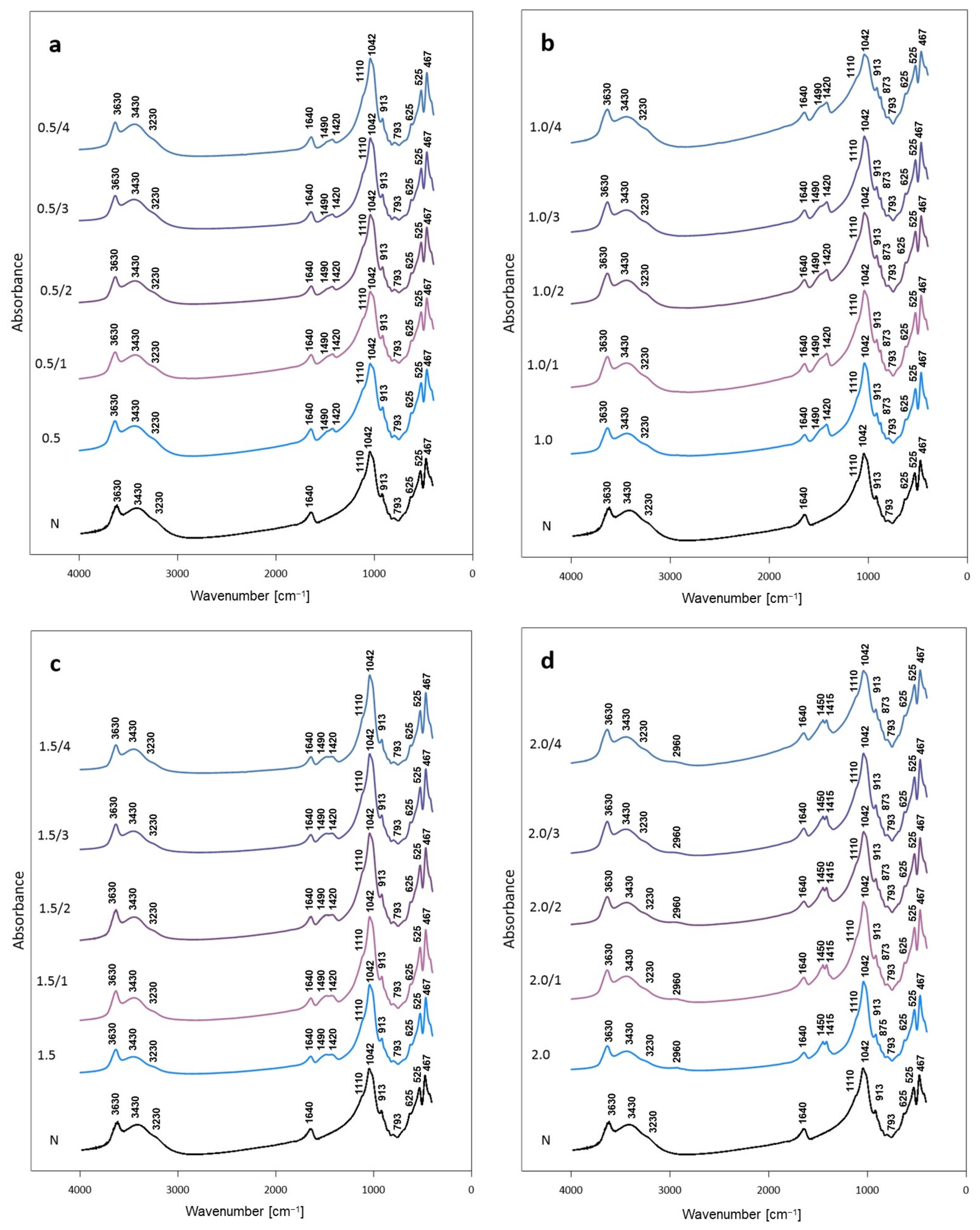
3.2.2. FTIR Analysis of Activated Bentonites
3.2.3. Influence of Na2CO3 Concentration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christidis, G.E. The concept of layer charge of smectites and its implications for important smectite-water properties. In Layered Mineral Structures and Their Application in Advanced Technologies; Brigatti, M.F., Mottana, A., Eds.; Mineralogical Society: London, UK, 2011; pp. 239–260. [Google Scholar]
- Murray, H.H. Bentonite Applications. In Applied Clay Mineralogy: Occurrences, Processing and Applications of Kaolins, Bentonites, Palygorskite-Sepiolite, and Common Clays, 1st ed.; Murray, H.H., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 111–130. [Google Scholar]
- Heller-Kalai, L. Thermally modified clay minerals. In Handbook of Clay Science. Part A: Fundamentals, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 411–433. [Google Scholar]
- Harvey, C.C.; Lagaly, G. Industrial application. In Handbook of Clay Science. Part B: Techniques and Applications, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 451–490. [Google Scholar]
- Erbslöh, S. Improved Manufacture of Highly Swellable Inorganic Substances. GB Patent 447710, 12 June 1935. [Google Scholar]
- Erbslöh, S. Improved Manufacture of Highly Swellable Inorganic Substances. GB Patent 458240, 1 July 1936. [Google Scholar]
- He, H.; Frost, R.L.; Deng, F.; Zhu, J.; Wen, X.; Yuan, P. Conformation of Surfactant Molecules in the Interlayer of Montmorillonite Studied by 13C MAS NMR. Clays Clay Miner. 2004, 52, 350–356. [Google Scholar] [CrossRef] [Green Version]
- Yuan, P.; He, H.P.; Bergaya, F.; Wu, D.Q.; Zhou, Q.; Zhu, J.X. Synthesis and characterization of delaminated iron-pillared clay with meso-microporous structure. Micropor. Mesopor. Mater. 2006, 88, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Lebedenko, F.; Plée, D. Some considerations on the ageing of Na2CO3-activated bentonites. Appl. Clay Sci. 1988, 3, 1–10. [Google Scholar] [CrossRef]
- Volzone, C.; Garrido, L.B. The effect of some physico-chemical and mineralogical properties on the Na2CO3 activation of Argentine bentonites. Appl. Clay Sci. 1991, 6, 143–154. [Google Scholar] [CrossRef]
- Yildiz, N.; Sarikaya, Y.; Çalimli, A. The effect of the electrolyte concentration and pH on the rheological properties of the original and the Na2CO3-activated Kütahya bentonite. Appl. Clay Sci. 1999, 14, 319–327. [Google Scholar] [CrossRef]
- Volzone, C.; Garrido, L.B. Changes in suspension properties of structural modified montmorillonites. Cerâmica 2001, 47, 4–8. [Google Scholar] [CrossRef] [Green Version]
- Gougeon, R.D.; Soulard, M.; Miehé-Brendlé, J.; Chézeau, J.M.; Le Dred, R.; Jeandet, P.; Marchal, R. Analysis of Two Bentonites of Enological Interest before and after Commercial Activation by Solid Na2CO3. J. Agric. Food Chem. 2003, 51, 4096–4100. [Google Scholar] [CrossRef] [PubMed]
- Karagüzel, C.; Çetinel, T.; Boylu, F.; Çinku, K.; Çelik, M.S. Activation of (Na, Ca)-bentonites with soda and MgO and their utilization as drilling mud. Appl. Clay Sci. 2010, 48, 398–404. [Google Scholar] [CrossRef]
- Shah, L.A.; Khattak, N.S.; Valenzuela, M.G.S.; Manan, A.; Valenzuela Díaz, F.R. Preparation and characterization of purified Na-activated bentonite from Karak (Pakistan) for pharmaceutical use. Clay Miner. 2013, 48, 595–603. [Google Scholar] [CrossRef]
- Kaufhold, S.; Emmerich, K.; Dohrmann, R.; Steudel, A.; Ufer, K. Comparison of methods for distinguishing sodium carbonate activated from natural sodium bentonites. Appl. Clay Sci. 2013, 86, 23–37. [Google Scholar] [CrossRef]
- Boussen, S.; Sghaier, D.; Chaabani, F.; Jamoussi, B.; Messaoud, S.B.; Bennour, A. The rheological, mineralogical and chemical characteristic of the original and the Na2CO3-activated Tunisian swelling clay (Aleg Formation) and their utilization as drilling mud. Appl. Clay Sci. 2015, 118, 344–353. [Google Scholar] [CrossRef]
- Mosbahi, M.; Tlili, A.; Khlifi, M.; Jamoussi, F. Basic activation of lower Eocene clay from Meknassy-Mezzouna basin (centerwestern Tunisia), synthesis of zeolite and clarification of soybean oils. Appl. Clay Sci. 2017, 138, 1–11. [Google Scholar] [CrossRef]
- Magzoub, M.I.; Nasser, M.S.; Hussein, I.A.; Benamor, A.; Onaizi, S.A.; Sultan, A.S.; Mahmoud, M.A. Effects of sodium carbonate addition, heat and agitation on swelling and rheological behavior of Ca-bentonite colloidal dispersions. Appl. Clay Sci. 2017, 147, 176–183. [Google Scholar] [CrossRef]
- Mahmoud, M.; Mohamed, A.; Kamal, M.S.; Sultan, A.S.; Hussein, I.A. Upgrading Calcium-Bentonite to Sodium-Bentonite Using Seawater and Soda Ash. Energy Fuels 2019, 33, 10888–10894. [Google Scholar] [CrossRef]
- El Ouardi, Y.; Lenoble, V.; Branger, C.; Laatikainen, K.; Angeletti, B.; Ouammou, A. Enhancing clay adsorption properties: A comparison between chemical and combined chemical/thermal treatments. Groundw. Sustain. Dev. 2021, 12, 100544. [Google Scholar] [CrossRef]
- Kittrick, J.A. Interlayer Forces in Montmorillonite and Vermiculite. Soil Sci. Soc. Am. J. 1969, 33, 217. [Google Scholar] [CrossRef]
- Sato, T.; Watanabe, T.; Otsuka, R. Effects of Layer Charge, Charge Location, and Energy Change on Expansion Properties of Dioctahedral Smectites. Clays Clay Miner. 1992, 40, 103–113. [Google Scholar] [CrossRef]
- Laird, D.A. Model for crystalline swelling of 2:1 phyllosilicates. Clays Clay Miner. 1996, 44, 553–559. [Google Scholar] [CrossRef]
- Górniak, K.; Szydłak, T.; Gaweł, A.; Klimek, A.; Tomczyk, A.; Sulikowski, B.; Olejniczak, Z.; Motyka, J.; Serwicka, E.M.; Bahranowski, K. Commercial bentonite from the Kopernica deposit (Tertiary, Slovakia): A petrographic and mineralogical approach. Clay Miner. 2016, 51, 97–122. [Google Scholar] [CrossRef]
- Hendershot, W.H.; Lalande, H.; Duquette, M. Ion Exchange and Exchangeable Cations. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton FL, USA, 2008; pp. 225–234. [Google Scholar]
- Wacławska, I. Dehydration and dehydroxylation of smectites 1. Dehydration and dehydroxylation kinetics. Mineral. Pol. 1984, 15, 91–107. [Google Scholar]
- Fajnor, V.Š.; Jesenák, K. Differential thermal analysis of montmorillonite. J. Therm. Anal. 1996, 46, 489–493. [Google Scholar] [CrossRef]
- Dellisanti, F.; Calafato, A.; Pini, G.A.; Moro, D.; Ulian, G.; Valdrè, G. Effects of dehydration and grinding on the mechanical shear behaviour of Ca-rich montmorillonite. Appl. Clay Sci. 2018, 152, 239–248. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Llewellyn, P. Thermal analysis. In Handbook of Clay Science. Part B: Techniques and Applications, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 361–379. [Google Scholar]
- Lang, L.Z.; Xiang, W.; Huang, W.; Cui, D.S.; Schanz, T. An experimental study on oven-drying methods for laboratory determination of water content of a calcium-rich bentonite. Appl. Clay Sci. 2017, 150, 153–162. [Google Scholar] [CrossRef]
- Derkowski, A.; Drits, V.A.; McCarty, D.K. Rehydration of dehydrated-dehydroxylated smectite in a low water vapor environment. Am. Miner. 2012, 97, 110–127. [Google Scholar] [CrossRef]
- Dathe, F.; Strelnikova, V.; Werling, N.; Emmerich, K.; Dehn, F. Influence of lime, calcium silicate and portlandite on alkali activation of calcined common clays. Open Ceramics 2021, 7, 100152. [Google Scholar] [CrossRef]
- Madejová, J.; Gates, W.P.; Petit, S. IR spectra of clay minerals. In Infrared and Raman Spectroscopies of Clay Minerals. Developments in Clay Science; Gates, W.P., Klopproge, J.T., Madejová, J., Bergaya, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 8, pp. 107–149. [Google Scholar]
- Farmer, V.C. The layer silicates. In Infrared Spectra of Minerals; Farmer, V.C., Ed.; Mineralogical Society: London, UK, 1974; pp. 331–363. [Google Scholar]
- Andersen, F.A.; Brečević, L. Infrared spectra of amorphous and crystalline calcium carbonate. Acta Chem. Scand. 1991, 45, 1018–1024. [Google Scholar] [CrossRef]
- Tobler, D.J.; Rodriguez Blanco, J.D.; Sørensen, H.O.; Stipp, S.L.S.; Dideriksen, K. Effect of pH on Amorphous Calcium Carbonate Structure and Transformation. Cryst. Growth Des. 2016, 16, 4500–4508. [Google Scholar] [CrossRef]
- Estep, P.A.; Kovach, J.J.; Hiser, A.L.; Karr, C. Characterization of Carbonate Minerals in Oil Shales and Coals by Infrared Spectroscopy. In Spectrometry of Fuels; Friedel, R.A., Ed.; Springer: Boston, MA, USA, 1970; pp. 228–247. [Google Scholar]
- Böttcher, M.E.; Gehlken, P.L. Dehydration of natural gaylussite (Na2Ca(CO3)2∙5H2O) and pirssonite (Na2Ca(CO3)2∙2H2O) as illustrated by FTIR spectroscopy. Neues Jahrb. Mineral. Mon. 1996, 2, 73–91. [Google Scholar]
- Frost, R.L.; Dickfos, M. Hydrated double carbonates—A Raman and infrared spectroscopic study. Polyhedron 2007, 26, 4503–4508. [Google Scholar] [CrossRef] [Green Version]
- Bury, C.R.; Redd, R. The system sodium carbonate–calcium carbonate–water. J. Chem. Soc. 1933, 1160–1162. [Google Scholar] [CrossRef]
- Horgnies, M.; Chen, J.J.; Bouillon, C. Overview about the use of Fourier transform infrared spectroscopy to study cementitious materials. WIT Trans. Eng. Sci. 2013, 77, 251–262. [Google Scholar]
| Activation Procedure | Purpose | Reference |
|---|---|---|
| Mixture of Bent, water (24 or 34 wt.% of total dried matter) and Na2CO3 (1.5, 2.2 or 3.5 wt.% of clay) kneaded in a mill for 10 min and oven-dried at 80 °C for 3 h, ball-milled and sieved. | Study of ageing process with respect to rheological properties | [9] |
| Dry Mt added to saturated solution of Na2CO3 (100 meq/100 g Mt) homogenized at RT, left standing for 24 h and diluted to 6 wt.% aqueous suspension. | Study of rheological properties | [10,12] |
| 100 g of Ca-Bent and Na2CO3 (0.1, 2, 2.5, 5, 10 or 15 wt.% of clay) stirred in 800 mL boiling water for 1 h, clay fraction separated by multiple dispersion/sedimentation cycles. Supernatant concentrated by evaporation and the activated clay dried at 105 °C and ground. | Study of rheological properties | [11] |
| Bent moisturized with 40 wt.% water mixed with dry Na2CO3, mixture kneaded at RT and left to drying/curing under sunlight for a month. Alternatively, Na2CO3 blended with MgO. | Study of rheological properties (for application as drilling fluid) | [14] |
| Mixture of moisturized Bent and Na2CO3 (2, 3, 5, 10 wt.% of clay) kneaded at RT, left to drying/curing under sunlight for a month, mixed with water (30, 50 and 75 g/L) and the suspension aged for 24 h. | Study of rheological properties (for application as drilling fluid) | [17] |
| Na2CO3 (2, 4, 12 wt.% of clay) added to 6 wt.% suspension of Ca-Bent, stirred with or without heating at 70 °C, and aged for 24 h. | Study of rheological properties (for application as drilling fluid) | [19] |
| 22.5 g of Bent stirred in 350 mL of seawater, alkalized with NaOH to pH = 9, mixed with Na2CO3 (0.5, 1, 1.5 g), left under heating and stirring at 100 °C for 24 h, filtered and dried. | Study of rheological properties (for application as drilling fluid) | [20] |
| Mixture of 100 g of clay and Na2CO3 (2, 3, 5, 8 wt.% of clay) stirred in 1000 mL boiling water for 1 h, cooled and purified by multiple dispersion/sedimentation cycles. The activated clay collected by centrifugation, dried at 60 °C and ground. | Pharmaceutical application | [15] |
| Bent mixed with 2–5% Na2CO3 and various amounts of water (10–15% or excess), allowed to interact under shaking for 24 h, and dried at 60 °C. | Study of differences between natural and activated Na-Bent | [16] |
| Commercial Na-activated Bent obtained by treatment of wet raw Bent with solid Na2CO3 (3 wt.% of clay) at 80 °C. | Study of protein sorption (for application in winemaking) | [13] |
| Mixture of 5 g of clay and Na2CO3 (2, 3, 5, 10 wt.% of clay) stirred in 150 mL water for variable activation times (1, 2, 4 h), dried at 60 °C without washing. | Study of adsorptive properties (for application in edible oil clarification) | [18] |
| Na2CO3 (4 wt.% of clay) added to a suspension of Bent in 500 mL boiling water, stirred for 1 h, and cooled. The clay recovered by sedimentation, separated by filtering, washed, dried at 105 °C and calcined at 450 °C for 24 h. | Study of adsorptive properties (for waste water purification) | [21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahranowski, K.; Klimek, A.; Gaweł, A.; Serwicka, E.M. Rehydration Driven Na-Activation of Bentonite—Evolution of the Clay Structure and Composition. Materials 2021, 14, 7622. https://doi.org/10.3390/ma14247622
Bahranowski K, Klimek A, Gaweł A, Serwicka EM. Rehydration Driven Na-Activation of Bentonite—Evolution of the Clay Structure and Composition. Materials. 2021; 14(24):7622. https://doi.org/10.3390/ma14247622
Chicago/Turabian StyleBahranowski, Krzysztof, Agnieszka Klimek, Adam Gaweł, and Ewa M. Serwicka. 2021. "Rehydration Driven Na-Activation of Bentonite—Evolution of the Clay Structure and Composition" Materials 14, no. 24: 7622. https://doi.org/10.3390/ma14247622
APA StyleBahranowski, K., Klimek, A., Gaweł, A., & Serwicka, E. M. (2021). Rehydration Driven Na-Activation of Bentonite—Evolution of the Clay Structure and Composition. Materials, 14(24), 7622. https://doi.org/10.3390/ma14247622






