New Clayey Deposit and Their Potential as Raw Material for Red or Structured Ceramics: Technological Characterization
Abstract
:1. Introduction
2. Materials and Methods
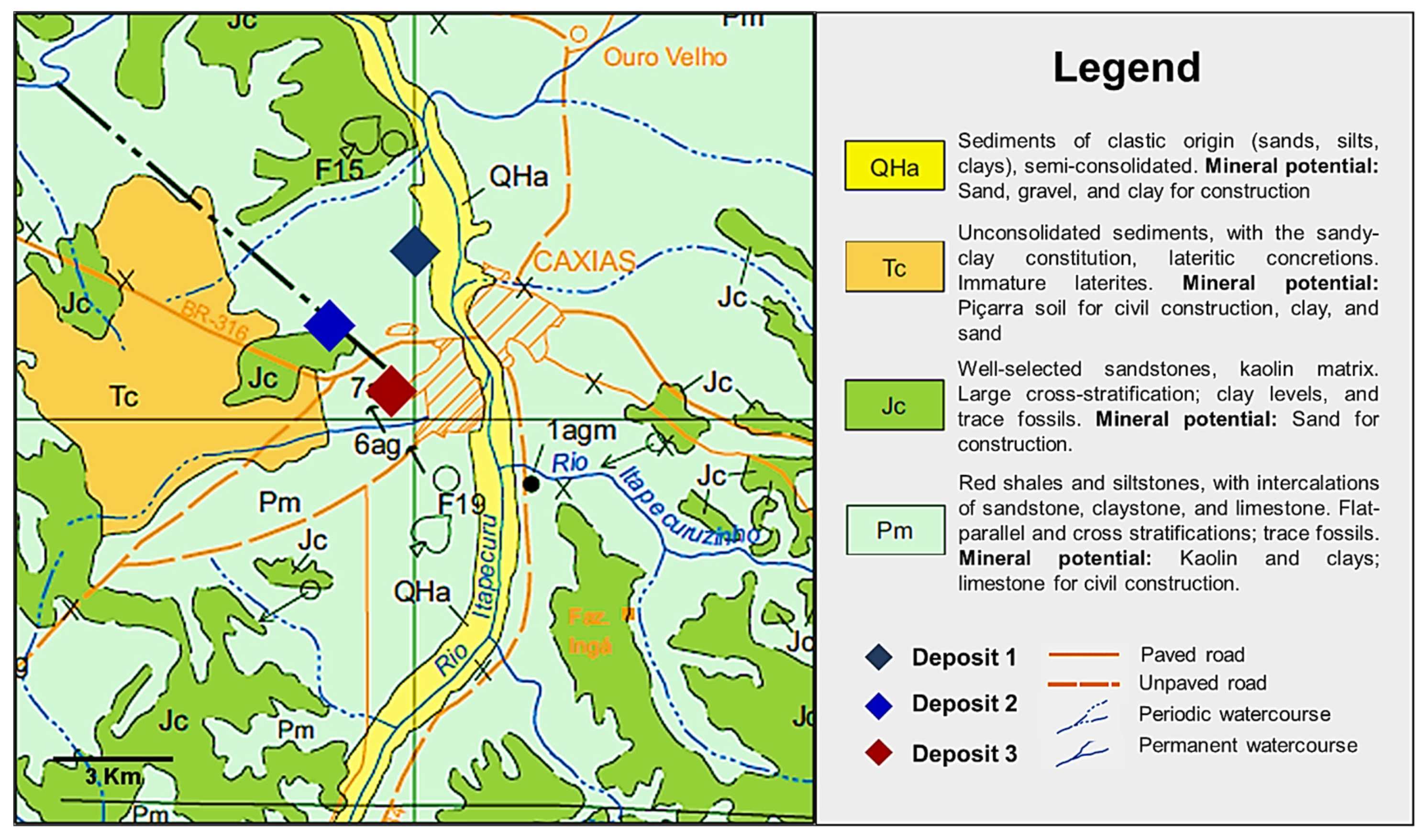
2.1. Materials
2.2. Methods
2.2.1. Sample Preparation
Separation and Characterization of the Clay Fraction (<2 µm)
Drying, Grinding, and Quartering of the Samples for Technological Tests
Preparation of the Specimens for Determination of Physicomechanical Properties
2.2.2. Analytical Techniques and Methods
2.2.3. Technological Tests
Cation Exchange Capacity (CEC)
Plastic Behavior
Physicomechanical Properties
3. Results and Discussion
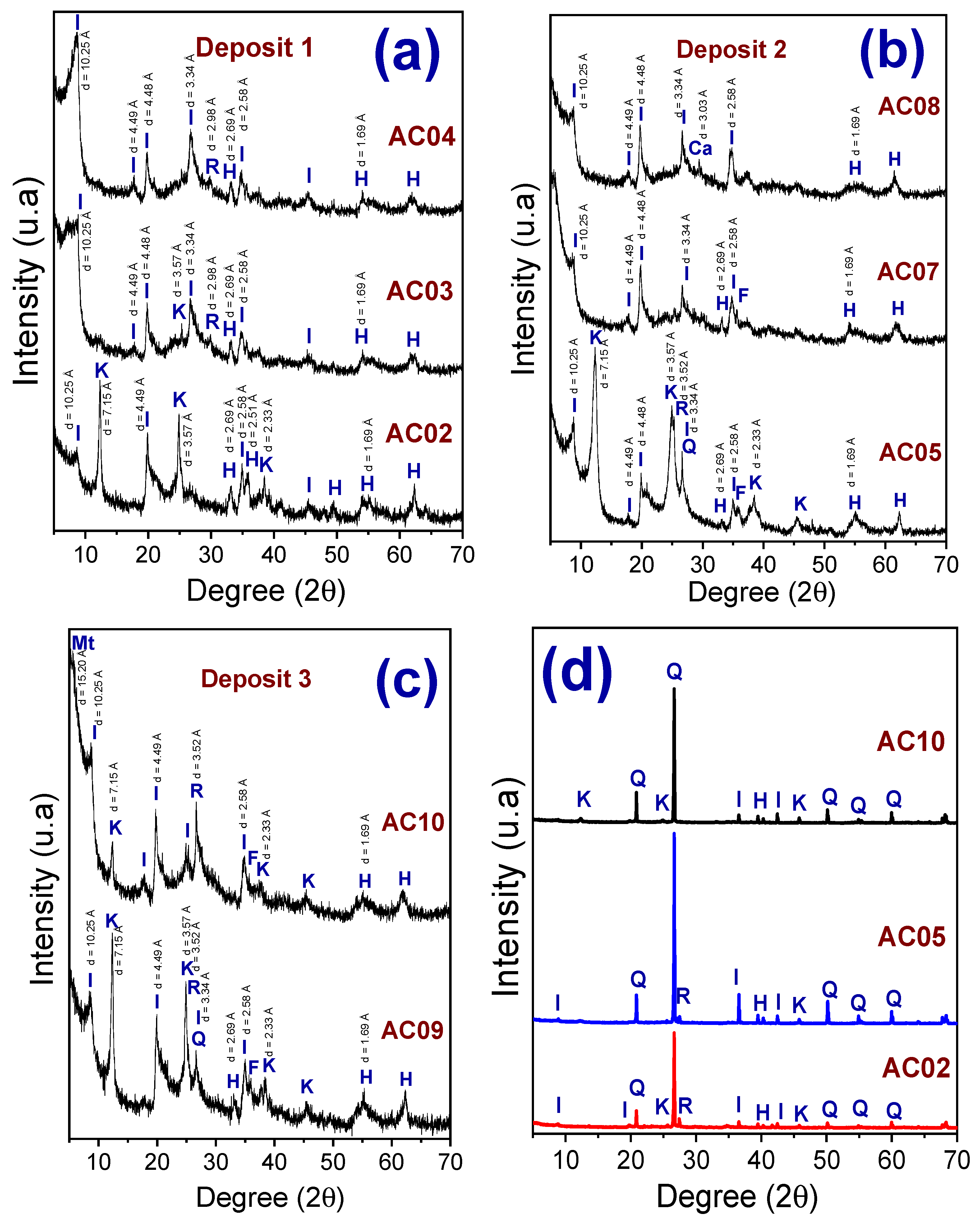
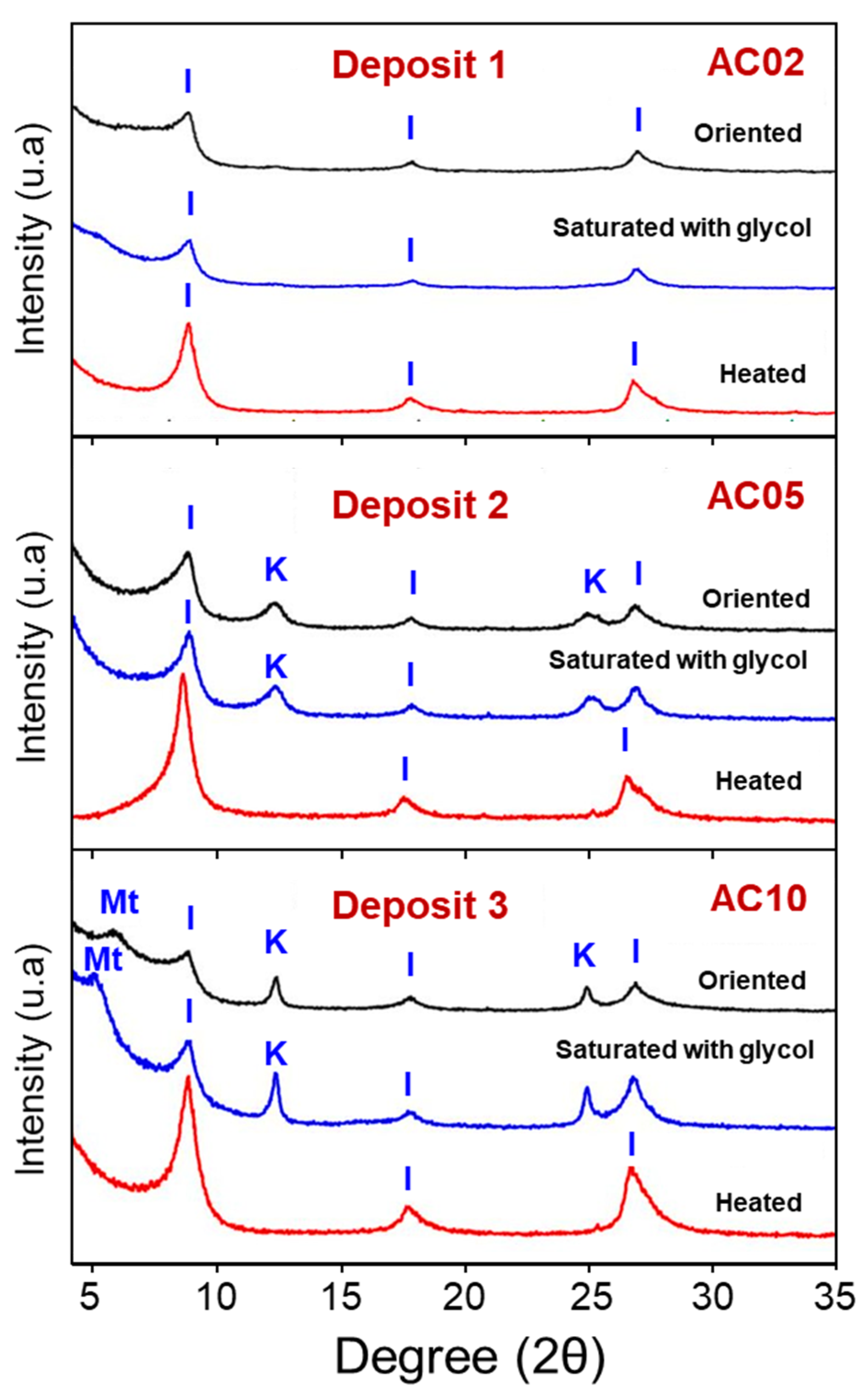
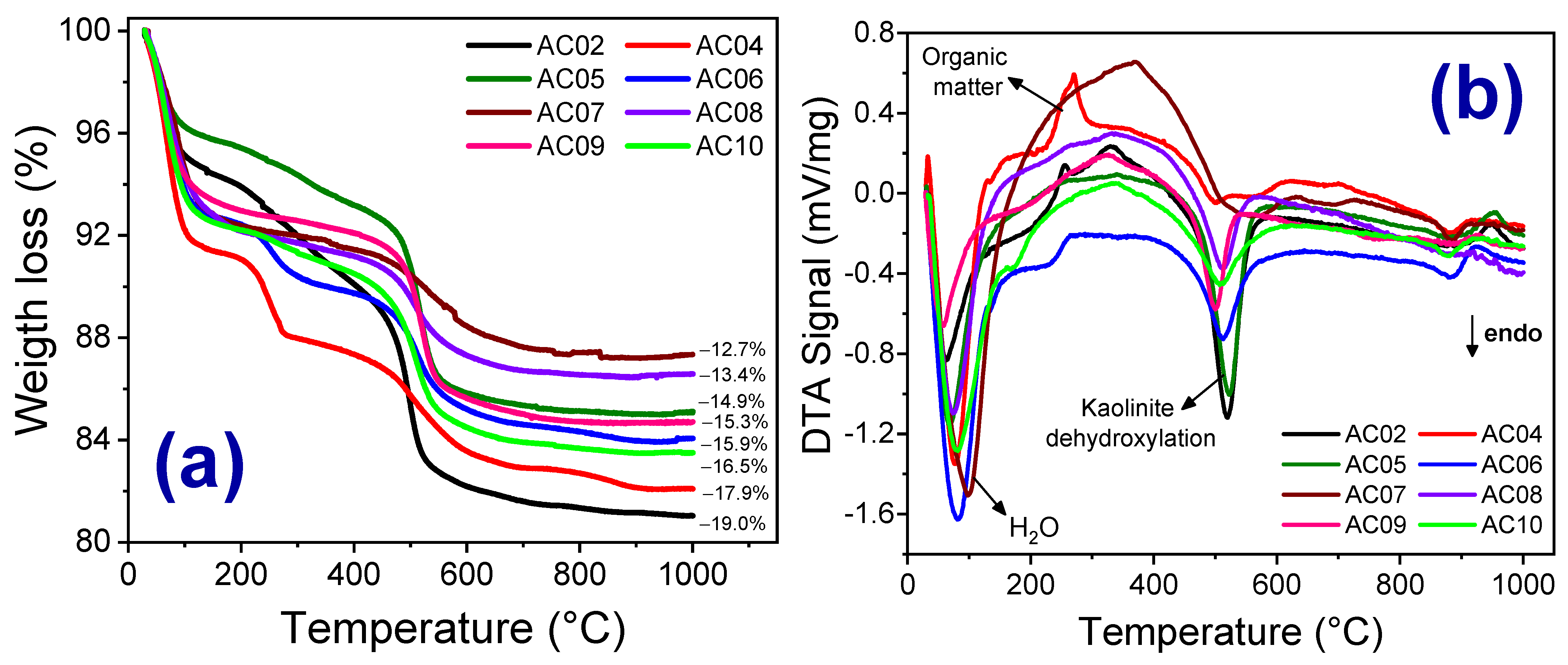
3.1. Mineralogical, Chemical Compositions and Thermal Behavior of the Clay Raw Materials
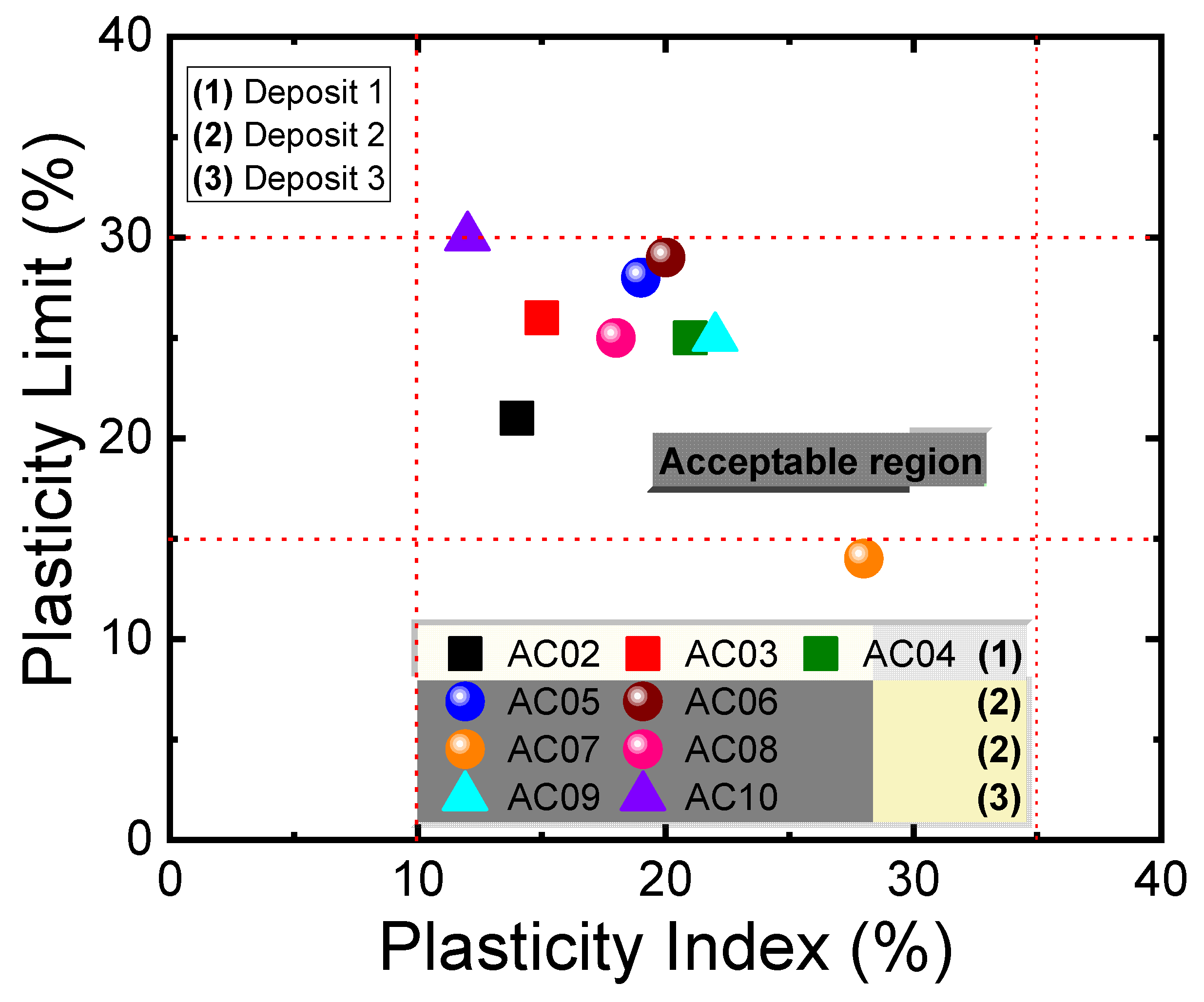
3.2. Particle Size Distribution and Plastic Behavior of the Clay Raw Materials
3.3. Cation Exchange Capacity (CEC) and Specific Area (SA) of the Clay Raw Materials
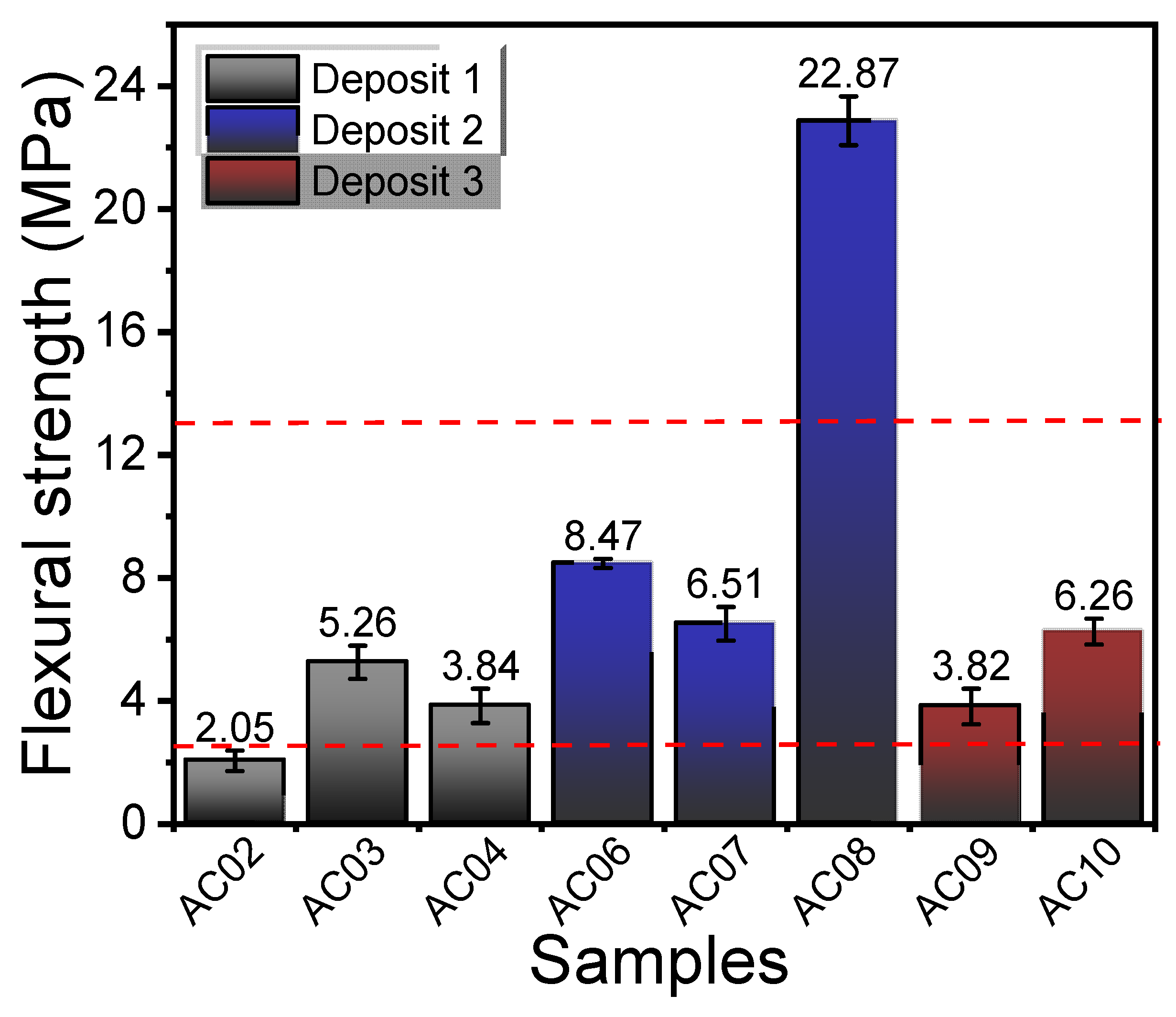
3.4. Linear Shrinkage after Firing, Water Absorption, Bulk Density, Apparent Porosity, and Flexural Strength
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- da Costa, F.P.; Fernandes, J.V.; de Melo, L.R.L.; Rodrigues, A.M.; Menezes, R.R.; de Araújo Neves, G. The potential for natural stones from northeastern brazil to be used in civil construction. Minerals 2021, 11, 440. [Google Scholar] [CrossRef]
- CBIC—Brazilian Chamber of the Construction Industry. GDP Brazil and Civil Construction. Available online: http://www.cbicdados.com.br/home/ (accessed on 14 May 2021).
- Lee, V.-G.; Yeh, T.-H. Sintering effects on the development of mechanical properties of fired clay ceramics. Mater. Sci. Eng. A 2008, 485, 5–13. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Srasra, E.; Zargouni, F. The use of Tunisian Barremian clay in the traditional ceramic industry: Optimization of ceramic properties. Appl. Clay Sci. 2008, 42, 125–129. [Google Scholar] [CrossRef]
- Anicer-National Association of the Ceramic Industry Sector Data. Available online: https://www.anicer.com.br/anicer/setor/ (accessed on 14 May 2021).
- ABCERAM-Brazilian Ceramic Association CERAMIC SEGMENTS (COMPANIES & PRODUCTS). Available online: https://abceram.org.br/segmentos-ceramicos/ (accessed on 14 May 2021).
- de Lima, A.G.B.; Delgado, J.M.P.Q.; Nascimento, L.P.C.; de Lima, E.S.; de Oliveira, V.A.B.; Silva, A.M.; Silva, J. Clay Ceramic Materials: From Fundamentals and Manufacturing to Drying Process Predictions. In Transport Processes and Separation Technologies; Springer Nature: Cham, Switzerland, 2020; pp. 1–28. ISBN 978-3-030-47856-8. [Google Scholar]
- Ribeiro, J.A.P.; Melo, F. Basic Geological Surveys of Brazil. Caxias, Sheet SB.23-X-B. Maranhão State, Brazil. Scale 1:250.000; CPRM: Fortaleza, Brazil, 2000. (In Portuguese) [Google Scholar]
- Türköz, M.; Tosun, H. The use of methylene blue test for predicting swell parameters of natural clay soils. Sci. Res. Essays 2011, 6, 1780–1792. [Google Scholar] [CrossRef]
- Sahin, H.; Gu, F.; Lytton, R.L. Development of Soil-Water Characteristic Curve for Flexible Base Materials Using the Methylene Blue Test. J. Mater. Civ. Eng. 2015, 27, 04014175. [Google Scholar] [CrossRef]
- ASTM C837-09. Standard Test Method for Methylene Blue Index of Clay; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar] [CrossRef]
- Ngun, B.K.; Mohamad, H.; Sulaiman, S.K.; Okada, K.; Ahmad, Z.A. Some ceramic properties of clays from central Cambodia. Appl. Clay Sci. 2011, 53, 33–41. [Google Scholar] [CrossRef]
- ASTM D4318-17e1. Standard Test Methods for Liquid Limit, Plastic Limit, and Plasticity Index of Soils; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar] [CrossRef]
- ASTM C674-13. Standard Test Methods for Flexural Properties of Ceramic Whiteware Materials; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar] [CrossRef]
- ASTM C20-00. Standard Test Methods for Apparent Porosity, Water Absorption, Apparent Specific Gravity, and Bulk Density of Burned Refractory Brick and Shapes by Boiling Water; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar] [CrossRef]
- da Costa, F.P.; Bezerra, I.M.T.; Fernandes, J.V.; Rodrigues, A.M.; Menezes, R.R.; Neves, G.d.A. Durability of Sustainable Ceramics Produced by Alkaline Activation of Clay Brick Residue. Sustainability 2021, 13, 10931. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; da Costa, F.P.; Beltrão, S.L.D.; Fernandes, J.V.; Menezes, R.R.; Neves, G.d.A. Development of Eco-Friendly Mortars Produced with Kaolin Processing Waste: Durability Behavior Viewpoint. Sustainability 2021, 13, 11395. [Google Scholar] [CrossRef]
- Osinubi, K.J.; Nwaiwu, C.M. Design of Compacted Lateritic Soil Liners and Covers. J. Geotech. Geoenviron. Eng. 2006, 132, 203–213. [Google Scholar] [CrossRef]
- da Costa, F.P.; Morais, C.R.d.S.; Pinto, H.C.; Rodrigues, A.M. Microstructure and physico-mechanical properties of Al2O3-doped sustainable glass-ceramic foams. Mater. Chem. Phys. 2020, 256, 123612. [Google Scholar] [CrossRef]
- da Costa, F.P.; Morais, C.R.d.S.; Rodrigues, A.M. Sustainable glass-ceramic foams manufactured from waste glass bottles and bentonite. Ceram. Int. 2020, 46, 17957–17961. [Google Scholar] [CrossRef]
- Muñoz, V.; Morales, O.; Letelier, G.; Mendívil, G. Fired clay bricks made by adding wastes: Assessment of the impact on physical, mechanical, and thermal properties. Constr. Build. Mater. 2016, 125, 241–252. [Google Scholar] [CrossRef]
- de Figueirêdo, J.M.R.; da Costa, F.P.; Fernandes, J.V.; Rodrigues, A.M.; Neves, G.d.A.; Menezes, R.R.; de Lima Santana, L.N. Development of Scheelite Tailings-Based Ceramic Formulations with the Potential to Manufacture Porcelain Tiles, Semi-Stoneware and Stoneware. Materials 2020, 13, 5122. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.V.; Guedes, D.G.; da Costa, F.P.; Rodrigues, A.M.; Neves, G.d.A.; Menezes, R.R.; Santana, L.N.d.L. Sustainable Ceramic Materials Manufactured from Ceramic Formulations Containing Quartzite and Scheelite Tailings. Sustainability 2020, 12, 9417. [Google Scholar] [CrossRef]
- Alcântara, A.C.S.; Beltrão, M.S.S.; Oliveira, H.A.; Gimenez, I.F.; Barreto, L.S. Characterization of ceramic tiles prepared from two clays from Sergipe—Brazil. Appl. Clay Sci. 2008, 39, 160–165. [Google Scholar] [CrossRef]
- Muñoz Velasco, P.; Morales Ortíz, M.P.; Mendívil Giró, M.A.; Muñoz Velasco, L. Fired clay bricks manufactured by adding wastes as sustainable construction material—A review. Constr. Build. Mater. 2014, 63, 97–107. [Google Scholar] [CrossRef]
- Sakizci, M.; Alver, B.E.; Yörükoğullari, E. Thermal behavior and immersion heats of selected clays from Turkey. J. Therm. Anal. Calorim. 2009, 98, 429–436. [Google Scholar] [CrossRef]
- Seynou, M.; Millogo, Y.; Ouedraogo, R.; Traoré, K.; Tirlocq, J. Firing transformations and properties of tiles from a clay from Burkina Faso. Appl. Clay Sci. 2011, 51, 499–502. [Google Scholar] [CrossRef]
- Vieira, C.M.F.; Sánchez, R.; Monteiro, S.N. Characteristics of clays and properties of building ceramics in the state of Rio de Janeiro, Brazil. Constr. Build. Mater. 2008, 22, 781–787. [Google Scholar] [CrossRef]
- Fajnor, V.Š.; Jesenák, K. Differential thermal analysis of montmorillonite. J. Therm. Anal. 1996, 46, 489–493. [Google Scholar] [CrossRef]
- FAJNOR, V.S. KUCHTA Effect of Degradation of Montmorillonite by Vibration Grinding on the DTA Curves in the Range 20–1500 °C. J. Therm. Anal. 1995, 45, 481–489. [Google Scholar] [CrossRef]
- Goswami, R.K.; Singh, B. Influence of fly ash and lime on plasticity characteristics of residual lateritic soil. Proc. Civ. Eng. Improv. 2005, 9, 175–182. [Google Scholar] [CrossRef]
- Boussen, S.; Sghaier, D.; Chaabani, F.; Jamoussi, B.; Bennour, A. Characteristics and industrial application of the Lower Cretaceous clay deposits (Bouhedma Formation), Southeast Tunisia: Potential use for the manufacturing of ceramic tiles and bricks. Appl. Clay Sci. 2016, 123, 210–221. [Google Scholar] [CrossRef]
- Dondi, M. Clay materials for ceramic tiles from the Sassuolo District Northern Apennines. Geology, composition and technological properties. Appl. Clay Sci. 1999, 15, 337–366. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, S.N.; Vieira, C.M.F. Influence of firing temperature on the ceramic properties of clays from Campos dos Goytacazes, Brazil. Appl. Clay Sci. 2004, 27, 229–234. [Google Scholar] [CrossRef]
- Hajjaji, W.; Hachani, M.; Moussi, B.; Jeridi, K.; Medhioub, M.; López-Galindo, A.; Rocha, F.; Labrincha, J.A.; Jamoussi, F. Mineralogy and plasticity in clay sediments from north-east Tunisia. J. African Earth Sci. 2010, 57, 41–46. [Google Scholar] [CrossRef]
- Meseguer, S.; Pardo, F.; Jordan, M.M.; Sanfeliu, T.; González, I. Ceramic behaviour of five Chilean clays which can be used in the manufacture of ceramic tile bodies. Appl. Clay Sci. 2010, 47, 372–377. [Google Scholar] [CrossRef]
- Baruah, B.; Mishra, M.; Bhattacharjee, C.R.; Nihalani, M.C.; Mishra, S.K.; Baruah, S.D.; Phukan, P.; Goswameea, R.L. The effect of particle size of clay on the viscosity build up property of mixed metal hydroxides (MMH) in the low solid-drilling mud compositions. Appl. Clay Sci. 2013, 80–81, 169–175. [Google Scholar] [CrossRef]
- Tlili, M.F.A.; Montacer, M.E.G.M. Mineralogical study of kaolinitic clys from Sidi El Bader in the far north of Tunisia. Appl. Clay Sci. Tunis. 2008, 39, 208–217. [Google Scholar] [CrossRef]
- Jeridi, K.; Hachani, M.; Hajjaji, W.; Moussi, B.; Medhioub, M.; López-Galindo, A.; Kooli, F.; Zargouni, F.; Labrincha, J.; Jamoussi, F. Technological behaviour of some Tunisian clays prepared by dry ceramic processing. Clay Miner. 2008, 43, 339–350. [Google Scholar] [CrossRef]
- Jordán, M.M.; Martín-Martín, J.D.; Sanfeliu, T.; Gómez-Gras, D.; de la Fuente, C. Mineralogy and firing transformations of Permo–Triassic clays used in the manufacturing of ceramic tile bodies. Appl. Clay Sci. 2009, 44, 173–177. [Google Scholar] [CrossRef]
- ASTM C1167-11. Standard Specification for Clay Roof Tiles; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar] [CrossRef]
- ASTM C216-1. Standard Specification for Facing Brick (Solid Masonry Units Made from Clay or Shale); ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar] [CrossRef]


| (a) Clays with Coarse Granulometry (<75 µm) (in wt%). | ||||||||||||
| Samples | B | Al2O3 | Fe2O3 | MgO | TiO2 | K2O | CaO | Na2O | P2O5 | SiO2/Al2O3 | LOI | |
| Deposit 1 | AC02 | 69.07 | 14.41 | 6.20 | 1.00 | 0.71 | 1.00 | 0.27 | 0.25 | 0.05 | 4.79 | 6.30 |
| AC03 | 72.01 | 13.82 | 5.12 | 0.64 | 0.63 | 1.29 | 0.05 | 0.11 | 0.11 | 5.21 | 6.11 | |
| AC04 | 72.55 | 11.69 | 3.50 | 1.54 | 0.51 | 3.50 | 0.10 | 0.18 | 0.14 | 6.20 | 6.04 | |
| Deposit 2 | AC05 | 86.67 | 8.09 | 1.00 | 0.29 | 0.32 | 1.03 | 0.05 | 0.09 | 0.05 | 10.71 | 2.98 |
| AC06 | 71.14 | 12.83 | 3.02 | 1.49 | 0.69 | 2.79 | 0.09 | 0.20 | 0.05 | 5.54 | 7.89 | |
| AC07 | 71.04 | 11.80 | 3.39 | 1.59 | 0.57 | 3.17 | 0.37 | 0.25 | 0.12 | 6.02 | 7.42 | |
| AC08 | 56.16 | 13.48 | 6.22 | 3.31 | 0.51 | 5.14 | 8.84 | 0.14 | 0.17 | 4.16 | 5.96 | |
| Deposit 3 | AC09 | 70.70 | 14.18 | 4.58 | 1.01 | 0.77 | 1.76 | 0.06 | 0.19 | 0.15 | 4.98 | 6.70 |
| AC10 | 68.70 | 12.99 | 3.72 | 1.77 | 0.66 | 3.17 | 0.27 | 0.18 | 0.10 | 5.28 | 8.36 | |
| (b) clays with fine granulometry (<2 µm) (in wt%). | ||||||||||||
| Samples | SiO2 | Al2O3 | Fe2O3 | MgO | TiO2 | K2O | CaO | Na2O | P2O5 | SiO2/Al2O3 | LOI | |
| Deposit 1 | AC02 | 42.15 | 27.61 | 11.52 | 1.35 | 0.82 | 1.90 | 0.07 | 0.10 | 0.10 | 1.53 | 13.99 |
| AC03 | 46.18 | 27.62 | 7.72 | 1.36 | 0.82 | 2.03 | 0.07 | 0.15 | 0.13 | 1.67 | 13.74 | |
| AC04 | 47.20 | 19.90 | 6.77 | 3.12 | 0.51 | 4.68 | 0.33 | 0.75 | 0.30 | 2.37 | 15.68 | |
| Deposit 2 | AC05 | 47.85 | 29.46 | 2.48 | 1.11 | 0.60 | 1.33 | 0.10 | 0.72 | 0.11 | 1.62 | 15.39 |
| AC06 | 48.63 | 21.26 | 5.13 | 2.66 | 0.62 | 3.18 | 0.14 | 0.57 | 0.13 | 2.29 | 18.40 | |
| AC07 | 49.22 | 19.97 | 6.84 | 4.13 | 0.64 | 4.80 | 0.66 | 0.73 | 0.55 | 2.46 | 12.33 | |
| AC08 | 47.21 | 17.24 | 6.32 | 4.55 | 0.60 | 4.62 | 1.95 | 0.45 | 0.12 | 2.74 | 16.48 | |
| Deposit 3 | AC09 | 45.59 | 25.34 | 3.15 | 2.34 | 0.65 | 3.46 | 0.19 | 0.67 | 0.17 | 1.80 | 18.39 |
| AC10 | 50.71 | 20.85 | 5.64 | 2.70 | 0.78 | 3.70 | 0.35 | 0.68 | 0.15 | 2.43 | 13.57 | |
| Samples | Thermal Events (°C) | |||||
|---|---|---|---|---|---|---|
| 50–200 °C | 200–425 °C | 425–600 °C | 850–930 °C | 930–960 °C | ||
| Endo | Endo | Exo | Endo | Endo | Exo | |
| AC02 | x x | --- | x | x x | --- | x |
| AC03 | x x | x | --- | x x | x | x |
| AC04 | x x | --- | x x | x | x x | --- |
| AC05 | x | --- | --- | x x | --- | x |
| AC06 | x x | --- | x | x x | x | x |
| AC07 | x | --- | x | x | x | --- |
| AC08 | x | --- | --- | x | x | --- |
| AC09 | x | --- | x | x x | x | x x |
| AC10 | x | --- | x | x | x x | x |
| Associated event | Loss of free and adsorbed water | Decomposition of goethite and hematite formation | Combustion of organic matter | Kaolinite dehydroxylation | Destruction of the crystalline lattice of the smectite clays | Formation of spinel |
| Samples | Particle Size Distribution (%) | Atterberg Limits (%) | |||||
|---|---|---|---|---|---|---|---|
| <45 µm | 45–75 µm | >75 µm | LL | PL | PI | ||
| Deposit 1 | AC02 | 68.9 | 14.1 | 17.0 | 35 | 21 | 14 |
| AC03 | 78.8 | 16.2 | 5.0 | 41 | 26 | 15 | |
| AC04 | 89.8 | 9.9 | 0.3 | 46 | 25 | 21 | |
| Deposit 2 | AC05 | 42.3 | 57.1 | 0.6 | 47 | 28 | 19 |
| AC06 | 97.3 | 2.4 | 0.3 | 49 | 29 | 20 | |
| AC07 | 89.8 | 7.1 | 3.1 | 42 | 14 | 28 | |
| AC08 | 94.1 | 2.5 | 3.4 | 43 | 25 | 18 | |
| Deposit 3 | AC09 | 88.1 | 5.4 | 6.54 | 47 | 25 | 22 |
| AC10 | 96.9 | 3.1 | 0.0 | 42 | 30 | 12 | |
| References | PL (%) | PI (%) | Plasticity Level |
|---|---|---|---|
| Dondi [33] | 18 ≤ PL ≤ 30 | 10 ≤ PI ≤ 35 | Acceptable |
| Boussen [32] | 20 ≤ PL ≤ 35 | 15 ≤ PI ≤ 45 | Acceptable |
| Ngun [12] | - | 10 ≤ PI ≤ 40 | Acceptable |
| Vieira [28] | 20 ≤ PL ≤ 30 | 10 ≤ PI ≤ 35 | Acceptable |
| Samples | Physico-Mechanical Properties | ||||
|---|---|---|---|---|---|
| LSF (%) | WA (%) | AP (%) | BD (g/cm3) | FS (MPa) | |
| AC02 | 0.1 ± 0.2 | 16.9 ± 0.5 | 31.2 ± 0.6 | 1.8 ± 0.1 | 2.1 ± 0.3 |
| AC03 | 1.5 ± 0.1 | 14.3 ± 0.5 | 27.2 ± 0.6 | 1.8 ± 0.1 | 5.3 ± 0.5 |
| AC04 | 0.0 ± 0.1 | 12.4 ± 0.5 | 24.0 ± 0.8 | 1.8 ± 0.1 | 3.8 ± 0.6 |
| AC05 | - | - | - | - | - |
| AC06 | 4.7 ± 0.3 | 11.6 ± 0.1 | 23.0 ± 0.4 | 2.0 ± 0.1 | 8.5 ± 0.2 |
| AC07 | 0.7 ± 0.1 | 13.4 ± 0.5 | 26.0± 0.9 | 2.0 ± 0.1 | 6.5 ± 0.6 |
| AC08 | 6.6 + 0.3 | 10.2 ± 0.1 | 20.4 ± 0.5 | 2.0 ± 0.1 | 22.9 ± 0.8 |
| AC09 | 0.3 ± 0.1 | 16.2 ± 0.9 | 29.5 ± 0.7 | 1.8 ± 0.1 | 3.8 ± 0.6 |
| AC10 | 0.2 ± 0.1 | 11.9 ± 0.1 | 25.0 ± 0.4 | 1.9 ± 0.1 | 6.3 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assunção, A.R.S.; Correia, G.S.; Vasconcelos, N.d.S.L.S.; Cabral, A.A.; Angélica, R.S.; da Costa, F.P.; Menezes, R.R.; de Araújo Neves, G.; Rodrigues, A.M.; Rivas-Mercury, J.M. New Clayey Deposit and Their Potential as Raw Material for Red or Structured Ceramics: Technological Characterization. Materials 2021, 14, 7672. https://doi.org/10.3390/ma14247672
Assunção ARS, Correia GS, Vasconcelos NdSLS, Cabral AA, Angélica RS, da Costa FP, Menezes RR, de Araújo Neves G, Rodrigues AM, Rivas-Mercury JM. New Clayey Deposit and Their Potential as Raw Material for Red or Structured Ceramics: Technological Characterization. Materials. 2021; 14(24):7672. https://doi.org/10.3390/ma14247672
Chicago/Turabian StyleAssunção, Ana Rosa S., Gricirene Sousa Correia, Nazaré do Socorro L. S. Vasconcelos, Aluísio Alves Cabral, Rômulo Simões Angélica, Fabiana Pereira da Costa, Romualdo Rodrigues Menezes, Gelmires de Araújo Neves, Alisson Mendes Rodrigues, and José Manuel Rivas-Mercury. 2021. "New Clayey Deposit and Their Potential as Raw Material for Red or Structured Ceramics: Technological Characterization" Materials 14, no. 24: 7672. https://doi.org/10.3390/ma14247672
APA StyleAssunção, A. R. S., Correia, G. S., Vasconcelos, N. d. S. L. S., Cabral, A. A., Angélica, R. S., da Costa, F. P., Menezes, R. R., de Araújo Neves, G., Rodrigues, A. M., & Rivas-Mercury, J. M. (2021). New Clayey Deposit and Their Potential as Raw Material for Red or Structured Ceramics: Technological Characterization. Materials, 14(24), 7672. https://doi.org/10.3390/ma14247672










