Spontaneous Formation and Fusion of Raspberry Vesicle Self-Assembled from Star Block Terpolymers in Aqueous Solution
Abstract
:1. Introduction
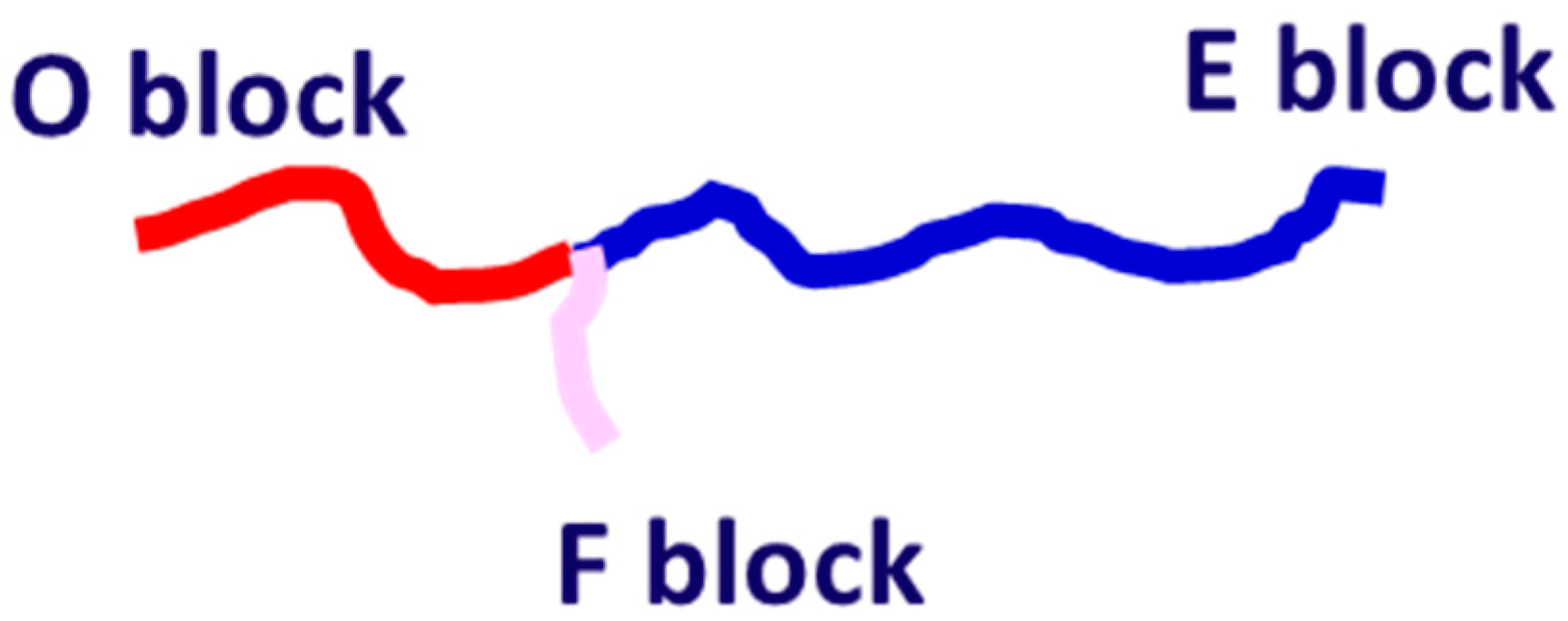
2. Simulation Methods
3. Results and Discussion
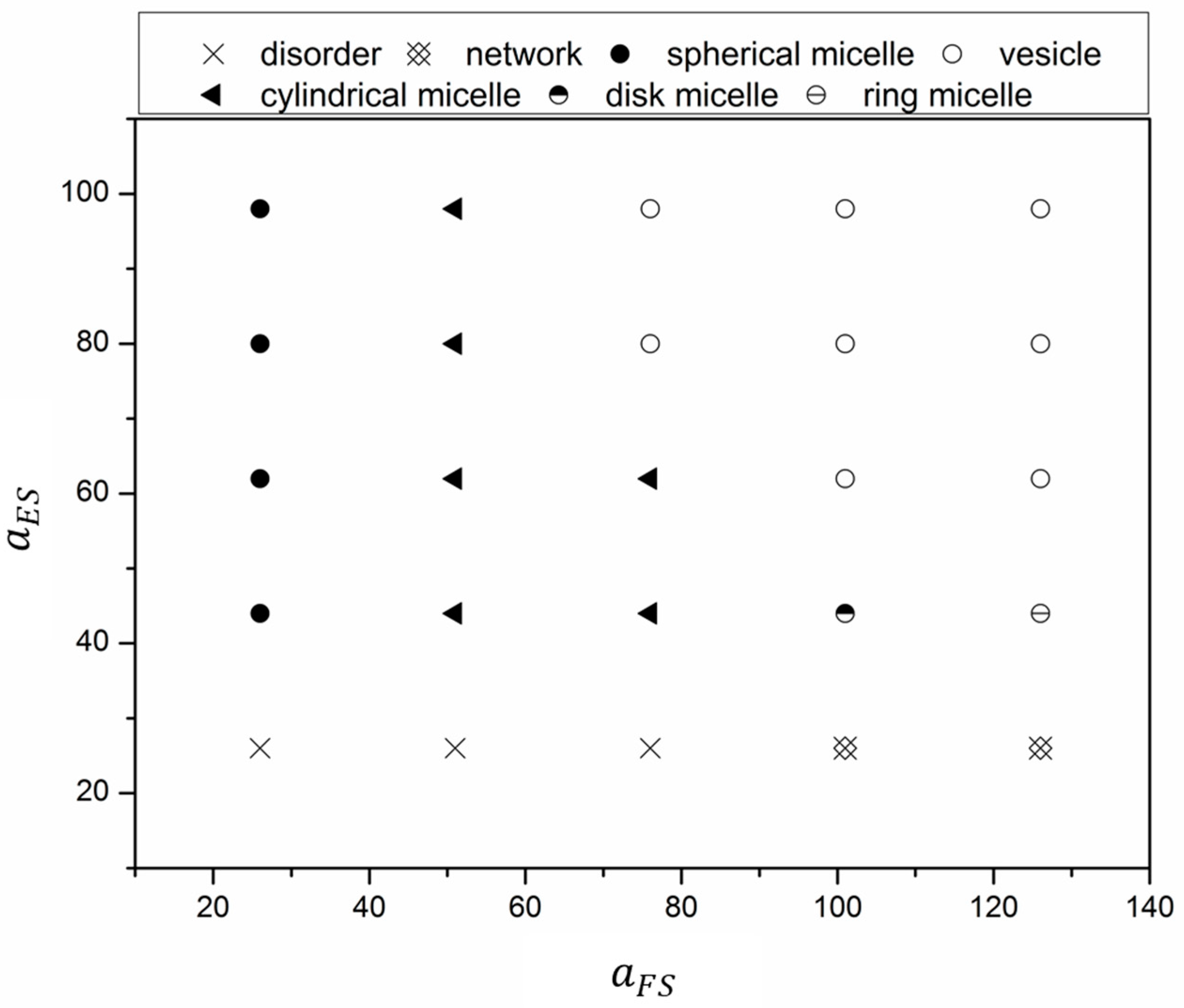
3.1. Effect of Block Solvophobicity on Vesicle Formation
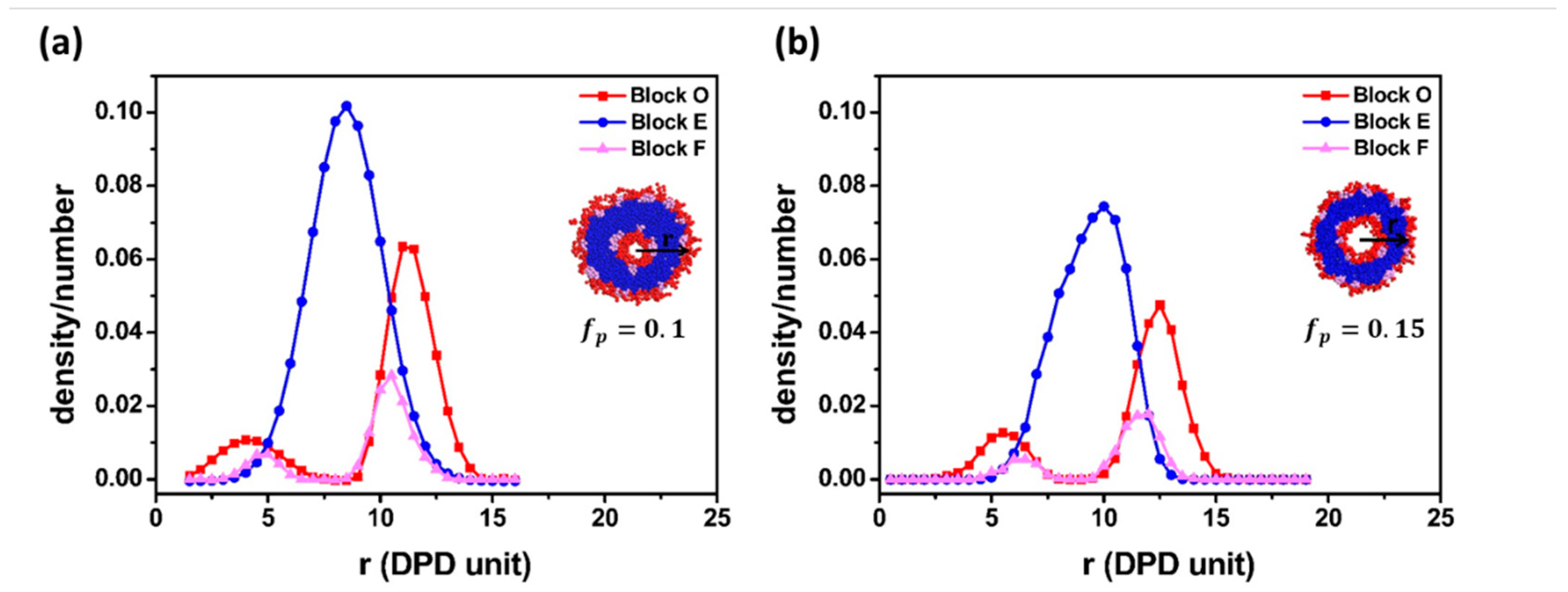
3.2. Vesicle Formation Mechanism
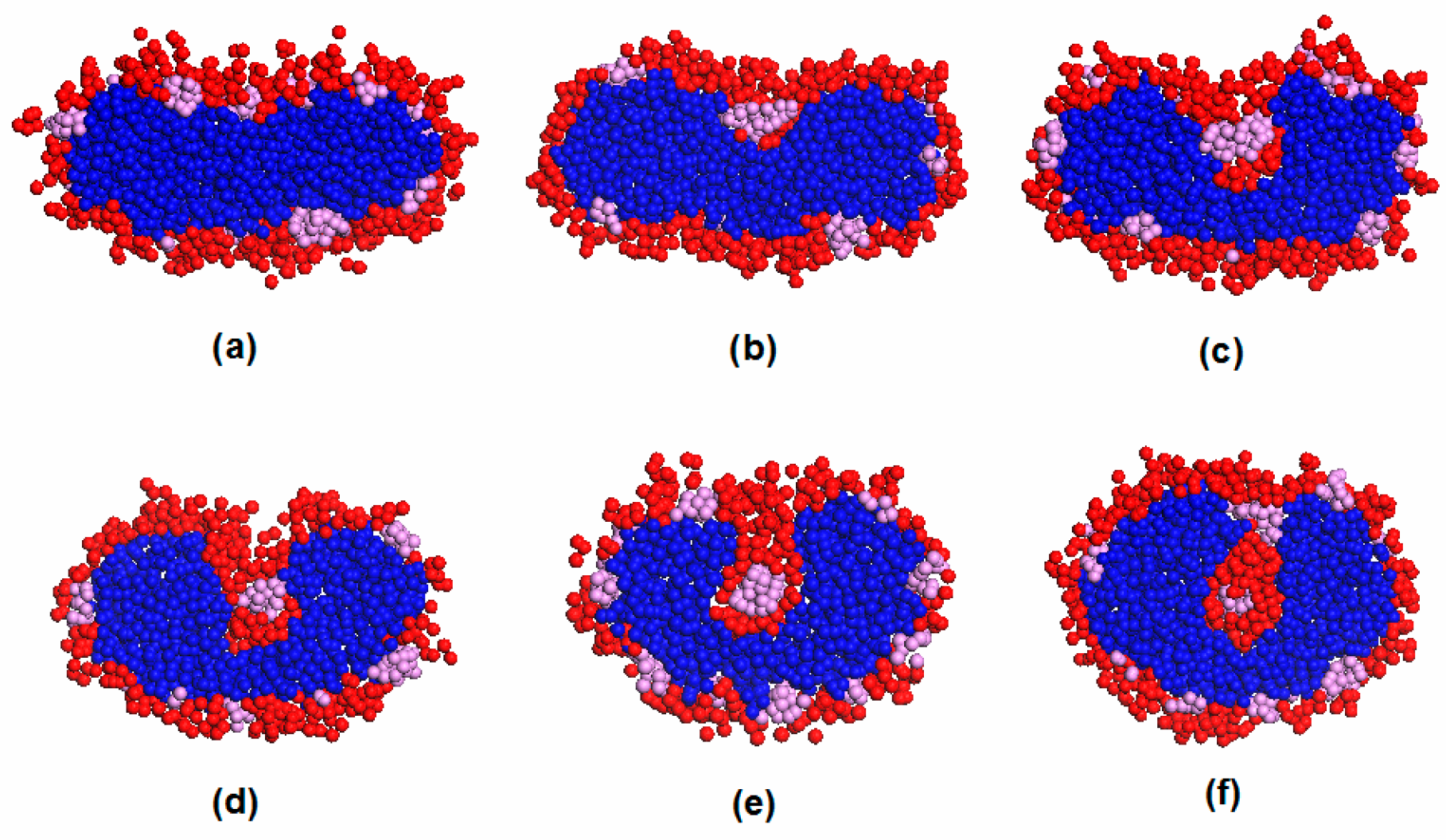
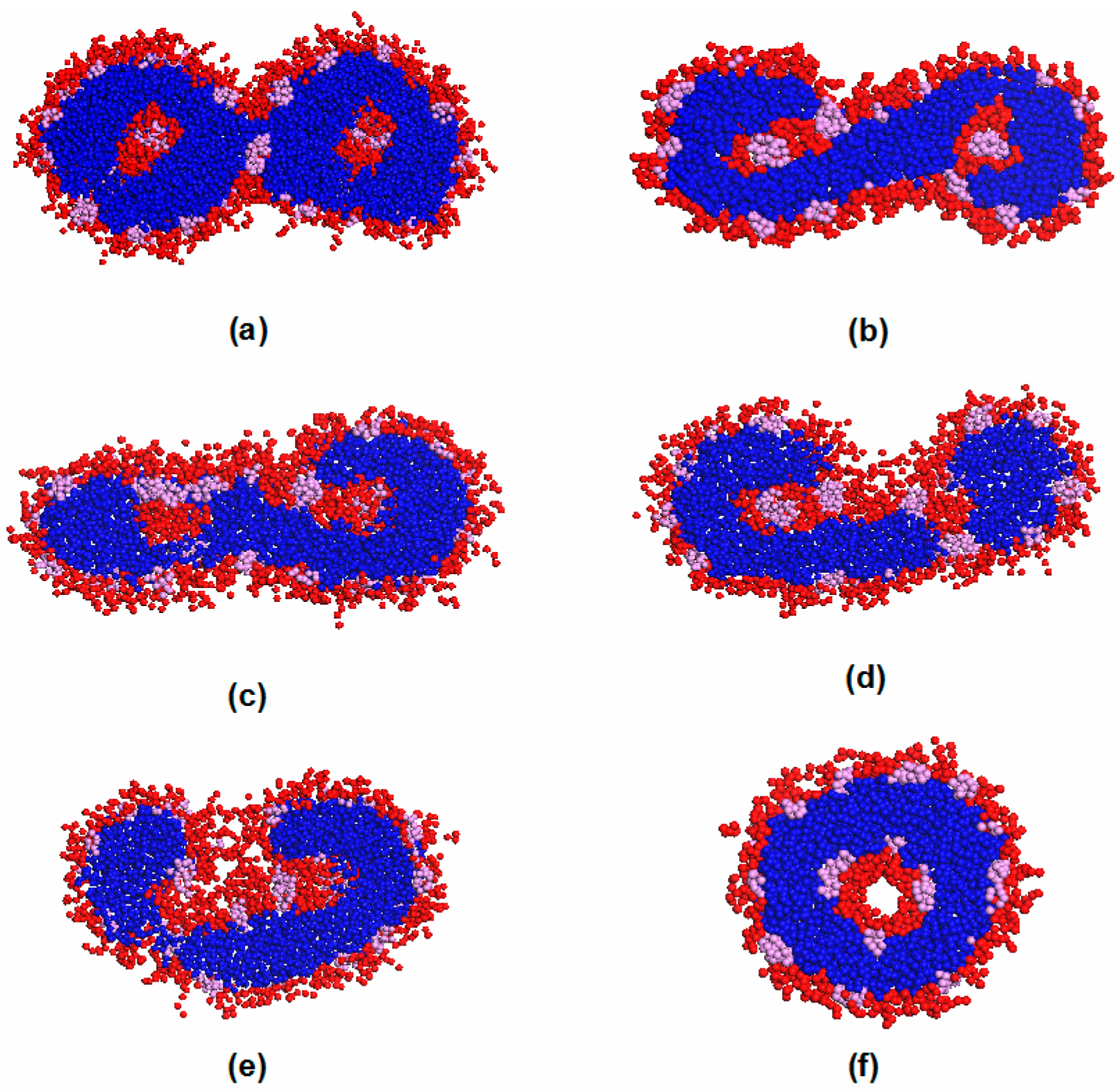
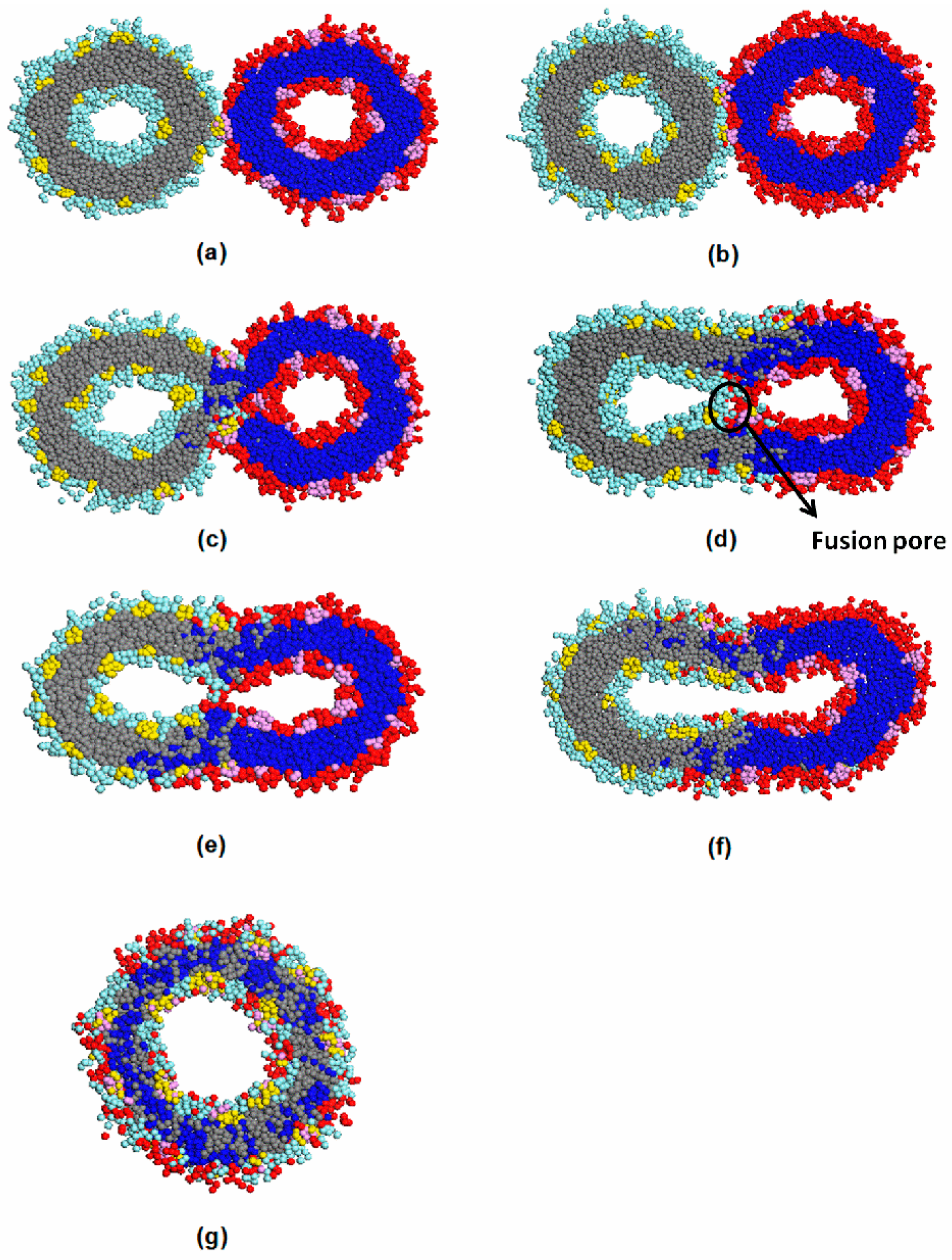
3.3. Vesicle Fusion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, S.Z.; Li, Z.W.; Zhao, H.Y. Patchy Micelles Based on Coassembly of Block Copolymer Chains and Block Copolymer Brushes on Silica Particles. Langmuir 2015, 31, 4129–4136. [Google Scholar] [CrossRef]
- Jo, I.S.; Lee, S.; Zhu, J.T.; Shim, T.S.; Yi, G.R. Soft patchy micelles. Curr. Opin. Colloid Interface Sci. 2017, 30, 97–105. [Google Scholar] [CrossRef]
- Schobel, J.; Hils, C.; Weckwerth, A.; Schlenk, M.; Bojer, C.; Stuart, M.C.A.; Breu, J.; Forster, S.; Greiner, A.; Karg, M.; et al. Strategies for the selective loading of patchy worm-like micelles with functional nanoparticles. Nanoscale 2018, 10, 18257–18268. [Google Scholar] [CrossRef] [Green Version]
- Nghiem, T.L.; Lobling, T.I.; Groschel, H. Supracolloidal chains of patchy micelles in water. Polym. Chem. 2018, 9, 1583–1592. [Google Scholar] [CrossRef]
- Hu, J.W.; Liu, G.J.; Nijkang, G. Hierarchical interfacial assembly of ABC triblock copolymer. J. Am. Chem. Soc. 2008, 130, 3236. [Google Scholar] [CrossRef] [PubMed]
- Rozynek, Z.; Mikkelsen, A.; Dommersnes, P.; Fossum, J.O. Electroformation of Janus and patchy capsules. Nat. Commun. 2014, 5, 3945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gummel, J.; Sztucki, M.; Narayanan, T.; Gradzielski, M. Concentration dependent pathways in spontaneous self-assembly of unilamellar vesicles. Soft Matter. 2011, 7, 5731–5738. [Google Scholar] [CrossRef] [Green Version]
- Adams, D.J.; Adams, S.; Atkins, D.; Butler, M.F.; Furzeland, S. Impact of mechanism of formation on encapsulation in block copolymer vesicles. J. Control Release 2008, 128, 165–170. [Google Scholar] [CrossRef]
- Han, Y.Y.; Yu, H.Z.; Du, H.B.; Jiang, W. Effect of Selective Solvent Addition Rate on the Pathways for Spontaneous Vesicle Formation of ABA Amphiphilic Triblock Copolymers. J. Am. Chem. Soc. 2010, 132, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.Y.; Xia, G.J.; Wang, R.; Xie, D.Q. Controlling the self-assembly pathways of amphiphilic block copolymers into vesicles. Soft Matter. 2012, 8, 7865–7874. [Google Scholar] [CrossRef]
- Korchagina, E.V.; Qiu, X.P.; Winnik, F.M. Effect of Heating Rate on the Pathway for Vesicle Formation in Salt-Free Aqueous Solutions of Thermosensitive Cationic Diblock Copolymers. Macromolecules 2013, 46, 2341–2351. [Google Scholar] [CrossRef]
- Xiao, Q.; Sherman, S.E.; Wilner, S.E.; Zhou, X.; Dazen, C.; Baumgart, T.; Reed, E.H.; Hammer, D.A.; Shinoda, W.; Klein, M.L.; et al. Janus dendrimersomes coassembled from fluorinated, hydrogenated, and hybrid Janus dendrimers as models for cell fusion and fission. Proc. Natl. Acad. Sci. USA 2017, 114, E7045–E7053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.Y.; Lin, Y.L.; Sheng, Y.J.; Tsao, H.K. Structural Characteristics and Fusion Pathways of Onion-Like Multilayered Polymersome Formed by Amphiphilic Comb-Like Graft Copolymers. Macromolecules 2013, 46, 5644–5656. [Google Scholar] [CrossRef]
- Bonifacino, J.S.; Glick, B.S. The mechanisms of vesicle budding and fusion. Cell 2004, 116, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.K.; Martin, A.D.; Floetenmeyer, M.; Parton, R.G.; Stenzel, M.H.; Thordarson, P. Faceted polymersomes: A sphere-to-polyhedron shape transformation. Chem. Sci. 2019, 10, 2725–2731. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Zhao, L.; Qian, H.J.; Lu, Z.Y. Coarse-grained simulation study on the self-assembly of miktoarm star-like block copolymers in various solvent conditions. Soft Matter. 2014, 10, 2245–2252. [Google Scholar] [CrossRef]
- Li, X.J.; Liu, Y.; Wang, L.; Deng, M.G.; Liang, H.J. Fusion and fission pathways of vesicles from amphiphilic triblock copolymers: A dissipative particle dynamics simulation study. Phys. Chem. Chem. Phys. 2009, 11, 4051–4059. [Google Scholar] [CrossRef]
- Muller, M.; Katsov, K.; Schick, M. A new mechanism of model membrane fusion determined from Monte Carlo simulation. Biophys. J. 2003, 85, 1611–1623. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.F.; Yan, D.Y. Real-time membrane fusion of giant polymer vesicles. J. Am. Chem. Soc. 2005, 127, 10468–10469. [Google Scholar] [CrossRef]
- Su, W.; Luo, Y.H.; Yan, Q.; Wu, S.; Han, K.; Zhang, Q.J.; Gu, Y.Q.; Li, Y.M. Photoinduced fusion of micro-vesicles self-assembled from azobenzene-containing amphiphilic diblock copolymers. Macromol. Rapid Comm. 2007, 28, 1251–1256. [Google Scholar] [CrossRef]
- Henderson, I.M.; Collins, A.M.; Quintana, H.A.; Montano, G.A.; Martinez, J.A.; Paxton, W.F. Lights on: Dye dequenching reveals polymersome fusion with polymer, lipid and stealth lipid vesicles. Polymer 2016, 83, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.L.; Chang, H.Y.; Sheng, Y.J.; Tsao, H.K. The fusion mechanism of small polymersomes formed by rod-coil diblock copolymers. Soft Matter. 2014, 10, 1500–1511. [Google Scholar] [CrossRef]
- Liu, Y.T.; Zhao, Y.; Liu, H.; Liu, Y.H.; Lu, Z.Y. Spontaneous Fusion between the Vesicles Formed by A(2n)(B-2)(n) Type Comb-Like Block Copolymers with a Semiflexible Hydrophobic Backbone. J. Phys. Chem. B 2009, 113, 15256–15262. [Google Scholar] [CrossRef]
- Li, Z.B.; Hillmyer, M.A.; Lodge, T.P. Laterally nanostructured vesicles, polygonal bilayer sheets, and segmented wormlike micelles. Nano Lett. 2006, 6, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Sheng, Y.J.; Tsao, H.K. Structural and mechanical characteristics of polymersomes. Soft Matter. 2014, 10, 6373–6381. [Google Scholar] [CrossRef]
- Moughton, A.O.; Sagawa, T.; Yin, L.G.; Lodge, T.P.; Hillmyer, M.A. Multicompartment Micelles by Aqueous Self-Assembly of mu-A(BC)(n) Miktobrush Terpolymers. Acs Omega 2016, 1, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Chambon, P.; Blanazs, A.; Battaglia, G.; Armes, S.P. Facile Synthesis of Methacrylic ABC Triblock Copolymer Vesicles by RAFT Aqueous Dispersion Polymerization. Macromolecules 2012, 45, 5081–5090. [Google Scholar] [CrossRef]
- Mable, C.J.; Fielding, L.A.; Derry, M.J.; Mykhaylyk, O.O.; Chambon, P.; Armes, S.P. Synthesis and pH-responsive dissociation of framboidal ABC triblock copolymer vesicles in aqueous solution. Chem. Sci. 2018, 9, 1454–1463. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Xu, R.; Wang, Z.L.; He, X.H. Kinetics of multicompartment micelle formation by self-assembly of ABC miktoarm star terpolymer in dilute solution. Soft Matter. 2012, 8, 11462–11470. [Google Scholar] [CrossRef]
- Guo, Y.Y.; di Mare, L.; Li, R.K.Y.; Wong, J.S.S. Structure of Amphiphilic Terpolymer Raspberry Vesicles. Polymers 2017, 9, 275. [Google Scholar] [CrossRef] [Green Version]
- Mable, C.J.; Warren, N.J.; Thompson, K.L.; Mykhaylyk, O.O.; Armes, S.P. Framboidal ABC triblock copolymer vesicles: A new class of efficient Pickering emulsifier. Chem. Sci. 2015, 6, 6179–6188. [Google Scholar] [CrossRef] [Green Version]
- Hoogerbrugge, P.J.; Koelman, J.M.V.A. Advances and challenges in smart and functional polymer vesicles. Europhys. Lett. 1992, 19, 155–160. [Google Scholar] [CrossRef]
- Groot, R.D.; Warren, P.B. Simulating Microscopic Hydrodynamic Phenomena with Dissipative Particle Dynamics. J. Chem. Phys. 1997, 107, 4423–4435. [Google Scholar] [CrossRef]
- Espaňol, P.; Warren, P.B. Dissipative particle dynamics: Bridging the gap between atomistic and mesoscopic simulation. Europhys. Lett. 1995, 30, 191–196. [Google Scholar]
- Mayer, A. What drives membrane fusion in eukaryotes? Trends Biochem. Sci. 2001, 26, 717–723. [Google Scholar] [CrossRef]
- Noguchi, H.; Takasu, M. Fusion pathways of vesicles: A Brownian dynamics simulation. J. Chem. Phys. 2001, 115, 9547–9551. [Google Scholar] [CrossRef]
- Marrink, S.J.; Mark, A. The Mechanism of Vesicle Fusion as Revealed by Molecular Dynamics Simulations. J. Am. Chem. Soc. 2003, 125, 11144–11145. [Google Scholar] [CrossRef] [Green Version]
- Kozlovsky, Y.; Kozlov, M.M. Stalk Model of Membrane Fusion: Solution of Energy Crisis. Biophys. J. 2002, 82, 882–895. [Google Scholar] [CrossRef] [Green Version]



| Type of Blocks | O | E | F | S |
|---|---|---|---|---|
| O | 25.0 | |||
| E | 38.5 | 25.0 | ||
| F | 89.4 | 78.0 | 25.0 | |
| S | 26.0 | 26.0–97.9 | 26.0–125.0 | 25.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Yang, S. Spontaneous Formation and Fusion of Raspberry Vesicle Self-Assembled from Star Block Terpolymers in Aqueous Solution. Materials 2021, 14, 7690. https://doi.org/10.3390/ma14247690
Guo Y, Yang S. Spontaneous Formation and Fusion of Raspberry Vesicle Self-Assembled from Star Block Terpolymers in Aqueous Solution. Materials. 2021; 14(24):7690. https://doi.org/10.3390/ma14247690
Chicago/Turabian StyleGuo, Yingying, and Shuyan Yang. 2021. "Spontaneous Formation and Fusion of Raspberry Vesicle Self-Assembled from Star Block Terpolymers in Aqueous Solution" Materials 14, no. 24: 7690. https://doi.org/10.3390/ma14247690
APA StyleGuo, Y., & Yang, S. (2021). Spontaneous Formation and Fusion of Raspberry Vesicle Self-Assembled from Star Block Terpolymers in Aqueous Solution. Materials, 14(24), 7690. https://doi.org/10.3390/ma14247690





