Microstructure and Oxidation Behavior of Fe-25Mn-9Al-8Ni-1C-xTi Alloy Prepared by Vacuum Arc Melting
Abstract
:1. Introduction
2. Preparation and Test Method
Preparation of Fe-25Mn-9Al-8Ni-1C-xTi Alloy
3. Results and Discussion
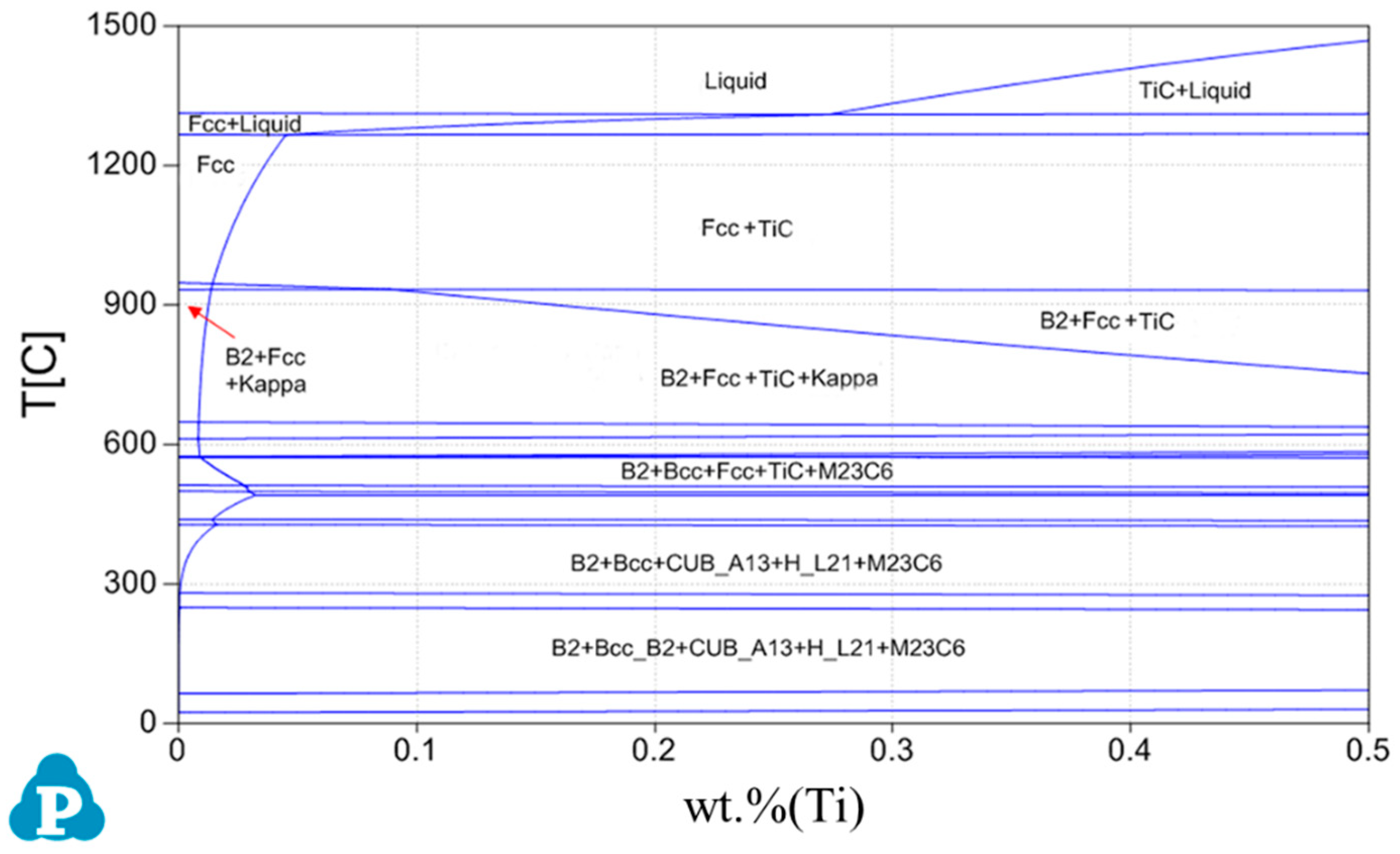
3.1. Phase Diagram Analysis
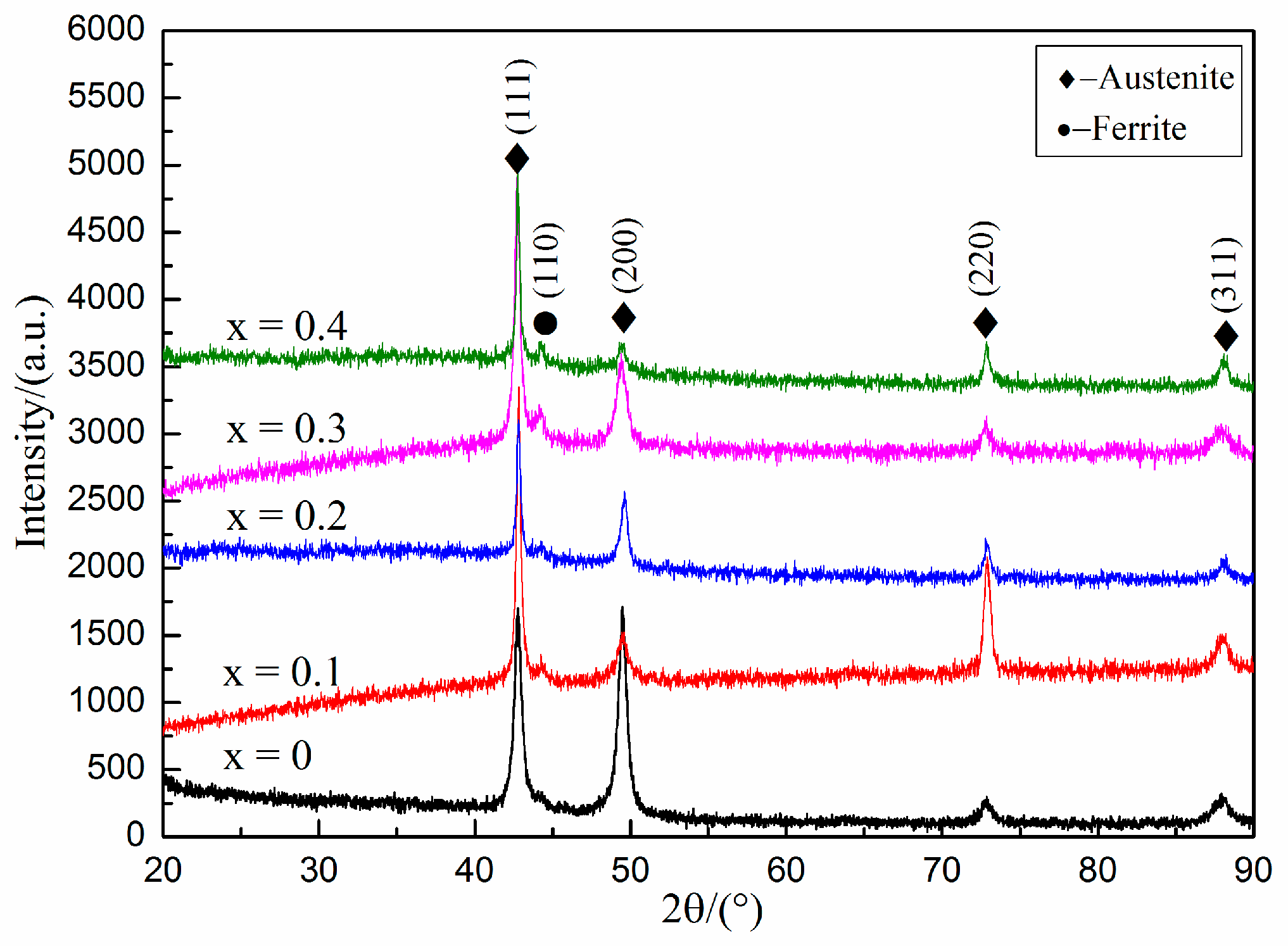
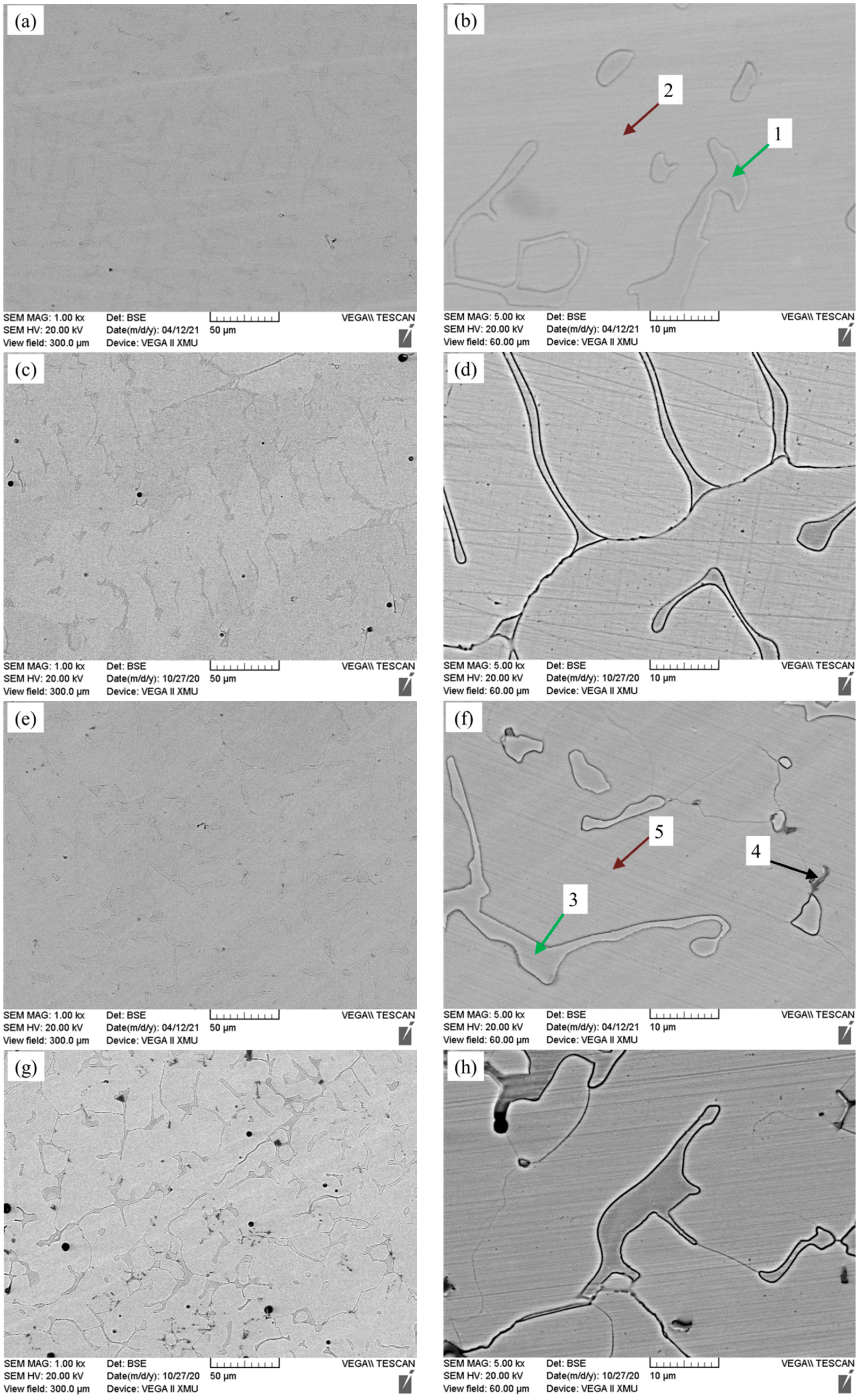
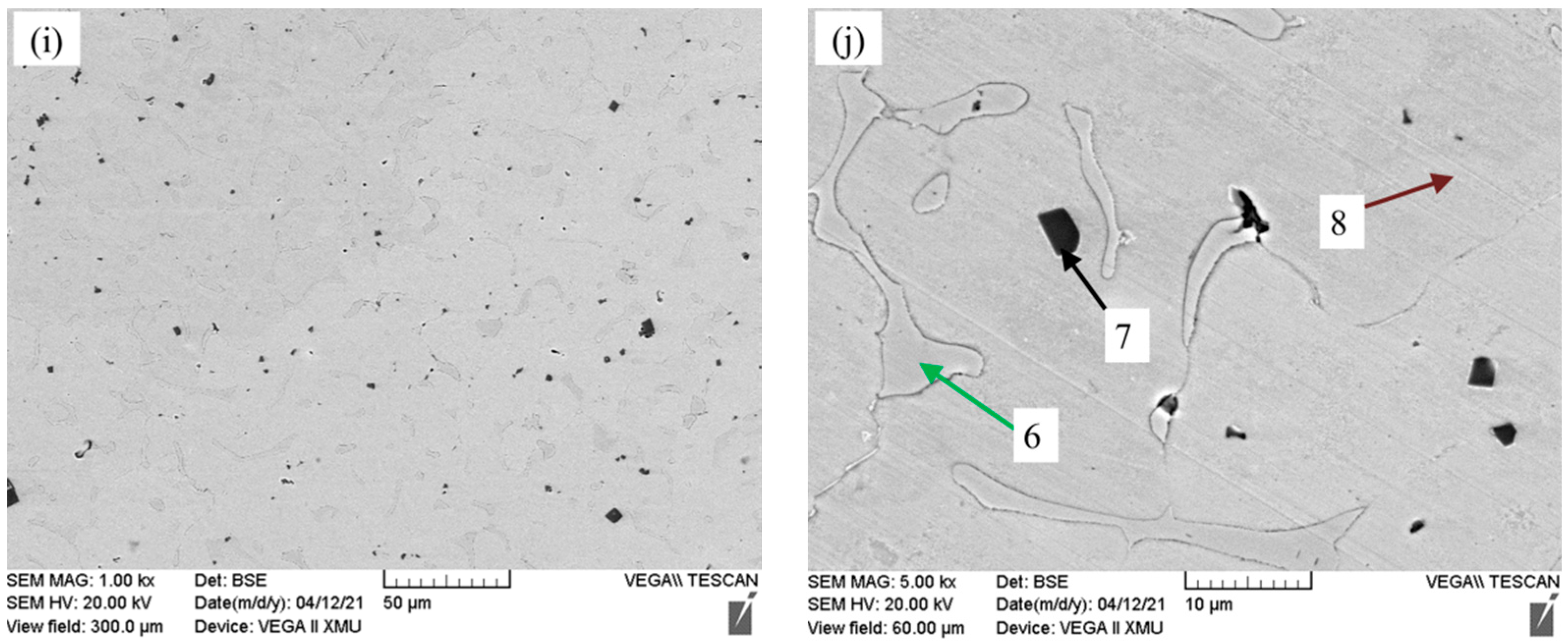
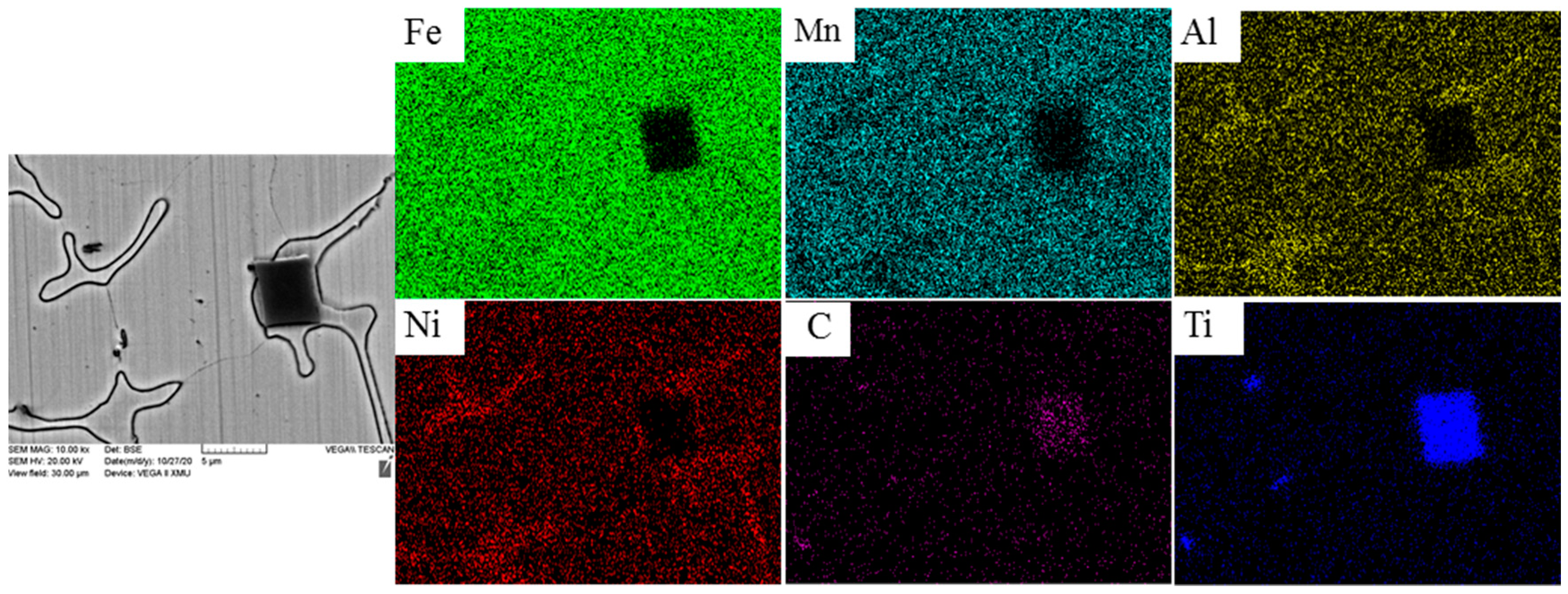
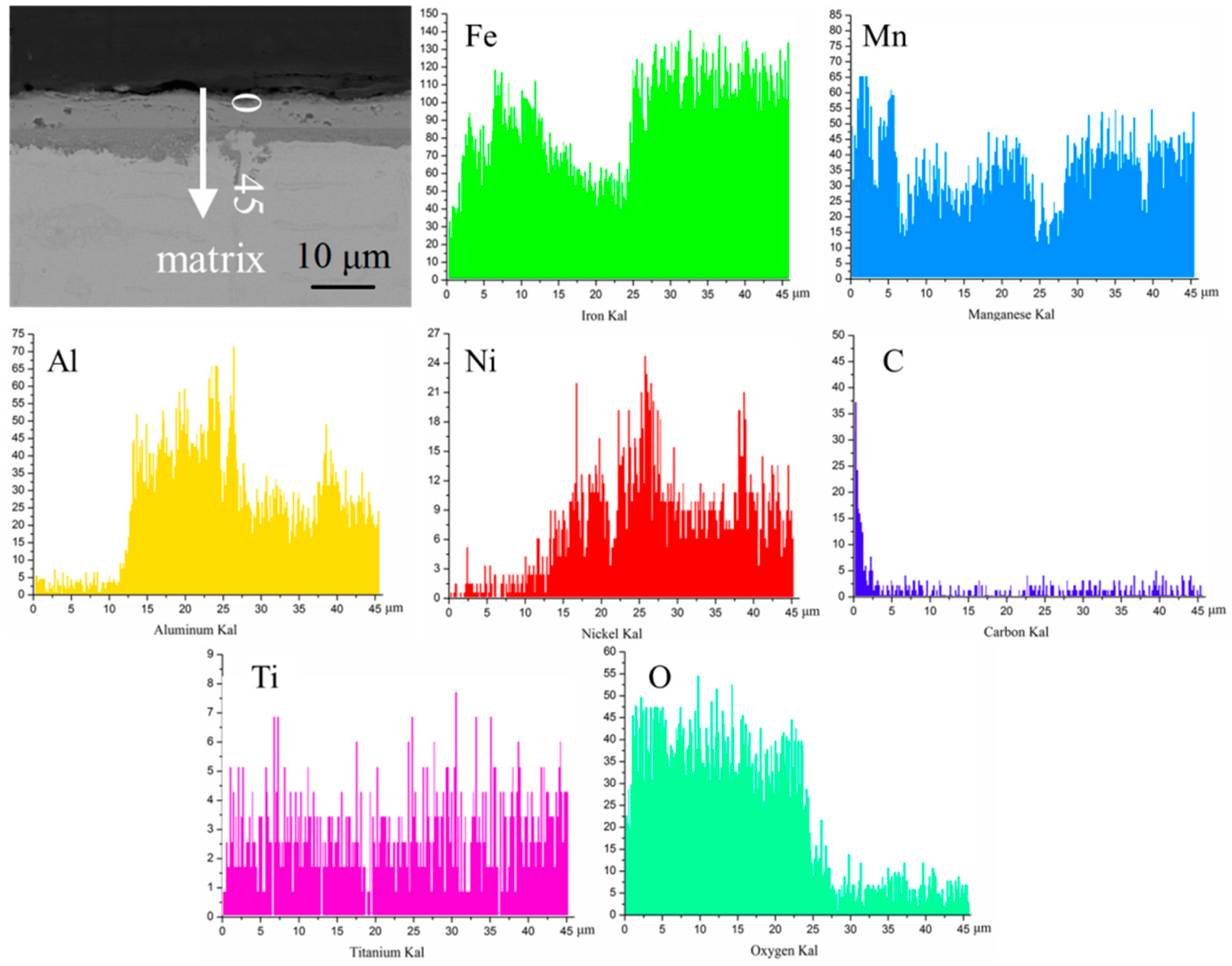
3.2. Microstructure Analysis
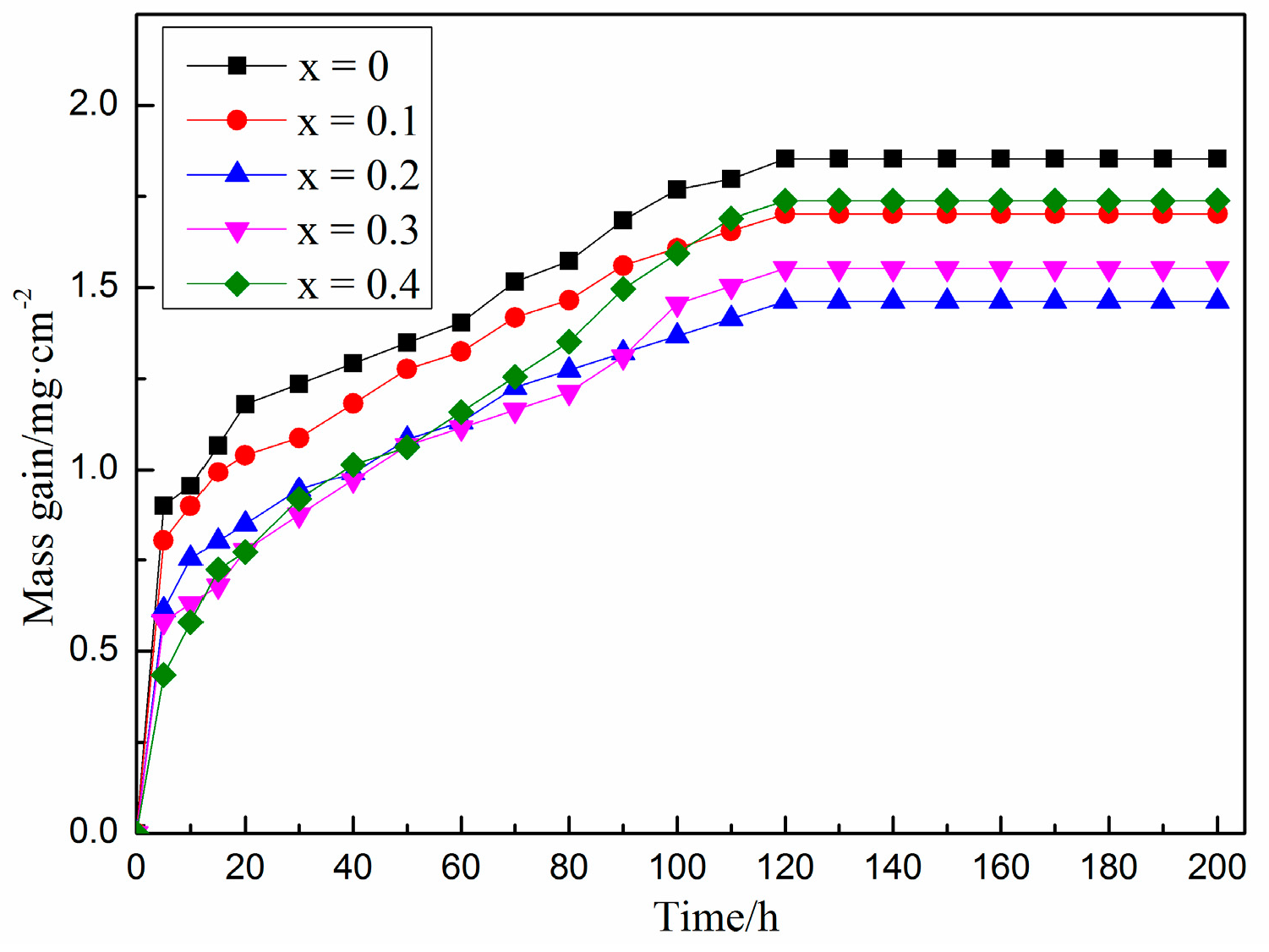
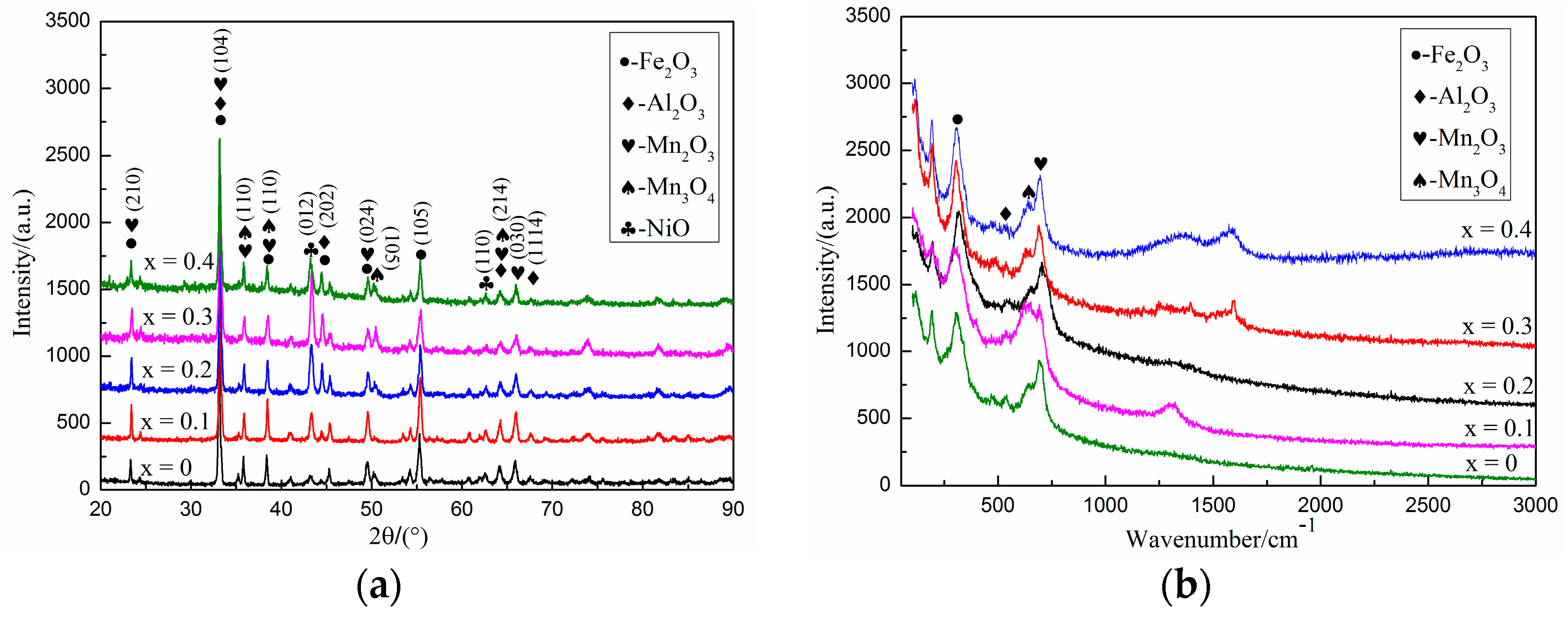
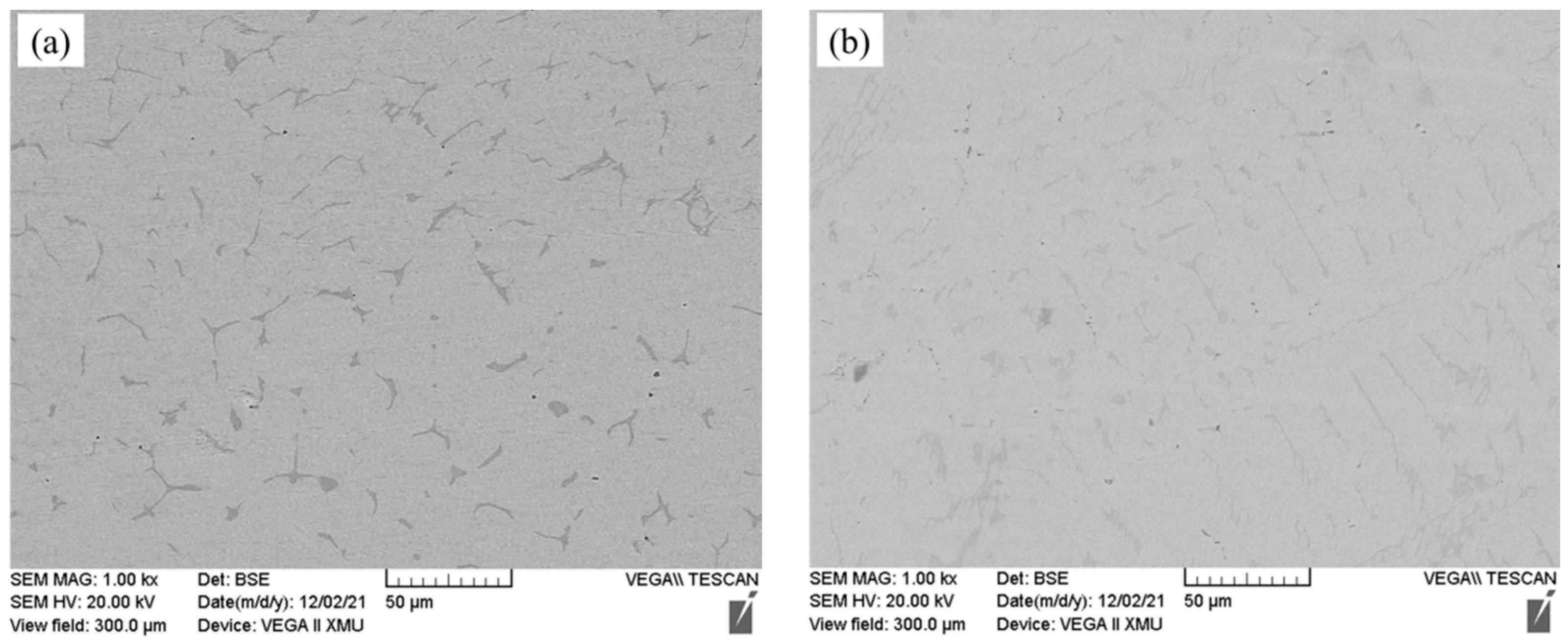
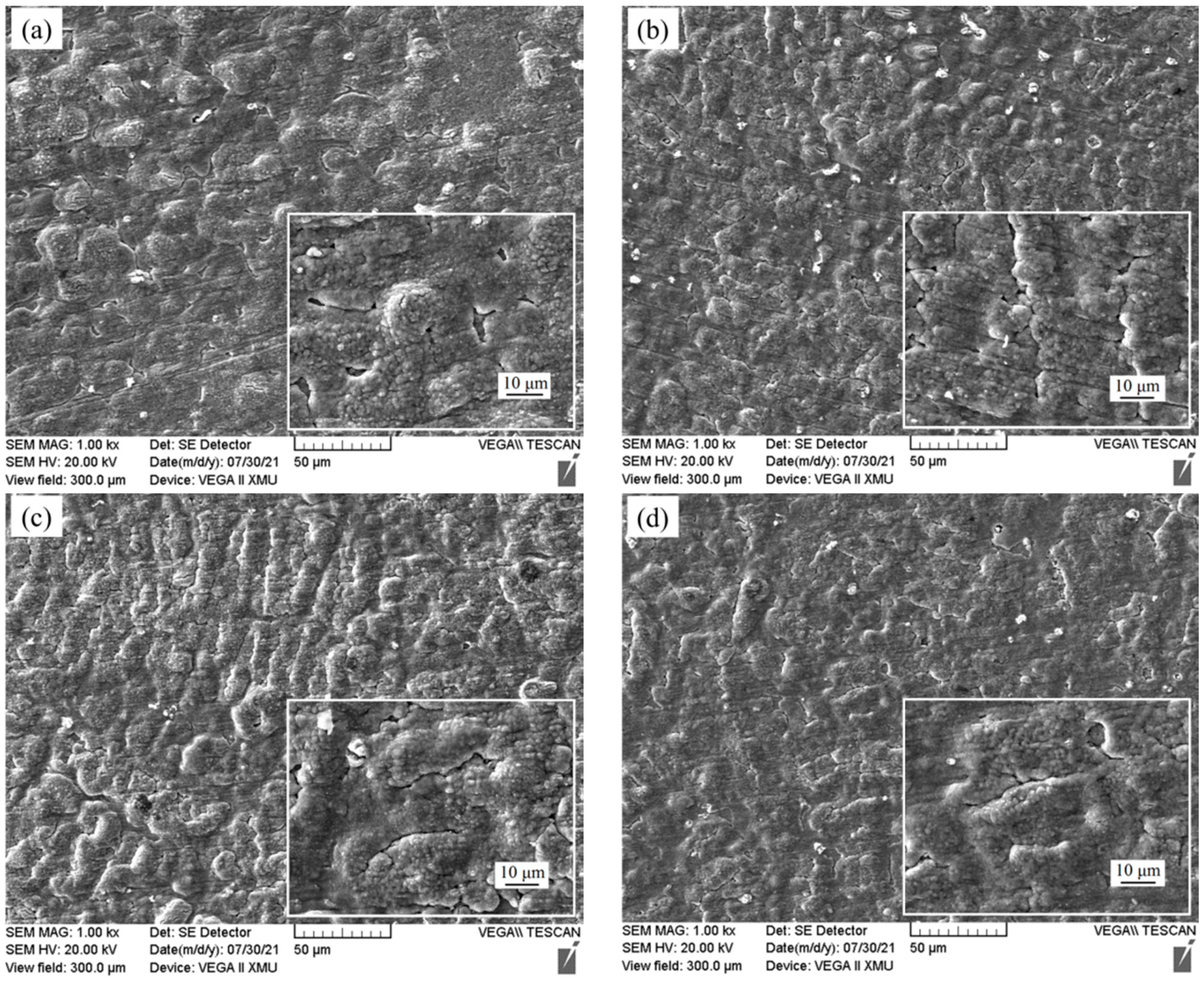
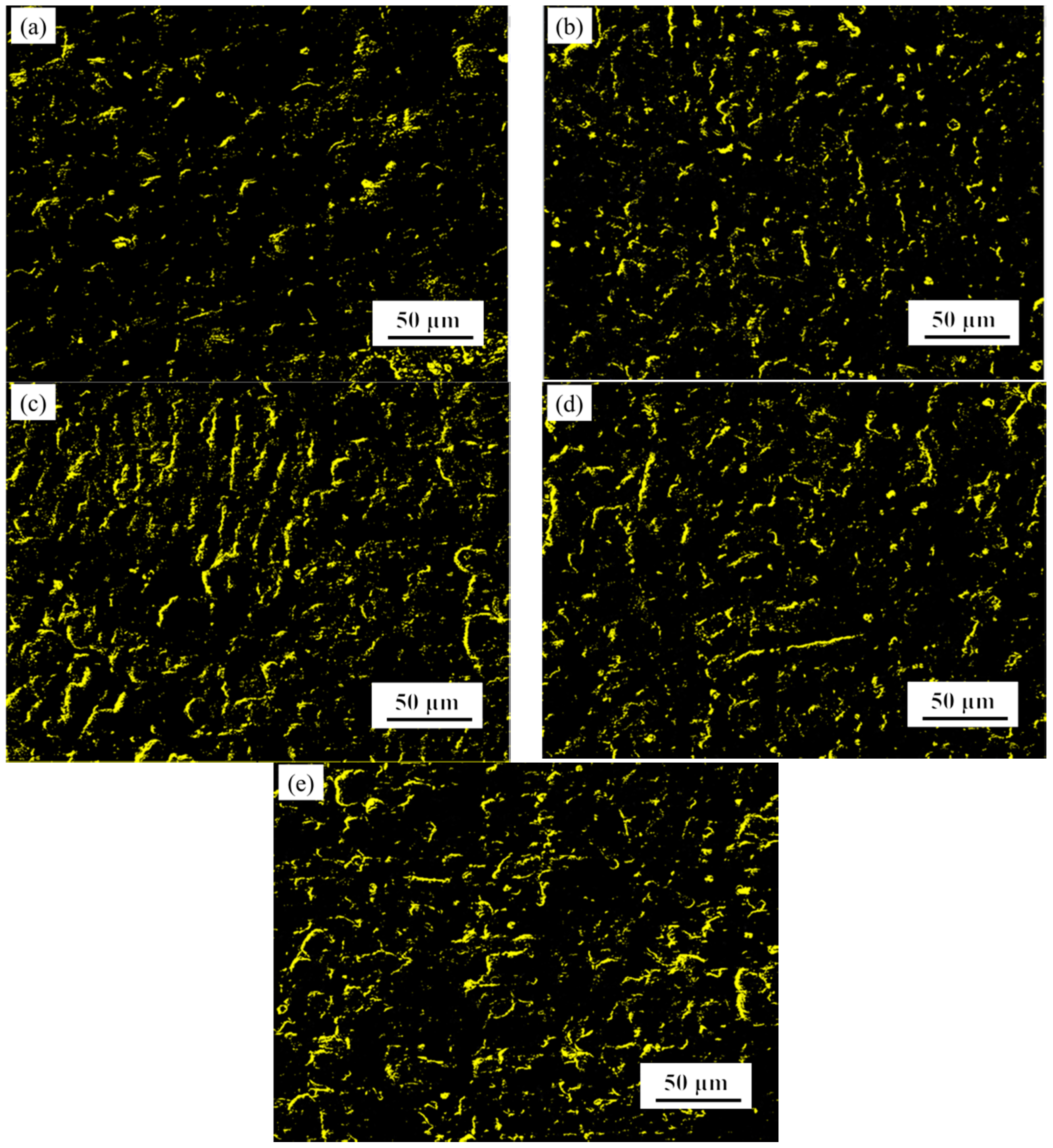
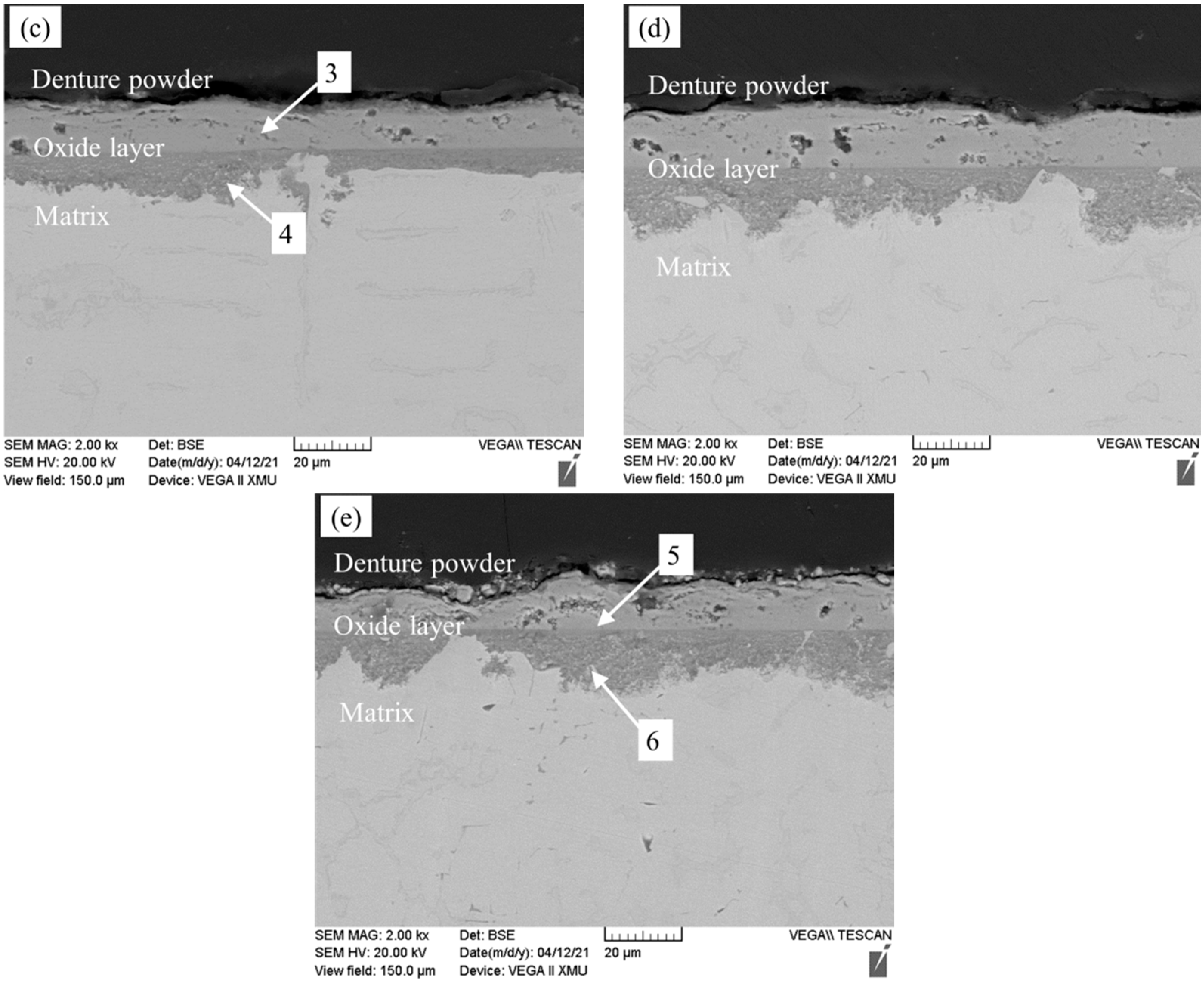
3.3. Oxidation Resistance Analysis
4. Conclusions
- (1)
- Fe-25Mn-9Al-8Ni-1C-xTi alloy mainly contains austenite, ferrite and a small amount of TiC. As the Ti content increases, the austenite content in the alloy gradually decreases, and the ferrite and TiC content gradually increases.
- (2)
- The oxidation performance test results of Fe-25Mn-9Al-8Ni-1C-xTi at 600 °C present that the addition of Ti element greatly reduces the weight gain of the alloy in the initial stage of oxidation. With the extension of the oxidation time and the further increase of the Ti content, the alloy oxidation weight gain shows a trend of first increasing and then decreasing. When the Ti content is 0.2 wt.%, the oxidation weight gain of the alloy in the stable oxidation period reaches the lowest value. The oxidation weight gain is 1.462 mg/cm2, which is 21.1% less than the weight gain per unit area of the alloy without Ti.
- (3)
- The oxide layer of Fe-25Mn-9Al-8Ni-1C-xTi alloy exhibits a double-layer structure. The outer oxidized layer mainly forms Fe oxide, and its structure is loose. The inner oxide layer is mainly the oxide formed by manganese and aluminum, which are Mn2O3 and Al2O3 respectively, and they are relatively dense. It can effectively prevent the diffusion of oxygen into the alloy. When the Ti content is 0.2 wt.%, the oxidized layer of the alloy is the thinnest at 25.467 μm, which is 3.943 μm (13.5%) less than the alloy without Ti addition.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- You, J.; Liu, Z.; Wang, Z.; Wang, D.; Xu, Y. Experimental analysis of inert gases in EGR on engine power and combustion characteristics in a stoichiometric dual fuel heavy-duty natural gas engine ignited with diesel. Appl. Therm. Eng. 2020, 180, 115860. [Google Scholar] [CrossRef]
- Pan, S.; Zeng, F.; Su, N.; Xian, Z. The effect of niobium addition on the microstructure and properties of cast iron used in cylinder head. J. Mater. Res. Technol. 2020, 9, 1509–1518. [Google Scholar] [CrossRef]
- Bai, Y.; Li, J.; Cheng, C.; Yang, Z. Study on microstructure and oxidation behavior of Fe–xMn–14Al–8Ni–C alloy prepared by vacuum arc melting. Mater. Res. Express 2020, 7, 096521. [Google Scholar] [CrossRef]
- Rashnoo, K.; Sharifi, M.J.; Azadi, M.; Azadi, M. Influences of reinforcement and displacement rate on microstructure, mechanical properties and fracture behaviors of cylinder-head aluminum alloy. Mater. Chem. Phys. 2020, 255, 123441. [Google Scholar] [CrossRef]
- Cihan, M.; Temizer, L.; Gven, M.; Karabas, M. Investigation of the effect of rare earth doped La2Zr2O7 based thermal barrier coating on performance and combustion characteristics of DI diesel engine. Surf. Coat. Technol. 2020, 403, 126437. [Google Scholar] [CrossRef]
- Kim, C.; Hong, H.U.; Jang, J.H.; Lee, B.H.; Park, S.J.; Moon, J.; Lee, C.H. Reverse partitioning of Al from κ-carbide to the γ-matrix upon Ni addition and its strengthening effect in Fe–Mn–Al–C lightweight steel. Mater. Sci. Eng. A 2021, 820, 141563. [Google Scholar] [CrossRef]
- Chen, P.; Li, W.; Yi, H. The κ-carbides in low-density Fe-Mn-Al-C steels: A review on their structure, precipitation and deformation mechanism. Met. Open Access Metall. J. 2020, 10, 1021. [Google Scholar] [CrossRef]
- Walnsch, A.; Kriegel, M.J.; Fischer, P.; Neumann, S.; Rafaja, D.; Leineweber, A. Nanoscale twinning and superstructures of martensite in the Fe–Mn–Al–Ni system. Materialia 2021, 16, 101062. [Google Scholar] [CrossRef]
- Pang, J.; Zhou, Z.; Zhao, Z.; Tang, D.; He, Q. Tensile behavior and deformation mechanism of Fe-Mn-Al-C low density steel with high strength and high plasticity. Met. Open Access Metall. J. 2019, 9, 897. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wang, Y.; Cheng, X.; Li, Z.; Gao, C.; Li, S. The effect of rolling and subsequent aging on microstructures and tensile properties of a Fe–Mn–Al–C austenitic steel. Mater. Sci. Eng. A 2021, 822, 141683. [Google Scholar] [CrossRef]
- Xing, J.; Wei, Y.; Hou, L. An overview of the effects of alloying elements on the properties of lightweight Fe-(15–35) Mn-(5–12) Al-(0.3–1.2) C steel. Met. Mater. Soc. 2018, 70, 929–937. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Y.; Zheng, W.; Liu, Y.; Ahmed, A.; Ji, G.; Song, C. Revisiting the formation mechanism of intragranular κ-carbide in austenite of a Fe-Mn-Al-Cr-C low-density steel. Scr. Mater. 2021, 199, 113836. [Google Scholar] [CrossRef]
- Xiong, R.; Peng, H.; Zhang, T.; Bae, J.W.; Kim, H.S.; Wen, Y. Superior strain-hardening by deformation-induced nano-HCP martensite in Fe–Mn–Si–C high-manganese steel. Mater. Sci. Eng. A 2021, 824, 141864. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, C. Microstructure and mechanical properties of 980MPa grade Fe-Mn-Al-C lightweight steel. Mater. Sci. Eng. 2017, 207, 012053. [Google Scholar] [CrossRef] [Green Version]
- Shyamal, S.; Das, S.R.; Jaskari, M.; Porter, D.A.; Karjalainen, L.P.; Sahu, P. Graded deformation in an Fe-Mn-Al-C steel under bending fatigue. Mater. Lett. 2021, 285, 129002. [Google Scholar] [CrossRef]
- Ren, P.; Chen, X.; Wang, C.; Zhou, Y.; Liu, Q. Evolution of microstructure, texture and mechanical properties of Fe–30Mn–11Al–1.2C low-density steel during cold rolling. Mater. Charact. 2021, 174, 111013. [Google Scholar] [CrossRef]
- Das, S.R.; Shyamal, S.; Sahu, T.; Kmi, J.I.; Chakraborti, P.C.; Porter, D.A.; Karjalainen, L.P.; Sahu, P. On the mechanism of cross-slip induced dislocation substructure formation in an high-Mn steel. Materialia 2021, 15, 101042. [Google Scholar] [CrossRef]
- Feng, Y.; Song, R.; Wang, Y.; Pei, Z. The synergistic effect of deformation twins and polycrystalline structure on strain hardening in a high-SFE Fe-Mn-Al-C austenitic cast steel in compression. Mater. Lett. 2020, 272, 127814. [Google Scholar] [CrossRef]
- Li, D.; Qian, L.; Wei, C.; Liu, S.; Zhang, F.; Meng, J. The role of Mn on twinning behavior and tensile properties of coarse- and fine-grained Fe–Mn–C twinning-induced plasticity steels. Mater. Sci. Eng. A 2020, 789, 139586. [Google Scholar] [CrossRef]
- Zou, Y.; Xu, Y.; Wang, G.; Han, Y.; Teng, H.; Han, D.; Qiu, M.; Yang, F.; Misra, R.D.K. Improved strength-ductility-toughness balance of a precipitation-strengthened low-carbon medium-Mn steel by adopting intercritical annealing-tempering process. Mater. Sci. Eng. A 2021, 802, 140636. [Google Scholar] [CrossRef]
- Li, K.; Yu, B.; Misra, R.D.K.; Han, G.; Tsai, Y.T.; Shao, C.; Shang, C.; Yang, J.; Zhang, Z. Strain rate dependence on the evolution of microstructure and deformation mechanism during nanoscale deformation in low carbon-high Mn TWIP steel. Mater. Sci. Eng. A 2019, 742, 116–123. [Google Scholar] [CrossRef]
- Lee, S.J.; Sun, Y.; Fujii, H. Stacking-fault energy, mechanical twinning and strain hardening of Fe-18Mn-0.6C-(0, 1.5)Al twinning-induced plasticity steels during friction stir welding. Acta Mater. 2018, 148, 235–248. [Google Scholar] [CrossRef]
- Anand, K.K.; Mahato, B.; Haase, C.; Kumar, A.; Chowdhury, G.S. Correlation of defect density with texture evolution during cold rolling of a Twinning-Induced Plasticity (TWIP) steel. Mater. Sci. Eng. A 2018, 711, 69–77. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H.; Kim, N.J. Brittle intermetallic compound makes ultrastrong low-density steel with large ductility. Nature 2015, 518, 77–79. [Google Scholar] [CrossRef]
- Rahnama, A.; Sridhar, S. Multiscale micromechanical model of duplex low density steel with B2 ordered precipitates. Mech. Mater. 2019, 131, 136–140. [Google Scholar] [CrossRef]
- Lee, J.Y.; Hong, J.S.; Kang, S.H.; Kang, S.H.; Lee, Y.K. The effect of austenite grain size on deformation mechanism of Fe-17Mn steel. Mater. Sci. Eng. A 2021, 809, 140972. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Cheng, X.; Li, Z.; Du, J.; Li, S. The effect of Ti-Mo-Nb on the microstructures and tensile properties of a Fe-Mn-Al-C austenitic steel. Mater. Sci. Eng. 2020, 780, 139220. [Google Scholar] [CrossRef]
- Han, Y.; Shi, J.; Xu, L.; Gao, W.; Dong, H. Effects of Ti addition and reheating quenching on grain refinement and mechanical properties in low carbon medium manganese martensitic steel. Mater. Des. 2012, 34, 427–434. [Google Scholar] [CrossRef]
- Sarkar, A.; Sanyal, S.; Bandyopadhyay, T.K.; Mandal, S. Implications of microstructure, Taylor factor distribution and texture on tensile properties in a Ti-added Fe-Mn-Al-Si-C steel. Mater. Sci. Eng. A 2019, 767, 138402. [Google Scholar] [CrossRef]
- Bai, Y.; Li, M.; Cheng, C.; Li, J.; Guo, Y.; Yang, Z. Study on Microstructure and In Situ Tensile Deformation Behavior of Fe-25Mn-xAl-8Ni-C Alloy Prepared by Vacuum Arc Melting. Metals 2021, 11, 814. [Google Scholar] [CrossRef]
- Chen, C. Study on Fabrication Processing and Properties of Fe-Mn-Al Alloy with Low Density and High Performance; Xi’an Technological University: Xi’an, China, 2020. (In Chinese) [Google Scholar]
- Barella, S.; Ciuffini, A.F.; Gruttadauria, A.; Mapelli, C.; Mombelli, D.; Longaretti, E. Corrosion and Oxidation Behavior of a Fe-Al-Mn-C Duplex Alloy. Materials 2019, 12, 2572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, W.; Wu, Z.; Xu, Y.; Ran, Q.; Xu, W.; Li, J.; Xiao, X. Internal oxidation behaviour of Fe-Mn-Al-C duplex light-weight steels with good combination of strength and ductility. Corros. Sci. 2017, 120, 148–157. [Google Scholar] [CrossRef]
- Liu, D.; Cai, M.; Ding, H.; Han, D. Control of inter/intra-granular k-carbides and its influence on overall mechanical properties of a Fe-11Mn-10Al-1.25C low density steel. Mater. Sci. Eng. A 2018, 715, 25–32. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, C.; Zhang, Y.; Li, J.; Song, C.; Zhai, Q. Microstructures, mechanical properties and deformation of near-rapidly solidified low-density Fe-20Mn-9Al-1.2C-xCr steels. Mater. Des. 2020, 186, 1083307. [Google Scholar] [CrossRef]
- Trindade, V.B.; Krupp, U.; Hanjarl, B.Z.; Yang, S.; Christ, H.J. Effect of alloy grain size on the high-temperature oxidation behavior of the austenitic steel TP 347. Mater. Res. 2005, 8, 371–375. [Google Scholar] [CrossRef]
- Chen, D.; Liu, M.; Liu, X.; Zhou, Y.; Wang, X.; Zhou, Y. Oxidation Behavior of 304 Stainless Steel with Modified Layer by Plasma Nitriding in High Temperature and Pressurized Water. Corros. Sci. 2021, 186, 109468. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Pakseresht, A.; Omidvar, H.; Shafiei, A. Investigation of the High-Temperature Oxidation Behavior of the Al0.5CoCrFeNi High Entropy Alloy. Surf. Interfaces 2020, 21, 100724. [Google Scholar] [CrossRef]




| Alloy | Variable-x | Industrial Pure Iron | 50%Fe-Mn Alloy | Aluminum Particles | Nickel Particles | Fe-5C Alloy | Titanium Particles |
|---|---|---|---|---|---|---|---|
| Fe-25Mn-9Al -8Ni-1C-xTi (0 ≤ x ≤ 0.4) | 0 | 13 | 50 | 9 | 8 | 20 | 0 |
| 0.1 | 12.9 | 50 | 9 | 8 | 20 | 0.1 | |
| 0.2 | 12.8 | 50 | 9 | 8 | 20 | 0.2 | |
| 0.3 | 12.7 | 50 | 9 | 8 | 20 | 0.3 | |
| 0.4 | 12.6 | 50 | 9 | 8 | 20 | 0.4 |
| Point | Element/(at.%) | |||||
|---|---|---|---|---|---|---|
| Fe | Mn | Al | Ni | C | Ti | |
| 1 | 43.53 | 14.54 | 26.26 | 15.58 | / | 0.09 |
| 2 | 44.39 | 17.42 | 12.09 | 5.00 | 21.08 | 0.02 |
| 3 | 40.32 | 13.22 | 21.82 | 11.10 | 13.46 | 0.08 |
| 4 | 12.36 | 7.65 | 2.44 | 1.78 | 51.36 | 24.41 |
| 5 | 45.17 | 16.22 | 12.60 | 4.79 | 20.98 | 0.24 |
| 6 | 39.01 | 12.82 | 22.91 | 13.09 | 12.02 | 0.15 |
| 7 | 1.73 | 1.13 | 0.70 | 0.33 | 48.59 | 47.52 |
| 8 | 41.65 | 17.74 | 12.56 | 5.48 | 22.57 | / |
| Point | Element/(at.%) | ||||||
|---|---|---|---|---|---|---|---|
| Fe | Mn | Al | Ni | C | Ti | O | |
| 1 | 9.41 | 30.51 | / | 0.18 | 2.24 | / | 57.66 |
| 2 | 15.58 | 19.19 | / | 0.07 | 5.69 | / | 59.47 |
| 3 | 11.07 | 29.56 | 0.08 | 0.15 | 3.54 | / | 55.60 |
| 4 | 13.62 | 23.53 | / | / | 6.33 | 0.07 | 56.45 |
| 5 | 8.00 | 22.68 | / | 0.11 | 3.83 | 0.05 | 65.51 |
| 6 | 12.68 | 20.15 | / | 0.03 | 7.06 | 0.02 | 60.06 |
| Alloy | Variable-x | Average Thickness (μm) |
|---|---|---|
| Fe-25Mn-9Al-8Ni-1C-xTi (0 ≤ x ≤ 0.4) | 0 | 29.410 |
| 0.1 | 28.454 | |
| 0.2 | 25.467 | |
| 0.3 | 26.476 | |
| 0.4 | 29.166 |
| Point | Element/(at.%) | ||||||
|---|---|---|---|---|---|---|---|
| Fe | Mn | Al | Ni | C | Ti | O | |
| 1 | 34.75 | 8.96 | 0.20 | 0.10 | 0.46 | / | 55.53 |
| 2 | 14.48 | 7.37 | 20.26 | 13.5 | 0.40 | / | 43.99 |
| 3 | 36.80 | 6.02 | 0.03 | 0.12 | 0.53 | / | 56.50 |
| 4 | 18.91 | 11.22 | 15.99 | 5.30 | 0.46 | 0.12 | 50.00 |
| 5 | 41.31 | 6.17 | 0.10 | 0.42 | 0.47 | / | 51.53 |
| 6 | 17.75 | 10.09 | 18.50 | 2.86 | 0.27 | 0.14 | 50.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Tian, K.; Li, J.; Yang, Z. Microstructure and Oxidation Behavior of Fe-25Mn-9Al-8Ni-1C-xTi Alloy Prepared by Vacuum Arc Melting. Materials 2021, 14, 7722. https://doi.org/10.3390/ma14247722
Bai Y, Tian K, Li J, Yang Z. Microstructure and Oxidation Behavior of Fe-25Mn-9Al-8Ni-1C-xTi Alloy Prepared by Vacuum Arc Melting. Materials. 2021; 14(24):7722. https://doi.org/10.3390/ma14247722
Chicago/Turabian StyleBai, Yaping, Keke Tian, Jianping Li, and Zhong Yang. 2021. "Microstructure and Oxidation Behavior of Fe-25Mn-9Al-8Ni-1C-xTi Alloy Prepared by Vacuum Arc Melting" Materials 14, no. 24: 7722. https://doi.org/10.3390/ma14247722
APA StyleBai, Y., Tian, K., Li, J., & Yang, Z. (2021). Microstructure and Oxidation Behavior of Fe-25Mn-9Al-8Ni-1C-xTi Alloy Prepared by Vacuum Arc Melting. Materials, 14(24), 7722. https://doi.org/10.3390/ma14247722






