Abstract
Over the past few decades, nanoparticles of iron oxide Fe3O4 (magnetite) gained significant attention in both basic studies and many practical applications. Their unique properties such as superparamagnetism, low toxicity, synthesis simplicity, high surface area to volume ratio, simple separation methodology by an external magnetic field, and renewability are the reasons for their successful utilisation in environmental remediation, biomedical, and agricultural applications. Moreover, the magnetite surface modification enables the successful binding of various analytes. In this work, we discuss the usage of core–shell nanoparticles and nanocomposites based on Fe3O4 for the modification of the GC electrode surface. Furthermore, this review focuses on the heavy metal ions electrochemical detection using Fe3O4-based nanoparticles-modified electrodes. Moreover, the most frequently used electrochemical methods, such as differential pulse anodic stripping voltammetry and measurement conditions, including deposition potential, deposition time, and electrolyte selection, are discussed.
1. Introduction
Nanotechnology has become a popular and rapidly developing field of science and industry since Nobel Prize winner R.P. Feynman’s breakthrough in 1959 [1]. A series of nanomaterials has been attracting researchers’ attention due to the significant features of these materials, such as excellent electrical, optical, magnetic, and catalytic properties [2]. The properties and potential applications of nanoparticles depend on their phases, sizes, and morphologies [3].
Recently, nanomaterials with magnetic properties, especially those comprising iron oxide Fe3O4, have gained considerable popularity. Magnetite (Fe3O4) nanoparticles have been widely used in many fields because of their unique electric and magnetic properties. Fe3O4 nanomaterials are found in many important applications in industrial areas, such as lithium-ion batteries [4,5], catalytic sorption [6], microwave absorption [7,8], and photocatalytic degradation [9,10,11]. Furthermore, magnetite-based nanocomposites are extensively used in biomedicine, in particular the photothermal killing of breast cancer cells [12], cell targeting and sorting, drug delivery vehicles [13,14], magnetic resonance [15,16], and fluorescence imaging [17]. Due to the increasing environmental pollution from heavy metals, nanomaterials based on magnetite are widely applied in environmental protection as metal ion adsorbents for metal ions remediation [18,19].
Magnetic Fe3O4 nanoparticles have been used as a basis for the development of many synthesis methods. There are plenty of Fe3O4 synthesis methods, including coprecipitation, sonochemical reaction, hydrothermal reaction, microemulsion and sol-gel synthesis, and cathodic electrochemical deposition [14,20,21,22,23]. Interestingly, an important characteristic of nanomagnetite is its surface modification ability, which increases its applicability [24]. The majority of synthesis methods are simple and quick in preparation. Furthermore, there are many synthetic methods that can be used to obtain different nanoparticle sizes [24,25,26,27,28] and shapes [28,29,30]. Nanomagnetite can be obtained in various sizes and shapes, including the most popular spherical nanoparticles and in cuboids, octahedrons, plates, tetrahedrons, concaves, octapods, multibranches, and nanorods [31,32,33,34]. Fe3O4 nanoparticles are the basic material for subsequent surface modifications creating core–shell structures, which affect the further extension of their applications in many fields.
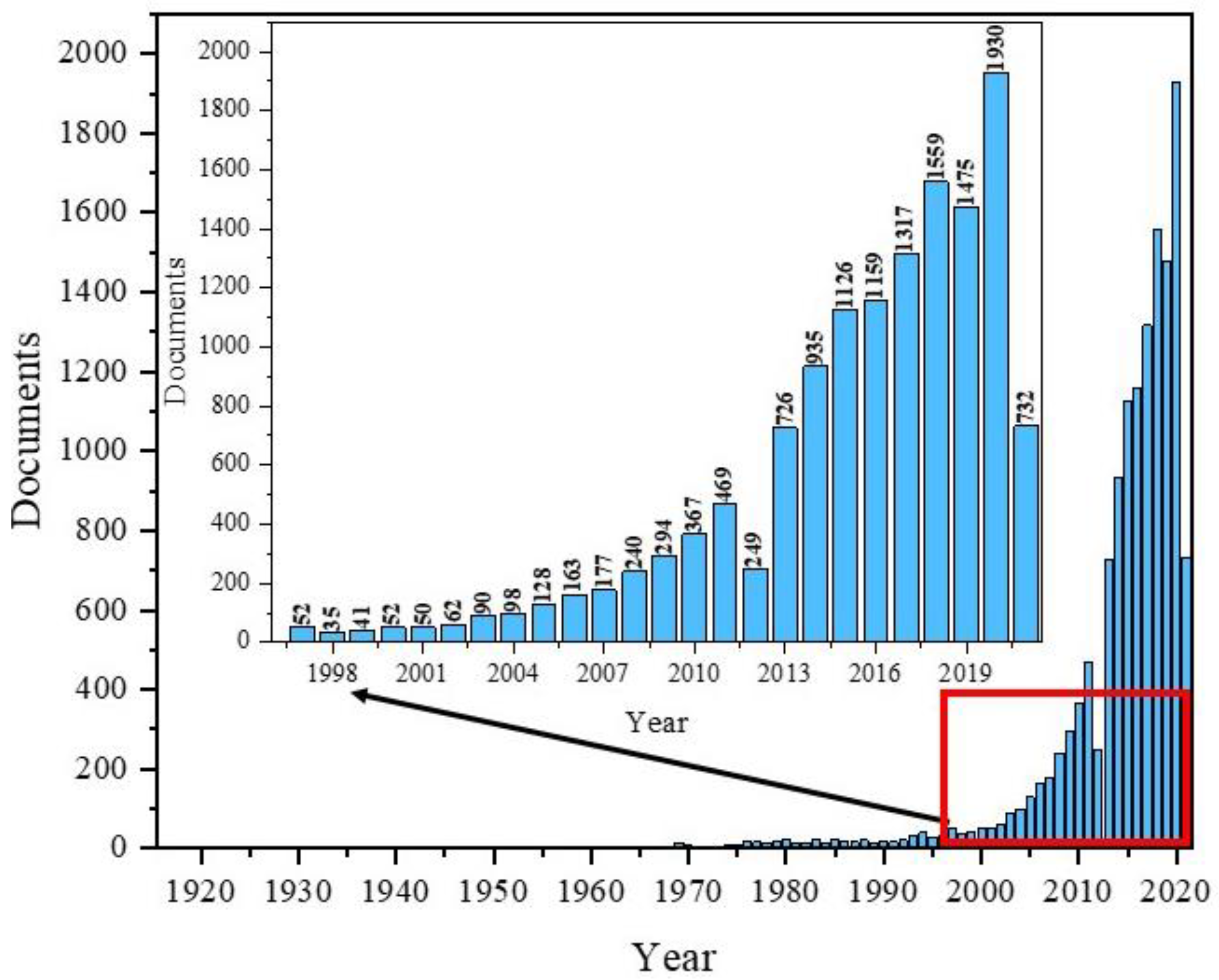
To the authors’ knowledge, the very first paper covering Fe3O4 was published in 1916 by the Americans, Sosman and Hostetter, and focused on iron oxides in general [35]. Over the next few decades, several articles appeared each year. Since the 1990s, we have observed a growing interest in nanomagnetites. The highest number of publications in the field with “Fe3O4” in the title appeared in 2020, totalling 1930 papers (Figure 1).
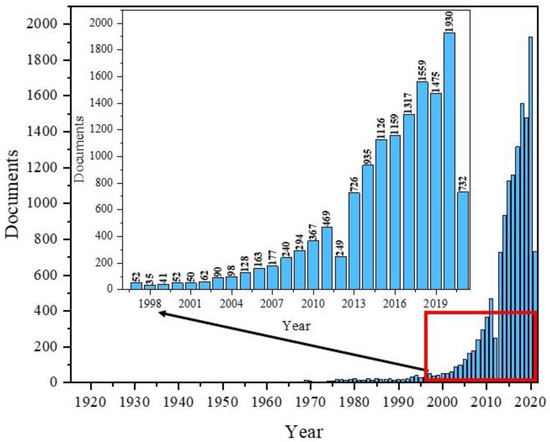
Figure 1.
Scheme of the number of publications with “Fe3O4” in title based on the year of publication using the Scopus base.
The data presented in Figure 1 shows the popularity of Fe3O4 and its dynamics as a topic of publication, especially during the most recent years. Additionally, composites based on Fe3O4 are used in many fields of science and industry, including magnetic separation, magnetic catalysis, environmental treatment, food analysis, target drug delivery systems, biosensors, magnetic resonance imagining, hyperthermia, and tissue engineering [36].
There are plenty of magnetite nanoparticles and hybrid structures used in the electrochemical detection of heavy metal ions providing an excellent basis for further functionalisation. The surface of Fe3O4 nanoparticles can be combined with nanoparticles of other metals (Au [37]); oxides (SiO2 [38,39] and TiO2 [40]) and additional conductive materials (GO [41]); complicated, organic functional groups (dendrimers [42] and polymers [43]); and biological particles fragments (DNA [44]). Fe3O4-based nanomaterials possess a high adsorption capacity, which makes them suitable for the electrochemical detection of metals [45].
The Fe3O4 nanoparticles are easy to oxidise and aggregate, which results in their low magnetic properties [46]; therefore, there is a need to coat bare nanomagnetite with polymer or inorganic shells. Additionally, the modification can increase the biocompatibility of these material [47].
In this work, we describe the recently published applications of a variety of functionalised Fe3O4 nanoparticles to electrode surface modifications to create a sensor for heavy metal ions. We discuss the preparation procedure of GCE for a sensor and the methods of electrode modification using Fe3O4 nanoparticles. We also present the most frequently used electrochemical techniques with their characteristic measurement parameters. Finally, we present a performance comparison of the recently developed heavy metal ion sensors.
2. Nano-Fe3O4 as Electrode Modifiers
Based on several decades of intensive research, Fe3O4 has become one of the best characterised metal oxides. Its cubic crystallographic system contains both Fe3+ and Fe2+ ions. Fe3O4 is a black solid with a density of 5.18 g·cm−3, Mohs hardness of 5, melting point range of 1583–1597 °C, and boiling point of 2623 °C. Its characteristic magnetic feature is the ferrimagnetism at room temperature and Neel (Curie) temperature of 850 °C [48].
In the past few years, Fe3O4 nanoparticles have become a focus of interest for numerous scientific groups. In the nano range (in diameter from 1 to 100 nm) smaller than 6 nm, magnetite particles indicate superparamagnetic properties, although their magnetic features strongly depend on the synthesis method [49]. Based on the gathered evidence, the nanomagnetites in most applications show the best characteristics in the range of 10–20 nm. Decreasing the nanoparticles’ size leads to an increase in the specific surface. Furthermore, the nanoparticles’ size strongly influences their magnetic moment and reaction to the magnetic field and depends on their size and shape [50]. Electrochemistry, and electrode modifications for the generation of a highly sensitive sensor, is one of the most rapidly developing fields of science. Electrochemical sensing is focused on the development of new electrode materials with better properties compared to commercial electrodes. The perfect sensor should exhibit a signal output proportional to the number of target species, high selectivity, sensitivity, repeatability, and rapid response [50].
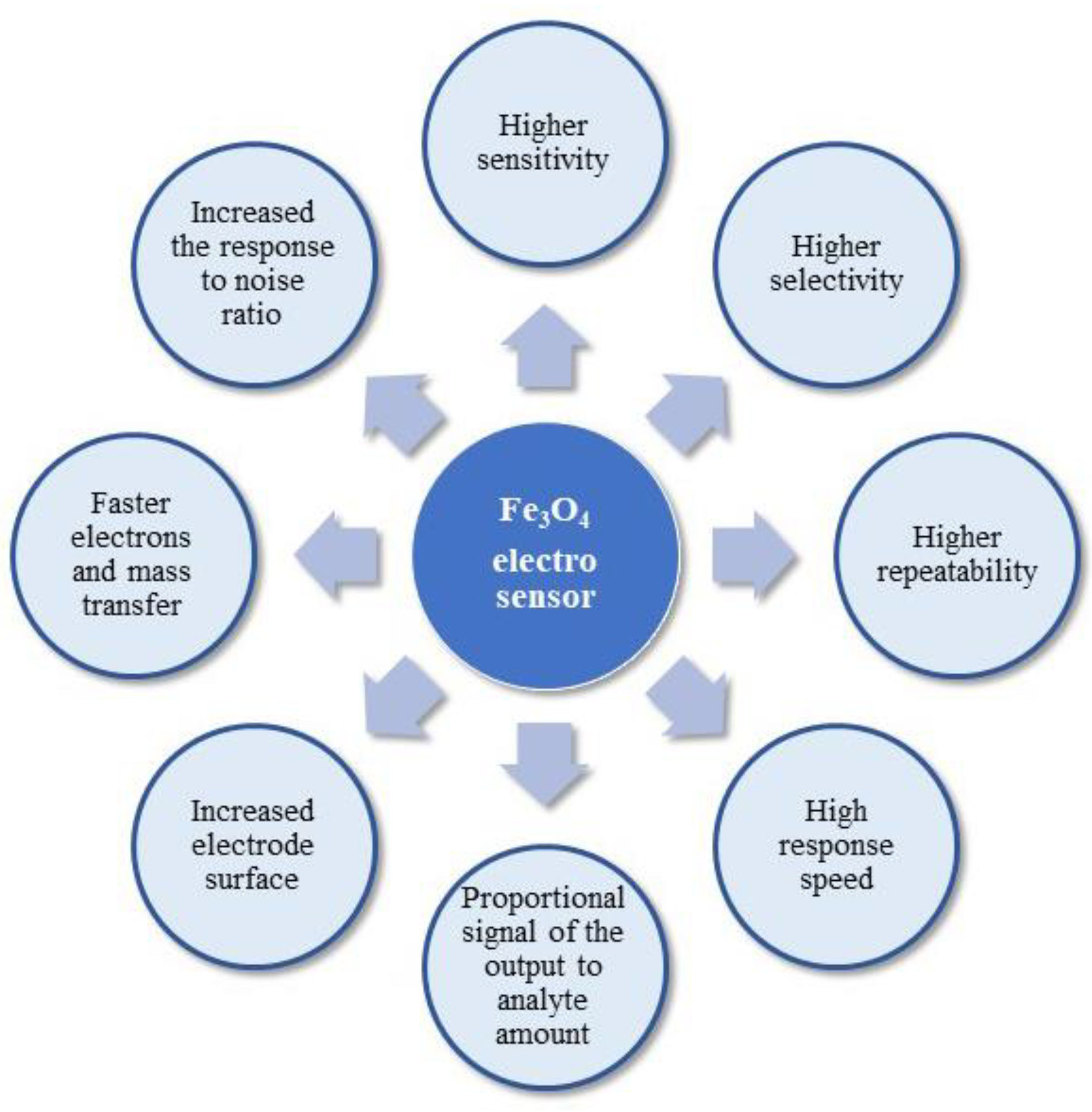
Nanomagnetite has been widely employed as a promising modifier due to its unique properties, low-cost, easy preparation, non-toxicity, excellent absorption capacity, catalytic properties, and inherent electrical conductivity [51]. The electrochemical performance of an electrode is closely related to the absorption capacity and the conductivity of the modified material. The imposition of Fe3O4 nanocomposites on the electrode surface causes the enhancement of the electrode area, enhancement of the rate of mass and electron transfer, improved selectivity and sensitivity, and, most importantly, increased response to the noise ratio [50]. Furthermore, Fe3O4-based electrochemical detection systems are characterised by small dimensions, costlessness, sensitivity, flexibility, and quickness in use [52]. The advantages of Fe3O4 usage as an electro-sensor are described in Figure 2.
Figure 2.
Scheme of the important electro-sensor features.
3. Recent Electrode Modifications with Magnetic Nanoparticles to Heavy Metal Ions Detection
The glassy carbon (GC) electrode is the most commonly used electrode for electroanalytical purposes due to its unique electrical conductivity, chemical stability, biocompatibility, and wide potential range and extremely low gas permeability [53]. Therefore, the GC electrode is an excellent material for modification to obtain a stable surface used as a biosensor. First of all, sensor development requires proper preparation of the electrode for further modification. Before each modification, the GC electrode usually needs to be polished to a shiny, mirror-like surface with wet alumina slurry—Al2O3 powder of different sizes, 1.0 μm, 0.3 μm, and 0.05 μm, using a polishing cloth and rinsing with water. Then, successive washing or sonications in absolute ethanol and ultrapure water are usually conducted, sometimes in a 1:1 (v/v) HNO3 solution, lasting at least a few minutes each [54]. Subsequently, the electrode surface is dried with nitrogen or at room temperature, and the electrode is ready for further use. As an exception, Miao et al. started the modification with GCE soaking in piranha solution (98% H2SO4:30% H2O2 = 3:1) for about 5 min to remove any adsorbed materials [44].
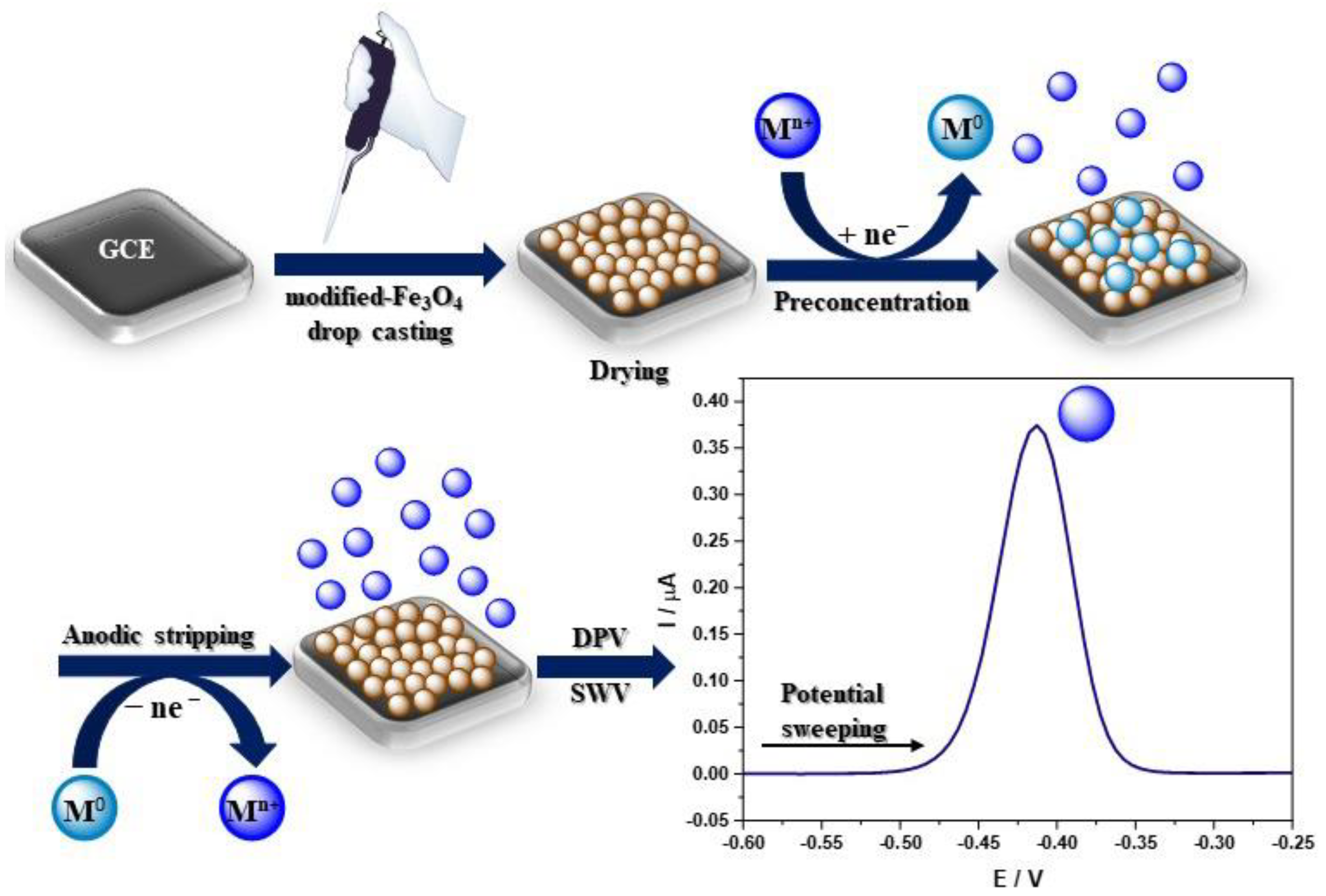
A homogeneous suspension of nanoparticles is necessary to modify the electrode, most often by sonication in deionized water, absolute ethanol, and sometimes an ethanol solution containing 0.25 wt% Nafion® [55] or IPA [56], or DMF [57] in a concentration of 1 mg/mL. Ultrasonic bath sonication lasts from 5 min to 2 h, but most often 30 min or until a uniform suspension is obtained. The most common method of electrode modification is drop-casting while the nanoparticles’ suspension is pipped on the electrode surface (Figure 3) [58]. The amount of applied nanoparticles depends on the active surface of the working electrode. After the modification, the electrode was dried at room temperature until the solvent completely evaporated, which usually takes from several minutes to a few hours. A different approach was presented by Kong et al., where 6 mL of Fe3O4@PANI nanoparticles suspension was pipetted onto an electrode, and after drying at 4 °C in a refrigerator, the electrode was coated with 3 mL of Nafion® solution (0.5 wt%) [59]. Moreover, Wang et al. created an unconventional sensor by adding the Fe3O4@PDA@MnO2 NPs homogenous suspension to the HCl solution (pH 3.0) with various concentrations of Pb2+. Then, nanoparticles with already adsorbed Pb2+ ions were completely transferred onto the mGCE for immediate electrochemical measurements (Figure 3) [60]. Recent findings concerning heavy metal ion detection with the GC electrode modified using Fe3O4-based nanocomposites are presented in Table 1. The authors focused only on reports published in the last 5 years related to heavy metal ions’ electrochemical analysis of GC electrodes modified with Fe3O4-based nanocomposites (Table 1).
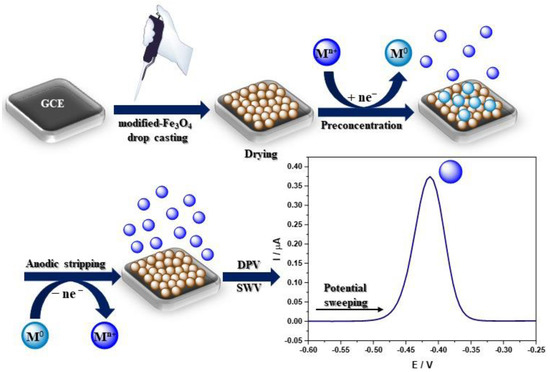
Figure 3.
Schematic visualisation of the sensor development and heavy metal ions electrochemical detection.

Table 1.
Selected studies on Fe3O4 nanoparticles in electrochemical sensors for heavy metal ions detection.
Electrochemical techniques, especially voltammetry, include electroanalytical methods for the determination of one or more analytes by measuring the current as a function of the potential. There are a few component techniques used to obtain information on the analyte, including CV, DPV, SWV, and stripping voltammetry [70]. Voltammetric techniques are widely used in heavy metal ions detection due to their precision and sensitivity. The most frequently chosen are DPV or alternative SWV techniques (Table 1.) due to their high sensitivity and lower detection limits, which are suitable for trace level analysis. However, square wave voltammetry is preferable for obtaining the response rate. The most frequently used method for quantitative analysis is stripping voltammetry. There are two types of stripping voltammetry, ASV and CSV, depending on the chosen concentration potential [71]. The two steps of stripping analysis include analyte deposition at the electrode surface (or in its volume, e.g., HDME [72]) and analyte quantification by potential sweeping [73]. During stripping experiments, a certain voltage is applied to the GC electrode to reduce the metal ions on the electrode surface into the elemental metal, following which linear voltammetry is performed from negative to positive to oxidate the preconcentrated metal back into ions (Figure 3). The ions detection is determined according to the oxidation current produced by the process. According to the literature, the ions detection mechanism can be illustrated with the following equations (M, metal; n, number of exchanged electrons) (Figure 3) [59]:
ne− + Mn+ → M0
M0 → Mn+ + ne−
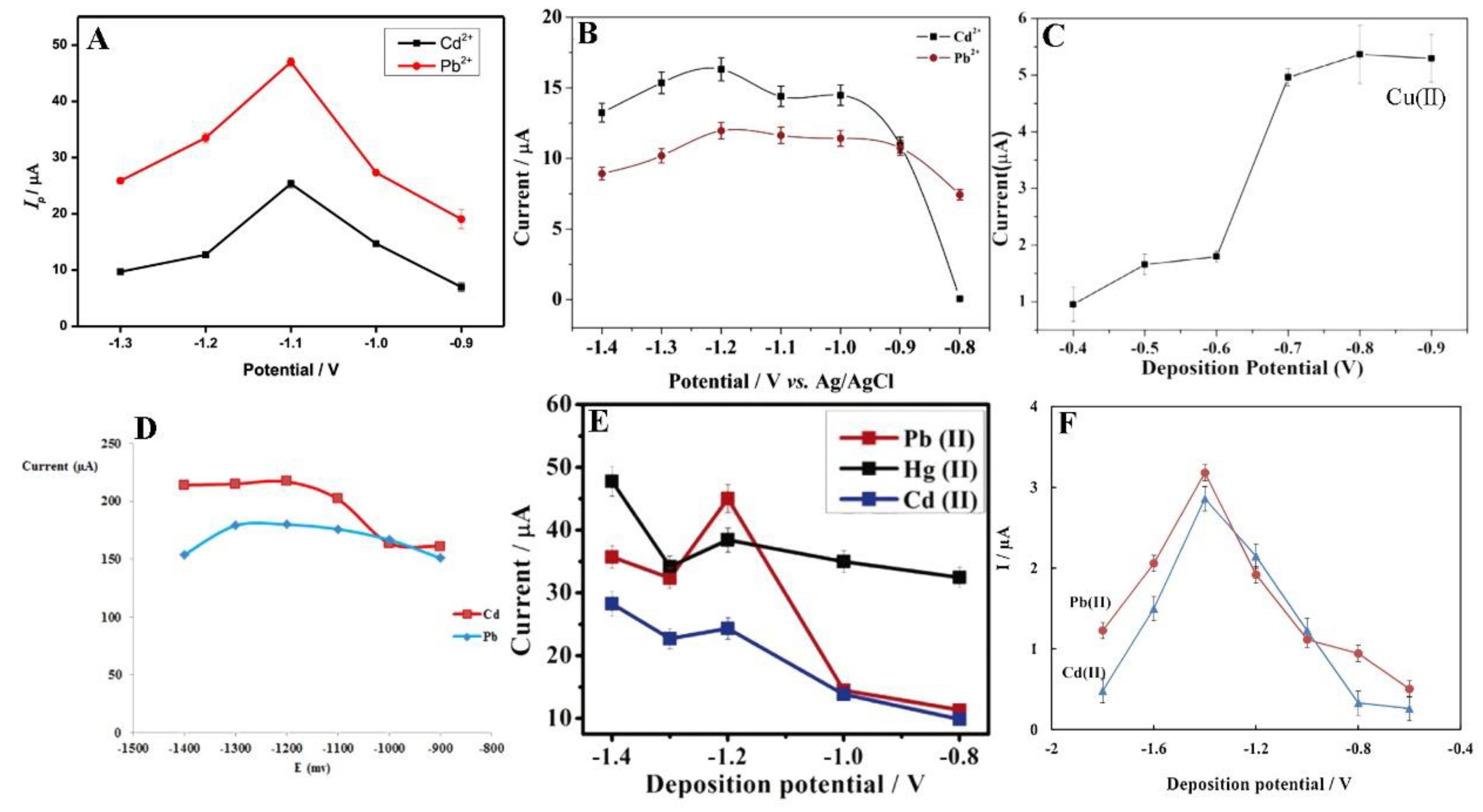
In the stripping analysis, the most significant parameters are potential and time of accumulation. Deposition potential should be slightly lower than the oxidation potentials of analytes. Obviously, each experiment is preceded by the optimisation of the measurement conditions. Nevertheless, in the case of metal ions, the anodic range with an optimum potential of −1.2 V, and sometimes lower to −1.4 V, is most commonly used [65,69]. Moreover, with an accumulation potential more negative than −1.2 V, a decrease in the current intensity was observed (Figure 4) [66]. The current intensity weakening can be attributed to the H2 evolution that deteriorates the working electrode surface activity [64,68].
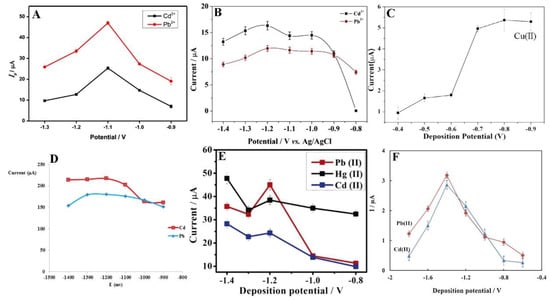
Figure 4.
Optimisation of deposition potential used in electrochemical determination of different ions such as Cd2+, Pb2+, and Hg2+ presented by Pu et al. [61] (A), Xu et al. [63] (B), Wei et al. [55] (C), Dahaghin et al. [66] (D), Deshmukh et al. [68] (E), and Baghayeri et al. [69] (F). All Figures are adapted from references [55,61,63,66,68,69] with permission from Elsevier.
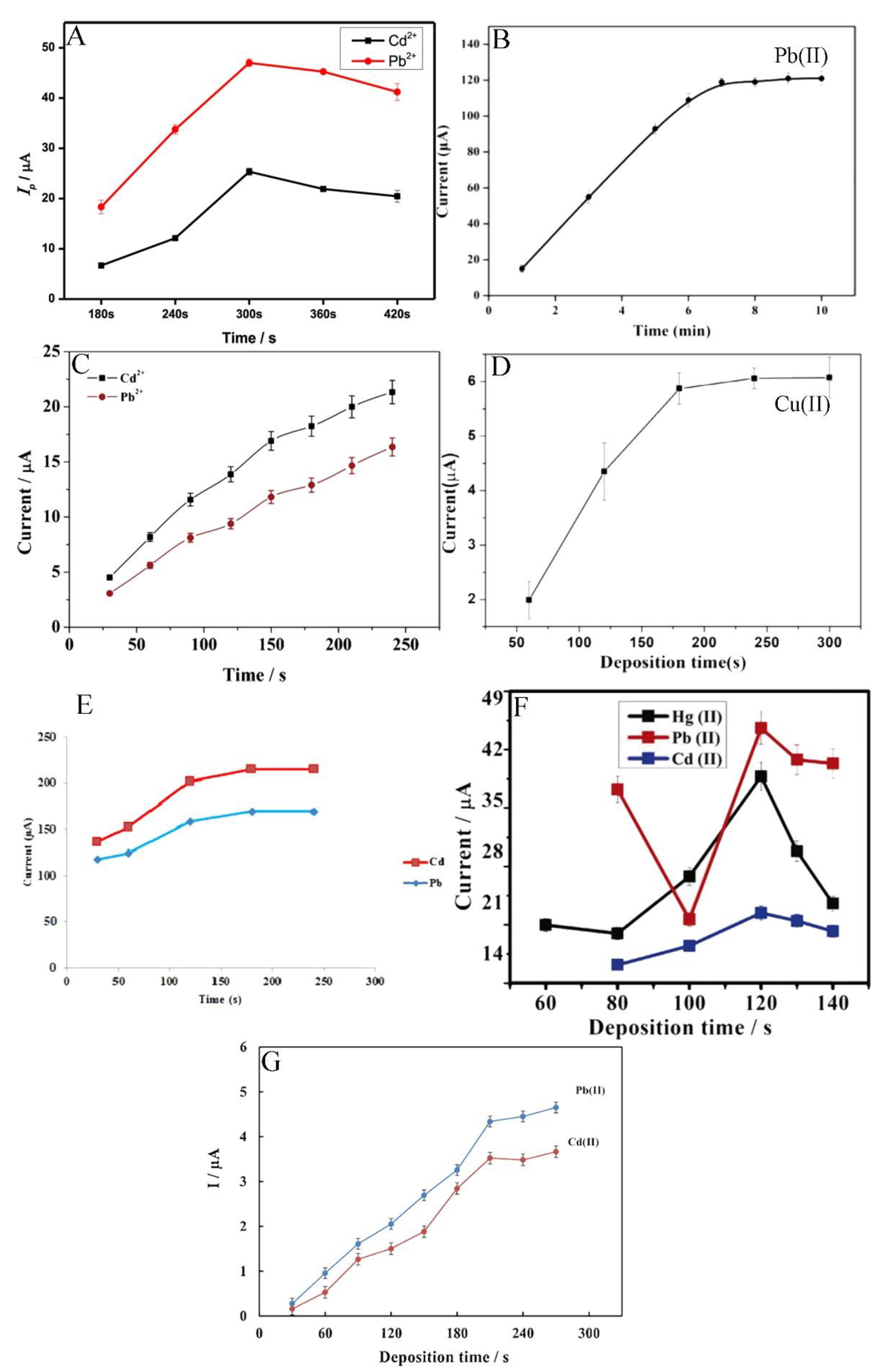
The metal ions’ electro-reduction time on the electrode surface starts from 120 s and reaches up to 480 s (Figure 5) [54,60]. However, the most common concentration time is within 180 s. For example, Pu et al. examined the effect of accumulation times within the range of 180 s to 420 s and observed that the peak currents of Cd2+ and Pb2+ increase linearly as the deposition time increases from 180 s to 300 s, after which the peak currents achieved plateau. Consequently, the deposition time of 300 s was used in all subsequent experiments [61]. This is caused by the saturation of selective sites on the electrode surface with the ions. Because of this phenomenon, the electrode surface does not tend to absorb more species [64].
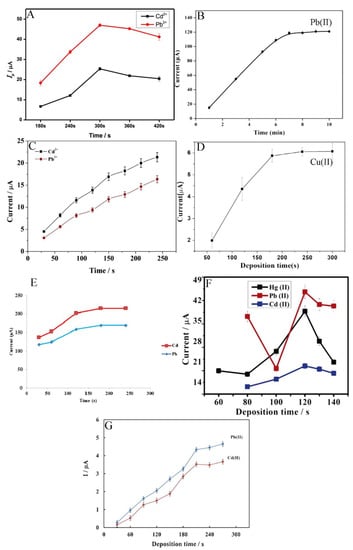
Figure 5.
Optimisation of deposition time of different ions such as Cd2+, Pb2+, and Hg2+ determination by electrochemical methods presented by Pu et al. [61] (A), Wang et al. [60] (B), Xu et al. [63] (C), Wei et al. [55] (D), Dahaghin et al. [66] (E), Deshmukh et al. [68] (F), and Baghayeri et al. [69] (G). All Figures are adapted from references [55,60,61,63,66,68,69] with permission from Elsevier.
Additionally, after the stripping experiment, Fan et al., Wu et al., and Pu et al. introduced heavy metal ions oxidation in the measurement method to remove the residual metals and clean the electrode surface by applying the desorption potential: 0.9 V for 150 s, 1.0 V for 210 s, and 0.2 V for 120 s [54,61,62].
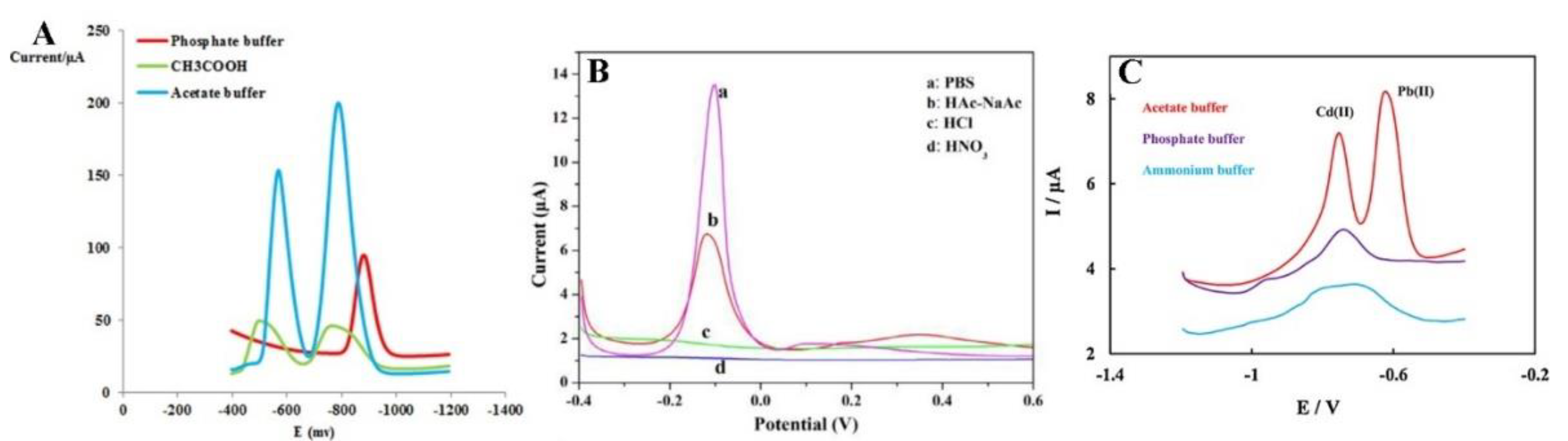
Another extremely important parameter optimised during the analysis of metal ions is the selection of an appropriate electrolyte. The selection of a suitable supporting electrolyte and its pH guarantees the achievement of excellent electrochemical responses as well-formed, high-intensity current peaks. The electrolyte type affects the formation of various metals’ peaks (Figure 6). In heavy metal ions analysis, 0.1 M NaNO3 [56], 1 M HCl [60], and PBS [55] were selected, but a 0.1 M acetate buffer solution NaAc/HAc was the most commonly used and delivered the best results. The highest and best-defined peaks of metal ions are observed in the acetate buffer (Figure 6). The explanation of this phenomenon is complex and affected by many factors. Firstly, different electrodes may exhibit different electrochemical properties in the same electrolyte because an electrical double-layer is formed on the electrode as a result of an interaction between the cations or anions present in the solution. The double-layer model is described by many papers, including the Helmholtz, Gouy–Chapman, and Stern–Grahame models [74,75,76]. The authors of this review suggest that the increase of electrochemical signals of measured ions observed in the acetate buffer solution (NaAc/HAc) is caused not only by the appropriate pH but also because the acetate buffer enables the binding reaction as a result of intermolecular ion binding on the surface of the Fe3O4-modified electrode. The acetate buffer enables the reduction of ions by the intermolecular ion binding both the positively [55] and negatively charged species [69] and due to their interaction with the organic ligand [66]. Additionally, the authors suggest that the hydroxyl groups in the carboxylic group of acetic acid serve as active sites to adsorb heavy metal ions on the modified surface.
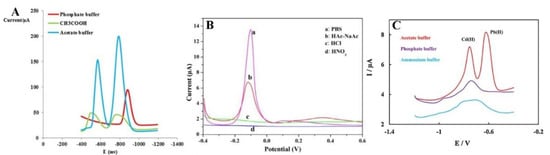
Figure 6.
Electrolyte selection in determination of Pb2+ and Cu2+, by Dahaghin et al. [66] (A), Pb2+ by Wei et al. [55] (B), and Cd2+ and Pb2+ detection by Bagahayeri et al. [69] (C). All Figures are adapted from references [55,66,69] with permission from Elsevier.
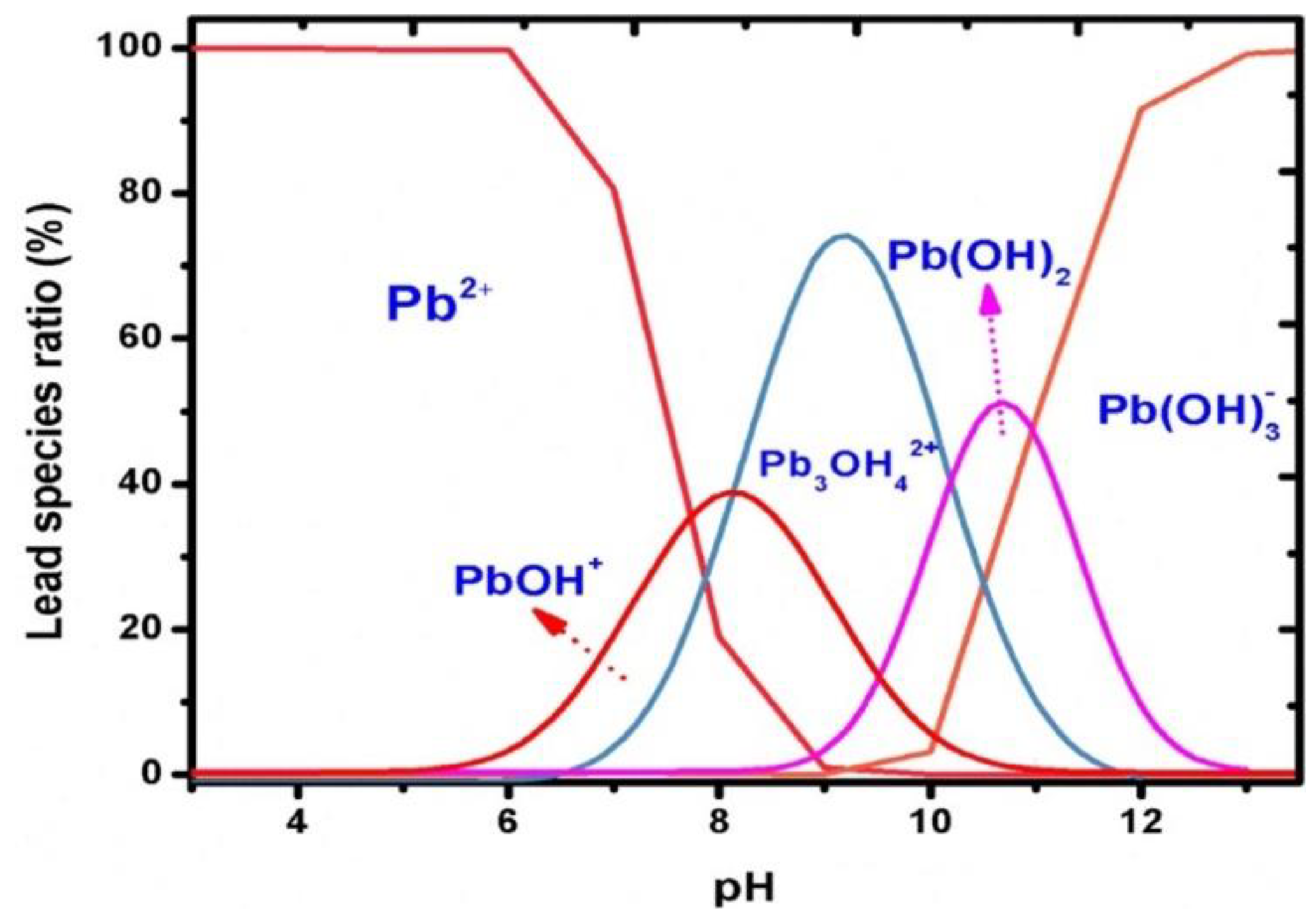
The pH value of the supporting electrolyte is the factor that inherently affects the intensity of the peaks of metal ions. The optimisation of the pH value is usually carried out in an acidic environment because above pH 7, the vast majority of metals form hydroxides, with the highest probability in the range of pH 4 and 6. However, the peaks with the highest intensity are obtained at pH 5 to 5.5 and less often within the range of pH 4 to 4.5. Qureashi et al. described the formation of Pb2+ ions occurring in a solution depending on the pH (Figure 7) [56].
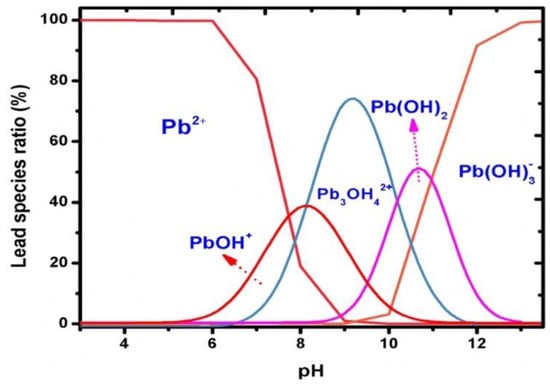
Figure 7.
Distribution of the lead species as a function of pH presented by Qureashi et al. [56]. Figure is adapted from reference [56] with permission from Elsevier.
Figure 7 shows that Pb2+ ions dominate in an acidic medium with a pH lower than 6. Subsequently, when the pH value increases to 8, the formation of a Pb(OH)+ complex occurs, and with a further increase to pH 9, the Pb3OH42+ species is predominant. Pb(OH)2 shows maximum adsorption in a pH range from 9 to 11. The Pb(OH)3− anionic complex exists in a pH range higher than 12 [56]. Similar relationships can be presented for other ions, for example Cd2+ [77] and Cu2+ [72].
Due to the ions species distribution, we can state that bi-positive ions adsorption experiments should be performed below pH 7. At the same time, an extremely low pH value may cause physiological changes in the Fe3O4 adsorbent. Based on these assumptions, the optimal pH for heavy metal ions analysis is in the range of pH 4 to 6.
The presence of magnetic nanoparticles on the GC electrode surface increases the sensor sensitivity even to the nano range. The electrode modification with bare Fe3O4 carried out by Fan et al. resulted in the development of a sensor with a limit of detection range of 119 nM, 154 nM, 83.9 nM, and 76.5 nM for Pb2+, Cd2+, Hg2+, and Cu2+, respectively [54]. The presence of functional groups on the nanomagnetite surface or additional modifiers increases the sensitivity even further. The highest sensitivity was reached by He et al., who created an electrochemical sensor by the immobilisation of Fe3O4/GN composite integrated with garlic extract (GE) onto the GC surface for the determination of Pb2+ in wastewater. The sensor exhibited two dynamic linear ranges including 0.001 to 0.5 nM and 0.5 to 1000 nM with an excellent low detection limit of 0.0123 pM (S/N = 3) and quantification limit (LOQ) of 0.41 pM (S/N = 10) [67]. A slightly lower sensitivity was obtained by Wu et al. [62], Kong, et al. [59], Baghayeri et al. [69], Dahaghin et al. [66], and Hu et al. [63] within tenths and hundredths of a nanomole for the variety of heavy metal ions. Among those mentioned in Table 1, the detection limit remained at the highest level for the sensors developed by Desmukh et al. They achieved LOD values in the range of 0.1, 0.05 μM, and 0.01 μM for individual analysis of Hg2+, Pb2+, and Cd2+ ions, respectively, whereas the LOD values for the simultaneous analysis of these ions were found to be 0.3 μM, 0.04 μM, and 0.2 μM, respectively, with the use of a sensor based on Fe3O4 nanoparticles capped with terephthalic acid [68].
4. Conclusions and Perspectives
This review covers a general discussion of trace metal electrochemical sensors based on the magnetic iron oxide, Fe3O4. Magnetite nanoparticles, which are a considerable part of the world of nanomaterials, have gained extensive significance in many fields of science. A multitude of applications has been progressed for the use of magnetite nanoparticles. Thanks to their properties, these nanoparticles are successfully used in many fields of chemistry, e.g., for remediation of contaminants using an external magnetic field, but are mainly used as electrode modifiers in electrochemistry. Modifiers based on Fe3O4 were used to create plenty of electrochemical sensors for various analytes detection, including heavy metal ions. This overview of recently published articles indicates that the GCE was the most commonly used conventional electrode surface for modification. However, SWASV and DPASW were the most commonly used measurement techniques for the detection of heavy metal ions.
In conclusion, the many possibilities and simplicity of the magnetite nanoparticles’ surface functionalisation provide the basis for the development of even more sensitive and selective sensors for heavy metal ions detection in the future. We can expect a possible development of commercial electrochemical sensors through the integration of standard electrodes with Fe3O4-based nanoparticles.
The Fe3O4 nanoparticles’ properties and the possibility of surface functionalisation are the foundation for obtaining new sensory platforms for a variety of analytes, not only for heavy metal ions detection. This phenomenon not only creates an opportunity to use nanomagnetite in many fields of chemistry but also in science in general.
Author Contributions
Conceptualisation, A.K.-K.; investigation, A.K.-K., T.O. and P.N.; writing—original draft preparation, A.K.-K.; writing—review and editing, A.K.-K., T.O. and P.N.; visualisation, A.K.-K.; supervision, A.K.-K. and P.N.; funding acquisition, A.K.-K. and T.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was created thanks to the University of Gdansk within the project supporting young scientists and PhD students (grant No. BMN 539-T050-B890-21).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analysed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 2-CBT | benzothiazole-2-carboxaldehyde |
| ASV | anodic stripping voltammetry |
| CS | chitosan |
| CSV | cathodic stripping voltammetry |
| CV | cyclic voltammetry |
| DMF | N,N-Dimethylformamide |
| DPASV | differential pulse anodic stripping voltammetry |
| DPV | differential pulse voltammetry |
| F-MWCNTs | fluorinated multi-walled carbon nanotubes |
| GCE | glassy carbon electrode |
| GE | garlic extract |
| GN | graphene |
| GO | graphene oxide |
| GSH | glutathione |
| HDME | hanging drop mercury electrode |
| IPA | isopropyl alcohol |
| LOD | limit of detection |
| LOQ | limit of quantification |
| LSG | laser scribed graphene |
| mGCE, MGCE | magnetic glassy carbon electrode |
| MWCNTs | multi-walled carbon nanotubes |
| NaAc/HAc | acetate buffer solution |
| NPs | nanoparticles |
| PANI | polyaniline |
| PDA | polydopamine |
| PMDA | poly methyldopa |
| S/N | signal to noise ratio |
| SWAdCSV | square wave adsorptive cathodic stripping voltammetry |
| SWASV | square wave anodic stripping voltammetry |
| SWV | square wave voltammetry |
| TA | terephthalic acid |
| PDA | polydopamine |
| PMDA | poly methyldopa |
| SWAdCSV | square wave adsorptive cathodic stripping voltammetry |
| SWASV | square wave anodic stripping voltammetry |
| TA | terephthalic acid |
References
- Mansoori, G.A.; Soelaiman, T.F. Nanotechnology—An Introduction for the Standards Community. JAI 2005, 2, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Capek, I. (Ed.) Chapter 1 Nanotechnology and Nanomaterials. In Studies in Interface Science; Nanocomposite Structures and Dispersions; Elsevier: Amsterdam, The Netherlands, 2006; Volume 23, pp. 1–69. [Google Scholar]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Jiang, Z.-J.; Yang, L.; Cheng, S.; Liu, M. A High-Performance Anode for Lithium Ion Batteries: Fe3O4 Microspheres Encapsulated in Hollow Graphene Shells. J. Mater. Chem. A 2015, 3, 11847–11856. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhao, H.; Wang, J.; Lv, P.; Zhang, T.; Xia, Q. Nanostructured Fe3O4@C as Anode Material for Lithium-Ion Batteries. J. Power Sources 2014, 248, 15–21. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Huang, Y.; Ma, Y.; Wang, C.; Chen, M.; Chen, Y. Supercapacitor Devices Based on Graphene Materials. J. Phys. Chem. C 2009, 113, 13103–13107. [Google Scholar] [CrossRef]
- Adebayo, L.L.; Soleimani, H.; Yahya, N.; Abbas, Z.; Wahaab, F.A.; Ayinla, R.T.; Ali, H. Recent Advances in the Development of Fe3O4-BASED Microwave Absorbing Materials. Ceram. Int. 2020, 46, 1249–1268. [Google Scholar] [CrossRef]
- Huang, L.; Liu, X.; Yu, R. Enhanced Microwave Absorption Properties of Rod-Shaped Fe2O3/Fe3O4/MWCNTs Composites. Prog. Nat. Sci. Mater. Int. 2018, 28, 288–295. [Google Scholar] [CrossRef]
- Zazouli, M.A.; Ghanbari, F.; Yousefi, M.; Madihi-Bidgoli, S. Photocatalytic Degradation of Food Dye by Fe3O4–TiO2 Nanoparticles in Presence of Peroxymonosulfate: The Effect of UV Sources. J. Environ. Chem. Eng. 2017, 5, 2459–2468. [Google Scholar] [CrossRef]
- Mercyrani, B.; Hernandez-Maya, R.; Solis-Lopez, M.; Th-Th, C.; Velumani, S. Photocatalytic Degradation of Orange G Using TiO2/Fe3O4 Nanocomposites. J. Mater. Sci. Mater. Electron. 2018, 29, 15436–15444. [Google Scholar] [CrossRef]
- Golshan, M.; Zare, M.; Goudarzi, G.; Abtahi, M.; Babaei, A.A. Fe3O4@HAP-Enhanced Photocatalytic Degradation of Acid Red73 in Aqueous Suspension: Optimization, Kinetic, and Mechanism Studies. Mater. Res. Bull. 2017, 91, 59–67. [Google Scholar] [CrossRef]
- Choi, K.-H.; Nam, K.C.; Cho, G.; Jung, J.-S.; Park, B.J. Enhanced Photodynamic Anticancer Activities of Multifunctional Magnetic Nanoparticles (Fe3O4) Conjugated with Chlorin E6 and Folic Acid in Prostate and Breast Cancer Cells. Nanomaterials 2018, 8, 722. [Google Scholar] [CrossRef] [Green Version]
- Dukenbayev, K.; Korolkov, I.V.; Tishkevich, D.I.; Kozlovskiy, A.L.; Trukhanov, S.V.; Gorin, Y.G.; Shumskaya, E.E.; Kaniukov, E.Y.; Vinnik, D.A.; Zdorovets, M.V.; et al. Fe3O4 Nanoparticles for Complex Targeted Delivery and Boron Neutron Capture Therapy. Nanomaterials 2019, 9, 494. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Li, B.; Qiao, Y. Fe3O4 Nanoparticles in Targeted Drug/Gene Delivery Systems. Materials 2018, 11, 324. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, H. Fe3O4-Based Nanotheranostics for Magnetic Resonance Imaging-Synergized Multifunctional Cancer Management. Nanomedicine 2019, 14, 1493–1512. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Cao, X.; Liu, G.H.; Hong, R.Y.; Chen, Y.M.; Chen, X.F.; Li, H.Z.; Xu, B.; Wei, D.G. Synthesis of Fe3O4 Magnetic Fluid Used for Magnetic Resonance Imaging and Hyperthermia. J. Magn. Magn. Mater. 2011, 323, 2953–2959. [Google Scholar] [CrossRef]
- Xi, P.; Cheng, K.; Sun, X.; Zeng, Z.; Sun, S. Magnetic Fe3O4 Nanoparticles Coupled with a Fluorescent Eu Complex for Dual Imaging Applications. Chem. Commun. 2012, 48, 2952–2954. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, L.; Erto, A.; Moreno-Piraján, J.C. Magnetite Nanoparticles for Removal of Heavy Metals from Aqueous Solutions: Synthesis and Characterization. Adsorption 2013, 19, 465–474. [Google Scholar] [CrossRef]
- Kulpa, A.; Ryl, J.; Skowierzak, G.; Koterwa, A.; Schroeder, G.; Ossowski, T.; Niedziałkowski, P. Comparison of Cadmium Cd2+ and Lead Pb2+ Binding by Fe3O4@SiO2-EDTA Nanoparticles—Binding Stability and Kinetic Studies. Electroanalysis 2020, 32, 588–597. [Google Scholar] [CrossRef]
- Xue-Mei, L.; Gaojie, X.; Yue, L.; Tao, H. Magnetic Fe3O4 Nanoparticles: Synthesis and Application in Water Treatment. Nanosci. Nanotechnol. Asia 2011, 1, 14–24. [Google Scholar]
- Niculescu, A.-G.; Chircov, C.; Grumezescu, A.M. Magnetite Nanoparticles: Synthesis Methods—A Comparative Review. Methods 2021. In Press. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liao, Y.; Zhang, D.; Wen, T.; Zhong, Z. A Review of Fe3O4 Thin Films: Synthesis, Modification and Applications. J. Mater. Sci. Technol. 2018, 34, 1259–1272. [Google Scholar] [CrossRef]
- Ajinkya, N.; Yu, X.; Kaithal, P.; Luo, H.; Somani, P.; Ramakrishna, S. Magnetic Iron Oxide Nanoparticle (IONP) Synthesis to Applications: Present and Future. Materials 2020, 13, 4644. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.U.; Akram, M.W.; Udego, I.O.; Li, H.; Niu, X. Surface Modification of Magnetic Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef] [Green Version]
- Kalantari, K.; Ahmad, M.B.; Shameli, K.; Hussein, M.Z.B.; Khandanlou, R.; Khanehzaei, H. Size-Controlled Synthesis of Fe3O4 Magnetic Nanoparticles in the Layers of Montmorillonite. J. Nanomater. 2014, 2014, e739485. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Zheng, C.; Zhang, F.; Yang, Y.; Wu, G.; Yu, A.; Guan, N. Size-Controlled Synthesis of Magnetite (Fe3O4) Nanoparticles Coated with Glucose and Gluconic Acid from a Single Fe(III) Precursor by a Sucrose Bifunctional Hydrothermal Method. J. Phys. Chem. C 2009, 113, 16002–16008. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H. Size-Controlled Synthesis of Magnetite Nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Guo, Z.; Gao, F.; Gao, Q.; Wang, D.; Liaw, B.; Cai, Q.; Sun, X.; Wang, X.; Zhao, L. Shape-, Size- and Structure-Controlled Synthesis and Biocompatibility of Iron Oxide Nanoparticles for Magnetic Theranostics. Theranostics 2018, 8, 3284–3307. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Liu, X.; Shi, R.; Zhou, K.; Shi, Y.; Ma, R.; Takayama-Muromachi, E.; Qiu, G. Shape-Controlled Synthesis and Magnetic Properties of Monodisperse Fe3O4 Nanocubes. Cryst. Growth Des. 2010, 10, 2888–2894. [Google Scholar] [CrossRef]
- Fatima, H.; Lee, D.-W.; Yun, H.J.; Kim, K.-S. Shape-Controlled Synthesis of Magnetic Fe3O4 Nanoparticles with Different Iron Precursors and Capping Agents. RSC Adv. 2018, 8, 22917–22923. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Sun, H.; Yan, X.; Zhong, W.; Shen, W.; Zhang, Y.; Cheng, X. Cube Fe3O4 Nanoparticles Embedded in Three-Dimensional Net Porous Carbon from Silicon Oxycarbide for High Performance Supercapacitor. Ceram. Int. 2020, 46, 24805–24815. [Google Scholar] [CrossRef]
- Lei, W.; Liu, Y.; Si, X.; Xu, J.; Du, W.; Yang, J.; Zhou, T.; Lin, J. Synthesis and Magnetic Properties of Octahedral Fe3O4 via a One-Pot Hydrothermal Route. Phys. Lett. A 2017, 381, 314–318. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Wang, H.; Hai, J.; Wang, B. Se Atom-Induced Synthesis of Concave Spherical Fe3O4@Cu2O Nanocrystals for Highly Efficient MRI–SERS Imaging-Guided NIR Photothermal Therapy. Part. Part. Syst. Charact. 2018, 35, 1800197. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, Z.; Bao, J.; Wang, Z.; Hu, J.; Chi, X.; Ni, K.; Wang, R.; Chen, X.; Chen, Z.; et al. Octapod Iron Oxide Nanoparticles as High-Performance T2 Contrast Agents for Magnetic Resonance Imaging. Nat. Commun. 2013, 4, 2266. [Google Scholar] [CrossRef] [PubMed]
- Sosman, R.B.; Hostetter, J.C. The Oxides of Iron. I. Solid Solution in the System Fe2O3-Fe3O4. J. Am. Chem. Soc. 1916, 38, 807–833. [Google Scholar] [CrossRef]
- Liu, S.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Preparation, Surface Functionalization and Application of Fe3O4 Magnetic Nanoparticles. Adv. Colloid Interface Sci. 2020, 281, 102165. [Google Scholar] [CrossRef] [PubMed]
- Riahifar, V.; Haghnazari, N.; Keshavarzi, F.; Nasri, F. Design a High Sensitive Electrochemical Sensor Based on Immobilized Cysteine on Fe3O4@Au Core-Shell Nanoparticles and Reduced Graphene Oxide Nanocomposite for Nitrite Monitoring. Microchem. J. 2021, 166, 106217. [Google Scholar] [CrossRef]
- Pirsa, S.; Asadzadeh, F. Synthesis of Fe3O4/SiO2/Polypyrrole Magnetic Nanocomposite Polymer Powder: Investigation of Structural Properties and Ability to Purify of Edible Sea Salts. Adv. Powder Technol. 2021, 32, 1233–1246. [Google Scholar] [CrossRef]
- Patil, S.; Tandon, R.; Tandon, N. A Current Research on Silica Coated Ferrite Nanoparticle and Their Application: Review. Curr. Res. Green Sustain. Chem. 2021, 4, 100063. [Google Scholar] [CrossRef]
- ZabihiSahebi, A.; Koushkbaghi, S.; Pishnamazi, M.; Askari, A.; Khosravi, R.; Irani, M. Synthesis of Cellulose Acetate/Chitosan/SWCNT/Fe3O4/TiO2 Composite Nanofibers for the Removal of Cr(VI), As(V), Methylene Blue and Congo Red from Aqueous Solutions. Int. J. Biol. Macromol. 2019, 140, 1296–1304. [Google Scholar] [CrossRef]
- El-Shafai, N.M.; Abdelfatah, M.M.; El-Khouly, M.E.; El-Mehasseb, I.M.; El-Shaer, A.; Ramadan, M.S.; Masoud, M.S.; El-Kemary, M.A. Magnetite Nano-Spherical Quantum Dots Decorated Graphene Oxide Nano Sheet (GO@Fe3O4): Electrochemical Properties and Applications for Removal Heavy Metals, Pesticide and Solar Cell. Appl. Surf. Sci. 2020, 506, 144896. [Google Scholar] [CrossRef]
- Luan, L.; Tang, B.; Liu, Y.; Wang, A.; Zhang, B.; Xu, W.; Niu, Y. Selective Capture of Hg(II) and Ag(I) from Water by Sulfur-Functionalized Polyamidoamine Dendrimer/Magnetic Fe3O4 Hybrid Materials. Sep. Purif. Technol. 2021, 257, 117902. [Google Scholar] [CrossRef]
- Chen, D.; Shen, Y.; Wang, S.; Chen, X.; Cao, X.; Wang, Z.; Li, Y. Efficient Removal of Various Coexisting Organic Pollutants in Water Based on β-Cyclodextrin Polymer Modified Flower-like Fe3O4 Particles. J. Colloid Interface Sci. 2021, 589, 217–228. [Google Scholar] [CrossRef]
- Miao, P.; Tang, Y.; Wang, L. DNA Modified Fe3O4@Au Magnetic Nanoparticles as Selective Probes for Simultaneous Detection of Heavy Metal Ions. ACS Appl. Mater. Interfaces 2017, 9, 3940–3947. [Google Scholar] [CrossRef]
- Munonde, T.S.; Nomngongo, P.N. Nanocomposites for Electrochemical Sensors and Their Applications on the Detection of Trace Metals in Environmental Water Samples. Sensors 2021, 21, 131. [Google Scholar] [CrossRef] [PubMed]
- Rebodos, R.L.; Vikesland, P.J. Effects of Oxidation on the Magnetization of Nanoparticulate Magnetite. Langmuir 2010, 26, 16745–16753. [Google Scholar] [CrossRef]
- Ahadpour Shal, A.; Jafari, A. Study of Structural and Magnetic Properties of Superparamagnetic Fe3O4–ZnO Core–Shell Nanoparticles. J. Supercond Nov. Mag. 2014, 27, 1531–1538. [Google Scholar] [CrossRef]
- Parkinson, G.S. Iron Oxide Surfaces. Surf. Sci. Rep. 2016, 71, 272–365. [Google Scholar] [CrossRef] [Green Version]
- Teja, A.S.; Koh, P.-Y. Synthesis, Properties, and Applications of Magnetic Iron Oxide Nanoparticles. Prog. Cryst. Growth Charact. Mater. 2009, 55, 22–45. [Google Scholar] [CrossRef]
- Mollarasouli, F.; Zor, E.; Ozcelikay, G.; Ozkan, S.A. Magnetic Nanoparticles in Developing Electrochemical Sensors for Pharmaceutical and Biomedical Applications. Talanta 2021, 226, 122108. [Google Scholar] [CrossRef]
- Chimezie, A.B.; Hajian, R.; Yusof, N.A.; Woi, P.M.; Shams, N. Fabrication of Reduced Graphene Oxide-Magnetic Nanocomposite (RGO-Fe3O4) as an Electrochemical Sensor for Trace Determination of As(III) in Water Resources. J. Electroanal. Chem. 2017, 796, 33–42. [Google Scholar] [CrossRef]
- Garkani Nejad, F.; Tajik, S.; Beitollahi, H.; Sheikhshoaie, I. Magnetic Nanomaterials Based Electrochemical (Bio)Sensors for Food Analysis. Talanta 2021, 228, 122075. [Google Scholar] [CrossRef] [PubMed]
- Bystron, T.; Sramkova, E.; Dvorak, F.; Bouzek, K. Glassy Carbon Electrode Activation—A Way towards Highly Active, Reproducible and Stable Electrode Surface. Electrochim. Acta 2019, 299, 963–970. [Google Scholar] [CrossRef]
- Fan, H.-L.; Zhou, S.-F.; Gao, J.; Liu, Y.-Z. Continuous Preparation of Fe3O4 Nanoparticles through Impinging Stream-Rotating Packed Bed Reactor and Their Electrochemistry Detection toward Heavy Metal Ions. J. Alloy Compd. 2016, 671, 354–359. [Google Scholar] [CrossRef]
- Wei, P.; Li, Z.; Zhao, X.; Song, R.; Zhu, Z. Fe3O4/SiO2/CS Surface Ion-Imprinted Polymer Modified Glassy Carbon Electrode for Highly Sensitivity and Selectivity Detection of Toxic Metal Ions. J. Taiwan Inst. Chem. Eng. 2020, 113, 107–113. [Google Scholar] [CrossRef]
- Qureashi, A.; Pandith, A.H.; Bashir, A.; Manzoor, T.; Malik, L.A.; Sheikh, F.A. Citrate Coated Magnetite: A Complete Magneto Dielectric, Electrochemical and DFT Study for Detection and Removal of Heavy Metal Ions. Surf. Interfaces 2021, 23, 101004. [Google Scholar] [CrossRef]
- Bai, F.; Zhang, X.; Hou, X.; Liu, H.; Chen, J.; Yang, T. Individual and Simultaneous Voltammetric Determination of Cd(II), Cu(II) and Pb(II) Applying Amino Functionalized Fe3O4@Carbon Microspheres Modified Electrode. Electroanalysis 2019, 31, 1448–1457. [Google Scholar] [CrossRef]
- Kaliyaraj Selva Kumar, A.; Zhang, Y.; Li, D.; Compton, R.G. A Mini-Review: How Reliable Is the Drop Casting Technique? Electrochem. Commun. 2020, 121, 106867. [Google Scholar] [CrossRef]
- Kong, Y.; Wu, T.; Wu, D.; Zhang, Y.; Wang, Y.; Du, B.; Wei, Q. An Electrochemical Sensor Based on Fe3O4@PANI Nanocomposites for Sensitive Detection of Pb2+ and Cd2+. Anal. Methods 2018, 10, 4784–4792. [Google Scholar] [CrossRef]
- Wang, L.; Lei, T.; Ren, Z.; Jiang, X.; Yang, X.; Bai, H.; Wang, S. Fe3O4@PDA@MnO2 Core-Shell Nanocomposites for Sensitive Electrochemical Detection of Trace Pb(II) in Water. J. Electroanal. Chem. 2020, 864, 114065. [Google Scholar] [CrossRef]
- Pu, Y.; Wu, Y.; Yu, Z.; Lu, L.; Wang, X. Simultaneous Determination of Cd2+ and Pb2+ by an Electrochemical Sensor Based on Fe3O4/Bi2O3/C3N4 Nanocomposites. Talanta Open 2021, 3, 100024. [Google Scholar] [CrossRef]
- Wu, W.; Jia, M.; Zhang, Z.; Chen, X.; Zhang, Q.; Zhang, W.; Li, P.; Chen, L. Sensitive, Selective and Simultaneous Electrochemical Detection of Multiple Heavy Metals in Environment and Food Using a Lowcost Fe3O4 Nanoparticles/Fluorinated Multi-Walled Carbon Nanotubes Sensor. Ecotoxicol. Environ. Saf. 2019, 175, 243–250. [Google Scholar] [CrossRef]
- Xu, Z.; Fan, X.; Ma, Q.; Tang, B.; Lu, Z.; Zhang, J.; Mo, G.; Ye, J.; Ye, J. A Sensitive Electrochemical Sensor for Simultaneous Voltammetric Sensing of Cadmium and Lead Based on Fe3O4/Multiwalled Carbon Nanotube/Laser Scribed Graphene Composites Functionalized with Chitosan Modified Electrode. Mater. Chem. Phys. 2019, 238, 121877. [Google Scholar] [CrossRef]
- Nodehi, M.; Baghayeri, M.; Veisi, H. Preparation of GO/Fe3O4@PMDA/AuNPs Nanocomposite for Simultaneous Determination of As3+ and Cu2+ by Stripping Voltammetry. Talanta 2021, 230, 122288. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Jia, M.; Wang, Z.; Zhang, W.; Zhang, Q.; Liu, G.; Zhang, Z.; Li, P. Simultaneous Voltammetric Determination of Cadmium(II), Lead(II), Mercury(II), Zinc(II), and Copper(II) Using a Glassy Carbon Electrode Modified with Magnetite (Fe3O4) Nanoparticles and Fluorinated Multiwalled Carbon Nanotubes. Microchim. Acta 2019, 186, 97. [Google Scholar] [CrossRef] [PubMed]
- Dahaghin, Z.; Kilmartin, P.A.; Mousavi, H.Z. Simultaneous Determination of Lead(II) and Cadmium(II) at a Glassy Carbon Electrode Modified with GO@Fe3O4@benzothiazole-2-Carboxaldehyde Using Square Wave Anodic Stripping Voltammetry. J. Mol. Liq. 2018, 249, 1125–1132. [Google Scholar] [CrossRef]
- He, B.; Shen, X.; Nie, J.; Wang, X.; Liu, F.; Yin, W.; Hou, C.; Huo, D.; Fa, H. Electrochemical Sensor Using Graphene/Fe3O4 Nanosheets Functionalized with Garlic Extract for the Detection of Lead Ion. J. Solid State Electrochem. 2018, 11, 3515–3525. [Google Scholar] [CrossRef]
- Deshmukh, S.; Kandasamy, G.; Upadhyay, R.K.; Bhattacharya, G.; Banerjee, D.; Maity, D.; Deshusses, M.A.; Roy, S.S. Terephthalic Acid Capped Iron Oxide Nanoparticles for Sensitive Electrochemical Detection of Heavy Metal Ions in Water. J. Electroanal. Chem. 2017, 788, 91–98. [Google Scholar] [CrossRef]
- Baghayeri, M.; Amiri, A.; Maleki, B.; Alizadeh, Z.; Reiser, O. A Simple Approach for Simultaneous Detection of Cadmium(II) and Lead(II) Based on Glutathione Coated Magnetic Nanoparticles as a Highly Selective Electrochemical Probe. Sens. Actuators B Chem. 2018, 273, 1442–1450. [Google Scholar] [CrossRef]
- Kimmel, D.W.; LeBlanc, G.; Meschievitz, M.E.; Cliffel, D.E. Electrochemical Sensors and Biosensors. Anal. Chem. 2012, 84, 685–707. [Google Scholar] [CrossRef] [Green Version]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A Review on Various Electrochemical Techniques for Heavy Metal Ions Detection with Different Sensing Platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef]
- Kulpa, A.; Ryl, J.; Schroeder, G.; Koterwa, A.; Sein Anand, J.; Ossowski, T.; Niedziałkowski, P. Simultaneous Voltammetric Determination of Cd2+, Pb2+, and Cu2+ Ions Captured by Fe3O4@SiO2 Core-Shell Nanostructures of Various Outer Amino Chain Length. J. Mol. Liq. 2020, 314, 113677. [Google Scholar] [CrossRef]
- Companys, E.; Galceran, J.; Pinheiro, J.P.; Puy, J.; Salaün, P. A Review on Electrochemical Methods for Trace Metal Speciation in Environmental Media. Curr. Opin. Electrochem. 2017, 3, 144–162. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.S.; Tanaya Das, H.; Maiyalagan, T.; Elumalai, P. Influence of Designed Electrode Surfaces on Double Layer Capacitance in Aqueous Electrolyte: Insights from Standard Models. Appl. Surf. Sci. 2018, 449, 445–453. [Google Scholar] [CrossRef]
- Kiamahalleh, M.V.; Zein, S.H.S.; Najafpour, G.; Sata, S.A.; Buniran, S. Multiwalled Carbon Nanotubes Based Nanocomposites for Supercapacitors: A Review of Electrode Materials. NANO 2012, 07, 1230002. [Google Scholar] [CrossRef]
- Śmietana, M.; Niedziałkowski, P.; Białobrzeska, W.; Burnat, D.; Sezemsky, P.; Koba, M.; Stranak, V.; Siuzdak, K.; Ossowski, T.; Bogdanowicz, R. Study on Combined Optical and Electrochemical Analysis Using Indium-Tin-Oxide-Coated Optical Fiber Sensor. Electroanalysis 2019, 31, 398–404. [Google Scholar] [CrossRef]
- Huang, X.; Chen, T.; Zou, X.; Zhu, M.; Chen, D.; Pan, M. The Adsorption of Cd(II) on Manganese Oxide Investigated by Batch and Modeling Techniques. Int. J. Environ. Res. Public Health 2017, 14, 1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).