Enhanced Degradation of Rhodamine B by Metallic Organic Frameworks Based on NH2-MIL-125(Ti) under Visible Light
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.1.1. Materials
2.1.2. Preparation of NH2-MIL-125(Ti) and 15% M/Ti-MOFs (M: Ni, Co, Fe)
2.2. Instrumentation
2.3. Photocatlytic Test
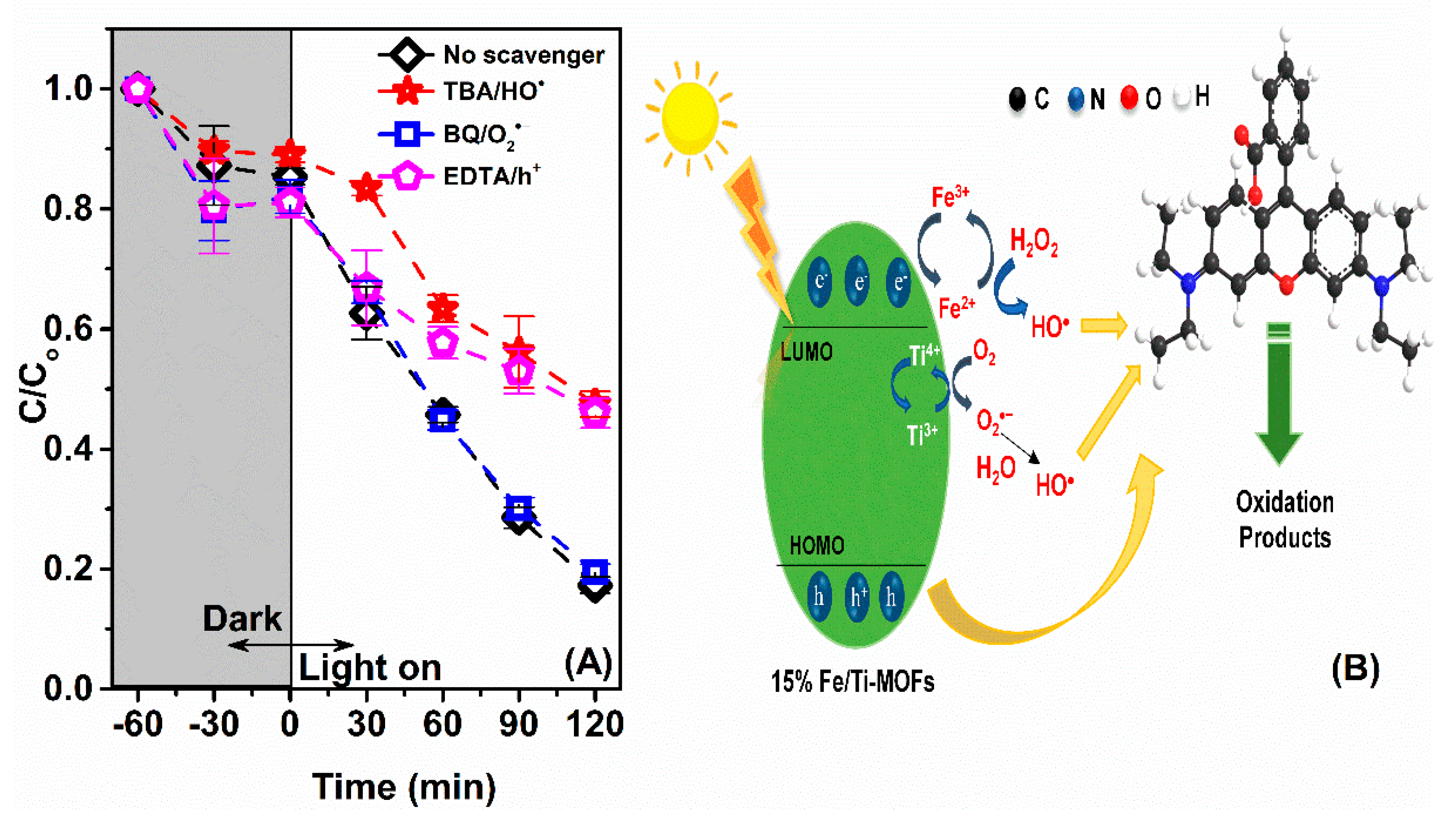
2.4. Trapping Test
3. Results and Discussion
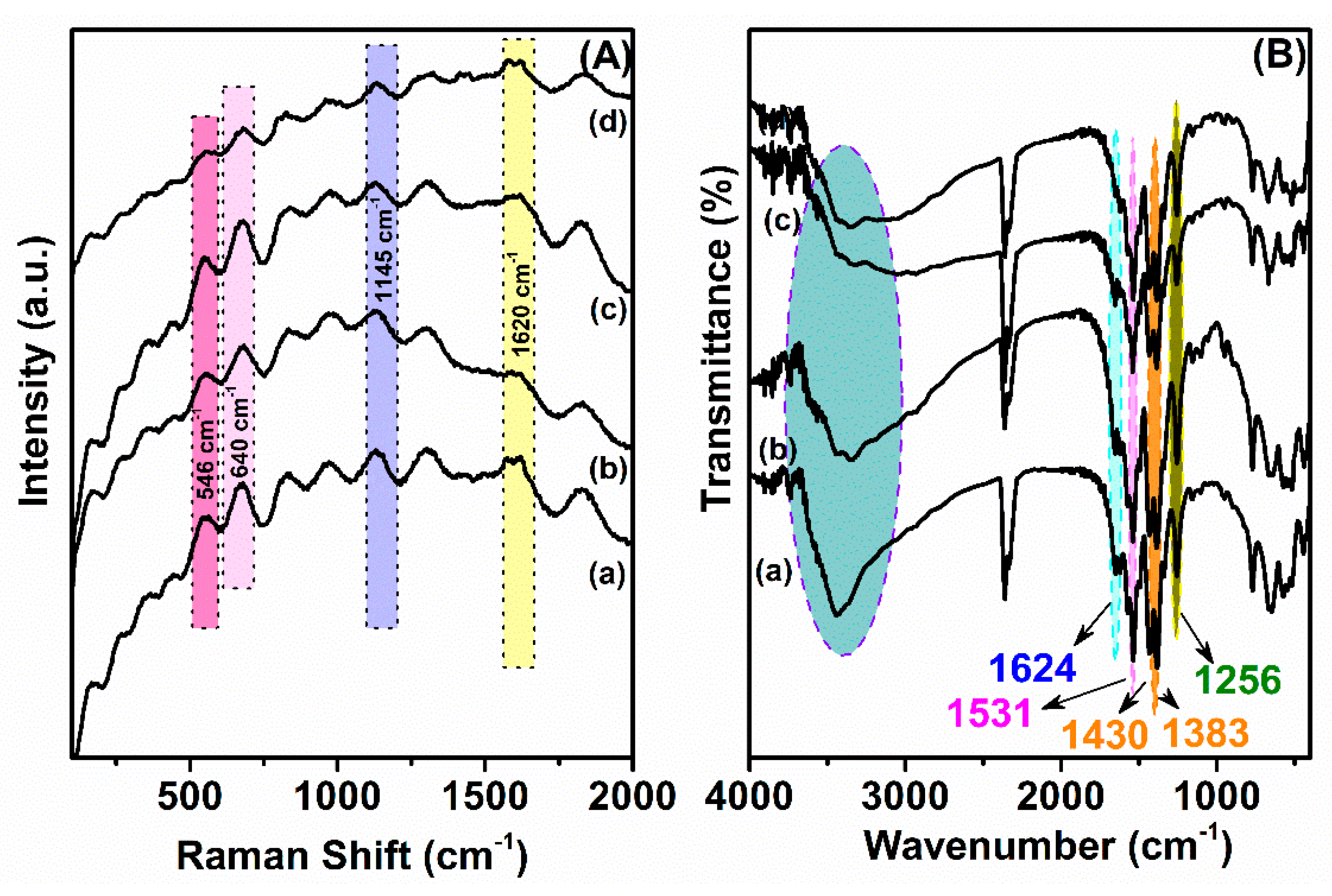
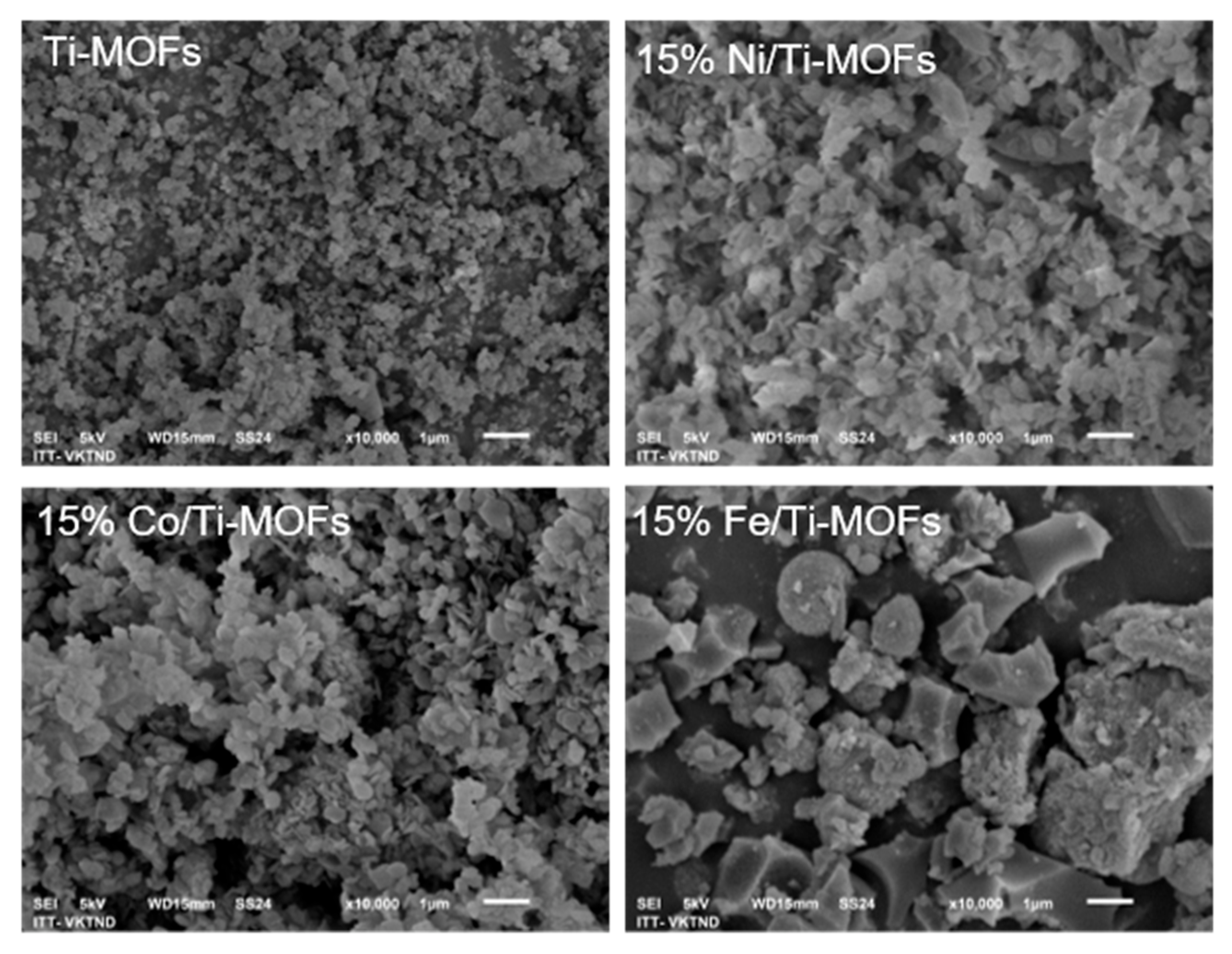
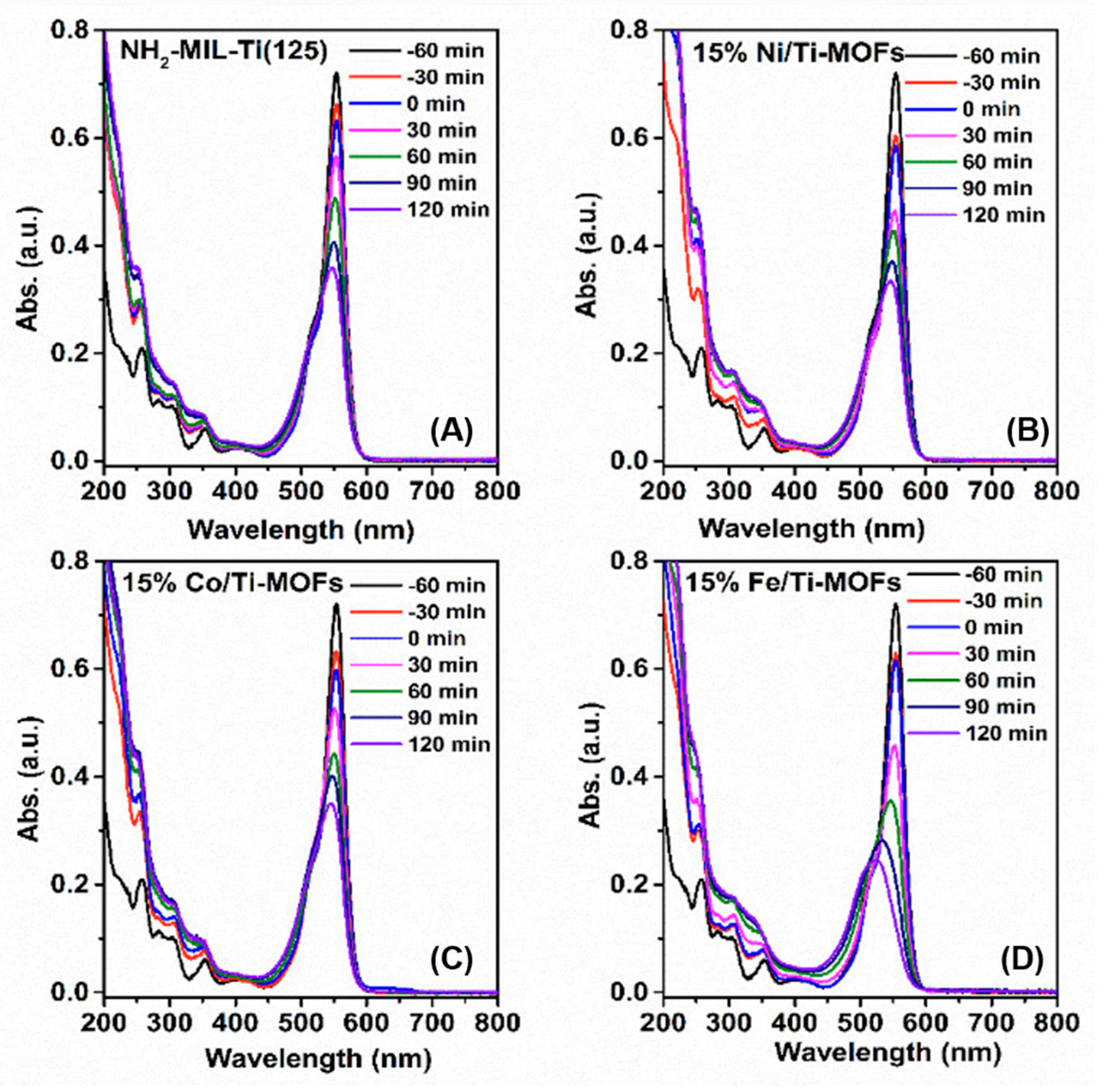
3.1. Characterization
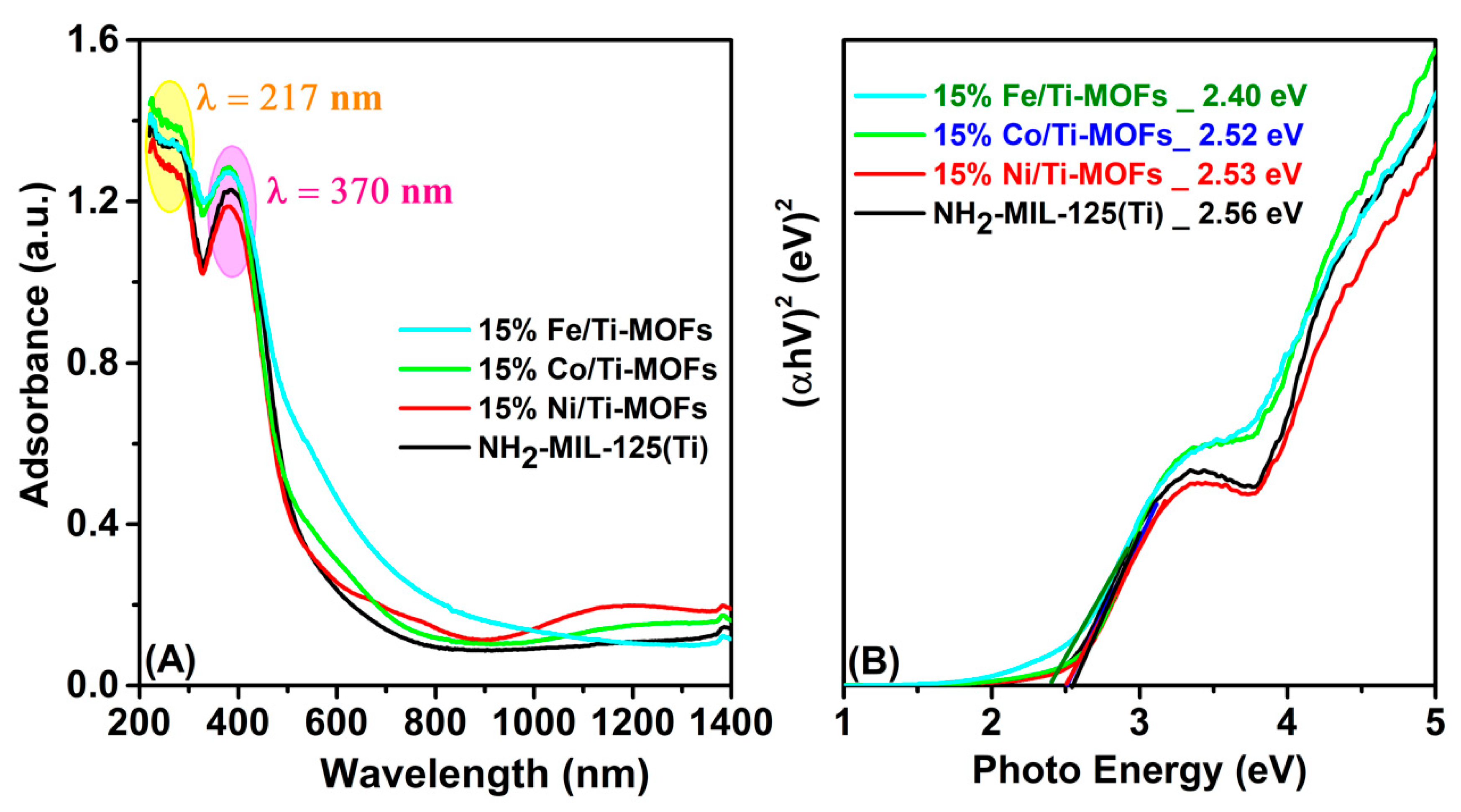
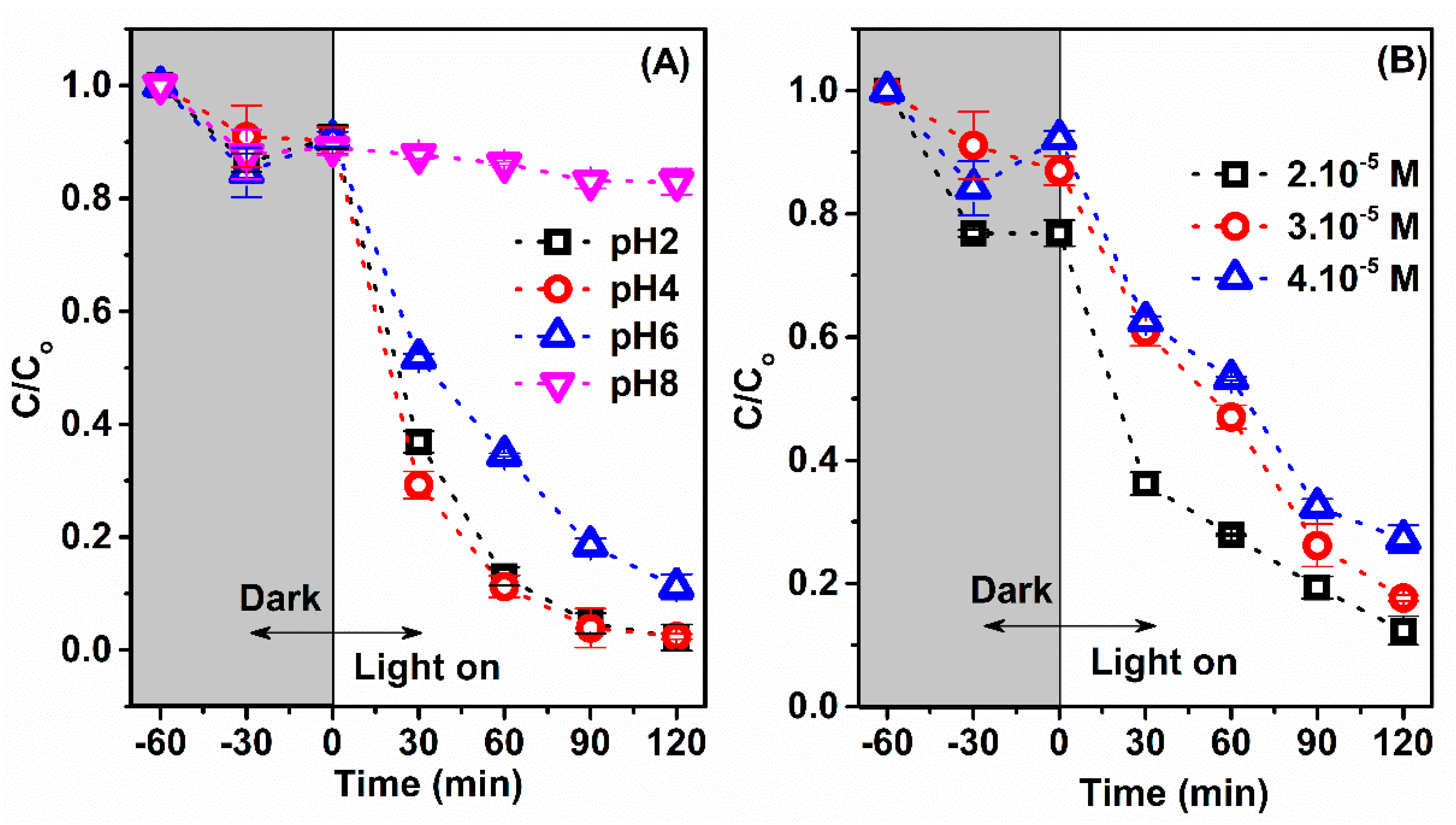
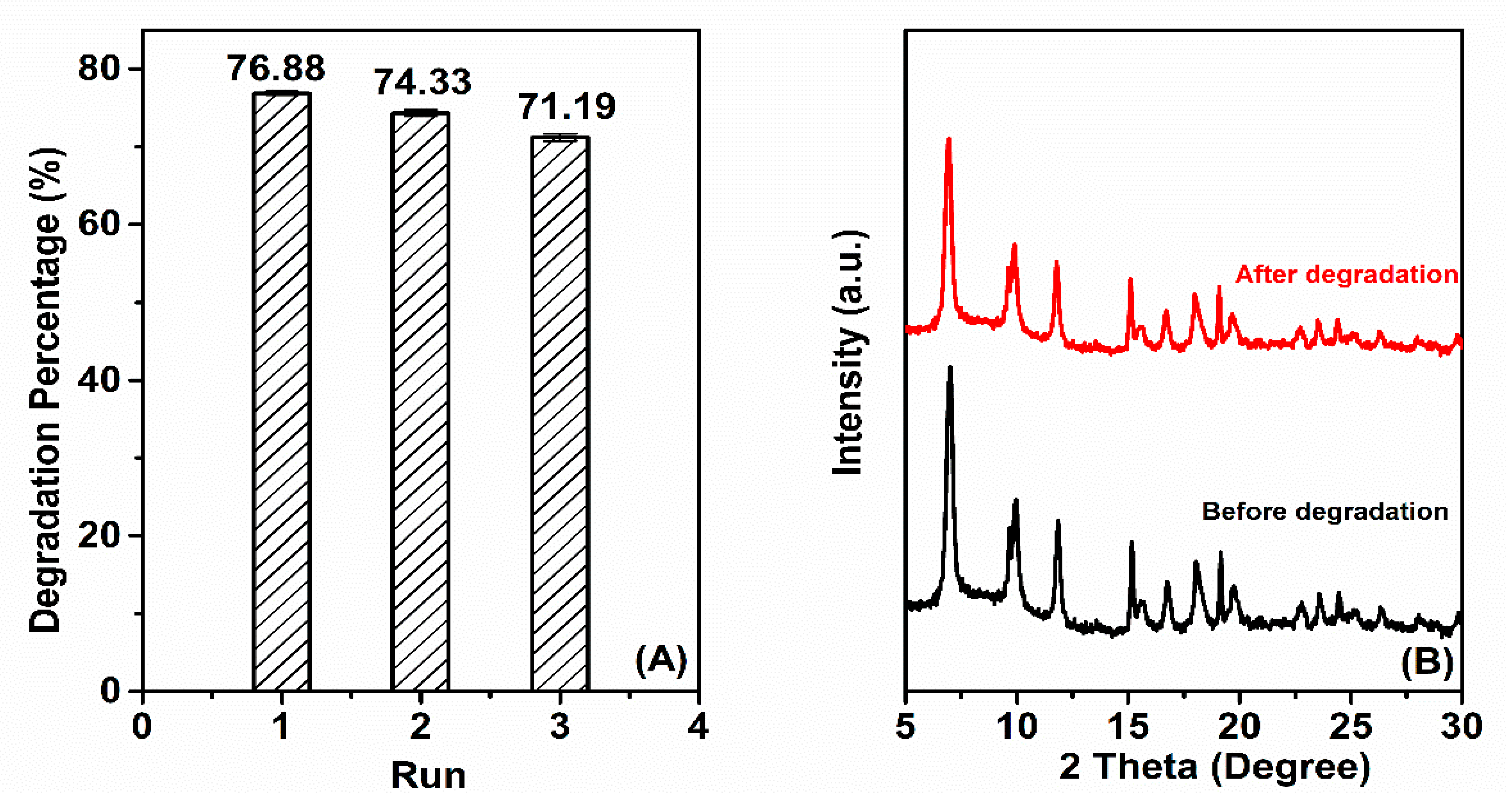
3.2. Photocatalytic Activity and Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vieira, Y.; Leichtweis, J.; Foletto, E.L.; Silvestri, S. Reactive oxygen species-induced heterogeneous photocatalytic degradation of organic pollutant Rhodamine B by copper and zinc aluminate spinels. J. Chem. Technol. Biotechnol. 2020, 95, 791–797. [Google Scholar] [CrossRef]
- Nohynek, G.; Hueber-Becker, F.; Meuling, W.; Dufour, E.; Bolt, H.; DeBie, A. Occupational exposure of hairdressers to [14C]-para-phenylenediamine-containing oxidative hair dyes. Toxicol. Lett. 2007, 172, S30–S31. [Google Scholar] [CrossRef]
- Cassano, A.; Molinari, R.; Romano, M.; Drioli, E. Treatment of aqueous effluents of the leather industry by membrane processes. J. Memb. Sci. 2001, 181, 111–126. [Google Scholar] [CrossRef]
- Albano, G.; Aronica, L.A.; Biver, T.; Detti, R.; Pucci, A. Tris -Ethynylphenyl-amine Fluorophores: Synthesis, Characterisation and Test of Performances in Luminescent Solar Concentrators. ChemistrySelect 2018, 3, 1749–1754. [Google Scholar] [CrossRef]
- Tavker, N.; Sharma, M. Enhanced photocatalytic activity of nanocellulose supported zinc oxide composite for RhB dye as well as ciprofloxacin drug under sunlight/visible light. AIP Conf. Proc. 2018, 1961, 030013. [Google Scholar] [CrossRef]
- Sun, D.; Ye, L.; Li, Z. Visible-light-assisted aerobic photocatalytic oxidation of amines to imines over NH2-MIL-125 (Ti). Appl. Catal. B Environ. 2015, 164, 428–432. [Google Scholar] [CrossRef]
- Wang, M.; Yang, L.; Yuan, J.; He, L.; Song, Y.; Zhang, H.; Zhang, Z.; Fang, S. Heterostructured Bi2S3@NH2-MIL-125(Ti) nanocomposite as a bifunctional photocatalyst for Cr(vi) reduction and rhodamine B degradation under visible light. RSC Adv. 2018, 8, 12459–12470. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.H.; Nguyen, T.D.; van Nguyen, T. Microwave-Assisted Solvothermal Synthesis and Photocatalytic Activity of Bismuth(III) Based Metal–Organic Framework. Top. Catal. 2020, 63, 1109–1120. [Google Scholar] [CrossRef]
- Zhao, Y.; Cai, W.; Chen, J.; Miao, Y.; Bu, Y. A Highly Efficient Composite Catalyst Constructed from NH2-MIL-125(Ti) and Reduced Graphene Oxide for CO2 Photoreduction. Front. Chem. 2019, 7, 789. [Google Scholar] [CrossRef] [PubMed]
- Ao, D.; Zhang, J.; Liu, H. Visible-light-driven photocatalytic degradation of pollutants over Cu-doped NH2-MIL-125(Ti). J. Photochem. Photobiol. A Chem. 2018, 364, 524–533. [Google Scholar] [CrossRef]
- Wu, Z.; Yuan, X.; Zhang, J.; Wang, H.; Jiang, L.; Zeng, G. Photocatalytic Decontamination of Wastewater Containing Organic Dyes by Metal-Organic Frameworks and their Derivatives. ChemCatChem 2017, 9, 41–64. [Google Scholar] [CrossRef]
- Zeng, L.; Guo, X.; He, C.; Duan, C. Metal–Organic Frameworks: Versatile Materials for Heterogeneous Photocatalysis. ACS Catal. 2016, 6, 7935–7947. [Google Scholar] [CrossRef]
- Hu, S.; Liu, M.; Li, K.; Zuo, Y.; Zhang, A.; Song, C.; Zhang, G.; Guo, X. Solvothermal synthesis of NH2 -MIL-125(Ti) from circular plate to octahedron. CrystEngComm 2014, 16, 9645–9650. [Google Scholar] [CrossRef]
- Hendon, C.H.; Tiana, D.; Fontecave, M.; Sanchez, C.; D’arras, L.; Sassoye, C.; Rozes, L.; Mellot-Draznieks, C.; Walsh, A. Engineering the Optical Response of the Titanium-MIL-125 Metal–Organic Framework through Ligand Functionalization. J. Am. Chem. Soc. 2013, 135, 10942–10945. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Liang, S.; Wu, W.; Liang, R.; Wu, L. Multifunctional NH2-mediated zirconium metal–organic framework as an efficient visible-light-driven photocatalyst for selective oxidation of alcohols and reduction of aqueous Cr(vi). Dalt. Trans. 2013, 42, 13649. [Google Scholar] [CrossRef]
- Liang, R.; Shen, L.; Jing, F.; Wu, W.; Qin, N.; Lin, R.; Wu, L. NH2 -mediated indium metal–organic framework as a novel visible-light-driven photocatalyst for reduction of the aqueous Cr(VI). Appl. Catal. B Environ. 2015, 162, 245–251. [Google Scholar] [CrossRef]
- Hu, S.; Liu, M.; Li, K.; Song, C.; Zhang, G.; Guo, X. Surfactant-assisted synthesis of hierarchical NH2 -MIL-125 for the removal of organic dyes. RSC Adv. 2017, 7, 581–587. [Google Scholar] [CrossRef] [Green Version]
- Vitillo, J.G.; Presti, D.; Luz, I.; Llabrés i Xamena, F.; Bordiga, S. Visible-Light-Driven Photocatalytic Coupling of Benzylamine over Titanium-Based MIL-125-NH2 Metal–Organic Framework: A Mechanistic Study. J. Phys. Chem. C. 2020, 124, 23707–23715. [Google Scholar] [CrossRef]
- Hanna, L.; Long, C.L.; Zhang, X.; Lockard, J.V. Heterometal incorporation in NH 2 -MIL-125(Ti) and its participation in the photoinduced charge-separated excited state. Chem. Commun. 2020, 56, 11597–11600. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yang, H.; Du, R.; Tu, G.; Xu, C.; Zhang, F.; Fan, M.; Zhu, W. Enhanced photocatalytic CO2 reduction over Co-doped NH 2 -MIL-125(Ti) under visible light. RSC Adv. 2017, 7, 42819–42825. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Avilés, A.; Peñas-Garzón, M.; Bedia, J.; Dionysiou, D.D.; Rodríguez, J.J.; Belver, C. Mixed Ti-Zr metal-organic-frameworks for the photodegradation of acetaminophen under solar irradiation. Appl. Catal. B Environ. 2019, 253, 253–262. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, H.; Wu, Y.; Zeng, G.; Chen, X.; Leng, L.; Wu, Z.; Li, H. One-pot self-assembly and photoreduction synthesis of silver nanoparticle-decorated reduced graphene oxide/MIL-125(Ti) photocatalyst with improved visible light photocatalytic activity. Appl. Organomet. Chem. 2016, 30, 289–296. [Google Scholar] [CrossRef]
- Karthik, P.; Vinoth, R.; Zhang, P.; Choi, W.; Balaraman, E.; Neppolian, B. π–π Interaction Between Metal–Organic Framework and Reduced Graphene Oxide for Visible-Light Photocatalytic H2 Production. ACS Appl. Energy Mater. 2018, 1, 1913–1923. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der inneren Struktur und der Größe von Kolloidteilchen mittels Röntgenstrahlen. In Kolloidchemie Ein Lehrbuch; Springer: Berlin/Heidelberg, Germany, 1912; pp. 387–409. [Google Scholar] [CrossRef]
- Llabres i Xamena, F.; Gascon, J. (Eds.) Metal Organic Frameworks as Heterogeneous Catalysts; Royal Society of Chemistry: Cambridge, UK, 2013. [Google Scholar] [CrossRef]
- Jin, D.; Xu, Q.; Yu, L.; Hu, X. Photoelectrochemical detection of the herbicide clethodim by using the modified metal-organic framework amino-MIL-125(Ti)/TiO 2. Microchim. Acta. 2015, 182, 1885–1892. [Google Scholar] [CrossRef]
- Rodríguez, N.A.; Savateev, A.; Grela, M.A.; Dontsova, D. Facile Synthesis of Potassium Poly(heptazine imide) (PHIK)/Ti-Based Metal–Organic Framework (MIL-125-NH2 ) Composites for Photocatalytic Applications. ACS Appl. Mater. Interfaces 2017, 9, 22941–22949. [Google Scholar] [CrossRef] [Green Version]
- Ahmadpour, N.; Sayadi, M.H.; Homaeigohar, S. A hierarchical Ca/TiO 2 /NH2 -MIL-125 nanocomposite photocatalyst for solar visible light induced photodegradation of organic dye pollutants in water. RSC Adv. 2020, 10, 29808–29820. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, D.; Chen, Y.; Huang, R.; Ding, Z.; Fu, X.; Li, Z. An Amine-Functionalized Titanium Metal-Organic Framework Photocatalyst with Visible-Light-Induced Activity for CO2 Reduction. Angew. Chemie Int. Ed. 2012, 51, 3364–3367. [Google Scholar] [CrossRef]
- Nasalevich, M.A.; Hendon, C.H.; Santaclara, J.G.; Svane, K.; van der Linden, B.; Veber, S.L.; Fedin, M.V.; Houtepen, A.J.; van der Veen, M.A.; Kapteijn, F.; et al. Electronic origins of photocatalytic activity in d0 metal organic frameworks. Sci. Rep. 2016, 6, 23676. [Google Scholar] [CrossRef]
- Maudhuit, A.; Raillard, C.; Héquet, V.; Le Coq, L.; Sablayrolles, J.; Molins, L. Adsorption phenomena in photocatalytic reactions: The case of toluene, acetone and heptane. Chem. Eng. J. 2011, 170, 464–470. [Google Scholar] [CrossRef]
- Jiang, Z.; Wei, W.; Mao, D.; Chen, C.; Shi, Y.; Lv, X.; Xie, J. Silver-loaded nitrogen-doped yolk–shell mesoporous TiO 2 hollow microspheres with enhanced visible light photocatalytic activity. Nanoscale 2015, 7, 784–797. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.; Wang, Y.; Pan, Q.; Zhong, B.; Luo, Y.; Cui, G.; Guo, X.; Sun, X. Enabling the electrocatalytic fixation of N 2 to NH 3 by C-doped TiO 2 nanoparticles under ambient conditions. Nanoscale Adv. 2019, 1, 961–964. [Google Scholar] [CrossRef] [Green Version]
- Yazdani, A.; Sayadi, M.H. Sonochemical degradation of azithromycin in aqueous solution. Environ. Heal. Eng. Manag. 2018, 5, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Pirsaheb, M.; Shahmoradi, B.; Beikmohammadi, M.; Azizi, E.; Hossini, H.; Ashraf, G.M. Photocatalytic degradation of Aniline from aqueous solutions under sunlight illumination using immobilized Cr:ZnO nanoparticles. Sci. Rep. 2017, 7, 1473. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.N.; Lei, S.; Jin, B.; Saint, C.; Chow, C.W.K. Optimisation of an annular photoreactor process for degradation of Congo Red using a newly synthesized titania impregnated kaolinite nano-photocatalyst. Sep. Purif. Technol. 2009, 67, 355–363. [Google Scholar] [CrossRef]

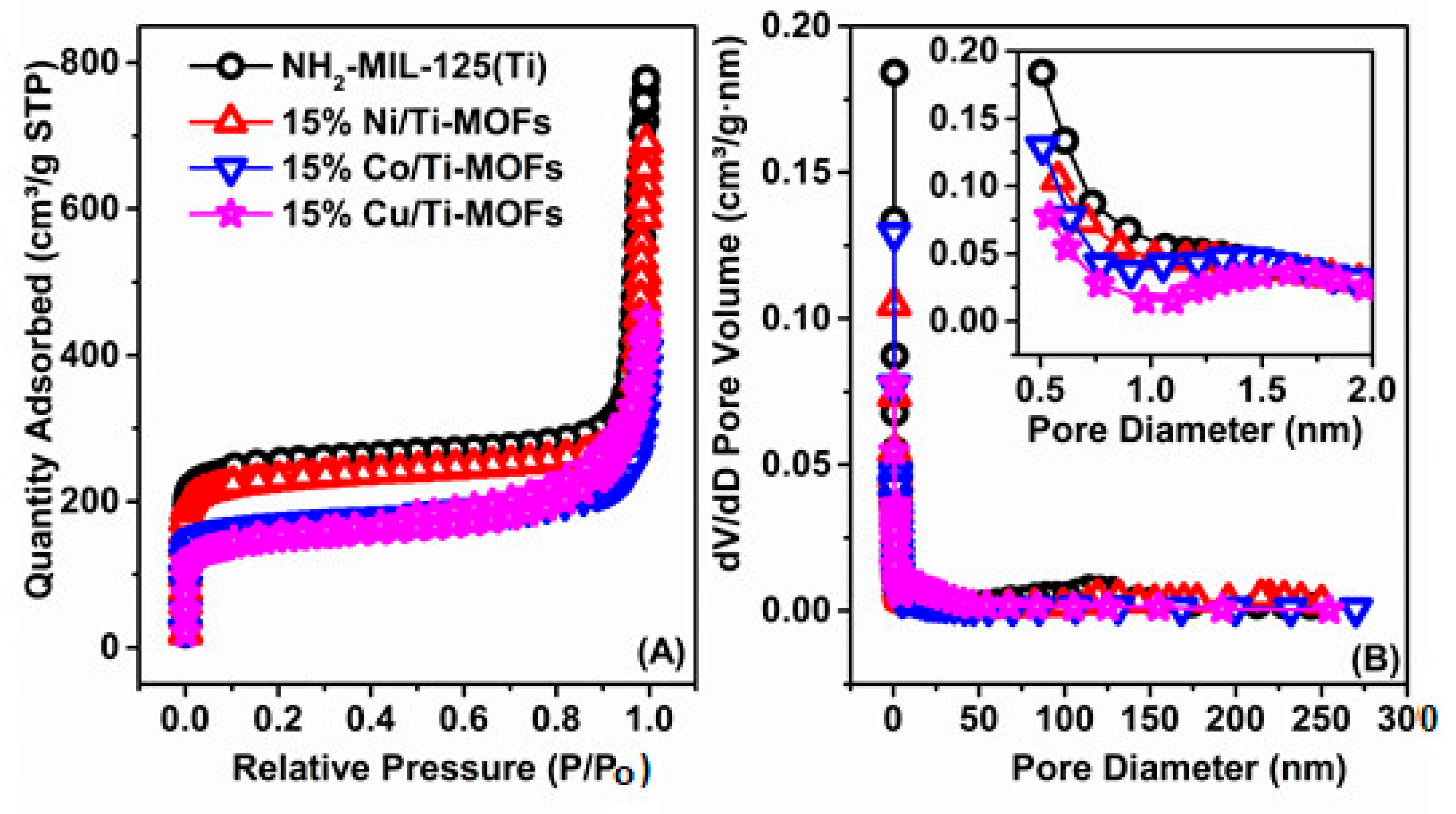

| Samples | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Average Pore Size (nm) |
|---|---|---|---|
| NH2-MIL-125(Ti) | 970.2 | 0.94 | 7.33 |
| 15% Ni/Ti−MOFs | 889.6 | 0.81 | 9.03 |
| 15% Co/Ti−MOFs | 640.7 | 0.47 | 4.92 |
| 15% Fe/Ti−MOFs | 554.9 | 0.52 | 7.63 |
| Sample | Band Gap (eV) | R2 | k1 (10−3·min−1) |
|---|---|---|---|
| NH2-MIL-125(Ti) | 2.56 | 0.9846 | 5.2 |
| 15% Ni/Ti-MOFs | 2.53 | 0.9869 | 5.35 |
| 15% Co/Ti-MOFs | 2.52 | 0.9936 | 5.41 |
| 15% Fe/Ti-MOFs | 2.40 | 0.9792 | 13.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen Thi, H.-T.; Tran Thi, K.-N.; Hoang, N.B.; Tran, B.T.; Do, T.S.; Phung, C.S.; Nguyen Thi, K.-O. Enhanced Degradation of Rhodamine B by Metallic Organic Frameworks Based on NH2-MIL-125(Ti) under Visible Light. Materials 2021, 14, 7741. https://doi.org/10.3390/ma14247741
Nguyen Thi H-T, Tran Thi K-N, Hoang NB, Tran BT, Do TS, Phung CS, Nguyen Thi K-O. Enhanced Degradation of Rhodamine B by Metallic Organic Frameworks Based on NH2-MIL-125(Ti) under Visible Light. Materials. 2021; 14(24):7741. https://doi.org/10.3390/ma14247741
Chicago/Turabian StyleNguyen Thi, Hong-Tham, Kim-Ngan Tran Thi, Ngoc Bich Hoang, Bich Thuy Tran, Trung Sy Do, Chi Sy Phung, and Kim-Oanh Nguyen Thi. 2021. "Enhanced Degradation of Rhodamine B by Metallic Organic Frameworks Based on NH2-MIL-125(Ti) under Visible Light" Materials 14, no. 24: 7741. https://doi.org/10.3390/ma14247741






