Waste to Wealth Strategy: Preparation and Properties of Lightweight Al2O3-SiO2-Rich Castables Using Aluminum Dross Waste
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
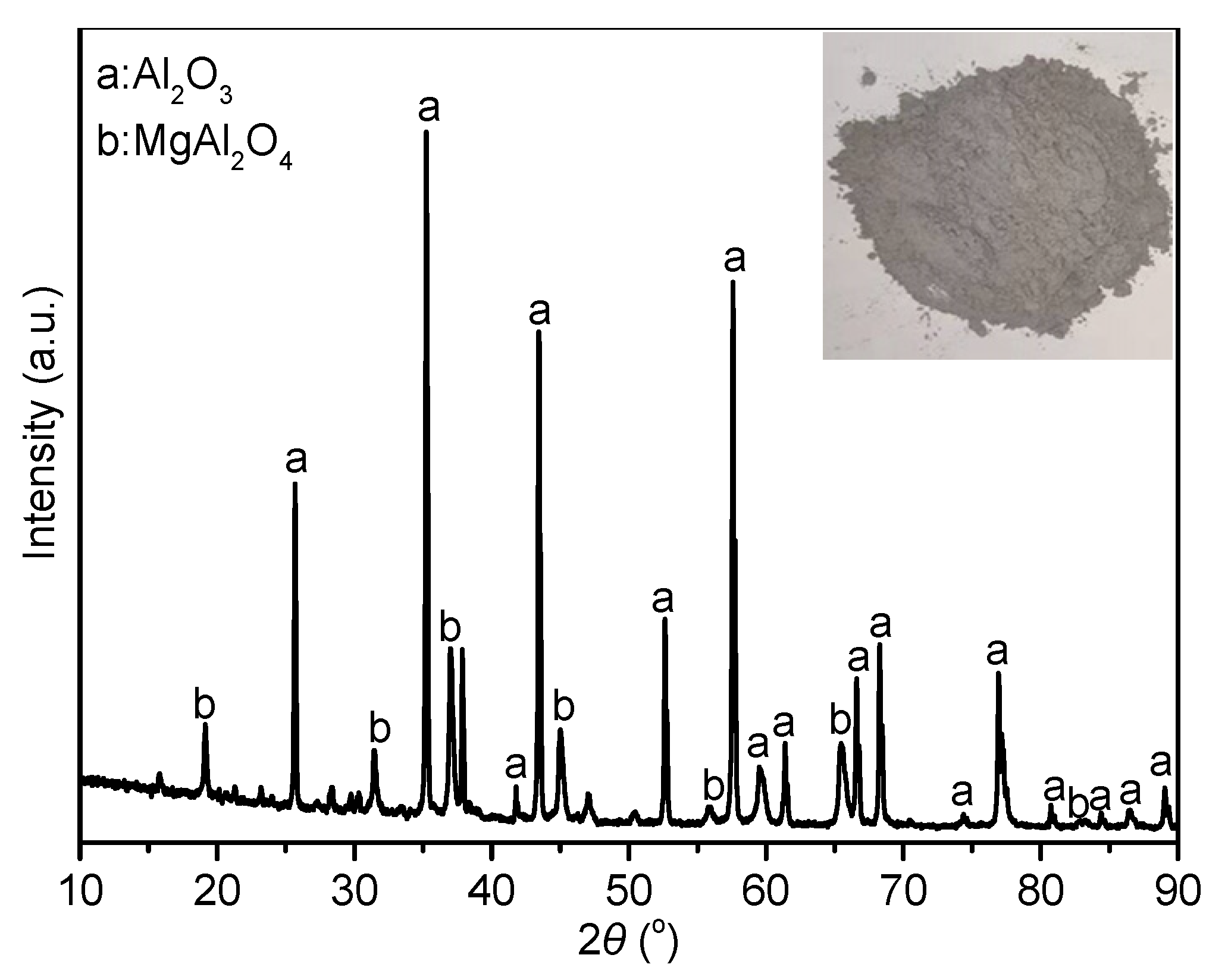
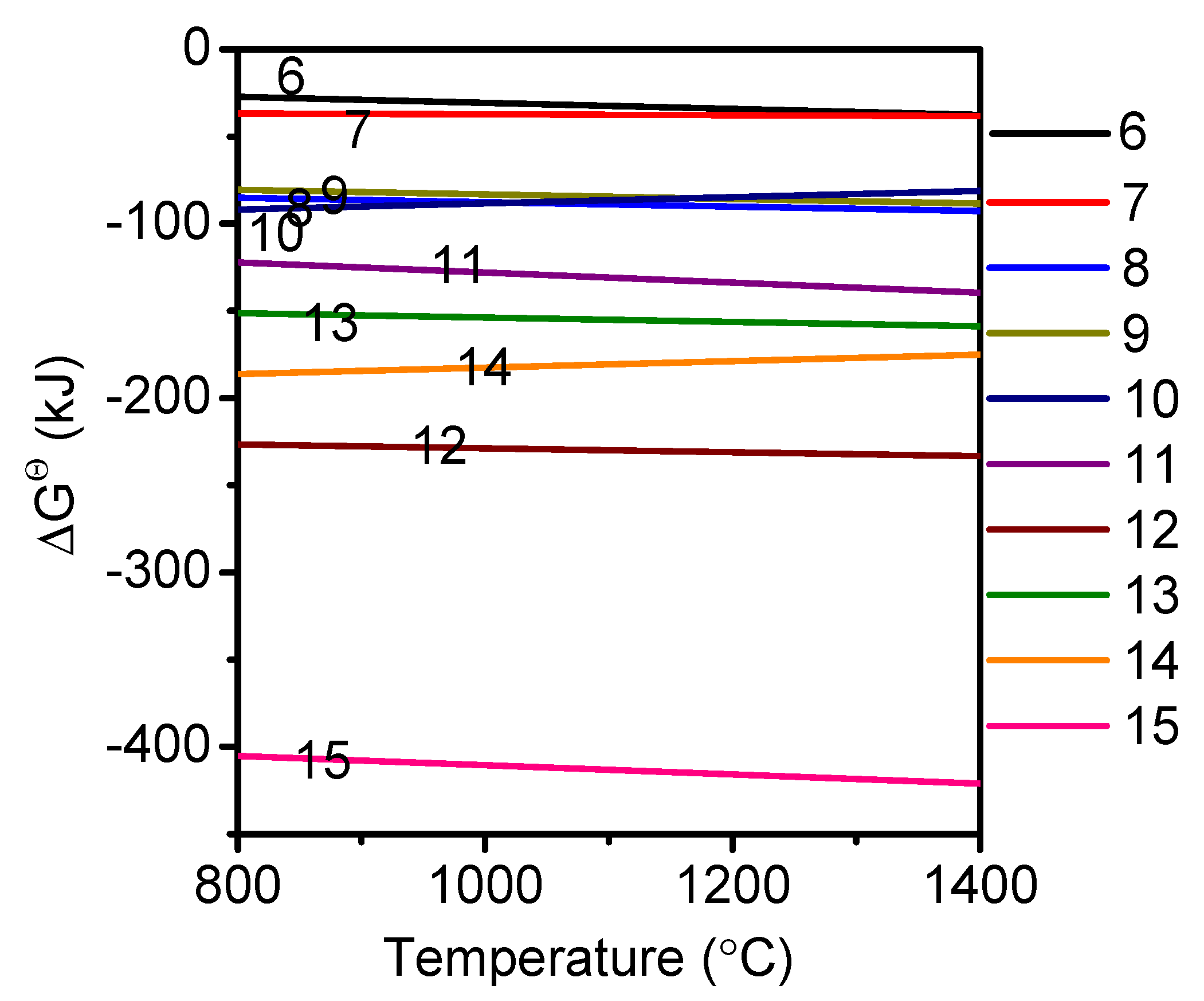
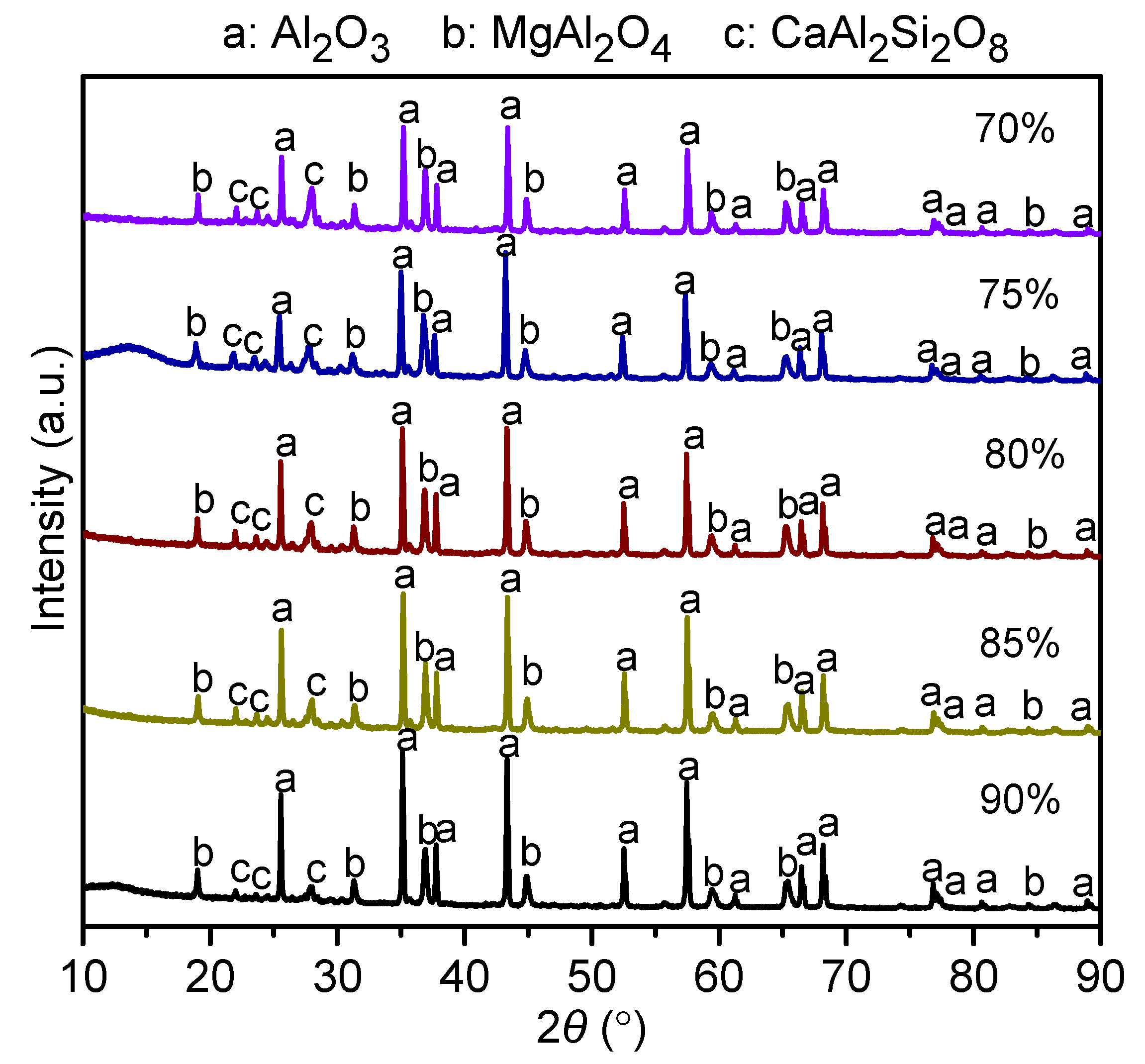
3.1. Phase Analysis
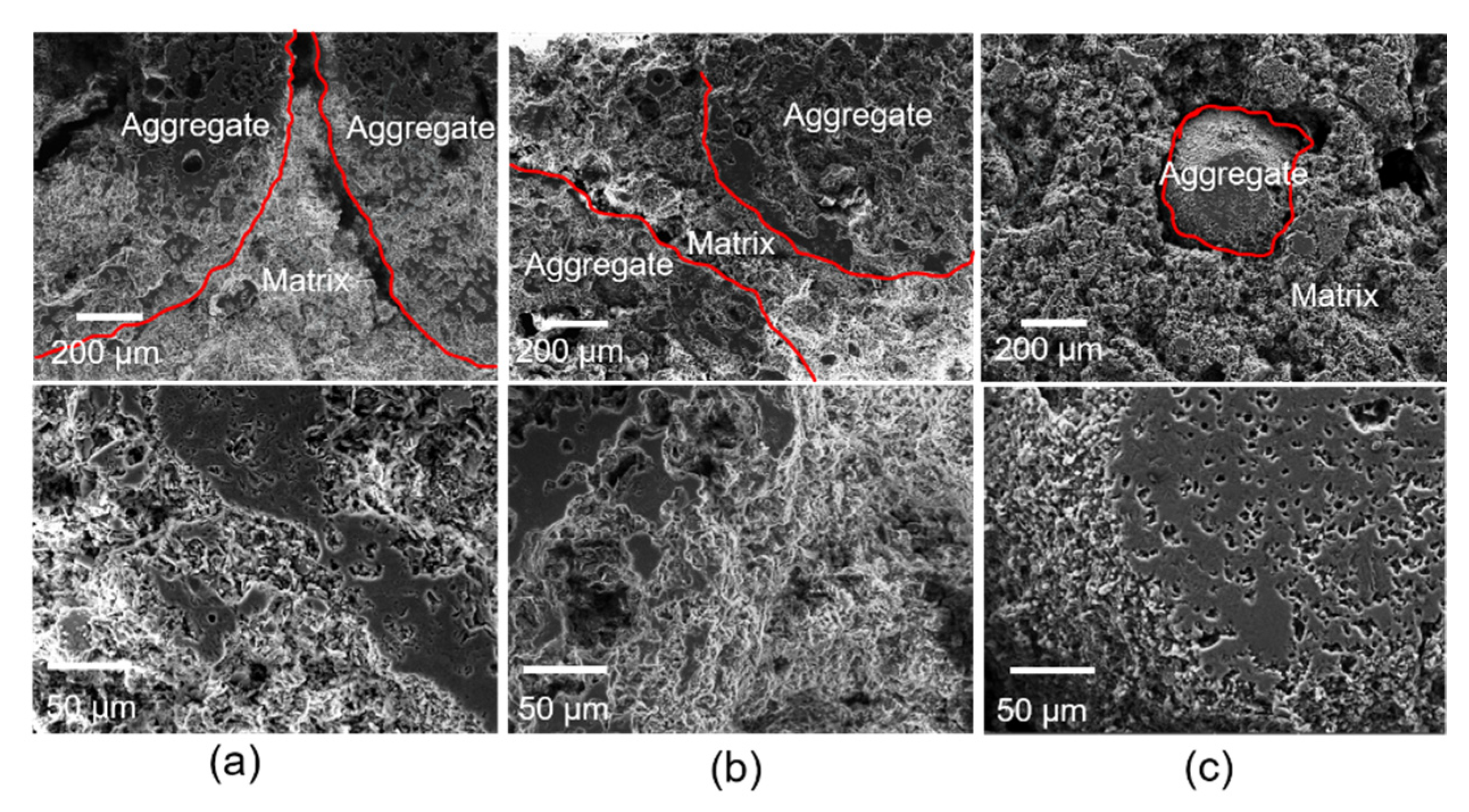
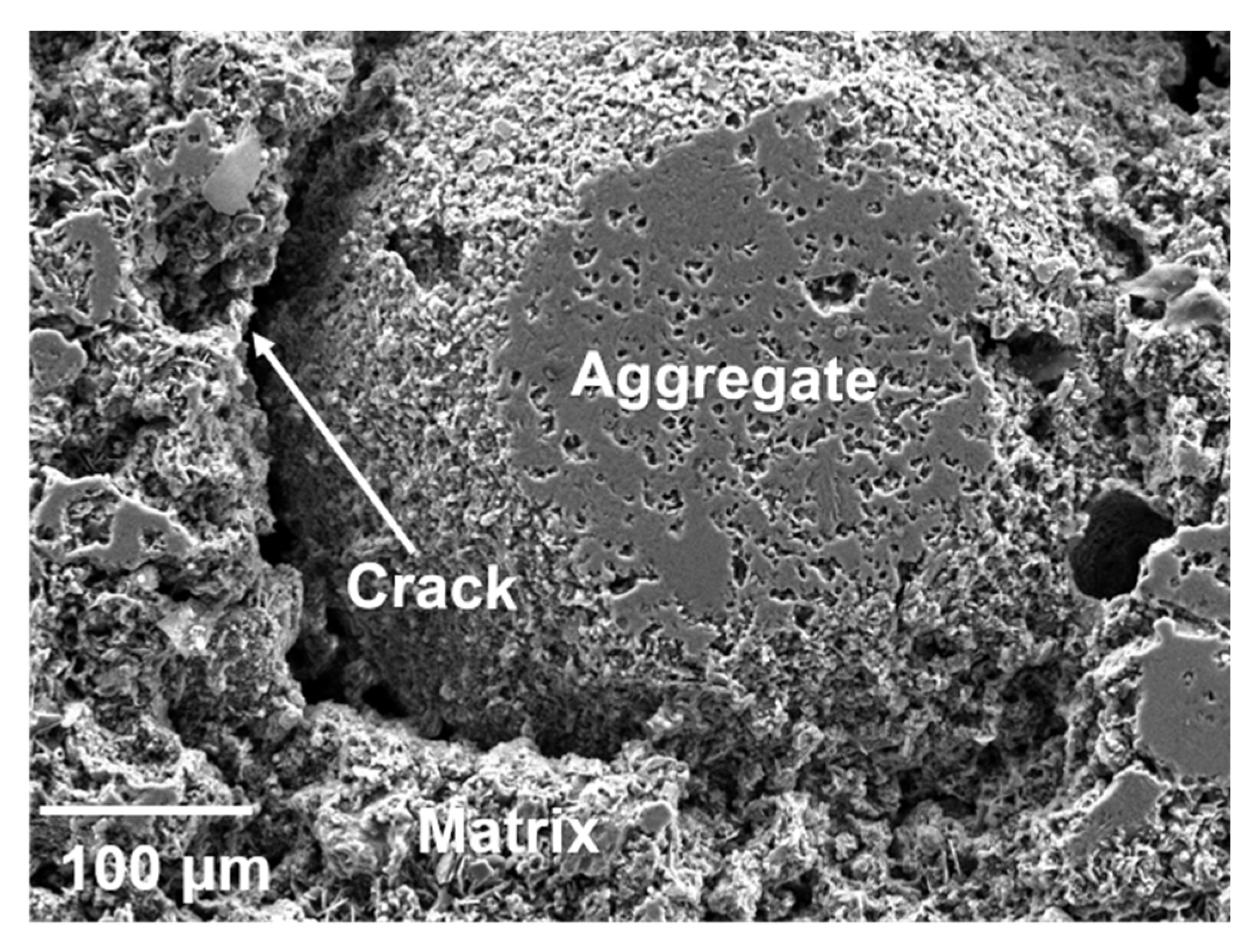
3.2. Microstructure
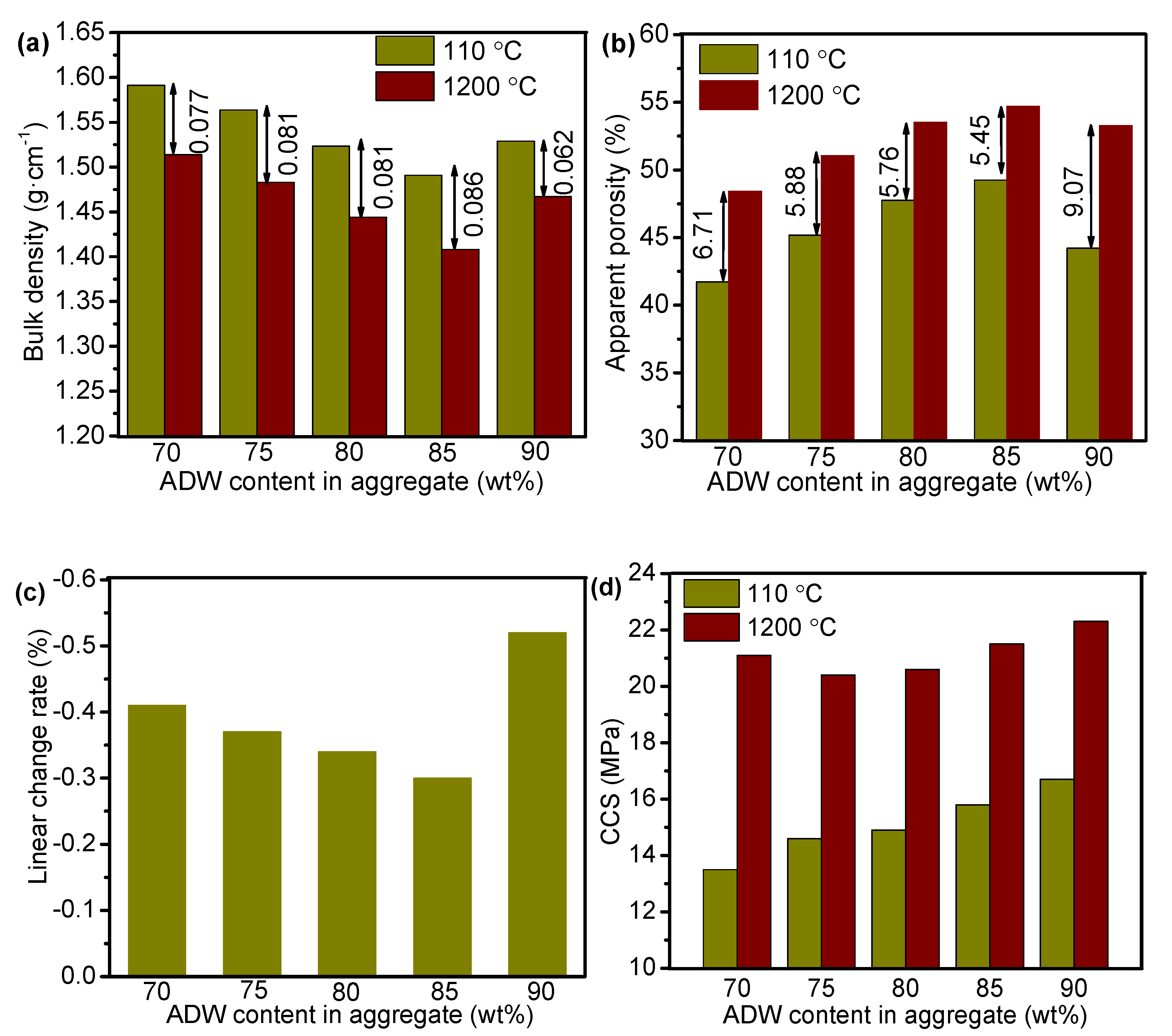
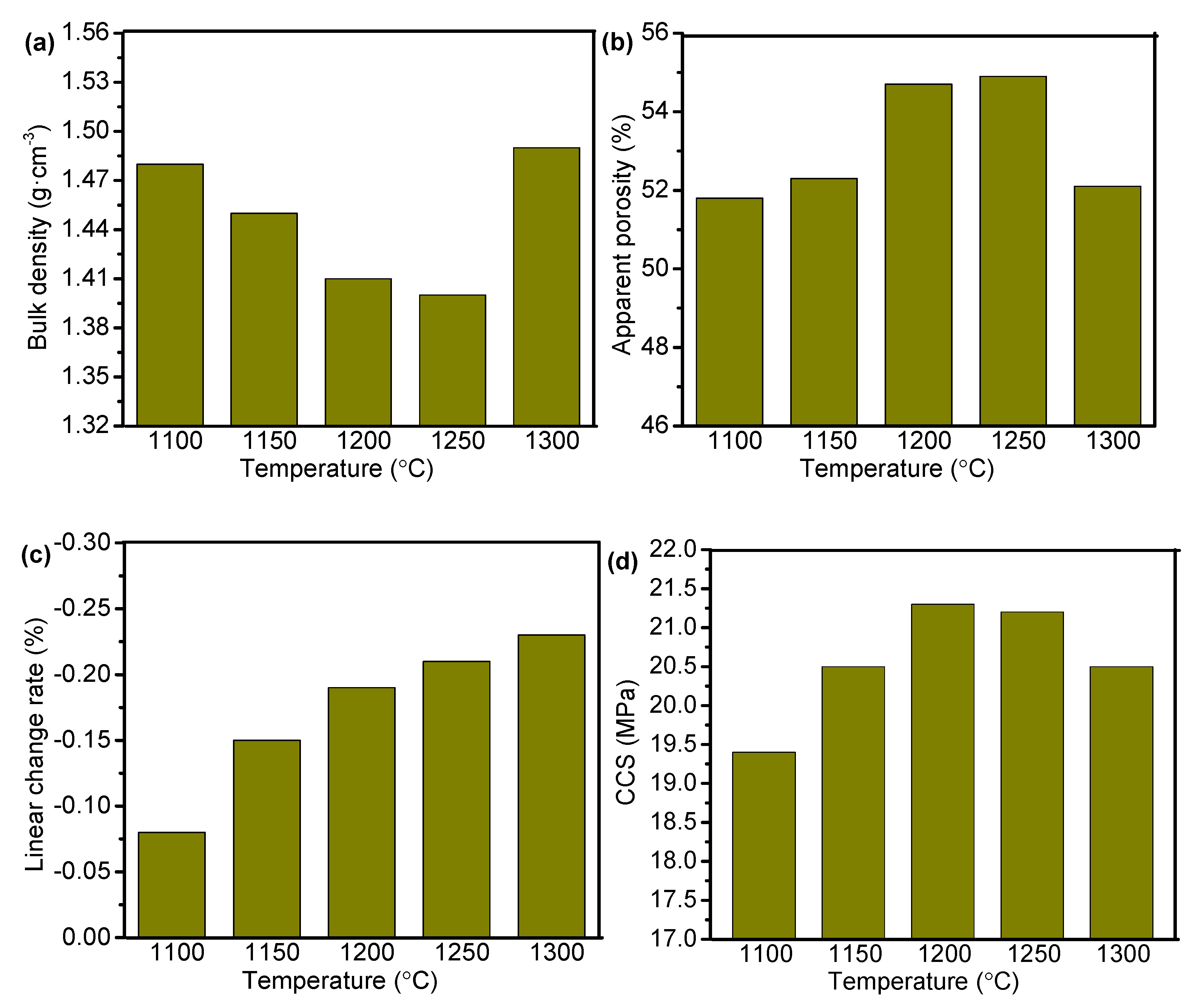
3.3. Densification and Mechanical Properties
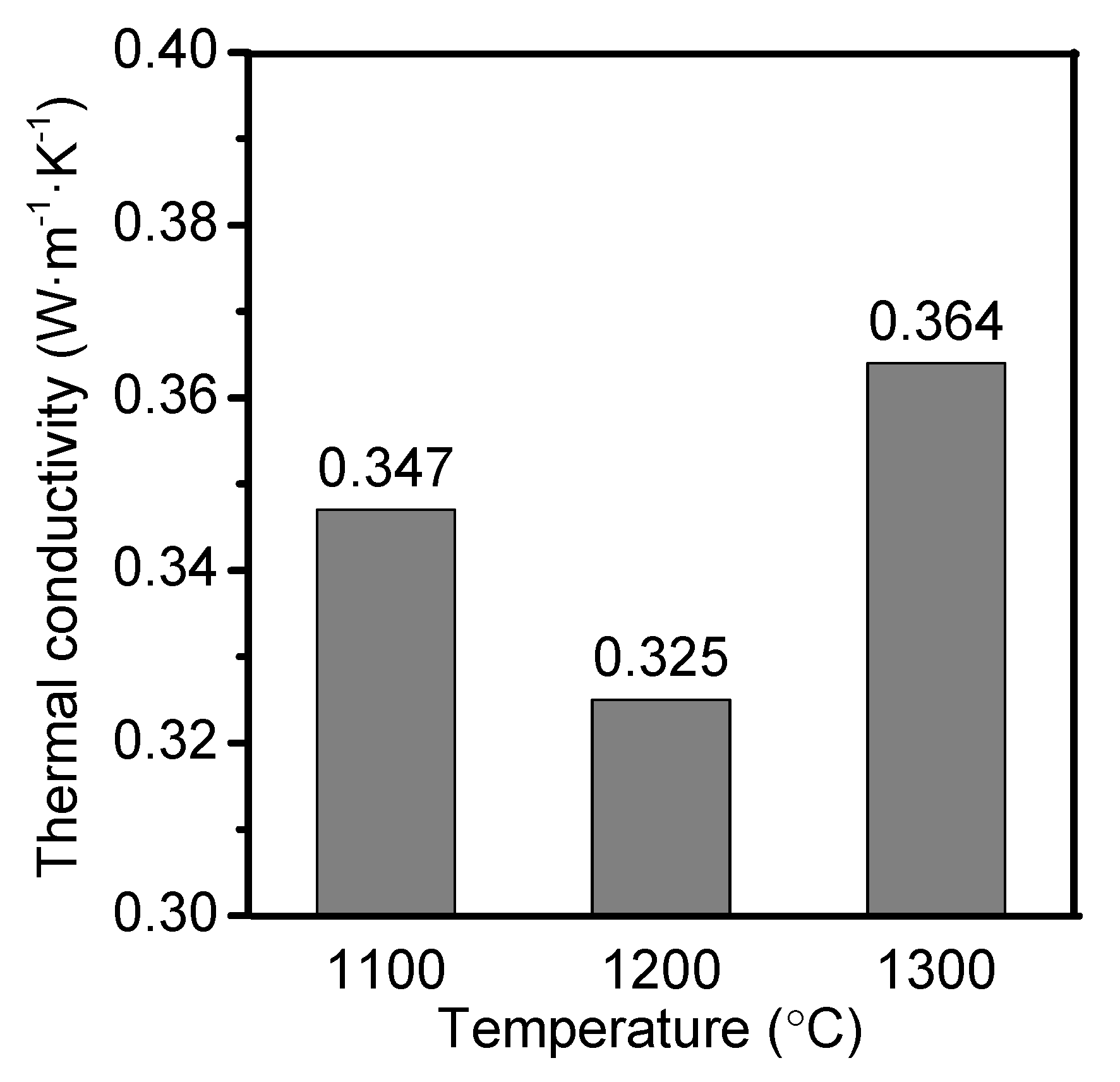
3.4. Thermal Conductivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, L.; Zhang, D.Y.; Liu, Y.; Chen, M.; Wang, N. Comparison of microstructure, thermo-mechanical property and corrosion resistance of bauxite-corundum refractory castables reinforced by two approaches. Ceram. Int. 2021, 47, 13660–13668. [Google Scholar] [CrossRef]
- Kakroudi, M.G.; Fogaing, E.Y.; Huger, M.; Gault, C.; Chotard, T. Influence of the thermal history on the mechanical properties of two alumina based castables. J. Eur. Ceram. Soc. 2009, 29, 3197–3204. [Google Scholar] [CrossRef]
- Pan, L.; He, Z.; Li, Y.; Zhu, T.; Wang, Q.; Li, B. Effects of cement content on the microstructural evolution and mechanical properties of cement-bonded corundum castables. Ceram. Int. 2020, 46, 4634–4642. [Google Scholar] [CrossRef]
- Patra, R.K.; Mukharjee, B.B. Influence of incorporation of granulated blast furnace slag as replacement of fine aggregate on properties of concrete. J. Clean. Prod. 2017, 165, 468–476. [Google Scholar] [CrossRef]
- Pawar, A.S.; Garud, D.B. Engineering properties of clay bricks with use of fly ash. Int. J. Res. Eng. Technol. 2014, 3, 75–80. [Google Scholar]
- Riaz, M.H.; Khitab, A.; Ahmed, S. Evaluation of sustainable clay bricks incorporating brick kiln dust. J. Build. Eng. 2019, 24, 100725. [Google Scholar] [CrossRef]
- Lopez-Perales, J.F.; Contreras, J.E.; Vazquez-Rodríguez, F.J.; Contreras, J.E.; Diaz-Tato, L. Partial replacement of a traditional raw material by blast furnace slag in developing a sustainable conventional refractory castable of improved physical-mechanical properties. J. Clean. Prod. 2021, 306, 127266. [Google Scholar] [CrossRef]
- Ramaswamy, P.; Tilleti, P.; Bhattacharjee, S.; Pinto, R.; Gomes, S.A. Synthesis of value added refractories from aluminium dross and zirconia composites. Mater. Today Proc. 2020, 22, 1264–1273. [Google Scholar] [CrossRef]
- Meshram, A.; Singh, K.K. Recovery of valuable products from hazardous aluminum dross: A review. Resour. Conserv. Recycl. 2018, 130, 95–108. [Google Scholar] [CrossRef]
- Liu, N.W.; Chou, M.S. Reduction of secondary aluminum dross by a waste pickling liquor containing ferrous chloride. Sustain. Environ. Res. 2013, 23, 61–67. [Google Scholar]
- Tripathy, A.K.; Mahalik, S.; Sarangi, C.K.; Tripathy, B.C.; Sanjay, K.; Bhattacharya, I.N. A pyro-hydrometallurgical process for the recovery of alumina from waste aluminium dross. Miner. Eng. 2019, 137, 181–186. [Google Scholar] [CrossRef]
- Tsakiridis, P.E. Aluminium salt slag characterization and utilization—A review. J. Hazard. Mater. 2012, 217, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Surul, O.; Bilir, T.; Gholampour, A. Recycle of ground granulated blast furnace slag and fly ash on eco-friendly brick production. Eur. J. Environ. Civ. Eng. 2020, 12, 1–19. [Google Scholar] [CrossRef]
- David, E.; Kopac, J. Aluminum recovery as a product with high added value using aluminum hazardous waste. J. Hazard. Mater. 2013, 261, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Liu, B.; Ekberg, C.; Zhang, S.G. Harmless disposal and resource utilization for secondary aluminum dross: A review. Sci. Total Environ. 2020, 760, 143968. [Google Scholar] [CrossRef]
- López, F.A.; Isabel, M.; Alguacil, F.J.; Ramírez, M.S.; Gonzalez, J. Synthesis of calcium aluminates from non-saline aluminum dross. Materials 2019, 12, 1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, M.; Martín, M.I.; Barberie, L.; Andreola, F.; Lancellotti, I.; Delgado, A.L. Valorisation of Al slag in the production of green ceramic tiles: Effect of experimental conditions on microstructure and crystalline phase composition. J. Am. Ceram. Soc. 2021, 104, 779–784. [Google Scholar] [CrossRef]
- Shen, H.L.; Liu, B.; Zhao, S.Z.; Zhang, J.J.; Yuan, J.S.; Zhang, Y.; Zhang, S. Iron oxide and jadeite nucleation in high alumina glass-ceramics prepared from secondary aluminum dross. Ceram. Int. 2021, 47, 21744–21750. [Google Scholar] [CrossRef]
- Ewais, E.; Besisa, N. Tailoring of magnesium aluminum titanate based ceramics from aluminum dross. Mater. Des. 2018, 141, 110–119. [Google Scholar] [CrossRef]
- El-Amir, A.A.M.; Attia, M.A.A.; Newishy, M.; Fend, T.; Ewais, E.M.M. Aluminium dross/soda lime glass waste-derived high-quality glass foam. J. Mater. Res. Technol. 2021, 15, 4940–4948. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Yu, J.K.; Yang, X.; Jin, E.D.; Yuan, L. Oxidation resistance and wetting behavior of MgO-C refractories: Effect of carbon content. Materials 2018, 11, 883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.J.; Zhou, J.H.; Guan, X.P.; Yang, J.J.; Li, N. Influence of chemical composition on structure and properties of light-burned magnesium-aluminum spinel. Bull. Chin. Ceram. Soc. 2021, 40, 1388–1394. [Google Scholar]
- Abou-Sekkina, M.M.; Abo-El-Enein, S.A.; Khalil, N.M.; Shalma, O.A. Phase composition of bauxite-based refractory castables. Ceram. Int. 2011, 37, 411–418. [Google Scholar] [CrossRef]




| Al2O3 | MgO | SiO2 | CaO | K2O | Na2O | Fe2O3 | TiO2 | MnO | F | |
|---|---|---|---|---|---|---|---|---|---|---|
| Aluminum dross | 82.50 | 8.65 | 1.75 | 2.32 | 0.43 | 1.48 | 0.38 | 0.92 | - | 1.57 |
| Clay | 13.21 | 2.93 | 75.73 | 5.64 | 0.45 | 0.03 | 0.97 | 0.88 | 0.16 | - |
| 70A | 75A | 80A | 85A | 90A | ||
|---|---|---|---|---|---|---|
| Castable aggregate | ADW | 35.0 | 37.5 | 40.0 | 42.5 | 45.0 |
| Clay | 15.0 | 12.5 | 10.0 | 7.5 | 5.0 | |
| Al2O3 powder | 10 | 10 | 10 | 10 | 10 | |
| Silica fume | 5 | 5 | 5 | 5 | 5 | |
| Clay powder | 5 | 5 | 5 | 5 | 5 | |
| ADW powder | 20 | 20 | 20 | 20 | 20 | |
| Calcium aluminate cement | 10 | 10 | 10 | 10 | 10 | |
| 85A | ZJQ120-1.5 | ||
|---|---|---|---|
| Bulk density (g/cm3) | 110 °C × 24 h | 1.41 | ≤1.5 |
| Cold crushing strength (MPa) | 110 °C × 24 h | 15.80 | ≥6 |
| Cold crushing strength (MPa) | 1200 °C × 3 h | 21.50 | ≥15 |
| Thermal conductivity (W·m−1·K−1) | - | 0.325 | ≤0.75 |
| Linear change rate (%) | 1200 °C × 3 h | −0.31 | −0.5~0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, N.; Li, Z.; Ding, Y.; Yang, H.; Zhang, J.; Fu, G. Waste to Wealth Strategy: Preparation and Properties of Lightweight Al2O3-SiO2-Rich Castables Using Aluminum Dross Waste. Materials 2021, 14, 7803. https://doi.org/10.3390/ma14247803
Su N, Li Z, Ding Y, Yang H, Zhang J, Fu G. Waste to Wealth Strategy: Preparation and Properties of Lightweight Al2O3-SiO2-Rich Castables Using Aluminum Dross Waste. Materials. 2021; 14(24):7803. https://doi.org/10.3390/ma14247803
Chicago/Turabian StyleSu, Nan, Zishen Li, Youdong Ding, Hongliang Yang, Jingzhou Zhang, and Gaofeng Fu. 2021. "Waste to Wealth Strategy: Preparation and Properties of Lightweight Al2O3-SiO2-Rich Castables Using Aluminum Dross Waste" Materials 14, no. 24: 7803. https://doi.org/10.3390/ma14247803





