Revealing the Microstructural Aspects of the Corrosion Dynamics in Rapidly Solidified Mg-Zn-Y Alloys Using the Acoustic Emission Technique
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
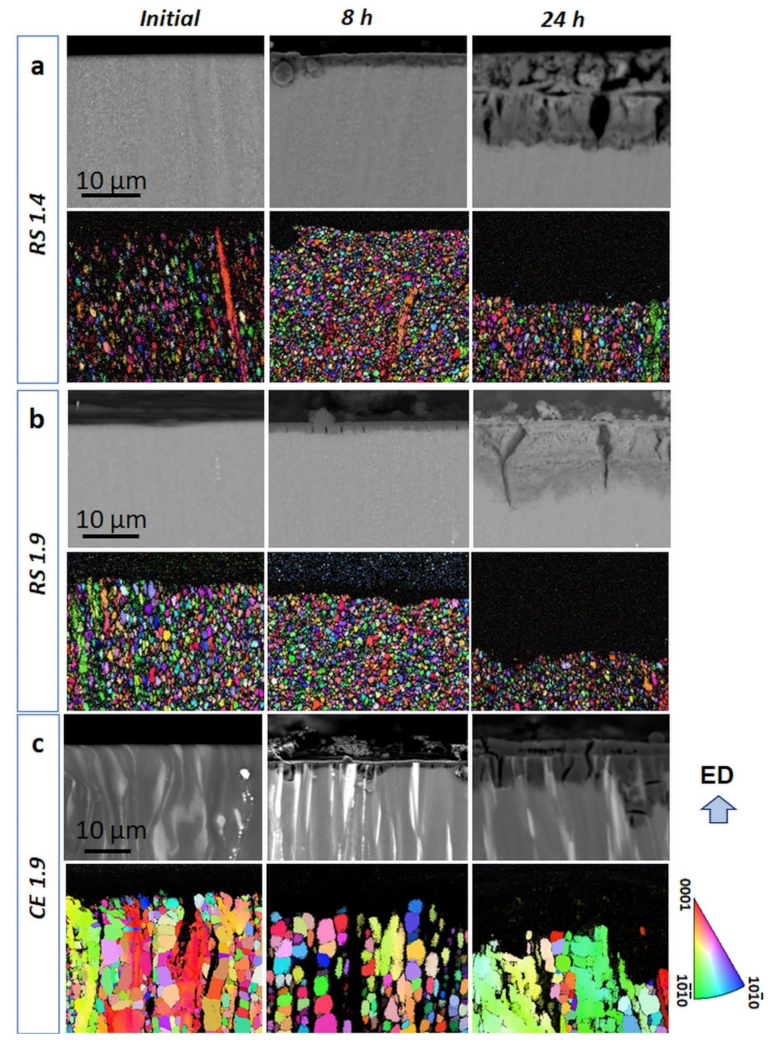
- Material processing significantly influences the microstructure. The application of the rapid solidification technique leads to the formation of ultrafine-grained (700–1200 nm) material with the solute segregated stacking faults dispersedly distributed in the grain interior. The rate of consolidation extrusion influences the dynamic recrystallization and leads to a difference in the internal strain distribution. In contrast, large LPSO laths form in the cast-extruded alloys.
- The RS ribbon-consolidated alloy extruded with a metal flow rate of 1.4 s−1 is characterized by a homogeneous distribution of the internal strain. At a higher metal flow rate (1.9 s−1), the distribution of the internal strain becomes uneven with higher values in worked non-dynamically recrystallized (DRX) grains than those in DRX grains.
- The presence of LPSO laths in the extruded alloy leads to severe anodic dissolution in the α-Mg grains next to the LPSO phase. In the RS alloys without the LPSO laths and, thus, suppressing the effect of potential difference, the strain inhomogeneity in the microstructure became more important for corrosion behavior.
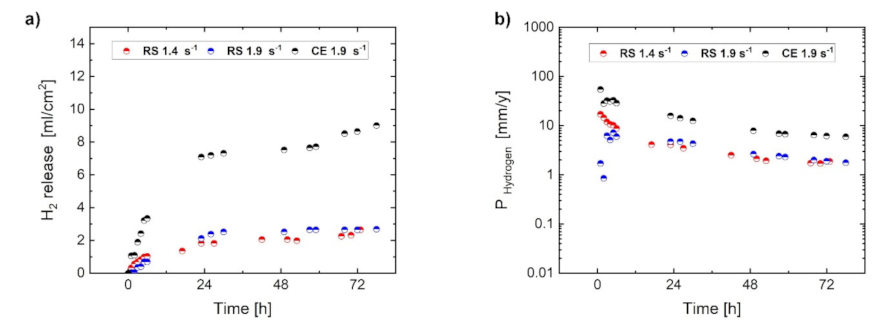
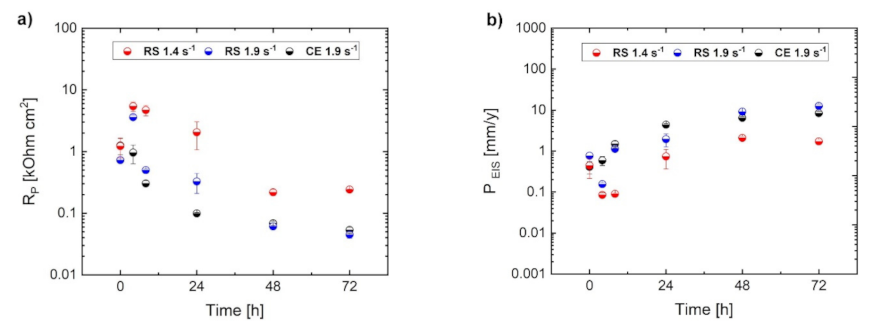
- An uneven distribution of the internal strain results in inhomogeneous development of the corrosion layer even in ultra-fine-grained material. Despite similar values of H2 release for RS materials, the instantaneous corrosion rate estimated from electrochemical measurements for the RS 1.9 specimen is slightly higher than that for the RS 1.4 specimen. This is given by the presence of strain inhomogeneity in the microstructure of RS 1.9, while the RS 1.4 specimen with even internal strain distribution exhibits a homogeneous formation and growth of the corrosion layer.
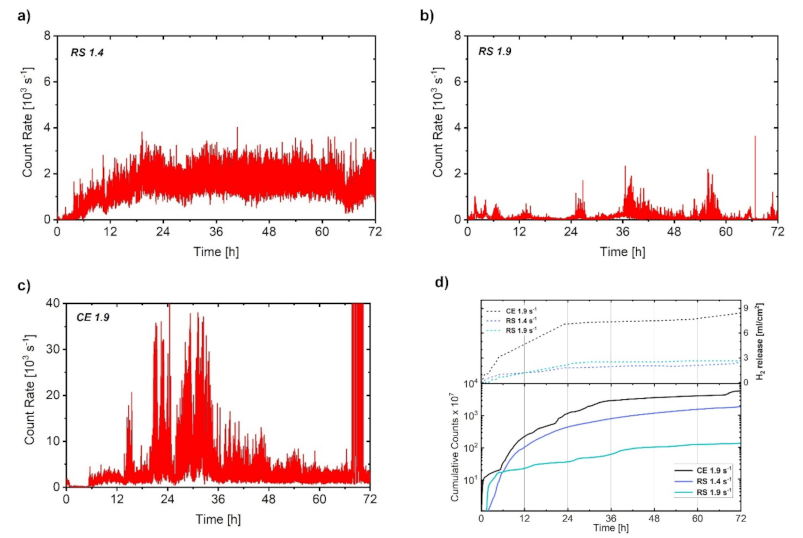
- The AE method was found to be capable of revealing the dynamics of the corrosion process. The uniform corrosion of the RS 1.4 was evidenced by a steady-level AE signal. In contrast, the AE response of the RS 1.9 and CE 1.9 specimens has an impulsive character, indicating inhomogeneous formation and breakdown of the corrosion layer.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adekanmbi, I.; Mosher, C.Z.; Lu, H.H.; Riehle, M.; Kubba, H.; Tanner, K.E. Mechanical behaviour of biodegradable AZ31 magnesium alloy after long term in vitro degradation. Mater. Sci. Eng. C 2017, 77, 1135–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brar, H.S.; Platt, M.O.; Sarntinoranont, M.; Martin, P.I.; Manuel, M.V. Magnesium as a biodegradable and bioabsorbable material for medical implants. JOM 2009, 61, 31–34. [Google Scholar] [CrossRef]
- Atrens, A.; Shi, Z.; Mehreen, S.U.; Johnston, S.; Song, G.-L.; Chen, X.; Pan, F. Review of Mg alloy corrosion rates. J. Magnes. Alloy. 2020, 8, 989–998. [Google Scholar] [CrossRef]
- Inoue, A.; Kawamura, Y.; Matsushita, M.; Hayashi, K.; Koike, J. Novel hexagonal structure and ultrahigh strength of magnesium solid solution in the Mg–Zn–Y system. J. Mater. Res. 2001, 16, 1894–1900. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, Y.; Hayashi, K.; Inoue, A.; Masumoto, T. Rapidly Solidified Powder Metallurgy Mg97Zn1Y2 Alloys with Excellent Tensile Yield Strength above 600 MPa. Mater. Trans. 2001, 42, 1172–1176. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, Y.; Kasahara, T.; Izumi, S.; Yamasaki, M. Elevated temperature Mg97Y2Cu1 alloy with long period ordered structure. Scr. Mater. 2006, 55, 453–456. [Google Scholar] [CrossRef]
- Yamasaki, M.; Hashimoto, K.; Hagihara, K.; Kawamura, Y. Effect of multimodal microstructure evolution on mechanical properties of Mg–Zn–Y extruded alloy. Acta Mater. 2011, 59, 3646–3658. [Google Scholar] [CrossRef]
- Abe, E.; Ono, A.; Itoi, T.; Yamasaki, M.; Kawamura, Y. Polytypes of long-period stacking structures synchronized with chemical order in a dilute Mg–Zn–Y alloy. Philos. Mag. Lett. 2011, 91, 690–696. [Google Scholar] [CrossRef]
- Egusa, D.; Abe, E. The structure of long period stacking/order Mg–Zn–RE phases with extended non-stoichiometry ranges. Acta Mater. 2012, 60, 166–178. [Google Scholar] [CrossRef]
- Garces, G.; Perez, P.; Cabeza, S.; Lin, H.K.; Kim, S.; Gan, W.; Adeva, P. Reverse tension/compression asymmetry of a Mg–Y–Zn alloys containing LPSO phases. Mater. Sci. Eng. A 2015, 647, 287–293. [Google Scholar] [CrossRef]
- Garces, G.; Muñoz-Morris, M.A.; Morris, D.G.; Jimenez, J.A.; Perez, P.; Adeva, P. The role of extrusion texture on strength and its anisotropy in a Mg-base alloy composed of the Long-Period-Structural-Order phase. Intermetallics 2014, 55, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Garcés, G.; Máthis, K.; Medina, J.; Horváth, K.; Drozdenko, D.; Oñorbe, E.; Dobroň, P.; Pérez, P.; Klaus, M.; Adeva, P. Combination of in-situ diffraction experiments and acoustic emission testing to understand the compression behavior of Mg-Y-Zn alloys containing LPSO phase under different loading conditions. Int. J. Plast. 2018, 106, 107–128. [Google Scholar] [CrossRef] [Green Version]
- Hagihara, K.; Kinoshita, A.; Sugino, Y.; Yamasaki, M.; Kawamura, Y.; Yasuda, H.Y.; Umakoshi, Y. Effect of long-period stacking ordered phase on mechanical properties of Mg97Zn1Y2 extruded alloy. Acta Mater. 2010, 58, 6282–6293. [Google Scholar] [CrossRef]
- Horváth, K.; Drozdenko, D.; Daniš, S.; Garcés, G.; Máthis, K.; Kim, S.; Dobroň, P. Characterization of Microstructure and Mechanical Properties of Mg–Y–Zn Alloys with Respect to Different Content of LPSO Phase. Adv. Eng. Mater. 2018, 20, 1700396. [Google Scholar] [CrossRef]
- Horváth, K.; Drozdenko, D.; Garcés, G.; Dobroň, P.; Máthis, K. Characterization of the acoustic emission response and mechanical properties of Mg alloy with LPSO phase. Mater. Sci. Forum 2017, 879, 762–766. [Google Scholar] [CrossRef]
- Hagihara, K.; Yokotani, N.; Umakoshi, Y. Plastic deformation behavior of Mg12YZn with 18R long-period stacking ordered structure. Intermetallics 2010, 18, 267–276. [Google Scholar] [CrossRef]
- Oñorbe, E.; Garcés, G.; Pérez, P.; Adeva, P. Effect of the LPSO volume fraction on the microstructure and mechanical properties of Mg–Y2X –Zn X alloys. J. Mater. Sci. 2012, 47, 1085–1093. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, M.; Shi, Z.; Atrens, A.; Furukawa, A.; Kawamura, Y. Influence of crystallographic orientation and Al alloying on the corrosion behavior of extruded a-Mg/LPSO two-phase Mg-Zn-Y alloys with multimodal microstructure. Corros. Sci. 2021. under review. [Google Scholar]
- Makar, G.L.; Kruger, J. Corrosion of magnesium. Int. Mater. Rev. 1993, 38, 138–153. [Google Scholar] [CrossRef]
- Hanawalt, J.D.; Nelson, C.E.; Peloubet, J.A. Corrosion studies of magnesium and its alloys. Trans. AIME 1942, 147, 273–299. [Google Scholar]
- Krajňák, T.; Minárik, P.; Stráská, J.; Gubicza, J.; Máthis, K.; Janeček, M. Influence of the initial state on the microstructure and mechanical properties of AX41 alloy processed by ECAP. J. Mater. Sci. 2019, 54, 3469–3484. [Google Scholar] [CrossRef]
- Krajňák, T.; Minárik, P.; Stráský, J.; Máthis, K.; Janeček, M. Mechanical properties of ultrafine-grained AX41 magnesium alloy at room and elevated temperatures. Mater. Sci. Eng. A 2018, 731, 438–445. [Google Scholar] [CrossRef]
- Minárik, P.; Jablonská, E.; Král, R.; Lipov, J.; Ruml, T.; Blawert, C.; Hadzima, B.; Chmelík, F. Effect of equal channel angular pressing on in vitro degradation of LAE442 magnesium alloy. Mater. Sci. Eng. C 2017, 73, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Minárik, P.; Král, R.; Čížek, J.; Chmelík, F. Effect of different c/a ratio on the microstructure and mechanical properties in magnesium alloys processed by ECAP. Acta Mater. 2016, 107, 83–95. [Google Scholar] [CrossRef]
- Minárik, P.; Veselý, J.; Král, R.; Bohlen, J.; Kubásek, J.; Janeček, M.; Stráská, J. Exceptional mechanical properties of ultra-fine grain Mg-4Y-3RE alloy processed by ECAP. Mater. Sci. Eng. A 2017, 708, 193–198. [Google Scholar] [CrossRef]
- Okouchi, H.; Seki, Y.; Sekigawa, T.; Hira, H.; Kawamura, Y. Nanocrystalline LPSO Mg-Zn-Y-Al Alloys with High Mechanical Strength and Corrosion Resistance. Mater. Sci. Forum 2010, 638, 1476–1481. [Google Scholar] [CrossRef]
- Garces, G.; Cabeza, S.; Barea, R.; Pérez, P.; Adeva, P. Maintaining High Strength in Mg-LPSO Alloys with Low Yttrium Content Using Severe Plastic Deformation. Materials 2018, 11, 733. [Google Scholar] [CrossRef] [Green Version]
- Suzawa, K.; Inoue, S.-i.; Yamasaki, M.; Kawamura, Y.; Miyanaga, M.; Yoshida, K.; Kawabe, N. Superplasticity in a Chip-Consolidated Mg97Zn1Y2 Alloy with LPSO Phase. Magnes. Technol. 2018, 1, 245–249. [Google Scholar]
- Yang, Y.; Chen, X.; Nie, J.; Wei, K.; Mao, Q.; Lu, F.; Zhao, Y. Achieving ultra-strong Magnesium–lithium alloys by low-strain rotary swaging. Mater. Res. Lett. 2021, 9, 255–262. [Google Scholar] [CrossRef]
- Trojanová, Z.; Drozd, Z.; Halmešová, K.; Džugan, J.; Škraban, T.; Minárik, P.; Németh, G.; Lukáč, P. Strain Hardening in an AZ31 Alloy Submitted to Rotary Swaging. Materials 2021, 14, 157. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, M.; Izumi, S.; Kawamura, Y. Development of High Strength and Highly Corrosion-Resistant Bulk Nanocrystalline Mg-Zn-Y Alloys with Long Period Stacking Ordered Phase. ECS Trans. 2009, 16, 81–88. [Google Scholar] [CrossRef]
- Yamasaki, M.; Izumi, S.; Kawamura, Y.; Habazaki, H. Corrosion and passivation behavior of Mg–Zn–Y–Al alloys prepared by cooling rate-controlled solidification. Appl. Surf. Sci. 2011, 257, 8258–8267. [Google Scholar] [CrossRef]
- Yamasaki, M.; Hayashi, N.; Izumi, S.; Kawamura, Y. Corrosion behavior of rapidly solidified Mg–Zn–rare earth element alloys in NaCl solution. Corros. Sci. 2007, 49, 255–262. [Google Scholar] [CrossRef]
- Jono, Y.; Yamasaki, M.; Kawamura, Y. Effect of LPSO Phase-Stimulated Texture Evolution on Creep Resistance of Extruded Mg–Zn–Gd Alloys. Mater. Trans. 2013, 54, 703–712. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, J.; Xu, C.; Jing, Y.; Zhuang, J.; Wu, R.; Zhang, M. Formation of stacking faults for improving the performance of biodegradable Mg–Ho–Zn alloy. Mater. Lett. 2014, 133, 158–162. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, J.; Kong, P.; Zhang, Z.; Jing, Y.; Zhuang, J.; Wang, W.; Zhang, L.; Xu, C.; Wu, R.; et al. Enhancing the performance of Mg-based implant materials by introducing basal plane stacking faults. J. Mater. Chem. B 2015, 3, 7386–7400. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, C.; Jing, Y.; Lv, S.; Liu, S.; Fang, D.; Zhuang, J.; Zhang, M.; Wu, R. New horizon for high performance Mg-based biomaterial with uniform degradation behavior: Formation of stacking faults. Sci. Rep. 2015, 5, 13933. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Kairy, S.K.; Dai, J.; Birbilis, N. A Closer Look at the Role of Nanometer Scale Solute-Rich Stacking Faults in the Localized Corrosion of a Magnesium Alloy GZ31K. J. Electrochem. Soc. 2018, 165, C310–C316. [Google Scholar] [CrossRef]
- Izumi, S.; Yamasaki, M.; Kawamura, Y. Relation between corrosion behavior and microstructure of Mg–Zn–Y alloys prepared by rapid solidification at various cooling rates. Corros. Sci. 2009, 51, 395–402. [Google Scholar] [CrossRef]
- Izumi, S.; Yamasaki, M.; Kawamura, Y. Influence of cooling rate on corrosion resistance of rapidly solidified Mg-Zn-Y alloys with a long period stacking ordered phase. ECS Trans. 2009, 16, 65–72. [Google Scholar] [CrossRef]
- Nie, Y.; Dai, J.; Li, X.; Zhang, X. Recent developments on corrosion behaviors of Mg alloys with stacking fault or long period stacking ordered structures. J. Magnes. Alloys 2021, 9, 1123–1146. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, J.; Dong, Q.; Ba, Z.; Wu, Y. Corrosion behavior and mechanical degradation of as-extruded Mg–Gd–Zn–Zr alloys for orthopedic application. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2020, 108, 698–708. [Google Scholar] [CrossRef]
- Drozdenko, D.; Yamasaki, M.; Máthis, K.; Dobroň, P.; Lukáč, P.; Kizu, N.; Inoue, S.-I.; Kawamura, Y. Optimization of mechanical properties of dilute Mg-Zn-Y alloys prepared by rapid solidification. Mater. Des. 2019, 181, 107984. [Google Scholar] [CrossRef]
- Knapek, M.; Minárik, P.; Čapek, J.; Král, R.; Kubásek, J.; Chmelík, F. Corrosion of pure magnesium and a WE43 magnesium alloy studied by advanced acoustic emission analysis. Corros. Sci. 2018, 145, 10–15. [Google Scholar] [CrossRef]
- Yuyama, S. Fundamental Aspects of Acoustic Emission Applications to the Problems Caused by Corrosion; Moran, G.C., Labine, P., Eds.; ASTM International: West Conshohocken, PA, USA, 1986; pp. 43–74. [Google Scholar]
- Zhang, Z.; Zhao, Z.; Bai, P.; Li, X.; Liu, B.; Tan, J.; Wu, X. In-situ monitoring of pitting corrosion of AZ31 magnesium alloy by combining electrochemical noise and acoustic emission techniques. J. Alloy. Compd. 2021, 878, 160334. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Kim, J.-Y.; Lee, S.-M.; Byeon, J.-W. Evaluation of Pitting Corrosion Mechanism of AZ31 Magnesium Alloy by Monitoring Acoustic Emission. Mat. Trans. 2017, 58, 123–126. [Google Scholar] [CrossRef] [Green Version]
- Minarik, P.; Landa, M.; Lesna, I.K.; Zemkova, M.; Jablonska, E.; Hadzima, B.; Janecek, M.; Kral, R. Interrelation of Microstructure and Corrosion Resistance in Biodegradable Magnesium Alloys with Aluminum, Lithium and Rare Earth Additions. Acta Phys. Pol. A 2015, 128, 491–496. [Google Scholar] [CrossRef]
- Hirano, M.; Yamasaki, M.; Hagihara, K.; Higashida, K.; Kawamura, Y. Effect of Extrusion Parameters on Mechanical Properties of Mg97Zn1Y2 Alloys at Room and Elevated Temperatures. Mater. Trans. 2010, 51, 1640–1647. [Google Scholar] [CrossRef] [Green Version]
- Field, D.P.; Merriman, C.C.; Allain-Bonasso, N.; Wagner, F. Quantification of dislocation structure heterogeneity in deformed polycrystals by EBSD. Model. Simul. Mater. Sci. Eng. 2012, 20, 024007. [Google Scholar] [CrossRef]
- King, A.D.; Birbilis, N.; Scully, J.R. Accurate Electrochemical Measurement of Magnesium Corrosion Rates; a Combined Impedance, Mass-Loss and Hydrogen Collection Study. Electrochim. Acta 2014, 121, 394–406. [Google Scholar] [CrossRef]
- Baril, G.; Galicia, G.; Deslouis, C.; Pébère, N.; Tribollet, B.; Vivier, V. An Impedance Investigation of the Mechanism of Pure Magnesium Corrosion in Sodium Sulfate Solutions. J. Electrochem. Soc. 2007, 154, C108–C113. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Yang, Q.; Javed, M.S.; Gong, Y.; Aslam, M.K.; Chen, C. The effects of NaF concentration on electrochemical and corrosion behavior of AZ31B magnesium alloy in a composite electrolyte. RSC Adv. 2017, 7, 5880–5887. [Google Scholar] [CrossRef] [Green Version]
- Stern, M.; Geaby, A.L. Electrochemical Polarization: I. A Theoretical Analysis of the Shape of Polarization Curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Levenberg, K. A method for the solution of certain non-linear problems in least squares. Q. Appl. Math. 1944, 2, 164–168. [Google Scholar] [CrossRef] [Green Version]
- Marquardt, D.W. An Algorithm for Least-Squares Estimation of Nonlinear Parameters. J. Soc. Ind. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- ASTM E750-04. Standard Practice for Characterizing Acoustic Emission Instrumentation. In Book of Standards; ASTM International: West Conshohocken, PA, USA, 2004. [Google Scholar]
- Mayama, T.; Noda, M.; Chiba, R.; Kuroda, M. Crystal plasticity analysis of texture development in magnesium alloy during extrusion. Int. J. Plast. 2011, 27, 1916–1935. [Google Scholar] [CrossRef] [Green Version]
- Sillekens, W.H.; Bohlen, J. Hydrostatic Extrusion of Magnesium Alloys; Woodhead Publishing: Sawston, UK, 2012. [Google Scholar]
- Garcés, G.; Requena, G.; Tolnai, D.; Pérez, P.; Adeva, P.; Stark, A.; Schell, N. Influence of rare-earth addition on the long-period stacking ordered phase in cast Mg–Y–Zn alloys. J. Mater. Sci. 2014, 49, 2714–2722. [Google Scholar] [CrossRef] [Green Version]
- Horvath, K.; Drozdenko, D.; Garces, G.; Mathis, K.; Dobron, P. Effect of Extrusion Ratio on Microstructure and Resulting Mechanical Properties of Mg Alloys with LPSO Phase. In Magnesium Technology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 29–34. [Google Scholar]
- Yamasaki, M.; Anan, T.; Yoshimoto, S.; Kawamura, Y. Mechanical properties of warm-extruded Mg–Zn–Gd alloy with coherent 14H long periodic stacking ordered structure precipitate. Scr. Mater. 2005, 53, 799–803. [Google Scholar] [CrossRef]
- Li, X.; Jiang, J.-H.; Zhao, Y.-H.; Ma, A.-B.; Wen, D.-J.; Zhu, Y.-T. Effect of equal-channel angular pressing and aging on corrosion behavior of ZK60 Mg alloy. Trans. Nonferrous Met. Soc. China 2015, 25, 3909–3920. [Google Scholar] [CrossRef]
- Song, D.; Ma, A.; Jiang, J.; Lin, P.; Yang, D.; Fan, J. Corrosion behavior of equal-channel-angular-pressed pure magnesium in NaCl aqueous solution. Corros. Sci. 2010, 52, 481–490. [Google Scholar] [CrossRef]



| Alloy | Mg | Zn | Y | Fe | Co | Ni | Cu |
|---|---|---|---|---|---|---|---|
| (at.%) | (at.%) | (at.%) | (ppm) | (ppm) | (ppm) | (ppm) | |
| Mg97.94Zn0.56Y1.5 | Bal. | 0.56 | 1.5 | 11 | – | 2 | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drozdenko, D.; Yamasaki, M.; Máthis, K.; Dobroň, P.; Inoue, S.-i.; Kawamura, Y. Revealing the Microstructural Aspects of the Corrosion Dynamics in Rapidly Solidified Mg-Zn-Y Alloys Using the Acoustic Emission Technique. Materials 2021, 14, 7828. https://doi.org/10.3390/ma14247828
Drozdenko D, Yamasaki M, Máthis K, Dobroň P, Inoue S-i, Kawamura Y. Revealing the Microstructural Aspects of the Corrosion Dynamics in Rapidly Solidified Mg-Zn-Y Alloys Using the Acoustic Emission Technique. Materials. 2021; 14(24):7828. https://doi.org/10.3390/ma14247828
Chicago/Turabian StyleDrozdenko, Daria, Michiaki Yamasaki, Kristián Máthis, Patrik Dobroň, Shin-ichi Inoue, and Yoshihito Kawamura. 2021. "Revealing the Microstructural Aspects of the Corrosion Dynamics in Rapidly Solidified Mg-Zn-Y Alloys Using the Acoustic Emission Technique" Materials 14, no. 24: 7828. https://doi.org/10.3390/ma14247828






