Microstructure Evolution of FeNiCoCrAl1.3Mo0.5 High Entropy Alloy during Powder Preparation, Laser Powder Bed Fusion, and Microplasma Spraying
Abstract
:1. Introduction
2. Materials and Methods
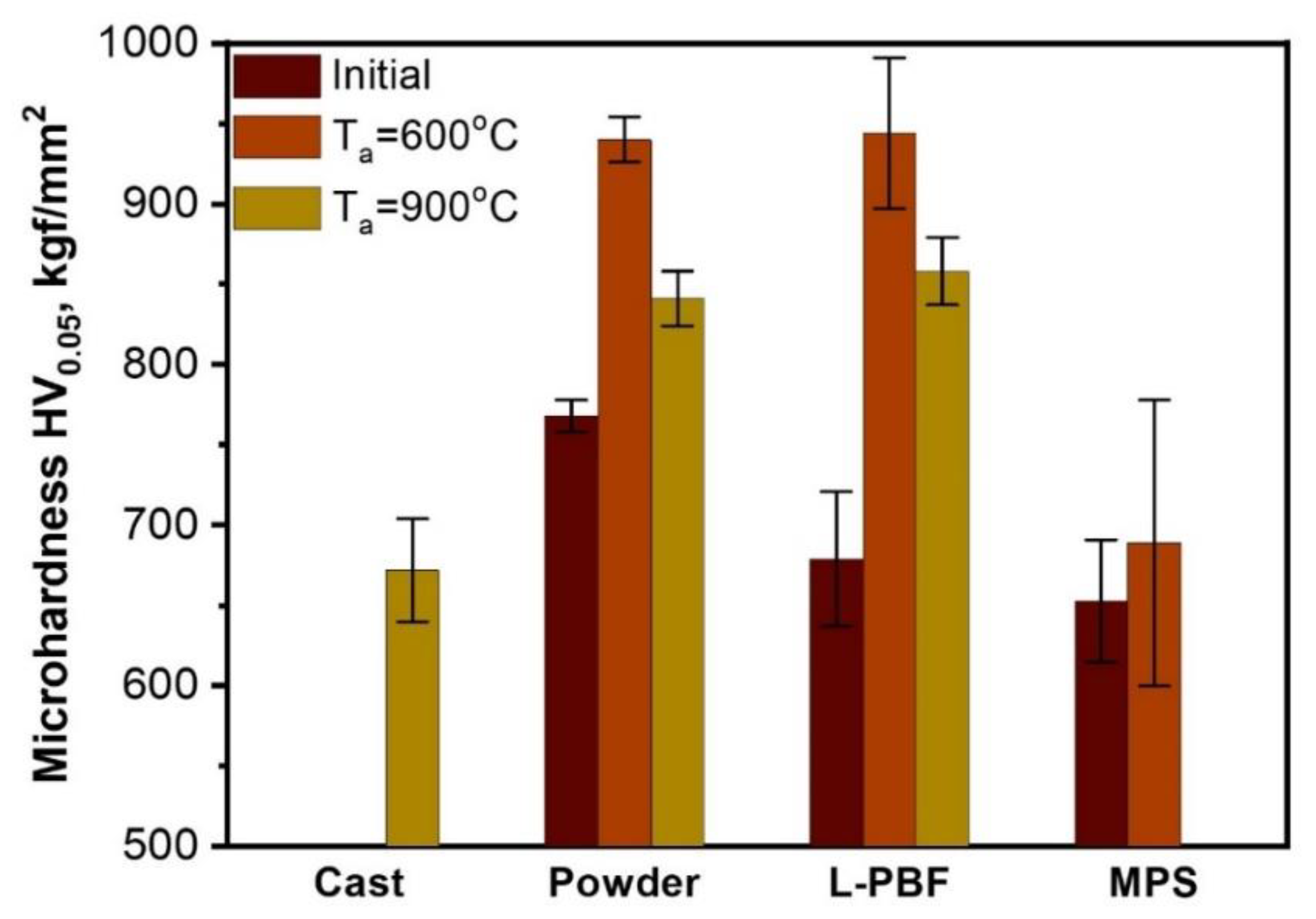
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Fang, W.; Yu, H.; Chang, R.; Zhang, X.; Ji, P.; Liu, B.; Li, J.; Qu, X.; Liu, Y.; Yin, F. Microstructure and mechanical properties of Cr-rich Co-Cr-Fe-Ni high entropy alloys designed by valence electron oncentration. Mater. Chem. Phys. 2019, 238, 121897. [Google Scholar] [CrossRef]
- Oh, S.M.; Hong, S.I. Microstructure and mechanical properties of equitomic CoCrFeCuNi high entropy alloy. Key Eng. Mater. 2018, 765, 149–154. [Google Scholar] [CrossRef]
- Chou, H.P.; Chang, Y.S.; Chen, S.K.; Yeh, J.W. Microstructure, thermophysical and electrical properties in AlxCoCrFeNi (0 ≤ x ≤2) high-entropy alloys. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2009, 163, 184–189. [Google Scholar] [CrossRef]
- Shun, T.T.; Chang, L.Y.; Shiu, M.H. Microstructures and mechanical properties of multiprincipal component CoCrFeNiTix alloys. Mater. Sci. Eng. A 2012, 556, 170–174. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Chen, Z.; Chen, Y.Z.; Shi, J.C.; Wang, Z.Y.; Wang, S.; Liu, F. Effect of Al content on high temperature oxidation resistance of AlxCoCrCuFeNi high entropy alloys (x=0, 0.5, 1, 1.5, 2). Vacuum 2019, 169, 108837. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Y.; Jin, X.; Du, X.; Li, B. Precipitation-hardened high entropy alloys with excellent tensile properties. Mater. Sci. Eng. A 2018, 732, 186–191. [Google Scholar] [CrossRef]
- Zhang, L.J.; Zhang, M.D.; Zhou, Z.; Fan, J.T.; Cui, P.; Yu, P.F.; Jing, Q.; Ma, M.Z.; Liaw, P.K.; Li, G.; et al. Effects of rare-earth element, Y, additions on the microstructure and mechanical properties of CoCrFeNi high entropy alloy. Mater. Sci. Eng. A 2018, 725, 437–446. [Google Scholar] [CrossRef] [Green Version]
- Fan, A.C.; Li, J.H.; Tsai, M.H. On the phase constituents of three CoCrFeNiX (X = V, Nb, Ta) high-entropy alloys after prolonged annealing. J. Alloys Compd. 2020, 823, 153524. [Google Scholar] [CrossRef]
- Li, C.; Ma, Y.; Hao, J.; Yan, Y.; Wang, Q.; Dong, C.; Liaw, P.K. Microstructures and mechanical properties of body-centered-cubic (Al,Ti)0.7(Ni,Co,Fe,Cr)5 high entropy alloys with coherent B2/L21 nanoprecipitation. Mater. Sci. Eng. A 2018, 737, 286–296. [Google Scholar] [CrossRef]
- Qin, G.; Chen, R.; Zheng, H.; Fang, H.; Wang, L.; Su, Y.; Guo, J.; Fu, H. Strengthening FCC-CoCrFeMnNi high entropy alloys by Mo addition. J. Mater. Sci. Technol. 2019, 35, 578–583. [Google Scholar] [CrossRef]
- Ma, H.; Shek, C.H. Effects of Hf on the microstructure and mechanical properties of CoCrFeNi high entropy alloy. J. Alloys Compd. 2020, 827, 154159. [Google Scholar] [CrossRef]
- Cai, Y.; Zhu, L.; Cui, Y.; Han, J. Manufacturing of FeCoCrNi + FeCoCrNiAl laminated high-entropy alloy by laser melting deposition (LMD). Mater. Lett. 2021, 289, 129445. [Google Scholar] [CrossRef]
- Yuan, B.; Dong, Y.; Li, C.; Yang, Y.; Zhang, P. Excellent strengthening effect of L12 precipitates on the selective laser melting Al0.3CoCrFeNiCu high entropy alloy via annealing treatment. Mater. Lett. 2021, 304, 130628. [Google Scholar] [CrossRef]
- Luo, S.; Gao, P.; Yu, H.; Yang, J.; Wang, Z.; Zeng, X. Selective laser melting of an equiatomic AlCrCuFeNi high-entropy alloy: Processability, non-equilibrium microstructure and mechanical behavior. J. Alloys Compd. 2019, 771, 387–397. [Google Scholar] [CrossRef]
- Chen, L.; Bobzin, K.; Zhou, Z.; Zhao, L.; Öte, M.; Königstein, T.; Tan, Z.; He, D. Wear behavior of HVOF-sprayed Al0.6TiCrFeCoNi high entropy alloy coatings at different temperatures. Surf. Coatings Technol. 2019, 358, 215–222. [Google Scholar] [CrossRef]
- Anupam, A.; Kottada, R.S.; Kashyap, S.; Meghwal, A.; Murty, B.S.; Berndt, C.C.; Ang, A.S.M. Understanding the microstructural evolution of high entropy alloy coatings manufactured by atmospheric plasma spray processing. Appl. Surf. Sci. 2020, 505, 144117. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.; Yan, S.; Yu, G.; Chen, J.; He, J.; Yin, F. Microstructure evolution and mechanical properties of atmosphere plasma sprayed AlCoCrFeNi high-entropy alloy coatings under post-annealing. J. Alloys Compd. 2021, 872, 159607. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Lampke, T. High-temperature wear behaviour of AlCoCrFeNiTi0.5 coatings produced by HVOF. Surf. Coatings Technol. 2020, 403, 126379. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Al Rashid, A.; Ur Rehman, E.; Atif, M. Laser deposition of high-entropy alloys: A comprehensive review. Opt. Laser Technol. 2022, 145, 107447. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Juan, C.C.; Sheu, T.S.; Chen, S.K.; Yeh, J.W. Effect of aluminum content on microstructure and mechanical properties of AlxCoCrFeMo0.5Ni high-entropy alloys. Jom 2013, 65, 1840–1847. [Google Scholar] [CrossRef]
- Juan, C.C.; Hsu, C.Y.; Tsai, C.W.; Wang, W.R.; Sheu, T.S.; Yeh, J.W.; Chen, S.K. On microstructure and mechanical performance of AlCoCrFeMo0.5Nix high-entropy alloys. Intermetallics 2013, 32, 401–407. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Sheu, T.S.; Yeh, J.W.; Chen, S.K. Effect of iron content on wear behavior of AlCoCrFexMo0.5Ni high-entropy alloys. Wear 2010, 268, 653–659. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Juan, C.C.; Wang, W.R.; Sheu, T.S.; Yeh, J.W.; Chen, S.K. On the superior hot hardness and softening resistance of AlCoCrxFeMo0,5Ni high-entropy alloys. Mater. Sci. Eng. A 2011, 528, 3581–3588. [Google Scholar] [CrossRef]
- Semikolenov, A.; Shalnova, S.; Klinkov, V.; Andreeva, V.; Salynova, M.; Larionova, T.; Tolochko, O. Effect of Al Content on Phase Compositions of FeNiCoCrMo0.5Alx High Entropy Alloy. Metals 2021, 11, 1734. [Google Scholar] [CrossRef]
- Gavrikov, I.S.; Chernyshev, B.D.; Kamynin, A.V.; Zhukov, A.S.; Chernyshev, D.L.; Kuznetsov, P.A. Fabrication of Powders of Alloy 25Kh15KA for Synthesizing Permanent Magnets by Selective Laser Melting. Met. Sci. Heat Treat. 2020, 62, 502–507. [Google Scholar] [CrossRef]
- Bobkova, T.I.; Chernysh, A.A.; Masailo, A.A.; Deev, A.A.; Klimov, V.N.; Yurkov, M.A. Structure and properties of the bronze-based functional coating obtained by gas-dynamic and microplasma spraying. Inorg. Mater. Appl. Res. 2017, 8, 861–869. [Google Scholar] [CrossRef]
- Cheng, K.C.; Chen, J.H.; Stadler, S.; Chen, S.H. Properties of atomized AlCoCrFeNi high-entropy alloy powders and their phase-adjustable coatings prepared via plasma spray process. Appl. Surf. Sci. 2019, 478, 478–486. [Google Scholar] [CrossRef]
- Evans, H.E.; Taylor, M.P. Diffusion cells and chemical failure of MCrAlY bond coats in thermal-barrier coating systems. Oxid. Met. 2001, 55, 17–34. [Google Scholar] [CrossRef]
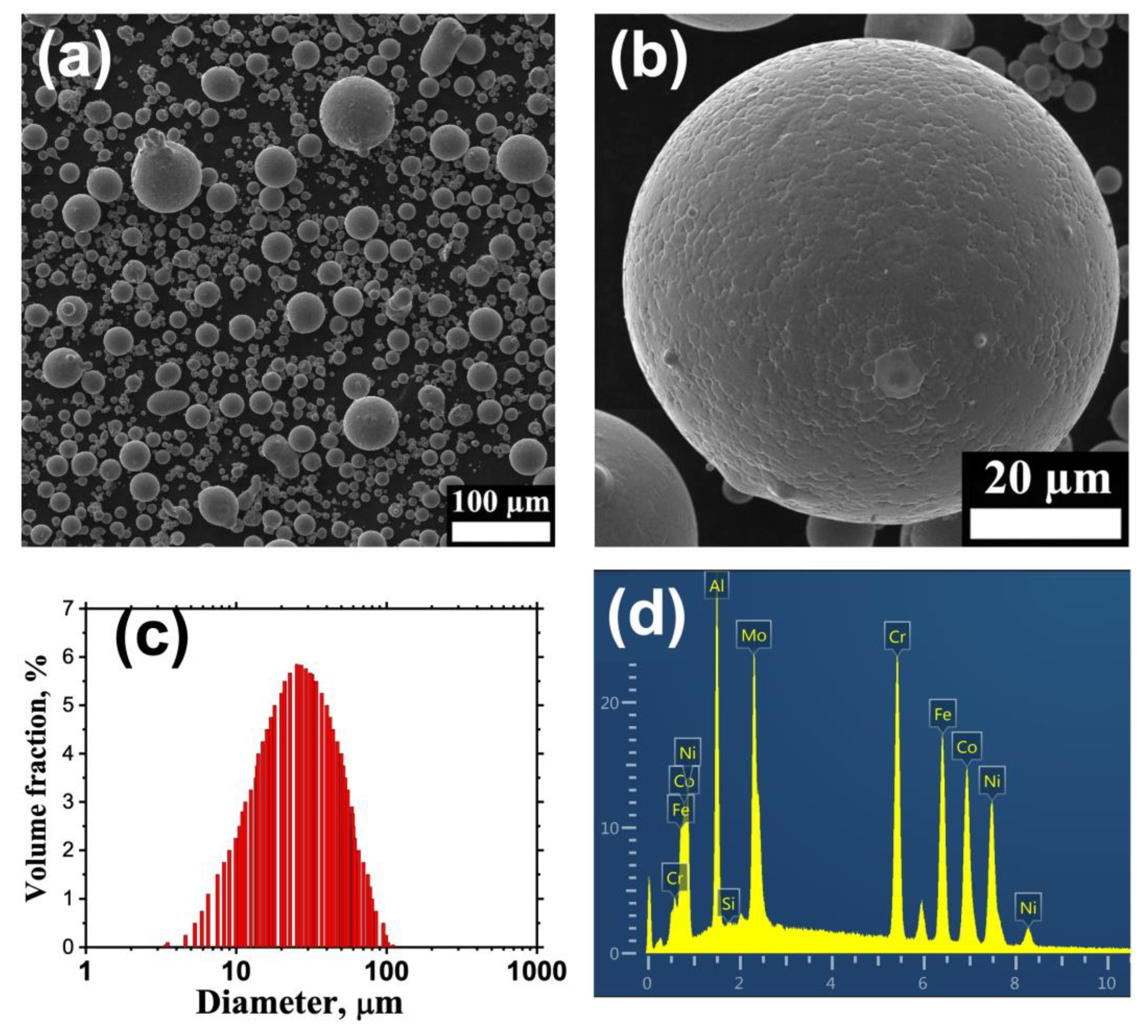
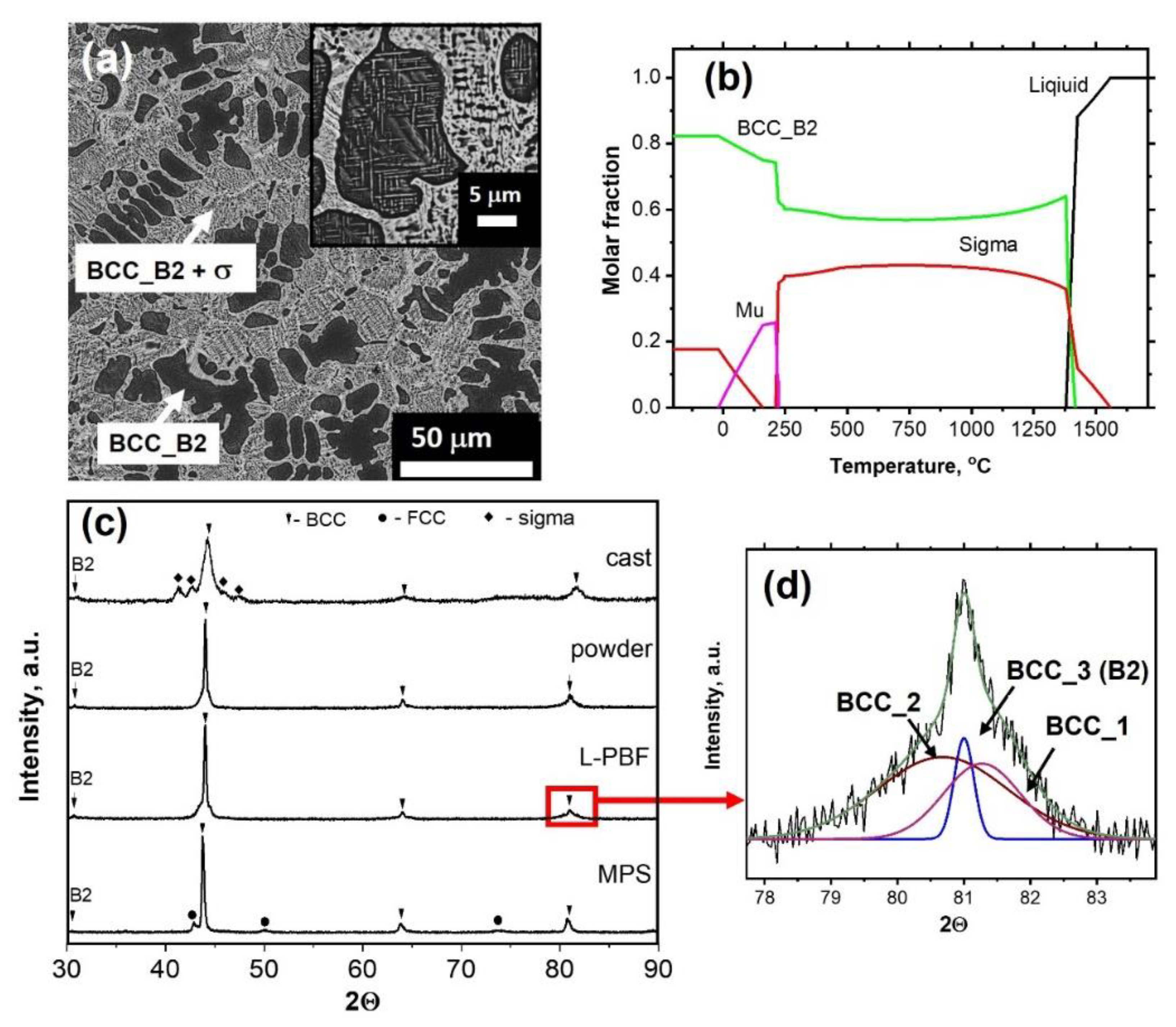
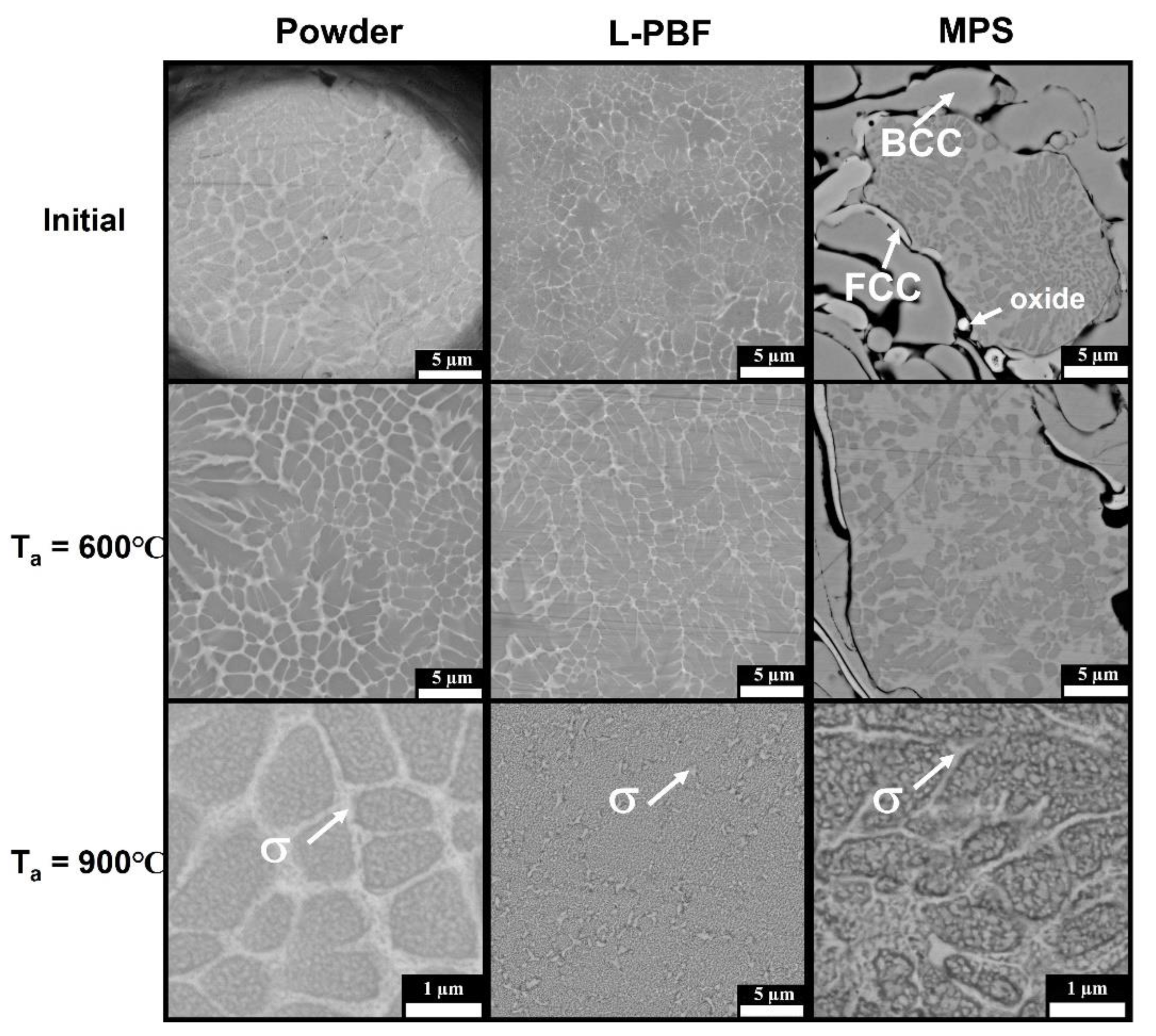

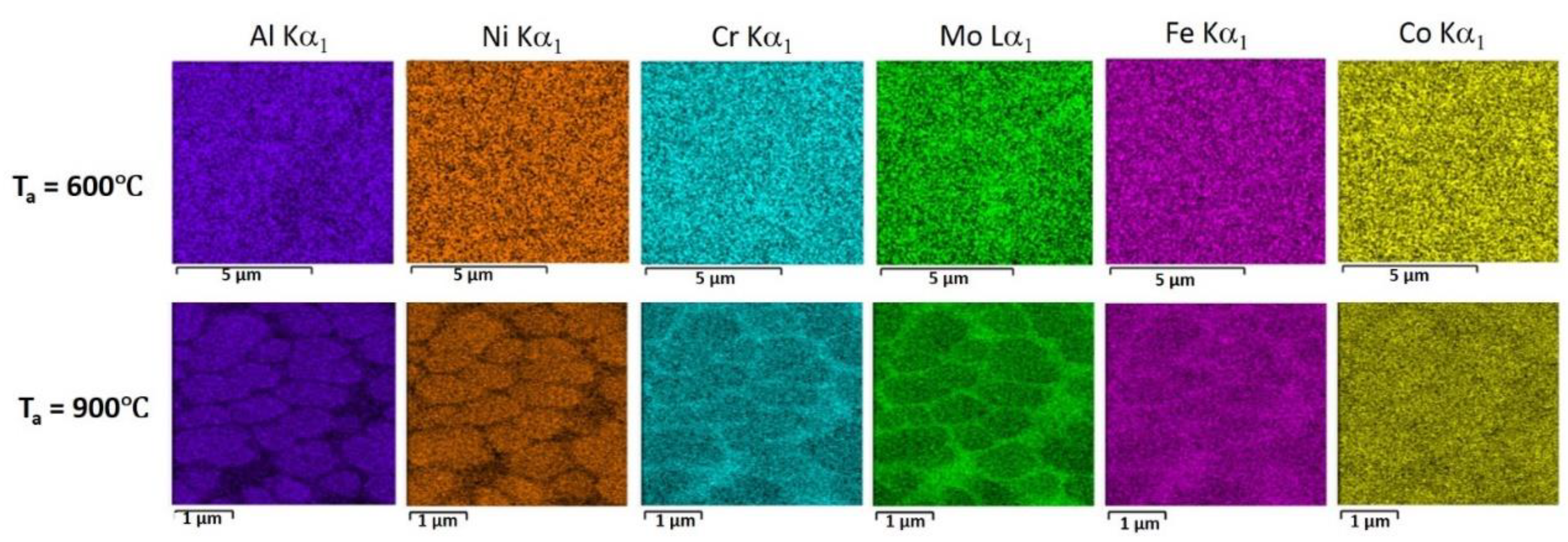

| Sample | Atomic Concentration, % | ||||||
|---|---|---|---|---|---|---|---|
| Fe | Ni | Co | Cr | Mo | Al | Si | |
| Cast | 17.2 | 17.3 | 17.1 | 17.0 | 9.3 | 22.1 | 0.14 |
| Powder | 17.2 | 17.3 | 17.2 | 16.9 | 9.3 | 22.0 | 0.19 |
| Sample | Phase | Atomic Concentration, * % | Lattice Parameter, Å | |||||
|---|---|---|---|---|---|---|---|---|
| Fe | Ni | Co | Cr | Mo | Al | |||
| Cast and homogenized | BCC_B2 Al,Ni-rich | 12.5 | 24.3 | 18.2 | 10.4 | 3.4 | 31.2 | 2.886 ± 0.005 |
| Interdendrite σ + BCC_B2 | 21.2 | 11.5 | 16.8 | 25.2 | 11.1 | 14.3 | - | |
| Powder | BCC_1 | 17.7 | 17.1 | 17.4 | 17.5 | 9.3 | 21.0 | 2.889 ± 0.005 |
| BCC_2 Mo-rich | 17.5 | 16.9 | 17.4 | 17.9 | 10.0 | 20.3 | 2.939 ± 0.006 | |
| BCC_3 (B2) Al-rich | 17.2 | 16.6 | 16.9 | 17.1 | 9.4 | 22.9 | 2.909 ± 0.001 | |
| MPS coating | BCC_1 | 17.8 | 17.1 | 17.5 | 17.4 | 9.1 | 21.1 | 2.878 ± 0.008 |
| BCC_2 Mo-rich | 17.7 | 16.1 | 16.7 | 18.5 | 12.0 | 18.6 | 2.932 ± 0.005 | |
| BCC_3 (B2) Al-rich | 16.9 | 16.9 | 17.0 | 16.9 | 9.8 | 22.6 | 2.907 ± 0.003 | |
| FCC | 20.7 | 20.8 | 21.1 | 17.6 | 11.7 | 8.0 | 3.632 ± 0.002 | |
| L-PBF | BCC_1 | 17.9 | 17.1 | 17.5 | 17.5 | 9.0 | 21.0 | 2.878 ± 0.008 |
| BCC_2 Mo-rich | 17.5 | 15.6 | 17.2 | 18.0 | 12.8 | 18.9 | 2.932 ± 0.005 | |
| BCC_3 (B2) Al-rich | 17 | 16.3 | 16.6 | 16.8 | 9.7 | 23.7 | 2.909 ± 0.002 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semikolenov, A.; Kuznetsov, P.; Bobkova, T.; Shalnova, S.; Klimova-Korsmik, O.; Klinkov, V.; Kobykhno, I.; Larionova, T.; Tolochko, O. Microstructure Evolution of FeNiCoCrAl1.3Mo0.5 High Entropy Alloy during Powder Preparation, Laser Powder Bed Fusion, and Microplasma Spraying. Materials 2021, 14, 7870. https://doi.org/10.3390/ma14247870
Semikolenov A, Kuznetsov P, Bobkova T, Shalnova S, Klimova-Korsmik O, Klinkov V, Kobykhno I, Larionova T, Tolochko O. Microstructure Evolution of FeNiCoCrAl1.3Mo0.5 High Entropy Alloy during Powder Preparation, Laser Powder Bed Fusion, and Microplasma Spraying. Materials. 2021; 14(24):7870. https://doi.org/10.3390/ma14247870
Chicago/Turabian StyleSemikolenov, Anton, Pavel Kuznetsov, Tatyana Bobkova, Svetlana Shalnova, Olga Klimova-Korsmik, Viktor Klinkov, Ilya Kobykhno, Tatyana Larionova, and Oleg Tolochko. 2021. "Microstructure Evolution of FeNiCoCrAl1.3Mo0.5 High Entropy Alloy during Powder Preparation, Laser Powder Bed Fusion, and Microplasma Spraying" Materials 14, no. 24: 7870. https://doi.org/10.3390/ma14247870
APA StyleSemikolenov, A., Kuznetsov, P., Bobkova, T., Shalnova, S., Klimova-Korsmik, O., Klinkov, V., Kobykhno, I., Larionova, T., & Tolochko, O. (2021). Microstructure Evolution of FeNiCoCrAl1.3Mo0.5 High Entropy Alloy during Powder Preparation, Laser Powder Bed Fusion, and Microplasma Spraying. Materials, 14(24), 7870. https://doi.org/10.3390/ma14247870








