New Approach for the Determination of Radiological Parameters on Hardened Cement Pastes with Coal Fly Ash
Abstract
:1. Introduction
- (1)
- Mechanical, mineralogical and structural characterisation of OPC + FA pastes
- (2)
- Determination of the radionuclide activity concentrations and ACI in both the anhydrous components and intact 28-day pastes
- (3)
- Validation of the method, comparing the radionuclide concentration and ACI values for the intact (unground) specimens and the ground samples of the same mixes.
2. Materials and Methods
2.1. Materials
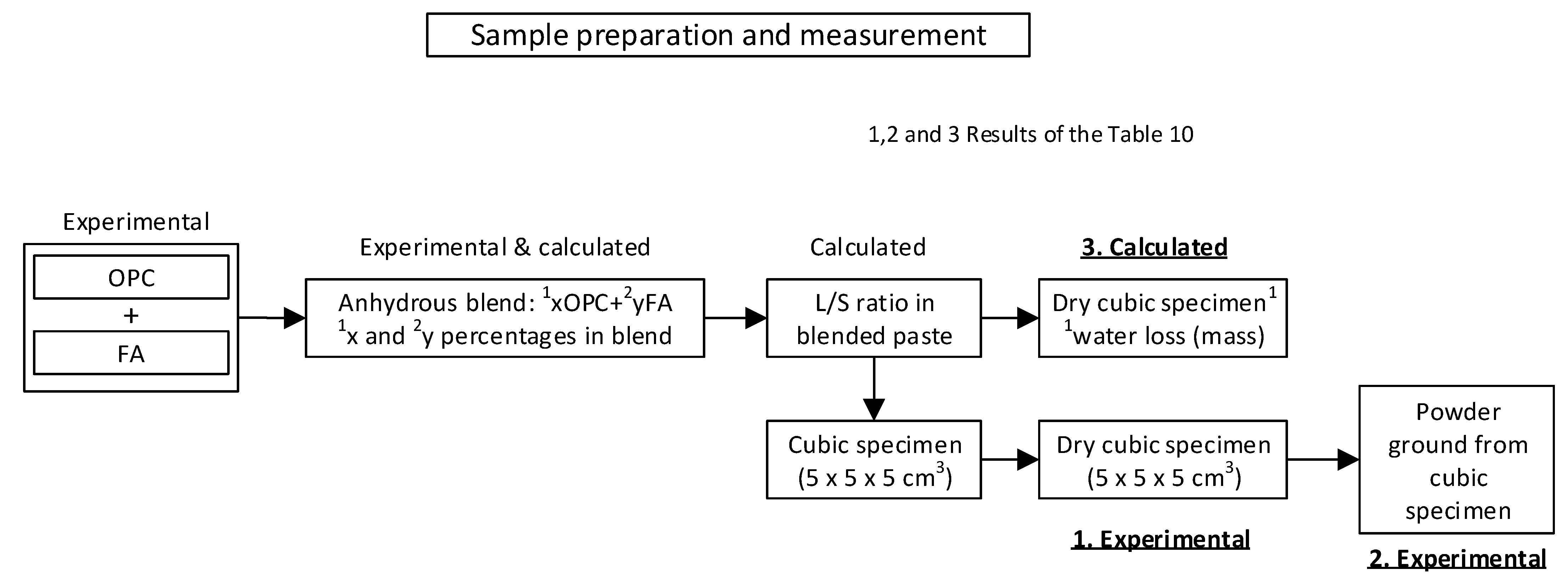
2.2. Preparation of Anhydrous OPC + FA Blends and Hydrated Pastes
2.3. Tests Conducted
- (a)
- Determination of compressive strength (three specimens per mix) on an Ibertest Autotest (Madrid, Spain) 15,000 kN test frame as recommended in European standard EN 196-2:2014 [32].
- (b)
- Determination of specimen porosity and water sorptivity. Total porosity was determined on a Micromeritics Autopore IV 9500 analyser (Norcross, GA, USA) designed for pressures of up to 36,000 psi, equivalent to a pore size of 0.0067 µm. Water sorptivity and density were found as described in European standard EN 12390-7:2009 [37].
- (c)
- Paste mineralogical and structural characterisation. Differential thermal and thermogravimetric analyses were conducted to determine paste mineralogy on a TA Instruments Q600 TGA-DSC-DTA. (New Castle, DE, USA) The test was run on platinum crucibles, ramping temperature at 10 °C/min from the ambient 25 ± 2 °C to 80 °C. After 1 h at the latter, temperature was raised at the same ramping rate to 900 °C. The entire test was conducted in a nitrogen atmosphere, flowing at 100 mL/min. 29Si MAS-NMR was recorded with a high-resolution solid-state Bruker Avance 400 spectrometer (Rheinstetten, Germany) equipped with a Fourier transform unit, at 79.49 MHz (9.4 T magnetic field) while spinning (10 kHz) the sample at a 54° 44” magic angle. A 6 µs pulse and 10 s recycle delay were used to maximise the intensity of the experimental signal. A total of 800 accumulations were acquired. Chemical shifts (δ, in ppm) were measured relative to tetra-methyl-silane (TMS, for 29Si). Spectra were fitted to Gaussian and Lorentzian peak shapes by applying non-linear least-squares iteration with Winfit software (Bruker). The 29Si MAS-NMR findings were used to find the degree of reaction (D.R.) in the Portland cement + FA blends with Equation (2) and the mean chain length (MCL) of the primary reaction product (C-S-H gel) from Equation (3) below:where the Q0 units (−70 ppm to −74 ppm) refer to the monomeric signals (SiO44−) generated by alite and belite, the anhydrous phases in the cement; and Q1 (−75 ppm to −81 ppm) to the dimeric units in C-S-H gel which, like the Q2 (1Al) units with a signal at −82 ppm, may indicate the presence of Al which replaces Si atoms in bridging sites. The interlayer uptake of alkaline or Ca2+ ions would balance the charges. The −82 ppm signal was disregarded in this study, given the overlap with the Q1 and Q2(0Al) (at around −85 ppm) signals, the latter denoting the presence of tetrahedral Si in bridging sites.
- (d)
- Radiological characterisation of cement pastes as intact cubic specimens and solid ground samples. Natural radionuclide activity concentration in the pastes was measured with gamma-ray spectrometry using two high purity germanium detectors: one featuring Pb-shielding and coaxial extended range detection and the other Fe-shielding and a broad energy system. The specifications for each are listed in the Supplementary Information, Supplementary S1 (Table S1). The electronic chain associated with the detectors included a Canberra Industries amplifier and the same firm’s ethernet acquisition interface module (AIM) analogue to digital converter. The spectra were analysed using their Genie 2000 software. 226Ra, 232Th (212Pb), and 40K gamma-ray emissions were identified on the grounds of their respective characteristic photo peaks at 186 keV (226Ra), 238 keV (212Pb) and 1460 keV (40K). 235U interference in the 226Ra 186 keV photo peak and 228Ac interference in the 1460 keV photo peak were eliminated by applying the algorithm developed in [38]. The energy efficiency curves for the 76 mm ∅, tall plastic dishes and 30 mm high plastic Petri dishes containing the ground samples as well as for the intact 5 cm cubic specimens were calculated for each type of sample (anhydrous; intact, hydrated 5 cm cubic specimens prepared with the various Portland cement blends; and the respective ground samples) with Canberra Industries LabSOCS software applying a procedure described elsewhere by the authors [21]. Three types of samples were analysed:
- (i)
- Anhydrous OPC and FA powders with a maximum particle size of 63 µm
- (ii)
- Intact, 5 cm cubic OPC paste specimens blended with FA at the five replacement ratios listed in Table 4 (top and bottom measurements)
- (iii)
- Powder samples with a particle size of under 63 µm, obtained by grinding the OPC + FA cubic paste specimens described in (ii).
3. Results and Discussion
3.1. Paste Mineralogical and Structural Characterisation. Mechanical Performance.
3.2. Radiological Characterisation of the Hardened Intact Specimens and Solid Powder Samples
4. Conclusions
- (1)
- This study validated a new method for determining 226Ra, 212Pb and 40K activity concentrations in intact 5 cm cubic specimens directly, i.e., with no need to grind the material as in present practice. The method was validated for cement pastes with variable (up to 30 wt %) amounts of a NORM waste, specifically type F coal fly ash, at 28 days of hydration.
- (2)
- No physical, mechanical, mineralogical or structural differences were observed between pastes bearing unblended OPC and those made from blends with up to 20%FA. In other words, the hydrated phases did not differ substantially in composition or content in the 28-day OPC + FA pastes bearing up to 30%FA (by cement weight), subsequently analysed with gamma-ray spectrometry.
- (3)
- The activity concentrations found for 226Ra, 212Pb and 40K in the hardened intact 5 cm cubic specimens were statistically equal to the values for the respective ground samples and to the theoretical concentrations calculated separately for each of the two components from the values observed for the respective anhydrous powders. Under the experimental conditions applied, 28-day unbound water loss had to be taken into consideration for the theoretical concentration calculations to yield accurate results. The findings showed Portland cement pastes blended with less than 30%FA to be radiologically safe.
- (4)
- They also attested to the technological validity of applying the new method for determining 226Ra, 232Th (212Pb) and 40K activity concentrations in intact 5 cm cubic specimens made with cement paste bearing up to 30%FA. The possibility of determining the activity concentrations needed to establish the ACI for cement-based materials on solid, unground samples introduces a new field of radiological research on cements, mortars and concretes actually used in construction.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Cement Production, 2010–2019. Available online: https://www.iea.org/data-and-statistics/charts/global-cement-production-2010-2019 (accessed on 16 September 2020).
- Singh, G.V.P.B.; Subramaniam, K.V.L. Production and characterization of low-energy Portland composite cement from post-industrial waste. J. Clean Prod. 2019, 239, 118024. [Google Scholar] [CrossRef]
- Scrivener, K.; John, V.M.; Gartner, E. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Puertas, F.; Alonso, M.; Torres-Carrasco, M.; Rivilla, P.; Gasco, C.; Yagüe, L.; Suárez, J.; Navarro, N. Radiological characterization of anhydrous/hydrated cements and geopolymers. Constr. Build. Mater. 2015, 101, 1105–1112. [Google Scholar] [CrossRef]
- Worrell, E.; Price, L.; Martin, N.; Hendriks, C.; Meida, L.O. Carbon dioxide emissions from the global cement industry. Annu. Rev. Energy Environ. 2001, 26, 303–329. [Google Scholar] [CrossRef]
- Kajaste, R.; Hurme, M. Cement industry greenhouse gas emissions—Management options and abatement cost. J. Clean. Prod. 2016, 112, 4041–4052. [Google Scholar] [CrossRef]
- Asokan, P.; Saxena, M.; Asolekar, S.R. Coal combustion residues—Environmental implications and recycling potentials. Resour. Conserv. Recycl. 2005, 43, 239–262. [Google Scholar] [CrossRef]
- He, Z.; Zhu, X.; Wang, J.; Mu, M.; Wang, Y. Comparison of CO2 emissions from OPC and recycled cement production. Constr. Build. Mater. 2019, 211, 965–973. [Google Scholar] [CrossRef]
- Sas, Z.; Doherty, R.; Kovács, T.; Soutsos, M.; Sha, W.; Schroeyers, W. Radiological evaluation of by-products used in construction and alternative applications; Part I. Preparation of a natural radioactivity database. Constr. Build. Mater. 2017, 150, 227–237. [Google Scholar] [CrossRef] [Green Version]
- Labrincha, J.; Puertas, F.; Schroeyers, W.; Kovler, K.; Pontikes, Y.; Nuccetelli, C.; Krivenko, P.; Kovalchuk, O.; Petropavlovsky, O.; Komljenovic, M.; et al. From NORM by-products to building materials. In Naturally Occurring Radioactive Materials in Construction; Woodhead Publishing: Cambridge, UK, 2017; pp. 183–252. [Google Scholar]
- Alonso, M.M.; Pasko, A.; Gascó, C.; Suarez, J.; Kovalchuk, O.; Krivenko, P.; Puertas, F. Radioactivity and Pb and Ni immobilization in SCM-bearing alkali-activated matrices. Constr. Build. Mater. 2018, 159, 745–754. [Google Scholar] [CrossRef]
- Maddalena, R.; Roberts, J.J.; Hamilton, A. Can Portland cement be replaced by low-carbon alternative materials? A study on the thermal properties and carbon emissions of innovative cements. J. Clean. Prod. 2018, 186, 933–942. [Google Scholar] [CrossRef]
- Gonzalez-Triviño, I.; Pascual-Cosp, J.; Moreno, B.; Benitez-Guerrero, M. Manufacture of ceramics with high mechanical properties from red mud and granite waste. Mater. Construcc. 2019, 69, e180. [Google Scholar] [CrossRef]
- García-Tenorio, R.; Bolívar, J.; Gazquez, M.J.; Mantero, J. Management of by-products generated by NORM industries: Towards their valorization and minimization of their environmental radiological impact. J. Radioanal. Nucl. Chem. 2015, 306, 641–648. [Google Scholar] [CrossRef]
- Chernysheva, N.; Lesovik, V.S.; Drebezgova, M.Y.; Shatalova, S.V.; Alaskhanov, A.H. Composite Gypsum Binders with Silica-containing Additives. IOP Conf. Ser. Mater. Sci. Eng. 2018, 327, 032015. [Google Scholar] [CrossRef]
- Ibragimov, R.; Fediuk, R. Improving the early strength of concrete: Effect of mechanochemical activation of the cementitious suspension and using of various superplasticizers. Constr. Build. Mater. 2019, 226, 839–848. [Google Scholar] [CrossRef]
- Directive, C. Council Directive 2013/51/EURATOM of 22 October 2013: Laying down requirements for the protection of the health of the general public with regard to radioactive substances in water intended for human consumption. Off. J. Eur. Union 2013, 7, 56. [Google Scholar]
- Kovler, K. Radiological constraints of using building materials and industrial by-products in construction. Constr. Build. Mater. 2009, 23, 246–253. [Google Scholar] [CrossRef]
- European Union. Radiación gamma procedente de los materiales de construcción. in Anexo XIII. Definición y uso del índice de concentración de actividad para la radiación gamma emitida por los materiales de construcción que se refiere al artículo 75. Diario Oficial de la Unión Europea 2013, 13, 1–73. [Google Scholar]
- Trevisi, R.; Leonardi, F.; Risica, S.; Nuccetelli, C. Updated database on natural radioactivity in building materials in Europe. J. Environ. Radioact. 2018, 187, 90–105. [Google Scholar] [CrossRef]
- Suárez-Navarro, J.A.; Moreno-Reyes, A.M.; Gascó, C.; Alonso, M.M.; Puertas, F. Gamma spectrometry and LabSOCS-calculated efficiency in the radiological characterisation of quadrangular and cubic specimens of hardened portland cement paste. Radiat. Phys. Chem. 2020, 171, 108709. [Google Scholar] [CrossRef]
- Temuujin, J.; Surenjav, E.; Ruescher, C.H.; Vahlbruch, J. Processing and uses of fly ash addressing radioactivity (critical review). Chemosphere 2019, 216, 866–882. [Google Scholar] [CrossRef]
- Stojanovska, Z.; Nedelkovski, D.; Ristova, M. Natural radioactivity and human exposure by raw materials and end product from cement industry used as building materials. Radiat. Meas. 2010, 45, 969–972. [Google Scholar] [CrossRef]
- Kovacs, T.; Bátor, G.; Schroeyers, W.; Labrincha, J.; Puertas, F.; Hegedus, M.; Nicolaides, D.; Sanjuán, M.; Krivenko, P.; Grubeša, I.; et al. From raw materials to NORM by-products. In Naturally Occurring Radioactive Materials in Construction; Woodhead Publishing: Cambridge, UK, 2017; pp. 135–182. [Google Scholar]
- Zielinski, R.A.; Finkelman, R.B. Radioactive Elements in Coal and Fly Ash: Abundance, Forms, and Environmental Significance; Fact Sheet; US Geological Survey: Reston, VA, USA, 1997; pp. 2327–6932. [Google Scholar] [CrossRef]
- Nisnevich, M.; Sirotin, G.; Schlesinger, T.; Eshel, Y. Radiological safety aspects of utilizing coal ashes for production of lightweight concrete. Fuel 2008, 87, 1610–1616. [Google Scholar] [CrossRef]
- Ignjatovic, I.; Sas, Z.; Dragaš, J.; Somlai, J.; Kovács, T. Radiological and material characterization of high volume fly ash concrete. J. Environ. Radioact. 2017, 168, 38–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinchón-Payá, S.; Piedecausa-García, B.; Hurtado-Bermúdez, S.; Sanjuán, M.; Chinchon, S. Radiological impact of cement, concrete and admixtures in Spain. Radiat. Meas. 2011, 46, 734–735. [Google Scholar] [CrossRef]
- Xinwei, L. Natural radioactivity in some building materials and by-products of Shaanxi, China. J. Radioanal. Nucl. Chem. 2004, 262, 775–777. [Google Scholar] [CrossRef]
- Ibrahim, N. Natural activities of 238U, 232Th and 40K in building materials. J. Environ. Radioact. 1999, 43, 255–258. [Google Scholar] [CrossRef]
- Turhan, S. Assessment of the natural radioactivity and radiological hazards in Turkish cement and its raw materials. J. Environ. Radioact. 2008, 99, 404–414. [Google Scholar] [CrossRef]
- AENOR. Method of Testing Cement—Part 2: Chemical Analysis of Cement; UNE-EN 196-2:2014; AENOR: Madrid, Spain, 2014. (In Spanish) [Google Scholar]
- AENOR. Method of Testing Cement—Part 6: Determination of Fineness; UNE-EN 196-6:2010; AENOR: Madrid, Spain, 2010. (In Spanish) [Google Scholar]
- Arjuman, P.; Silsbee, M.R.; Roy, D.M. Quantitative determination of the crystalline and amorphous phases in low calcium fly ash. In Proceedings of the 10th International Congress on the Chemistry of Cement, Gotherberg, Sweden, 2–6 June 1997; p. 4. [Google Scholar]
- Methods of Testing Cement—Part 3: Determination of Setting Times and Soundness; UNE-EN 196-3:2017; AENOR: Madrid, Spain, 2017. (In Spanish)
- AENOR. Cement—Part 1: Composition, Specifications and Conformity Criteria for Common Cements; UNE-EN 197-1; AENOR: Madrid, Spain, 2018. (In Spanish) [Google Scholar]
- AENOR. Testing Hardened Concrete—Part 7: Density of Hardened Concrete; UNE-EN 12390-7:2009; AENOR: Madrid, Spain, 2009. (In Spanish) [Google Scholar]
- Suárez-Navarro, J.; Gascó, C.; Alonso, M.M.; Blanco-Varela, M.T.; Lanzon, M.; Puertas, F. Use of Genie 2000 and Excel VBA to correct for γ-ray interference in the determination of NORM building material activity concentrations. Appl. Radiat. Isot. 2018, 142, 1–7. [Google Scholar] [CrossRef]
- Bambynek, W. Uncertainty assignment in radionuclide metrology. In Proceeding of the First International Summer School La Rabida, Huelva, Spain, 28 September–9 October 1987; Garcia-Leon, M., Madurga, G., Eds.; World Scientific: Hackensack, NJ, USA, 1987. [Google Scholar]
- Alonso, M.; Gascó, C.; Morales, M.M.; Suárez-Navarro, J.; Zamorano, M.; Puertas, F. Olive biomass ash as an alternative activator in geopolymer formation: A study of strength, radiology and leaching behaviour. Cem. Concr. Compos. 2019, 104, 103384. [Google Scholar] [CrossRef]
- Termkhajornkit, P.; Nawa, T.; Kurumisawa, K. Effect of water curing conditions on the hydration degree and compressive strengths of fly ash–cement paste. Cem. Concr. Compos. 2006, 28, 781–789. [Google Scholar] [CrossRef]
- Mikhail, R.S.; Abo-El-Enein, S.A.; Gabr, N.A. Hardened slag-cement pastes of various porosities I. Compressive strength, degree of hydration and total porosity. J. Appl. Chem. Biotechnol. 1974, 24, 735–743. [Google Scholar] [CrossRef]
- Alarcon-Ruiz, L.; Platret, G.; Massieu, E.; Ehrlacher, A. The use of thermal analysis in assessing the effect of temperature on a cement paste. Cem. Concr. Res. 2005, 35, 609–613. [Google Scholar] [CrossRef]
- Shah, V.; Scrivener, K.; Bhattacharjee, B.; Bishnoi, S. Changes in microstructure characteristics of cement paste on carbonation. Cem. Concr. Res. 2018, 109, 184–197. [Google Scholar] [CrossRef]
- Chhaiba, S.; Blanco-Varela, M.T.; Diouri, A.; Bougarrani, S. Characterization and hydration of cements and pastes obtained from raw mix containing Moroccan oil shale and coal waste as a raw material. Constr. Build. Mater. 2018, 189, 539–549. [Google Scholar] [CrossRef]
- Hongbo, Z.; Gou, H.; Haiyun, Z. Hydration/modification of fly ash in a fluidized bed. Mater. Chem. Phys. 2020, 251, 123068. [Google Scholar] [CrossRef]
- Soriano, L.; Payá, J.; Monzó, J.; Borrachero, M.; Tashima, M.M. High strength mortars using ordinary Portland cement–fly ash–fluid catalytic cracking catalyst residue ternary system (OPC/FA/FCC). Constr. Build. Mater. 2016, 106, 228–235. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Kim, I.-T.; Seo, K.-Y.; Park, H.-J. Strength and pore characteristics of OPC-slag cement paste mixed with polyaluminum chloride. Constr. Build. Mater. 2019, 223, 616–628. [Google Scholar] [CrossRef]
- El-Didamony, H.; Abd El-Aleem, S.; Ragab, A.E.-R. Hydration behavior of composite cement containing fly ash and nanosized-SiO2. Am. J. Nano Res. Appl. 2016, 4, 6–16. [Google Scholar]
- Elkhadiri, I.; Palacios, M.; Puertas, F. Effect of curing temperature on cement hydration. Ceram. Silik. 2009, 53, 65–75. [Google Scholar]
- Cong, X.; Kirkpatrick, R.J. 17O and 29Si MAS NMR study of β–C2S hydration and the structure of calcium-silicate hydrates. Cem. Concr. Res. 1993, 23, 1065–1077. [Google Scholar] [CrossRef]
- Andersen, M.D.; Jakobsen, H.J.; Skibsted, J. Characterization of white Portland cement hydration and the C-S-H structure in the presence of sodium aluminate by 27Al and 29Si MAS NMR spectroscopy. Cem. Concr. Res. 2004, 34, 857–868. [Google Scholar] [CrossRef]
- Gomes, S.; François, M. Characterization of mullite in silicoaluminous fly ash by XRD, TEM, and 29Si MAS NMR. Cem. Concr. Res. 2000, 30, 175–181. [Google Scholar] [CrossRef]
- Li, X.; Chen, Q.; Zhou, Y.; Tyrer, M.; Yü, Y. Stabilization of heavy metals in MSWI fly ash using silica fume. Waste Manag. 2014, 34, 2494–2504. [Google Scholar] [CrossRef] [PubMed]
- Jurado, J.M.; Muñiz-Valencia, R.; Alcázar, A.; Ceballos-Magaña, S.G.; González, J. Ajustando datos químicos con Excel: Un tutorial práctico. Educ. Quím. 2016, 27, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Sanjuán, M.Á.; Suarez-Navarro, J.A.; Argiz, C.; Mora, P. Assessment of radiation hazards of white and grey Portland cements. J. Radioanal. Nucl. Chem. 2019, 322, 1169–1177. [Google Scholar] [CrossRef]
- Trevisi, R.; Risica, S.; D’Alessandro, M.; Paradiso, D.; Nuccetelli, C. Natural radioactivity in building materials in the European Union: A database and an estimate of radiological significance. J. Environ. Radioact. 2012, 105, 11–20. [Google Scholar] [CrossRef] [PubMed]
- UNSCEAR. Sources and Effects of Ionizing Radiation; UNSCEAR 2000 Report to the General Assembly, with Scientigic Annexes. Volume I: Sources, United Nations Scientific Committee on the Effects of Atomic Radiation; United Nation Publication: New York, NY, USA, 2000. [Google Scholar]
- Sanjuán, M.Á.; Suárez-Navarro, J.; Argiz, C.; Mora, P. Assessment of natural radioactivity and radiation hazards owing to coal fly ash and natural pozzolan Portland cements. J. Radioanal. Nucl. Chem. 2020, 325, 381–390. [Google Scholar] [CrossRef]
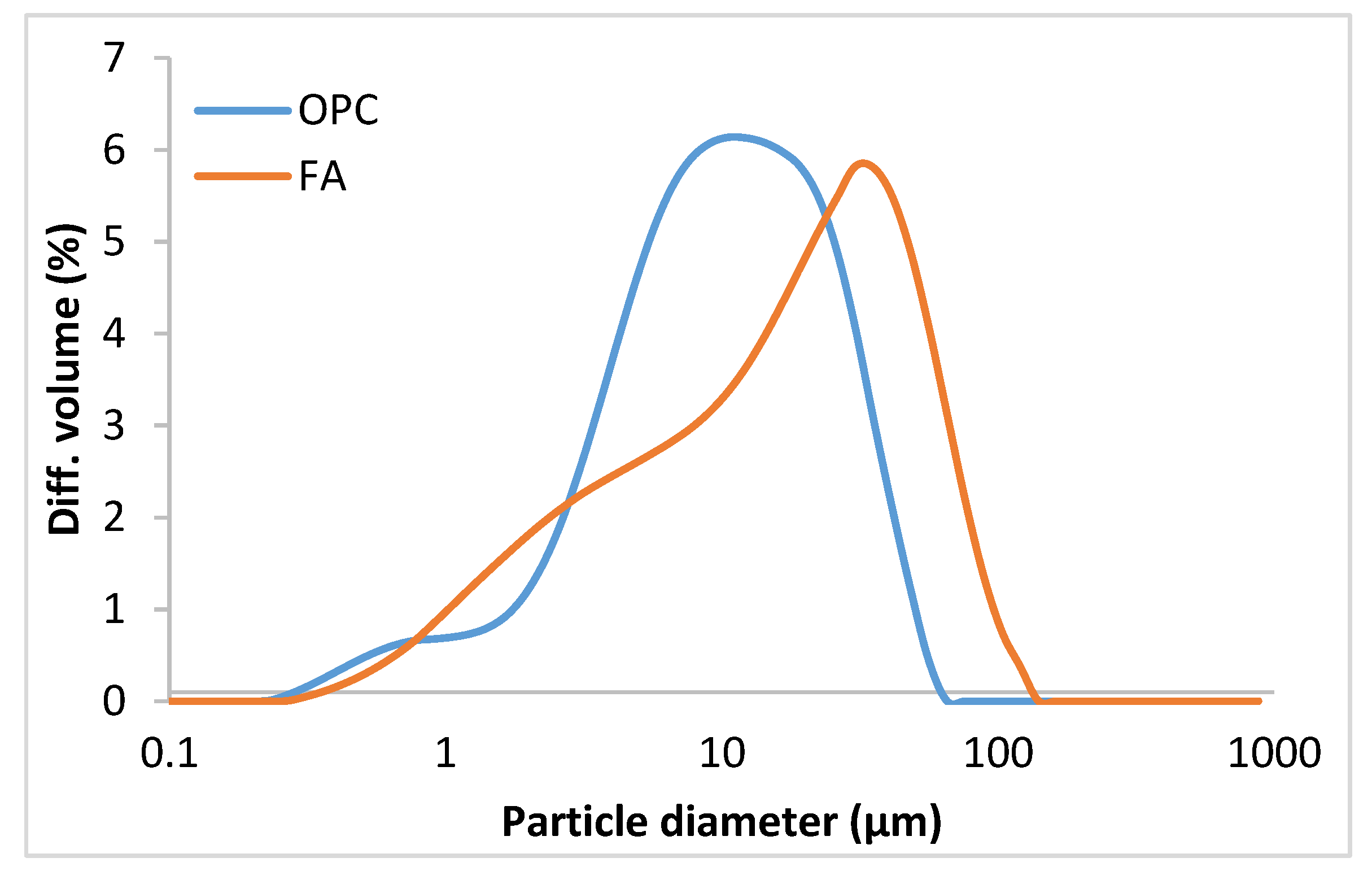


| Material | CaO | SiO2 | Al2O3 | Fe2O3 | K2O | MgO | Na2O | P2O5 | SO3 | TiO2 | Other | LoI 1 | I.R. 2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OPC | 64.47 | 20.29 | 5.67 | 2.35 | 0.97 | 0.84 | 0.11 | 0.14 | 2.91 | 0.24 | 0.17 | 2.97 | 1.07 |
| FA | 4.78 | 42.44 | 26.95 | 18.40 | 1.53 | 0.80 | 0.50 | 0.20 | 1.44 | 1.07 | 0.03 | 1.63 | 7.78 |
| Material | Dv10 (µm) | Dv50 (µm) | Dv90 (µm) | Blaine (m2/kg) |
|---|---|---|---|---|
| OPC | 2.34 | 9.31 | 27.01 | 404.68 |
| FA | 1.93 | 16.13 | 51.54 | 451.87 |
| Phases | Alite | Belite | Tricalcium Aluminate | Ferrite Phase | Gypsum | Bassanite | Calcite | |
| OPC | 64.21 | 13.16 | 8.98 | 5.82 | 1.77 | 1.64 | 4.42 | |
| Phases | Amorphous Phase | Quartz | Mullite | Hematite | Magnesium Ferrite | Magnetite | Maghemite | Calcite |
| FA | 62.09 | 7.97 | 20.43 | 2.41 | 3.99 | 1.65 | 0.82 | 0.64 |
| Cement | %OPC | %FA | l/s |
|---|---|---|---|
| OPC | 100 | - | 0.31 |
| OPC + 5%FA | 95 | 5 | 0.32 |
| OPC + 10%FA | 90 | 10 | 0.32 |
| OPC + 20%FA | 80 | 20 | 0.32 |
| OPC + 30%FA | 70 | 30 | 0.32 |
| Material | Compressive Strength (MPa) | Sorptivity (%) | Density (g/mL) | Total Porosity (%) |
|---|---|---|---|---|
| OPC | 69.4 ± 0.6 | 28.89 ± 0.2 | 1.84 | 17.88 |
| OPC + 5%FA | 72.1 ± 2.1 | 30.48 ± 0.2 | 1.79 | 18.94 |
| OPC + 10%FA | 71.8 ± 2.6 | 30.08 ± 0.2 | 1.80 | 17.16 |
| OPC + 20%FA | 69.3 ± 1.5 | 31.18 ± 0.2 | 1.78 | 18.44 |
| OPC + 30%FA | 61.2 ± 0.7 | 31.17 ± 0.2 | 1.77 | 22.52 |
| Material | 25–81.5 °C | Total Loss 81.5–900 °C | 81.5–350 °C | 350–550 °C | 550–900 °C |
|---|---|---|---|---|---|
| OPC | 6.30 | 15.94 | 5.39 | 5.24 | 5.31 |
| OPC + 5%FA | 6.25 | 15.73 | 5.75 | 5.58 | 4.40 |
| OPC + 10%FA | 6.07 | 15.31 | 5.66 | 5.02 | 4.63 |
| OPC + 20%FA | 6.06 | 15.10 | 6.07 | 5.12 | 4.34 |
| OPC + 30%FA | 5.86 | 14.10 | 5.64 | 4.56 | 3.90 |
| Material | Q0 | Q1 | Q2(0Al) | Q3(3Al) | Q4(0Al) | ||||
|---|---|---|---|---|---|---|---|---|---|
| OPC | −70.66 ppm I = 26.24% | −71.76 ppm I = 9.39% | −73.49 ppm I = 29.93% | −74.30 ppm I = 1.20% | −79.69 ppm I = 20.05% | −84.81 ppm I = 13.19% | - | - | - |
| OPC + 5%FA | −70.66 ppm I = 29.69% | −71.60 ppm I = 10.29% | −73.49 ppm I = 24.78% | −74.12 ppm I = 0.78% | −79.35 ppm I = 22.49% | −84.34 ppm I = 11.96% | - | - | - |
| OPC + 10%FA | −70.66 ppm I = 28.73% | −71.65 ppm I = 9.79% | −73.49 ppm I = 27.22% | −73.86 ppm I = 1.03% | −79.88 ppm I = 19.97% | −84.69 ppm I = 13.27% | - | - | - |
| OPC + 20%FA | −70.66 ppm I = 28.89% | −71.65 ppm I = 9.81% | −73.49 ppm I = 23.13% | −74.02 ppm I = 1.15% | −79.56 ppm I = 18.06% | −84.33 ppm I = 11.74% | - | −105.48 ppm I = 7.23% | - |
| OPC + 30%FA | −70.66 ppm I = 14.38% | −71.79 ppm I = 7.52% | −73.80 ppm I = 22.67% | −73.49 ppm I = 0.94% | −81.56 ppm I = 20.64% | −87.66 ppm I = 12.11% | −94.80 ppm I = 6.46% | −101.0 ppm I = 3.57% | −108.5 ppm I = 11.73% |
| Material | Q0 | Q1 + Q2 | Q2/Q1 | FA (n.r.) (a) | D.R. (b) | MCL (c) |
|---|---|---|---|---|---|---|
| OPC | 66.76 | 33.24 | 0.66 | - | 33.24 | 3.32 |
| OPC + 5%FA | 66.54 | 34.45 | 0.53 | −0 | 34.46 | 3.06 |
| OPC + 10%FA | 68.29 | 31.71 | 0.66 | −0 | 33.23 | 3.33 |
| OPC + 20%FA | 62.98 | 29.80 | 0.65 | 7.23 | 37.02 | 3.30 |
| OPC + 30%FA | 45.51 | 32.75 | 0.59 | 21.71 | 54.50 | 3.17 |
| Material | 226Ra (Bq·kg−1) | 212Pb (Bq·kg−1) | 40K (Bq·kg−1) |
|---|---|---|---|
| OPC | 19.0 ± 3.9 | 21.3 ± 3.2 | 235 ± 15 |
| FA | 164 ± 27 | 66.8 ± 7.6 | 292 ± 18 |
| OPC + 5%FA | 22.6 ± 4.7 | 23.3 ± 4.7 | 237 ± 15 |
| OPC + 10%FA | 30.7 ± 6.3 | 25.5 ± 3.8 | 241 ± 15 |
| OPC + 20%FA | 48.2 ± 8.2 | 29.9 ± 4.4 | 247 ± 15 |
| OPC + 30%FA | 64 ± 11 | 35.4 ± 4.3 | 251 ± 16 |
| 1. 5 cm Cubic (Intact) Specimens | 2. Powder Sample from Intact Specimens | 3. Theoretical Values | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Material | 40K | 238U Series | 232Th Series | ACI | 40K | 238U Series | 232Th Series | ACI | 40K | 238U Series | 232Th Series | ACI |
| 226Ra | 212Pb | 226Ra | 212Pb | 226Ra | 212Pb | |||||||
| OPC | 201 ± 9 | 20.0 ± 3.9 | 18.2 ± 1.5 | 0.225 ± 0.015 | 187 ± 12 | 13.2 ± 2.9 | 17.8 ± 2.1 | 0.195 ± 0.015 | 193 ± 12 | 15.4 ± 1.8 | 17.3 ± 2.0 | 0.199 ± 0.012 |
| OPC + 5%FA | 196 ± 9 | 23.3 ± 4.1 | 18.5 ± 1.5 | 0.236 ± 0.016 | 200 ± 14 | 27.0 ± 8.9 | 20.4 ± 2.4 | 0.259 ± 0.032 | 192 ± 11 | 21.0 ± 2.0 | 18.9 ± 1.9 | 0.229 ± 0.012 |
| OPC + 10%FA | 212 ± 10 | 38.7 ± 6.9 | 23.6 ± 2.0 | 0.320 ± 0.025 | 194 ± 14 | 32.8 ± 6.1 | 22.1 ± 2.6 | 0.284 ± 0.025 | 194 ± 11 | 26.8 ± 2.7 | 20.7 ± 1.9 | 0.260 ± 0.014 |
| OPC + 20%FA | 198 ± 9 | 38.9 ± 4.8 | 19.8 ± 1.6 | 0.295 ± 0.018 | 200 ± 14 | 370 ± 9.9 | 25 ± 3 | 0.318 ± 0.037 | 199 ± 10 | 38.5 ± 4.6 | 24.4 ± 2.0 | 0.316 ± 0.019 |
| OPC + 30%FA | 206 ± 11 | 56.8 ± 7.9 | 25.4 ± 2.2 | 0.384 ± 0.029 | 200 ± 13 | 52.0 ± 9.2 | 25 ± 3 | 0.366 ± 0.034 | 202.9±9.4 | 50.2 ± 6.6 | 28.1 ± 2.3 | 0.374 ± 0.025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno de los Reyes, A.M.; Suárez-Navarro, J.A.; Alonso, M.d.M.; Gascó, C.; Sobrados, I.; Puertas, F. New Approach for the Determination of Radiological Parameters on Hardened Cement Pastes with Coal Fly Ash. Materials 2021, 14, 475. https://doi.org/10.3390/ma14030475
Moreno de los Reyes AM, Suárez-Navarro JA, Alonso MdM, Gascó C, Sobrados I, Puertas F. New Approach for the Determination of Radiological Parameters on Hardened Cement Pastes with Coal Fly Ash. Materials. 2021; 14(3):475. https://doi.org/10.3390/ma14030475
Chicago/Turabian StyleMoreno de los Reyes, Ana María, José Antonio Suárez-Navarro, Maria del Mar Alonso, Catalina Gascó, Isabel Sobrados, and Francisca Puertas. 2021. "New Approach for the Determination of Radiological Parameters on Hardened Cement Pastes with Coal Fly Ash" Materials 14, no. 3: 475. https://doi.org/10.3390/ma14030475






