Superhydrophobic Coating Derived from Geothermal Silica to Enhance Material Durability of Bamboo Using Hexadimethylsilazane (HMDS) and Trimethylchlorosilane (TMCS)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Purification of Geothermal Silica
2.2. Superhydrophobic Solution Synthesis
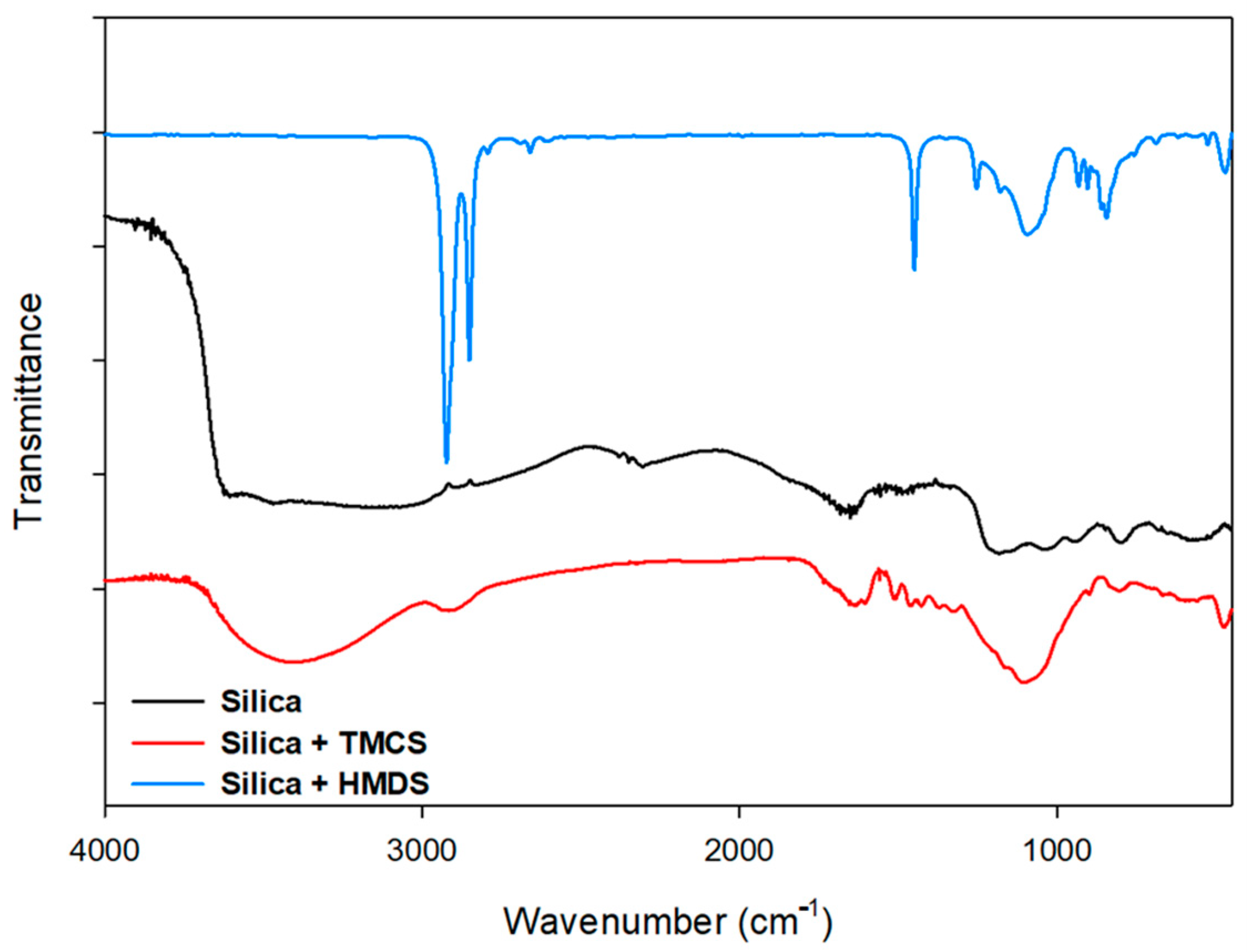
2.3. Product Characterization
2.4. Bamboo Durability Test
2.5. Durability of Superhydrophobic Coating Test
3. Results and Discussions
3.1. Morphology, Size, and Surface Area of Silica
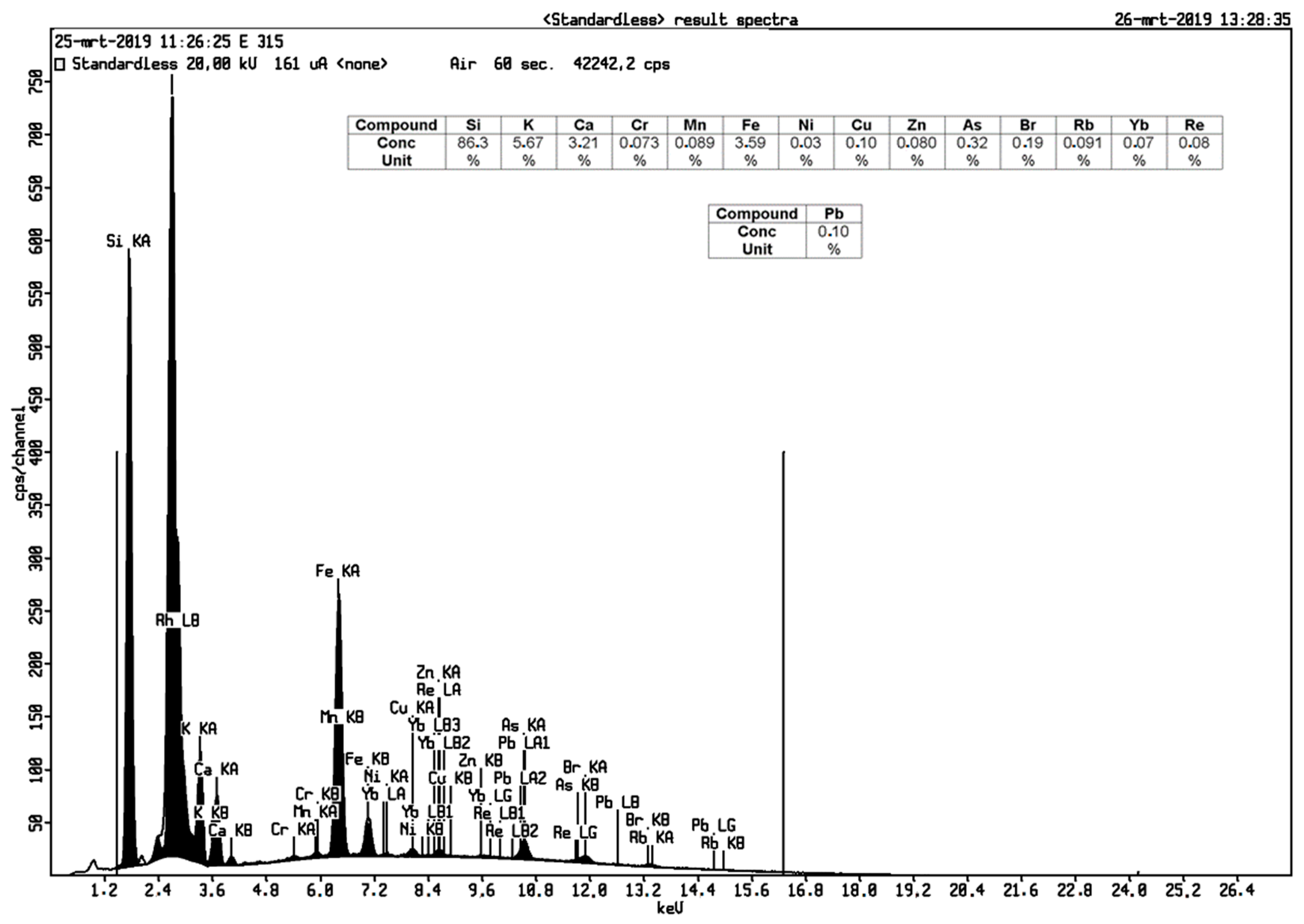
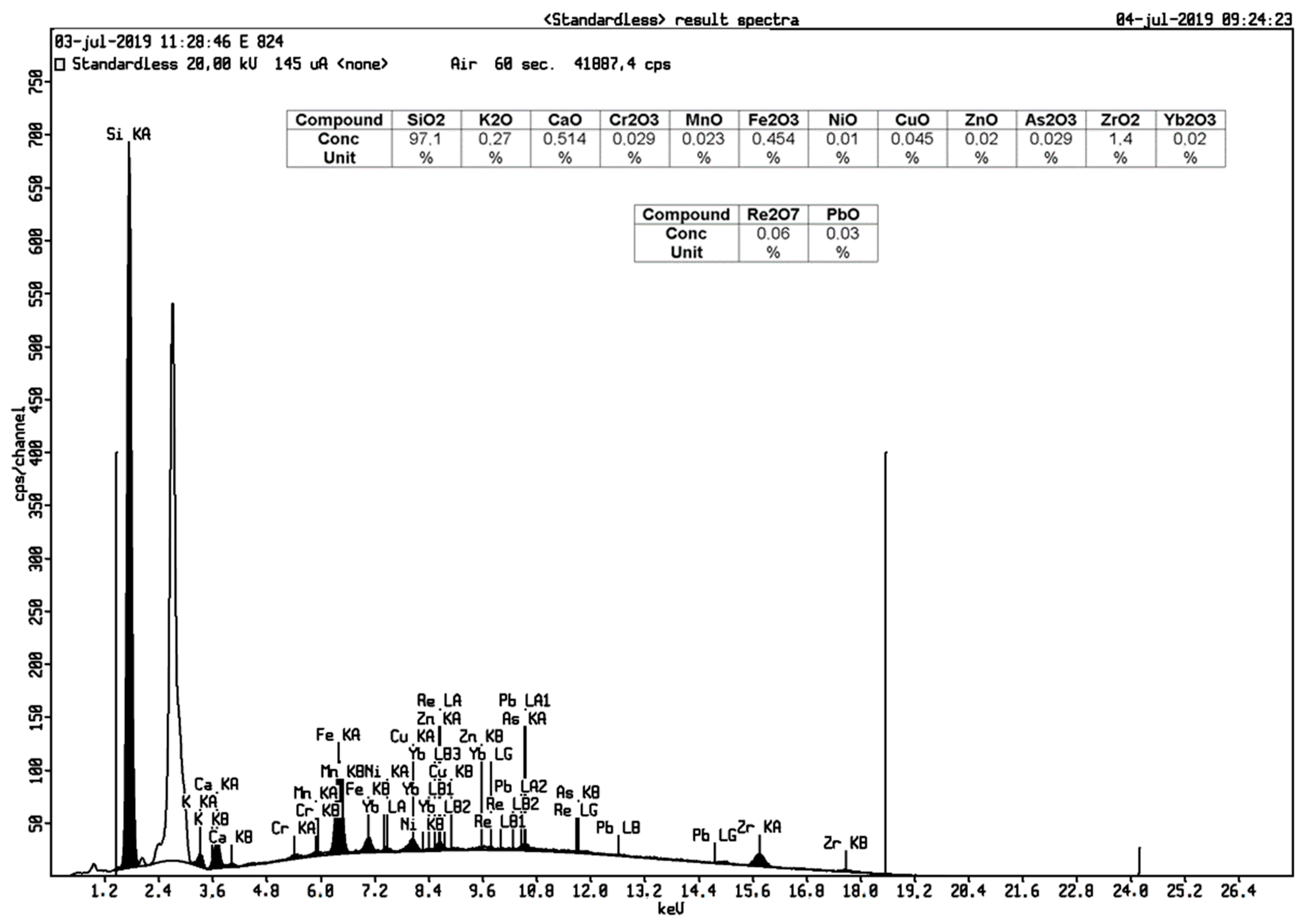
3.2. Mineral Contents in Geothermal Silica Scaling Waste
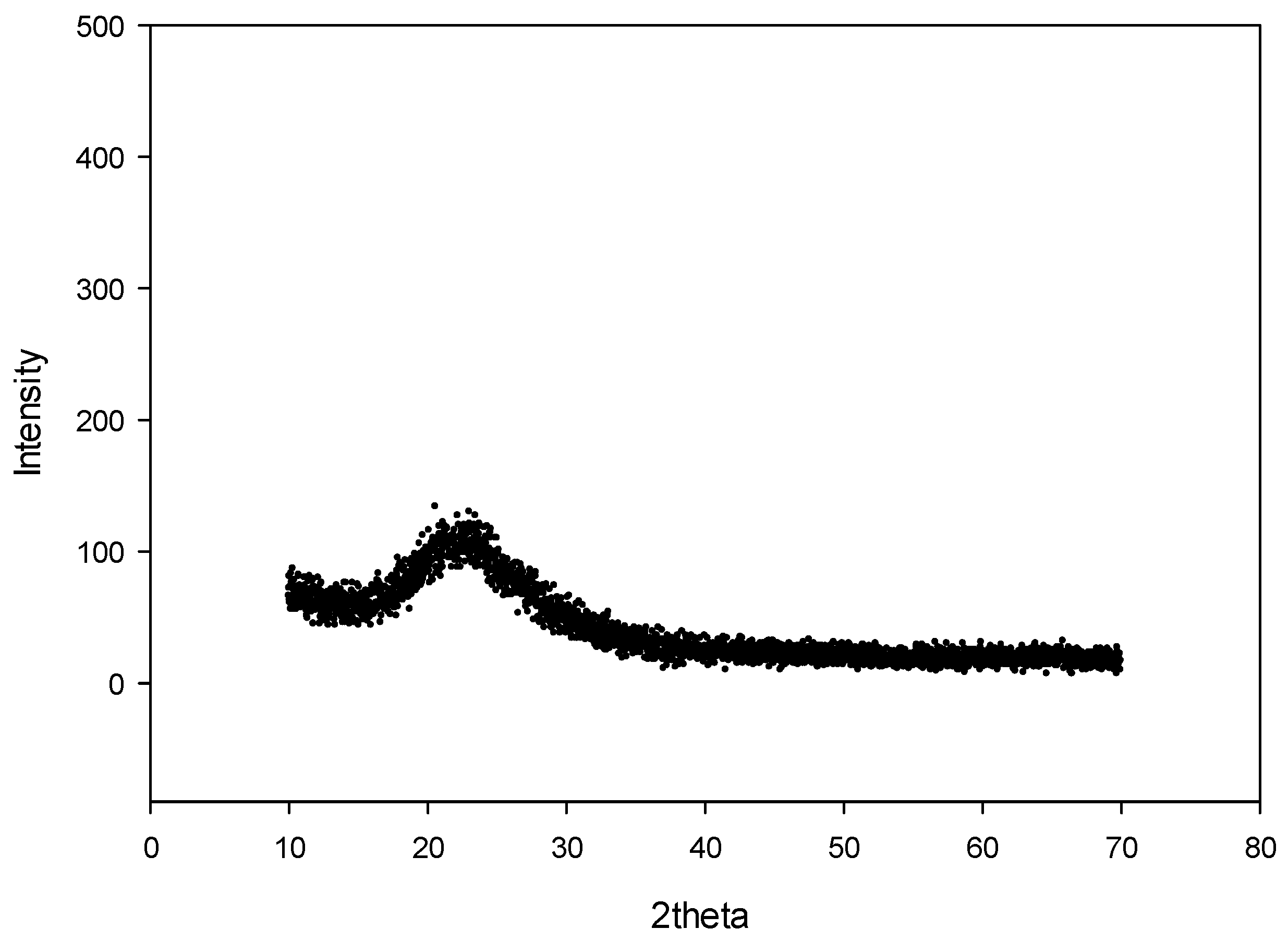
3.3. Structure of Geothermal Silica Scaling Waste Material
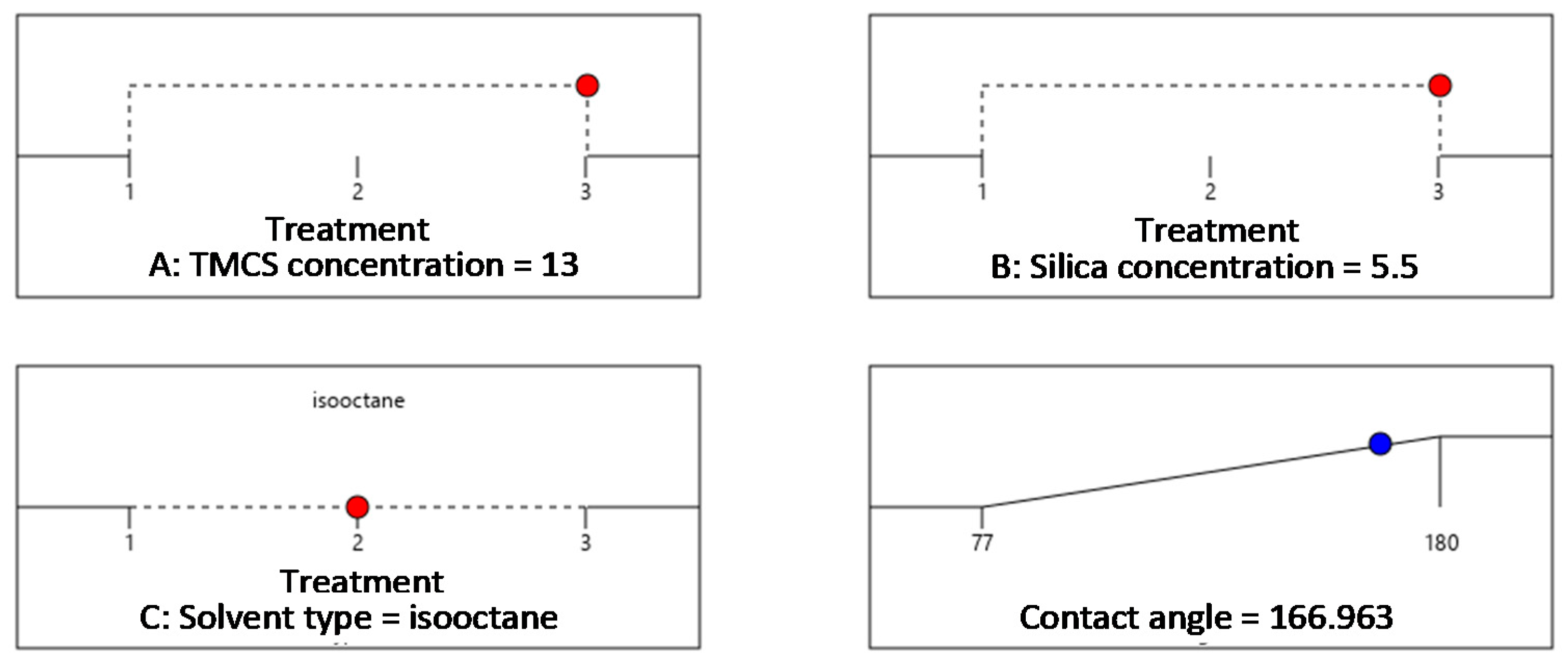
3.4. Analysis of Hydrophobicity, Contact Angle, and the Determination of Optimum Conditions
3.4.1. Effect of HMDS Concentration on Contact Angle
3.4.2. Effect of Type of Solvents used on Contact Angle
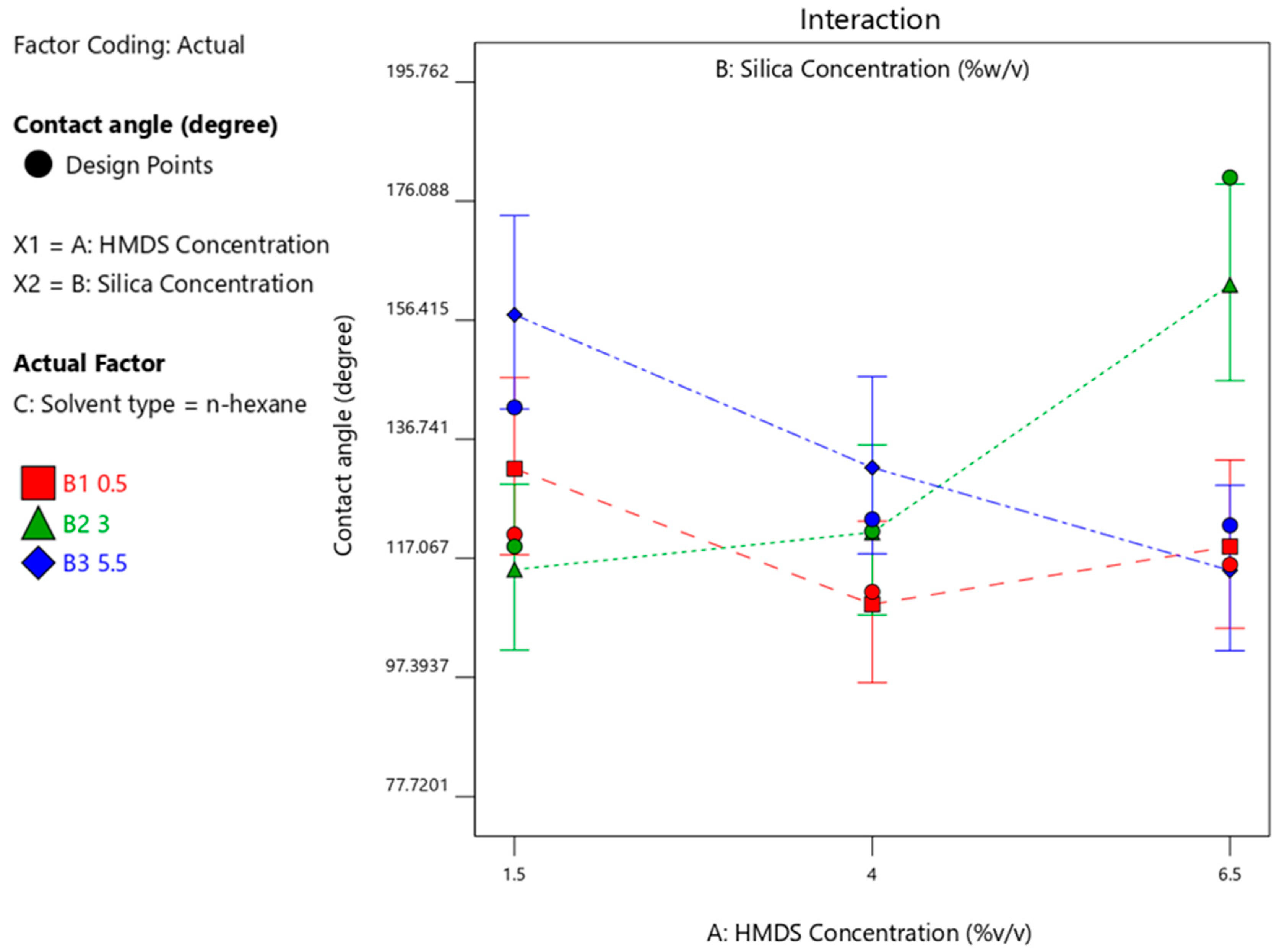
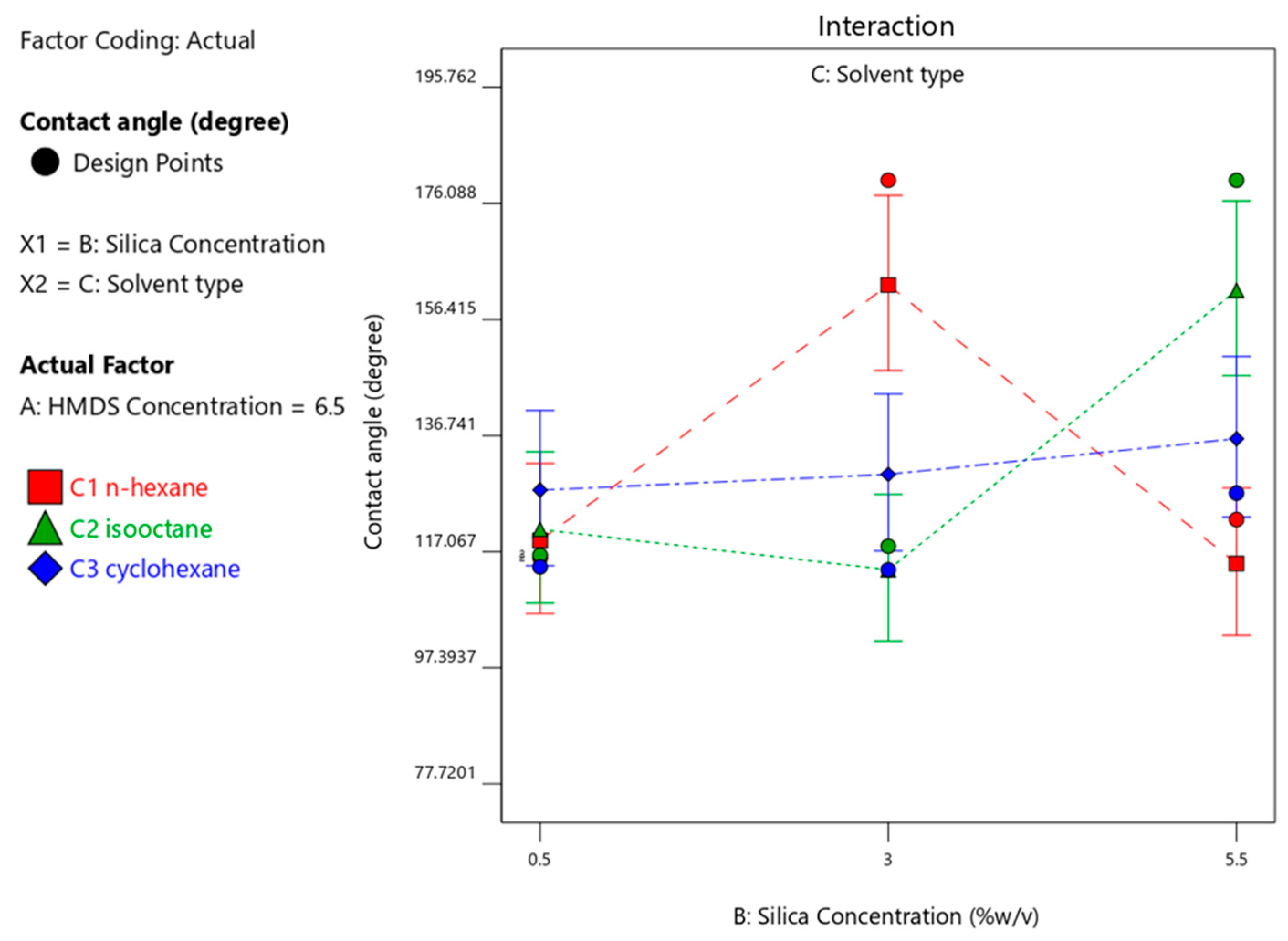
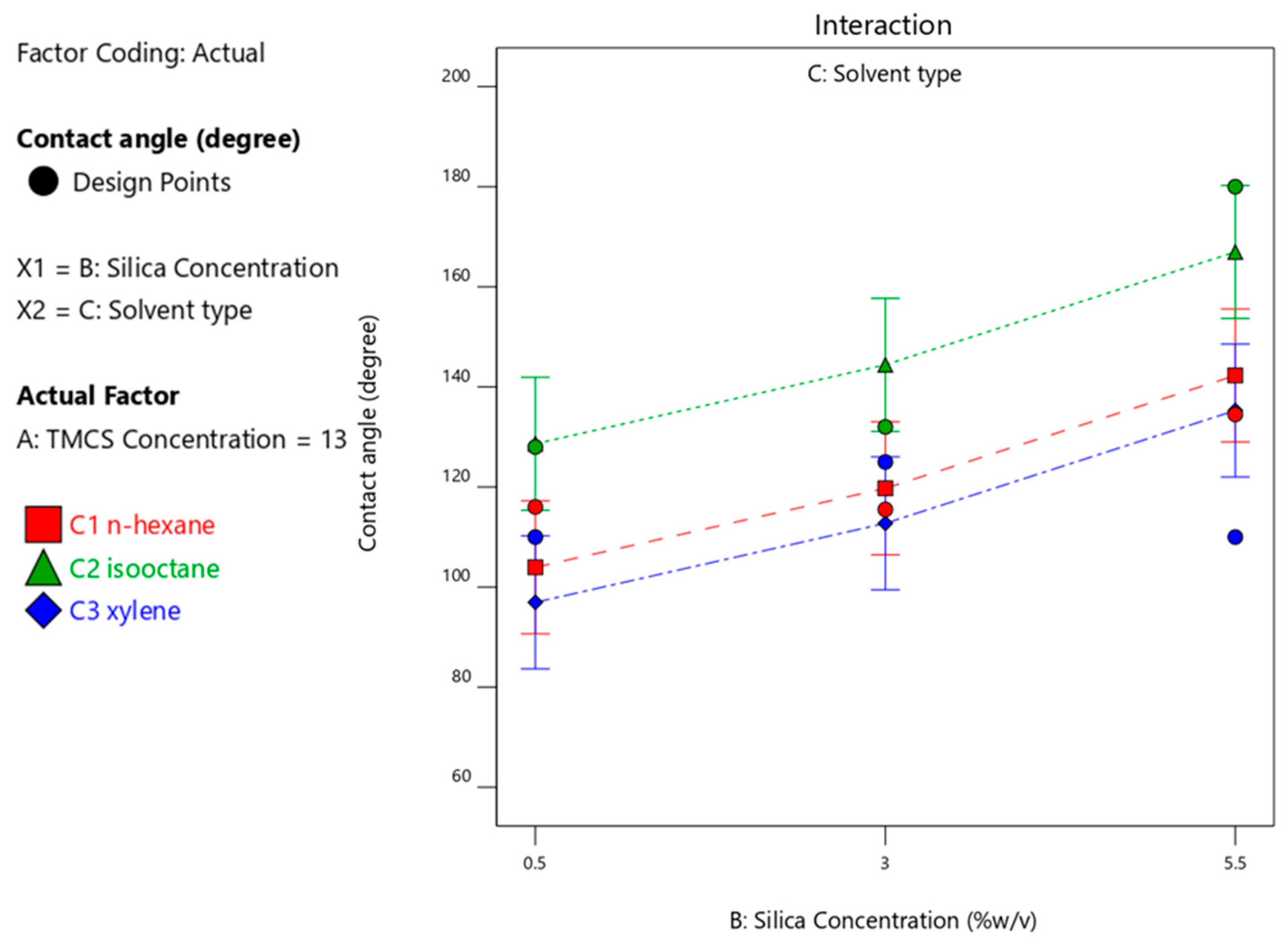
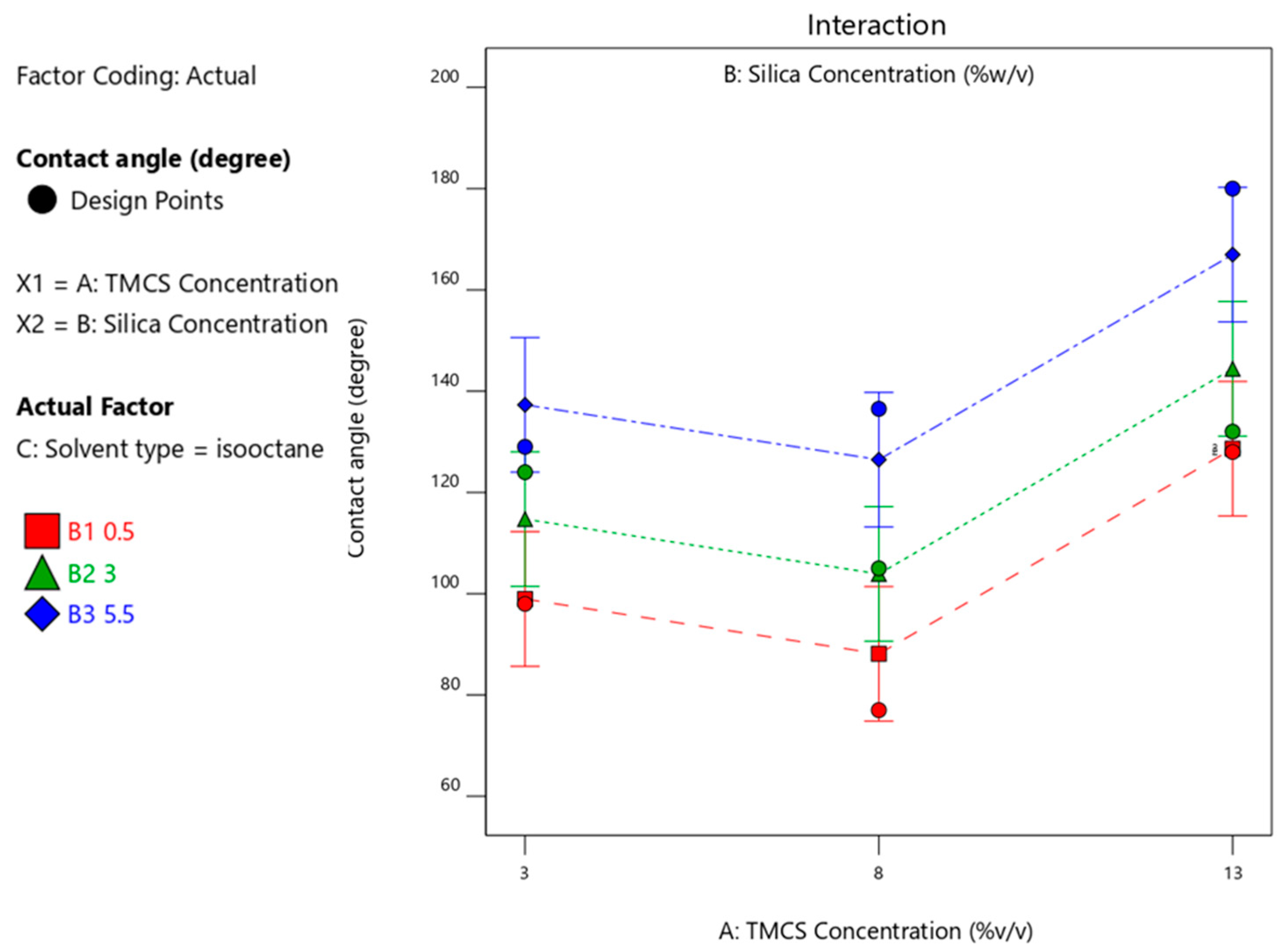
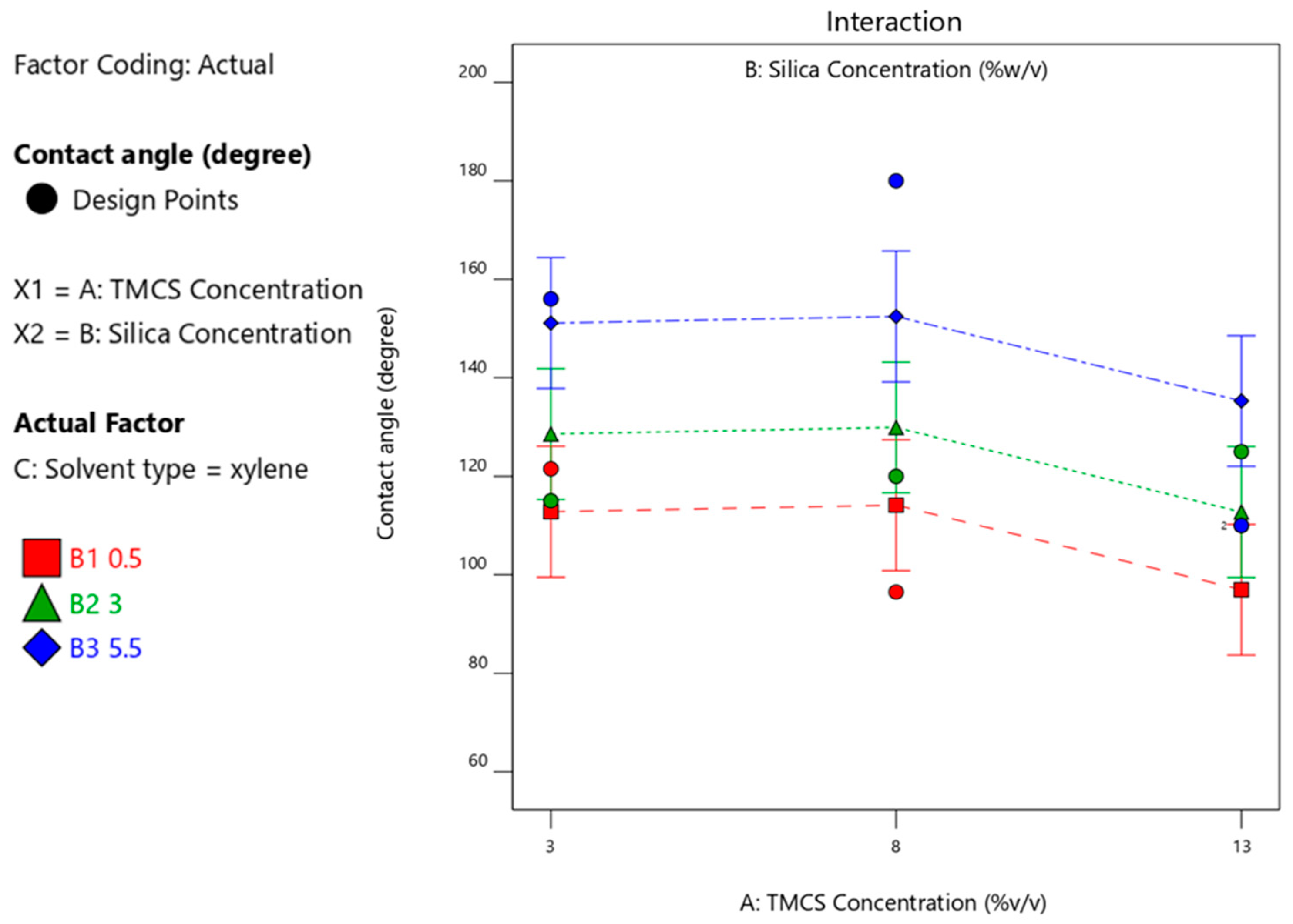
3.4.3. Effect of Silica Concentration on the Contact Angle Produced
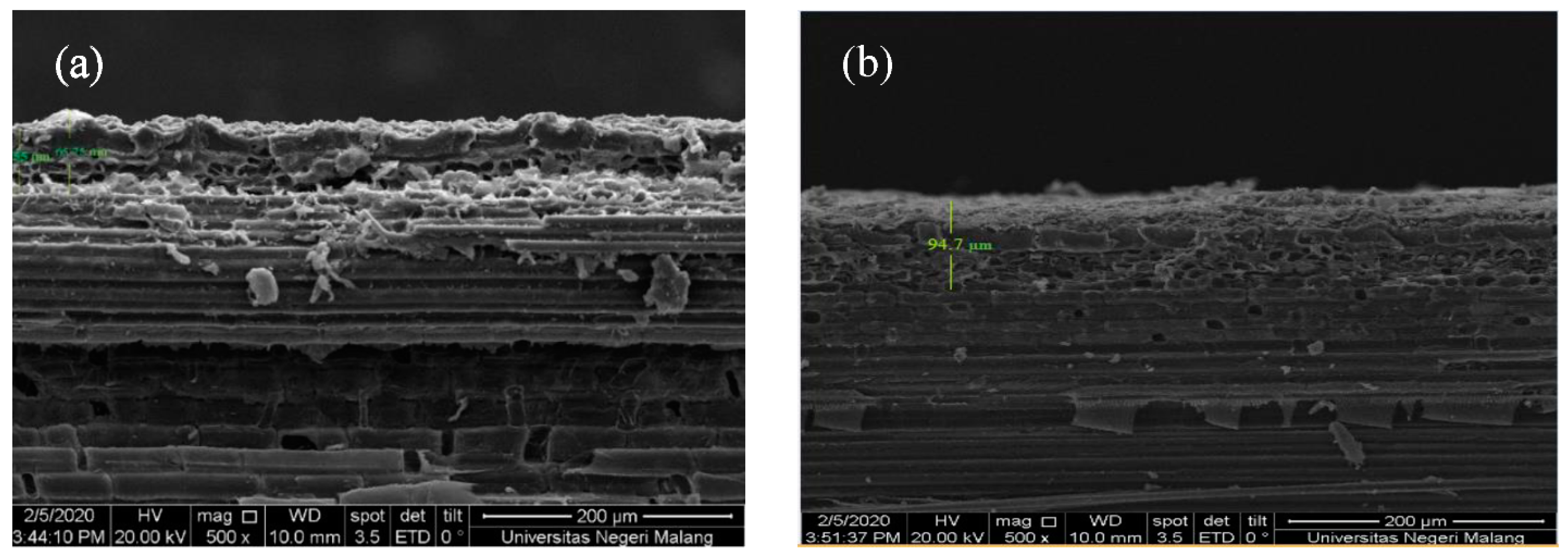
3.4.4. Durability Test on Bamboo
3.4.5. Durability Test of Superhydrophobic Coating
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karakurt, C.; Bayazit, Y. Freeze-thaw resistance of normal and high strength concretes produced with fly ash and silica fume. Adv. Mater. Sci. Eng. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, O.; Wei, D.S.; Liese, W.; Wollenberg, E. Fungal degradation of bamboo samples. Holzforschung 2011, 65, 883–888. [Google Scholar] [CrossRef]
- Wang, N.; Xiong, D. Comparison of micro-/nano-hierarchical and nano-scale roughness of silica membranes in terms of wetting behavior and transparency. Colloids Surf. A Physicochem. Eng. Asp. 2014, 446, 8–14. [Google Scholar] [CrossRef]
- Sun, T.; Hao, H.; Hao, W.T.; Yi, S.M.; Li, X.P.; Li, J.R. Preparation and antibacterial properties of titanium-doped ZnO from different zinc salts. Nanoscale Res. Lett. 2014, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, C.; Chen, Z.; Tiwari, M.K. All-organic superhydrophobic coatings with mechanochemical robustness and liquid impalement resistance. Nat. Mater. 2018, 17, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wei, W.; Li, Z.; Duan, W.; Han, H.; Xie, Q. A superhydrophobic fluorinated PDMS composite as a wearable strain sensor with excellent mechanical robustness and liquid impalement resistance. J. Mater. Chem. A 2020, 8, 3509–3516. [Google Scholar] [CrossRef]
- Shokuhfar, A.; Alzamani, M.; Eghdam, E.; Karimi, M.; Mastali, S. SiO2TiO2 Nanostructure Films on Windshields Prepared by Sol-Gel Dip-Coating Technique for Self-Cleaning and Photocatalytic Applications. Nanosci. Nanotechnol. 2012, 2, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, A.; Khudiyev, T.; Daglar, B.; Budunoglu, H.; Okyay, A.K.; Bayindir, M. Superhydrophobic and omnidirectional antireflective surfaces from nanostructured ormosil colloids. ACS Appl. Mater. Interfaces 2013, 5, 853–860. [Google Scholar] [CrossRef] [Green Version]
- Setyawan, H.; Yuwana, M.; Balgis, R. PEG-templated mesoporous silicas using silicate precursor and their applications in desiccant dehumidification cooling systems. Microporous Mesoporous Mater. 2015, 218, 95–100. [Google Scholar] [CrossRef]
- Ghuman, B.S. Preparation of HMDS from TMCS at Elevated Temperatures. J. Chem. Cheml. Sci. 2013, 3, 86–87. [Google Scholar]
- Vasimalla, S.; Subbarao, N.V.V.; Gedda, M.; Goswami, D.K.; Iyer, P.K. Effects of Dielectric Material, HMDS Layer, and Channel Length on the Performance of the Perylenediimide-Based Organic Field-Effect Transistors. ACS Omega 2017, 2, 2552–2560. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, H.; Omae, K.; Sakai, T.; Yamazaki, K.; Sakurai, H. Acute and subchronic inhalation toxicity of tetraethoxysilane (TEOS) in mice. Arch. Toxicol. 1994, 68, 277–283. [Google Scholar] [CrossRef]
- Purnomo, A.; Dalanta, F.; Oktaviani, A.D.; Silviana, S. Superhydrophobic coatings and self-cleaning through the use of geothermal scaling silica in improvement of material resistance. AIP Conf. Proc. 2018, 2026. [Google Scholar] [CrossRef]
- Holmberg, M.; Harlin, H.; Holmberg, M. Durability of Superhydrophobic Coatings—Sand Abrasion Test. Bachelor’s Thesis, Uppsala University, Uppsala, Sweden, 2016. [Google Scholar]
- Venkateswara Rao, A.; Latthe, S.S.; Nadargi, D.Y.; Hirashima, H.; Ganesan, V. Preparation of MTMS based transparent superhydrophobic silica films by sol-gel method. J. Colloid Interface Sci. 2009, 332, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Young, J.F. Humidity control in the laboratory using salt solutions—A review. J. Appl. Chem. 2007, 17, 241–245. [Google Scholar] [CrossRef]
- Sapuan, S.M.; Pua, F.L.; El-Shekeil, Y.A.; AL-Oqla, F.M. Mechanical properties of soil buried kenaf fibre reinforced thermoplastic polyurethane composites. Mater. Des. 2013, 50, 467–470. [Google Scholar] [CrossRef] [Green Version]
- Rafiee, E.; Shahebrahimi, S.; Feyzi, M.; Shaterzadeh, M. Optimization of synthesis and characterization of nanosilica produced from rice husk (a common waste material). Int. Nano Lett. 2012, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Musić, S.; Filipović-Vinceković, N.; Sekovanić, L. Precipitation of amorphous SiO2 particles and their properties. Braz. J. Chem. Eng. 2011, 28, 89–94. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, W. Study on amorphous silica powder properties. Adv. Mater. Res. 2012, 512–515, 2428–2433. [Google Scholar] [CrossRef]
- Teare, D.O.H.; Spanos, C.G.; Ridley, P.; Kinmond, E.J.; Roucoules, V.; Badyal, J.P.S.; Brewer, S.A.; Coulson, S.; Willis, C. Pulsed plasma deposition of super-hydrophobic nanospheres. Chem. Mater. 2002, 14, 4566–4571. [Google Scholar] [CrossRef]
- Snyder, L.R.; Kirkland, J.J.; Glajch, J.L. Practical HPLC Method Development, 2nd ed.; Wiley: New York, NY, USA, 1997; pp. 1–542. [Google Scholar]
- Silviana, S.; Darmawan, A.; Subagio, A.; Dalanta, F. Statistical approaching for superhydrophobic coating preparation using silica derived from geothermal solid waste. ASEAN J. Chem. Eng. 2019, 19, 91–99. [Google Scholar] [CrossRef]
- Cheng, Y.; Xia, M.; Luo, F.; Li, N.; Guo, C.; Wei, C. Effect of surface modification on physical properties of silica aerogels derived from fly ash acid sludge. Colloids Surf. A Physicochem. Eng. Asp. 2016, 490, 200–206. [Google Scholar] [CrossRef]
- Bhagat, S.D.; Kim, Y.H.; Suh, K.H.; Ahn, Y.S.; Yeo, J.G.; Han, J.H. Superhydrophobic silica aerogel powders with simultaneous surface modification, solvent exchange and sodium ion removal from hydrogels. Microporous Mesoporous Mater. 2008, 112, 504–509. [Google Scholar] [CrossRef]
- Khedkar, M.V.; Somvanshi, S.B.; Humbe, A.V.; Jadhav, K.M. Surface modified sodium silicate based superhydrophobic silica aerogels prepared via ambient pressure drying process. J. Non. Cryst. Solids 2019, 511, 140–146. [Google Scholar] [CrossRef]
- Aerogels, S.; Pierre, A.C.; Rigacci, A. Aerogels Handbook. Aerogels Handb. 2011, 21–45. [Google Scholar] [CrossRef]
- Thasma Subramanian, B.; Alla, J.P.; Essomba, J.S.; Nishter, N.F. Non-fluorinated superhydrophobic spray coatings for oil-water separation applications: An eco-friendly approach. J. Clean. Prod. 2020, 256, 120693. [Google Scholar] [CrossRef]
- Sarawade, P.B.; Kim, J.K.; Hilonga, A.; Kim, H.T. Production of low-density sodium silicate-based hydrophobic silica aerogel beads by a novel fast gelation process and ambient pressure drying process. Solid State Sci. 2010, 12, 911–918. [Google Scholar] [CrossRef]
- Latthe, S.S.; Terashima, C.; Nakata, K.; Sakai, M.; Fujishima, A. Development of sol-gel processed semi-transparent and self-cleaning superhydrophobic coatings. J. Mater. Chem. A 2014, 2, 5548–5553. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Domantovskiy, A.G.; Emelyanenko, A.M.; Pashinin, A.S.; Ionin, A.A.; Kudryashov, S.I.; Saltuganov, P.N. Femtosecond laser treatment for the design of electro-insulating superhydrophobic coatings with enhanced wear resistance on glass. ACS Appl. Mater. Interfaces 2014, 6, 2080–2085. [Google Scholar] [CrossRef]


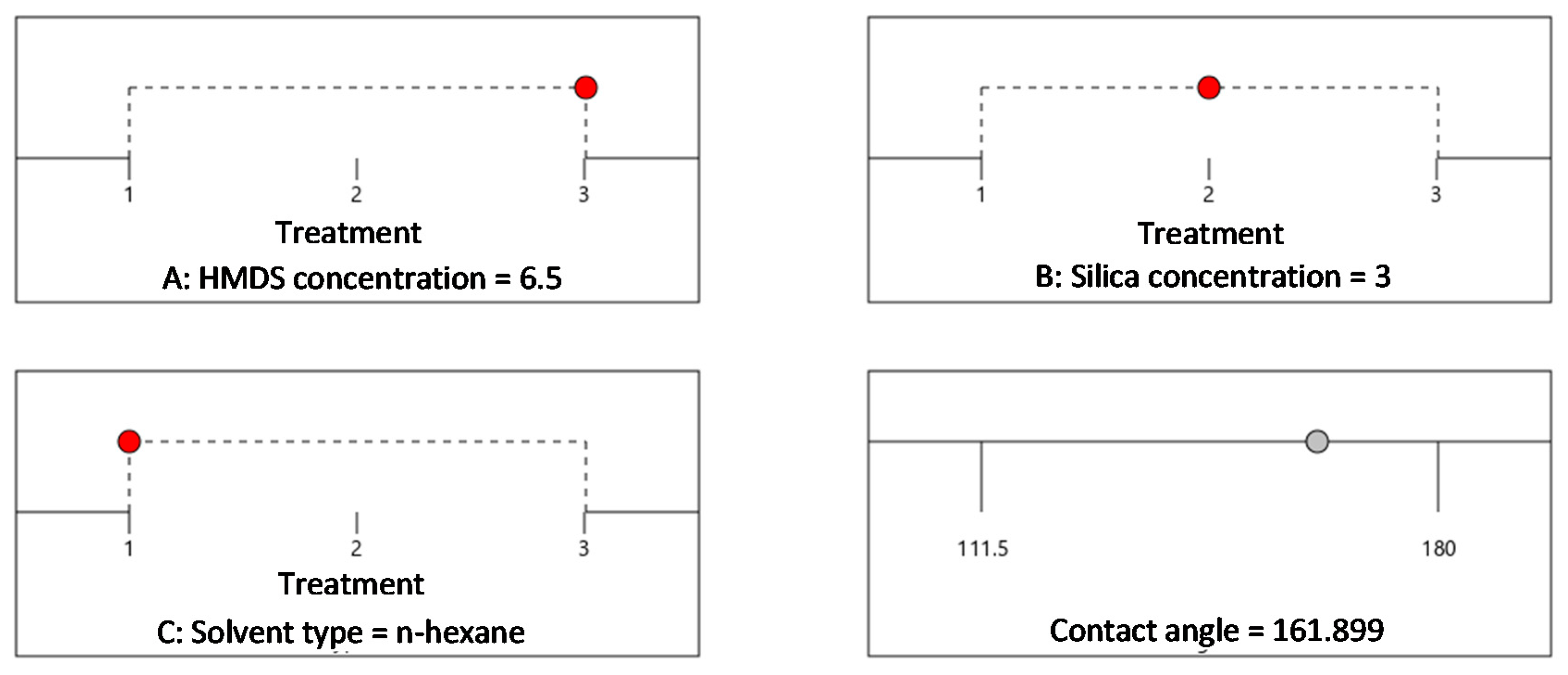





| Surface-Modifying Agent | Concentration (%-v/v) | Silica Concentration (%-w/v) | Solvent Type (Categorical Variables) |
|---|---|---|---|
| HMDS | 1.5 | 0.5 | cyclohexane |
| 4 | 3 | iso-octane | |
| 6 | 5.5 | n-hexane | |
| TMCS | 3 | 0.5 | n-hexane |
| 8 | 3 | xylene | |
| 13 | 5.5 | iso-octane |
| Element | Prior Treatment (%) | Post Treatment (%) |
|---|---|---|
| Si | 86.30 | 97.10 |
| Mn | 0.09 | 0.02 |
| Fe | 3.59 | 0.45 |
| Ca | 3.21 | 0.51 |
| Run | HDMS (%-v/v) | Silica Concentration (%-w/v) | Solvent Type | Contact Angle Degree (Average of 3 Measurements) |
|---|---|---|---|---|
| 1 | 4 | 5.5 | cyclohexane | 122 |
| 2 | 4 | 0.5 | cyclohexane | 123 |
| 3 | 4 | 3 | cyclohexane | 122.5 |
| 4 | 1.5 | 3 | cyclohexane | 128.5 |
| 5 | 1.5 | 0.5 | isooctane | 127.5 |
| 6 | 1.5 | 0.5 | cyclohexane | 124 |
| 7 | 1.5 | 0.5 | n-hexane | 121 |
| 8 | 6.5 | 3 | cyclohexane | 114 |
| 9 | 4 | 0.5 | isooctane | 122.5 |
| 10 | 4 | 5.5 | n-hexane | 123.5 |
| 11 | 1.5 | 5.5 | n-hexane | 142 |
| 12 | 1.5 | 5.5 | isooctane | 135 |
| 13 | 6.5 | 5.5 | cyclohexane | 127 |
| 14 | 1.5 | 5.5 | cyclohexane | 132.5 |
| 15 | 4 | 5.5 | isooctane | 125 |
| 16 | 6.5 | 3 | n-hexane | 180 |
| 17 | 4 | 0.5 | n-hexane | 111.5 |
| 18 | 6.5 | 0.5 | isooctane | 116.5 |
| 19 | 6.5 | 5.5 | isooctane | 180 |
| 20 | 6.5 | 0.5 | cyclohexane | 114.5 |
| 21 | 6.5 | 3 | isooctane | 118 |
| 22 | 4 | 3 | n-hexane | 121.5 |
| 23 | 6.5 | 5.5 | n-hexane | 122.5 |
| 24 | 6.5 | 0.5 | n-hexane | 116 |
| 25 | 1.5 | 3 | isooctane | 180 |
| 26 | 1.5 | 3 | n-hexane | 119 |
| 27 | 4 | 3 | isooctane | 112.5 |
| Run | TMCS (%v/v) | Silica (%-w/v) | Solvent Type | Contact Angle Degree (Average of 3 Measurements) |
|---|---|---|---|---|
| 1 | 13 | 0.5 | n-hexane | 116 |
| 2 | 8 | 0.5 | xylene | 96.5 |
| 3 | 13 | 5.5 | isooctane | 180 |
| 4 | 3 | 0.5 | isooctane | 98 |
| 5 | 3 | 0.5 | xylene | 121.5 |
| 6 | 8 | 5.5 | n-hexane | 121 |
| 7 | 8 | 3 | n-hexane | 118 |
| 8 | 8 | 5.5 | xylene | 180 |
| 9 | 3 | 5.5 | n-hexane | 135 |
| 10 | 8 | 3 | xylene | 120 |
| 11 | 13 | 3 | n-hexane | 115.5 |
| 12 | 3 | 3 | isooctane | 124 |
| 13 | 8 | 3 | isooctane | 105 |
| 14 | 3 | 3 | xylene | 115 |
| 15 | 8 | 0.5 | n-hexane | 79 |
| 16 | 8 | 0.5 | isooctane | 77 |
| 17 | 13 | 5.5 | n-hexane | 134.5 |
| 18 | 13 | 3 | xylene | 125 |
| 19 | 13 | 0.5 | isooctane | 128 |
| 20 | 13 | 5.5 | xylene | 110 |
| 21 | 13 | 3 | isooctane | 132 |
| 22 | 3 | 3 | n-hexane | 124.5 |
| 23 | 3 | 5.5 | xylene | 156 |
| 24 | 8 | 5.5 | isooctane | 136.5 |
| 25 | 13 | 0.5 | xylene | 110 |
| 26 | 3 | 0.5 | n-hexane | 111 |
| 27 | 3 | 5.5 | isooctane | 129 |
| Solvents | Polarity Index |
|---|---|
| n-Hexane | 0.1 |
| Isooctane | 0.1 |
| Cyclohexane | 0.2 |
| Xylene | 2.5 |
| Time (week) | Mass (g) | Moisture Content (%) | ||
|---|---|---|---|---|
| Coated 180° | Uncoated | Coated 180° | Uncoated | |
| 0 | 9.18 | 8.89 | 0 | 0 |
| 10 | 10.05 | 22.65 | 26 | 28 |
| Time (week) | Mass (g) | Moisture Content (%) | ||||
|---|---|---|---|---|---|---|
| Coated (180°) | Uncoated | Coated (112.5°) | Coated (180°) | Uncoated | Coated (112.5°) | |
| 0 | 9.09 | 9.08 | 3.48 | 0 | 0 | 0 |
| 10 | 9.33 | 17.01 | 3.93 | 19 | 40 | 21 |
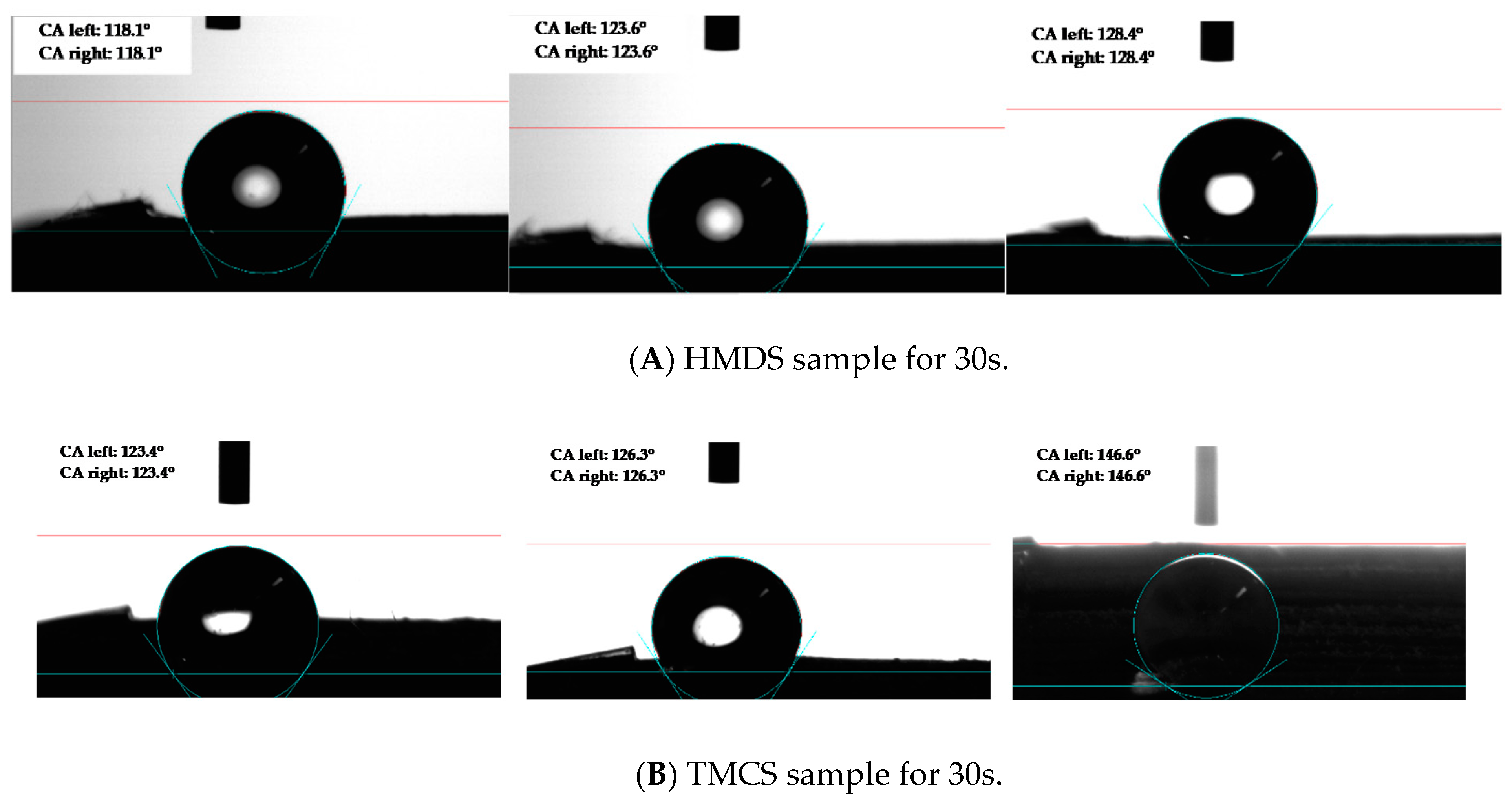
| Samples | 30 s (Average of 3 Measurements) | 120 s (Average of 3 Measurements) |
|---|---|---|
| HMDS | 123.4° | 120.7° |
| TMCS | 132° | 124.2° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silviana, S.; Darmawan, A.; Dalanta, F.; Subagio, A.; Hermawan, F.; Milen Santoso, H. Superhydrophobic Coating Derived from Geothermal Silica to Enhance Material Durability of Bamboo Using Hexadimethylsilazane (HMDS) and Trimethylchlorosilane (TMCS). Materials 2021, 14, 530. https://doi.org/10.3390/ma14030530
Silviana S, Darmawan A, Dalanta F, Subagio A, Hermawan F, Milen Santoso H. Superhydrophobic Coating Derived from Geothermal Silica to Enhance Material Durability of Bamboo Using Hexadimethylsilazane (HMDS) and Trimethylchlorosilane (TMCS). Materials. 2021; 14(3):530. https://doi.org/10.3390/ma14030530
Chicago/Turabian StyleSilviana, Silviana, Adi Darmawan, Febio Dalanta, Agus Subagio, Ferry Hermawan, and Hansel Milen Santoso. 2021. "Superhydrophobic Coating Derived from Geothermal Silica to Enhance Material Durability of Bamboo Using Hexadimethylsilazane (HMDS) and Trimethylchlorosilane (TMCS)" Materials 14, no. 3: 530. https://doi.org/10.3390/ma14030530
APA StyleSilviana, S., Darmawan, A., Dalanta, F., Subagio, A., Hermawan, F., & Milen Santoso, H. (2021). Superhydrophobic Coating Derived from Geothermal Silica to Enhance Material Durability of Bamboo Using Hexadimethylsilazane (HMDS) and Trimethylchlorosilane (TMCS). Materials, 14(3), 530. https://doi.org/10.3390/ma14030530








