Raltitrexed-Modified Gold and Silver Nanoparticles for Targeted Cancer Therapy: Cytotoxicity Behavior In Vitro on A549 and HCT-116 Human Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
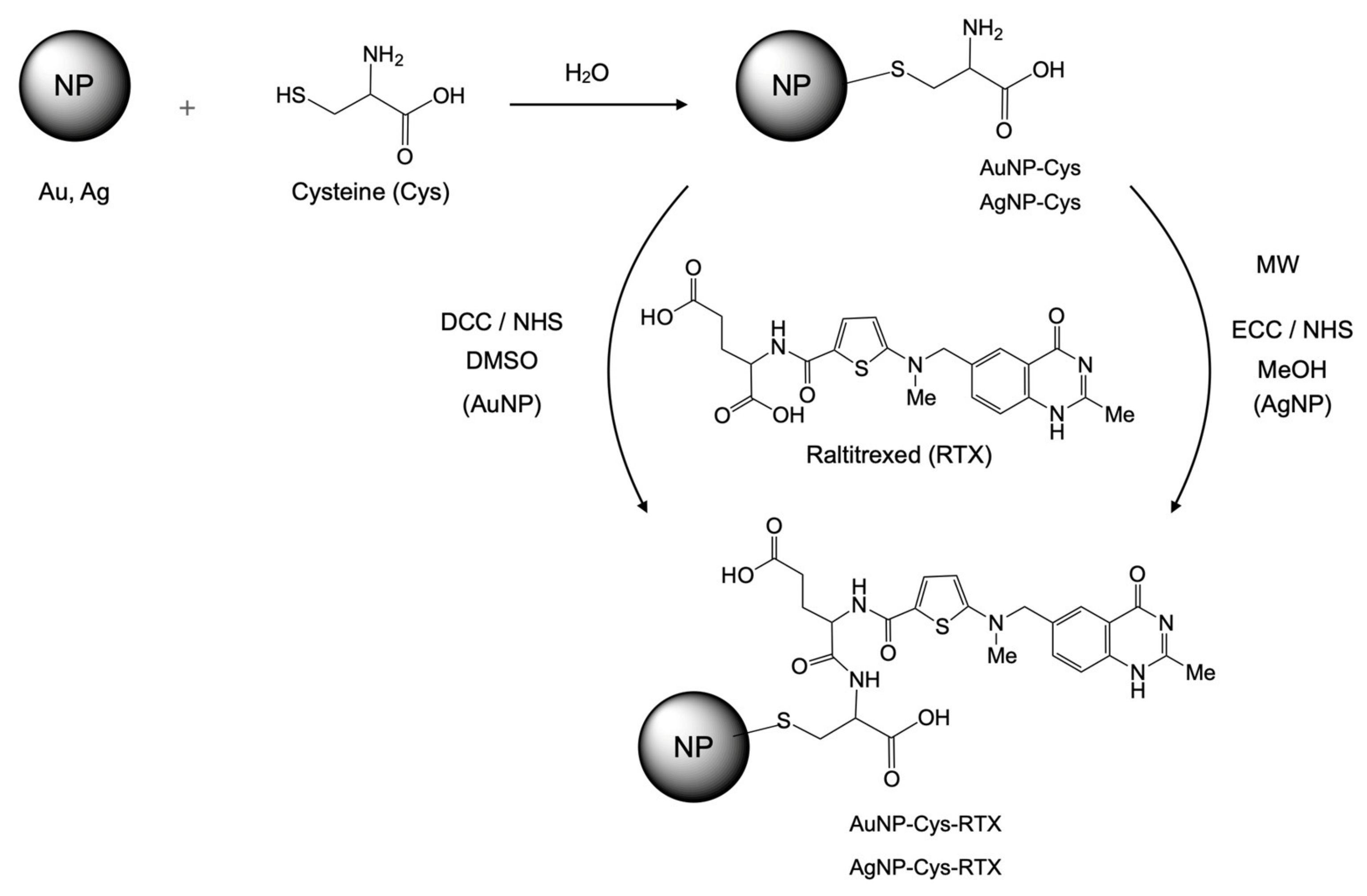
2.3. Synthesis and Physicochemical Characterization of the Nanoparticles
2.3.1. Synthesis of Gold Nanoparticles AuNPs
2.3.2. Synthesis of AuNP-Cys
2.3.3. Synthesis of AuNP-Cys-RTX
2.3.4. Synthesis of AgNP-Cys
2.3.5. Synthesis of Silver Nanoparticles AgNP-Cys-RTX
2.4. In Vitro Studies
2.4.1. Cell Culture HCT116 Human Colorectal Carcinoma
2.4.2. Cell Culture A549 Human Lung Adenocarcinoma
2.4.3. Cell Culture and Survival/Viability
3. Results
3.1. Characterization of Antifolate Hybrid Nanoparticles
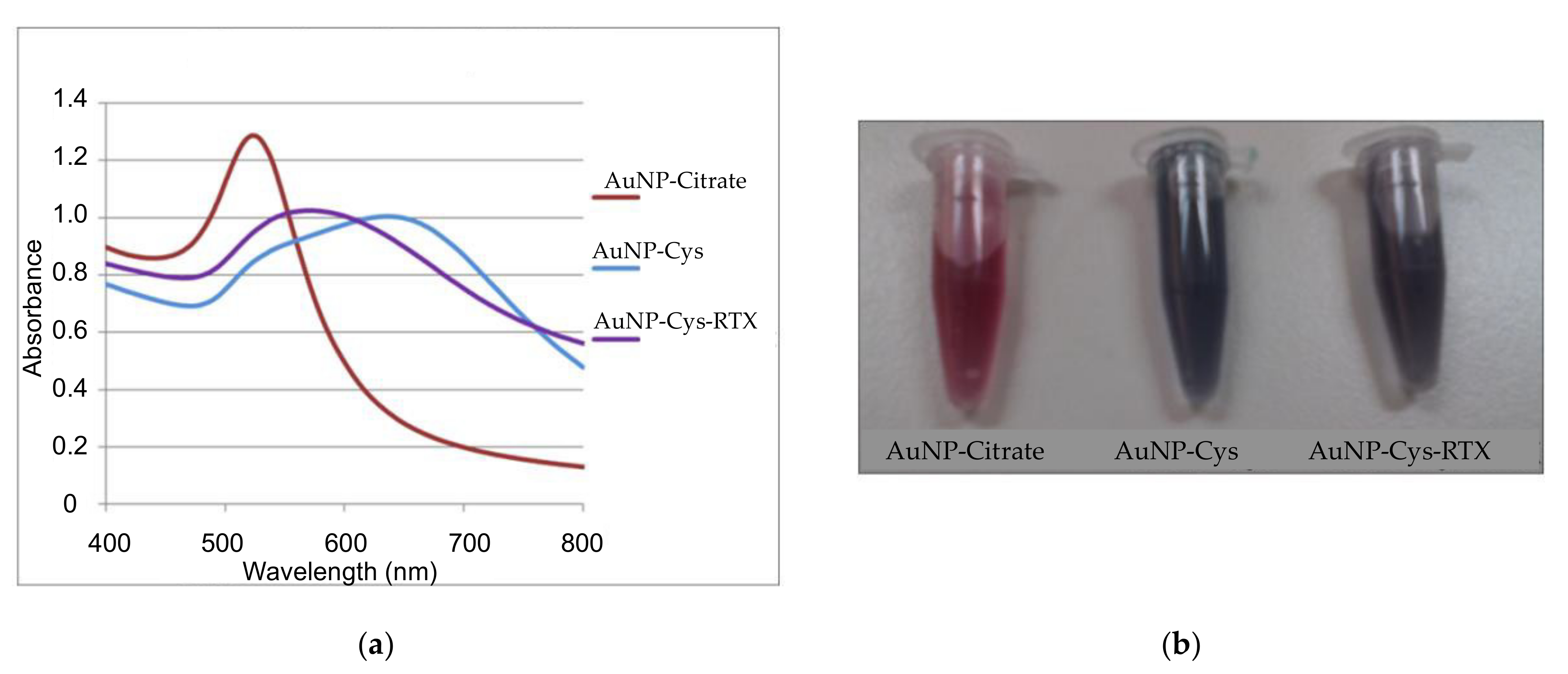
3.1.1. UV-Vis of AuNP-Cys-RTX
3.1.2. FTIR of AuNP-Cys-RTX and AgNP-Cys-RTX
3.1.3. Morphological Analysis of AuNP-Cys-RTX and AgNP-Cys-RTX
3.1.4. Zeta Potential (ζ) of AuNP-Cys-RTX and AgNP-Cys-RTX
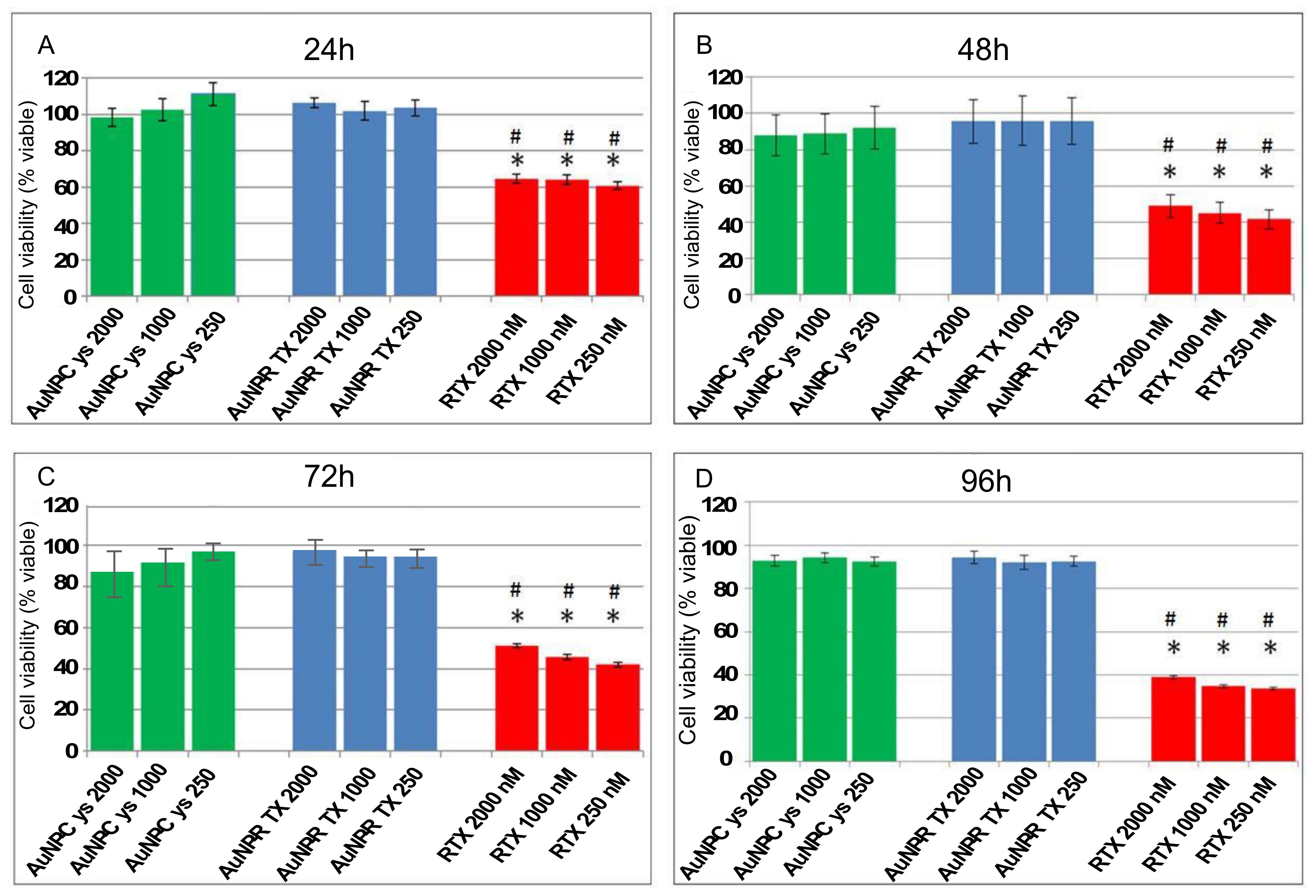
3.2. Cell Culture and Treatment for HCT 116 Cells with AuNP-Cys-RTX
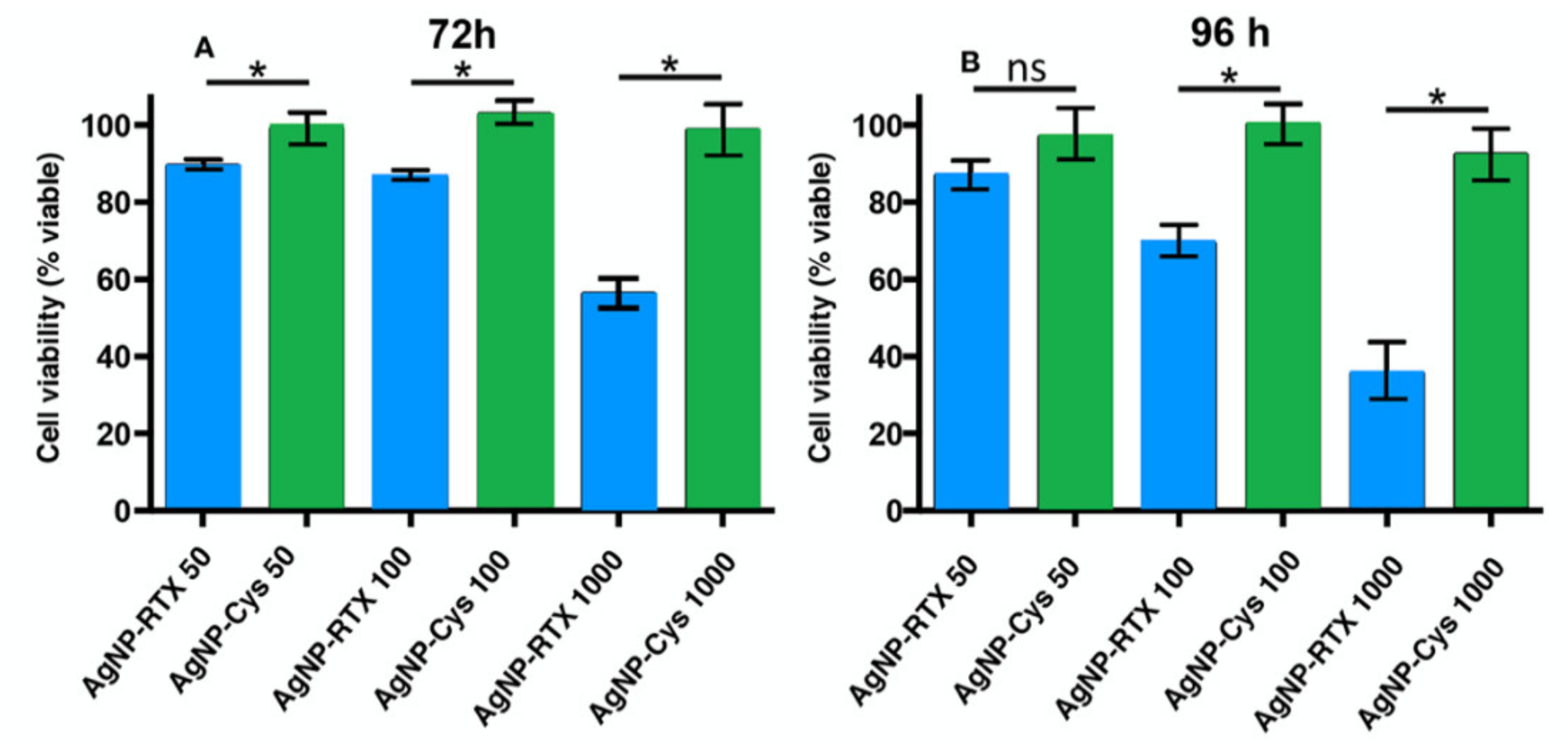
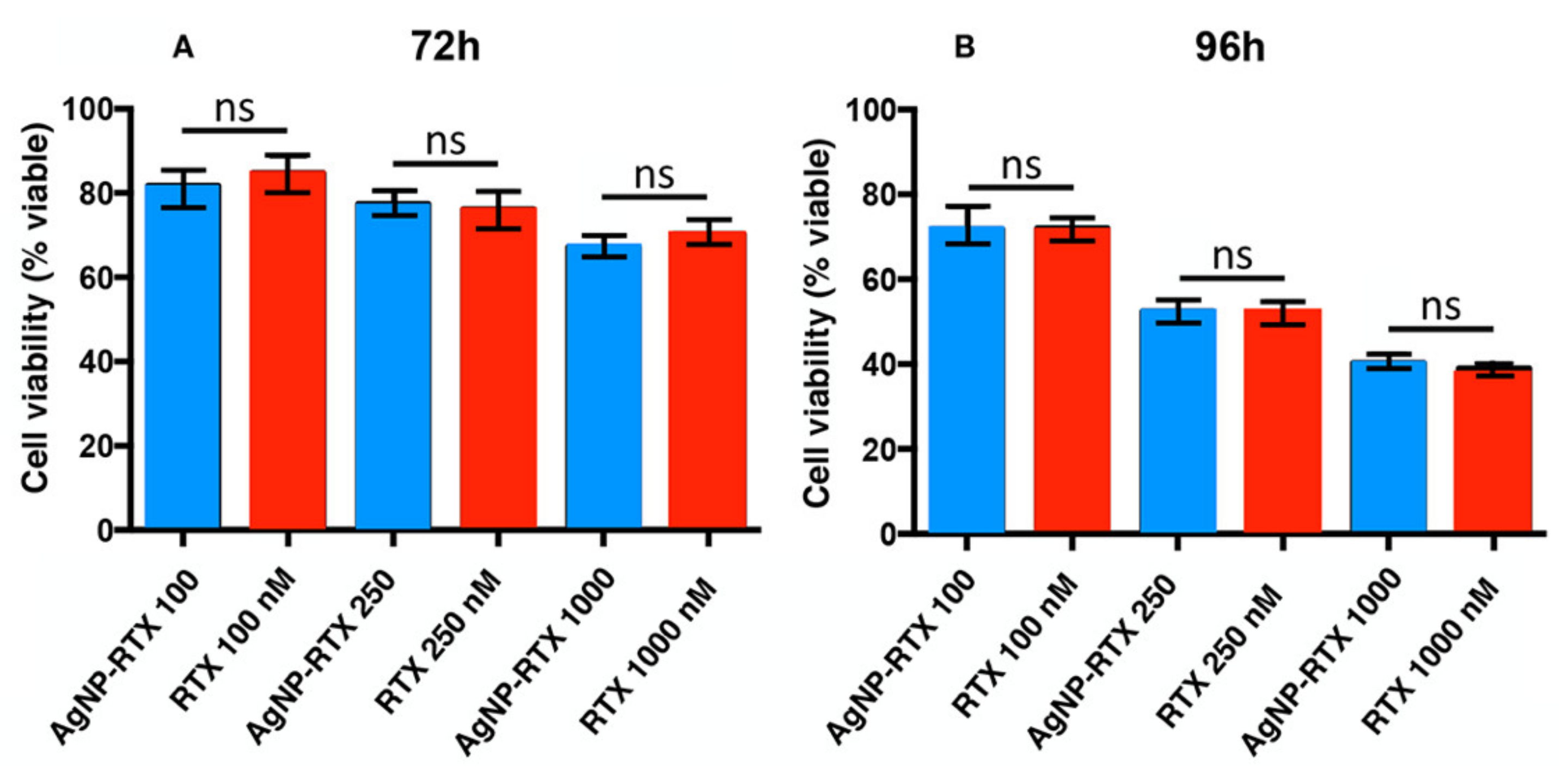
3.3. Cell Culture and Treatment for A549 Cells with AgNP-Cys-RTX
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Gu, F.X.; Langer, R.S.; Farokhzad, O.C.; Chan, J.M.; Wang, A.Z. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Habibi, N.; Quevedo, D.F.; Gregory, J.V.; Lahann, J. Emerging methods in therapeutics using multifunctional nanoparticles. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, K.; Kang, Q.; Zhao, X. Biogenic nanoparticles as immunomodulator for tumor treatment. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1646. [Google Scholar] [CrossRef]
- Alavijeh, A.A.; Barati, M.; Barati, M.; Dehkordi, H.A. The Potential of magnetic nanoparticles for diagnosis and treatment of cancer based on body magnetic field and organ-on-the-chip. Adv. Pharm. Bull. 2019, 9, 360–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohler, N.; Sun, C.; Fichtenholtz, A.; Gunn, J.; Fang, C.; Zhang, M. Methotrexate-immobilized poly(ethylene glycol) magnetic nanoparticles for MR imaging and drug delivery. Small 2006, 2, 785–792. [Google Scholar] [CrossRef]
- Bucharskaya, A.; Maslyakova, G.; Terentyuk, G.; Yakunin, A.; Avetisyan, Y.; Bibikova, O.; Tuchina, E.; Khlebtsov, B.; Khlebtsov, N.; Tuchin, V. Towards effective photothermal/photodynamic treatment using plasmonic gold nanoparticles. Int. J. Mol. Sci. 2016, 17, 1295. [Google Scholar] [CrossRef]
- Wadajkar, A.S.; Dancy, J.G.; Carney, C.P.; Hampton, B.S.; Ames, H.M.; Winkles, J.A.; Woodworth, G.F.; Kim, A.J. Leveraging Surface Plasmon Resonance to Dissect the Interfacial Properties of Nanoparticles: Implications for Tissue Binding and Tumor Penetration. Nanomedicine 2019, 20, 102024. [Google Scholar] [CrossRef]
- Kang, B.; Mackey, M.A.; El-Sayed, M.A. Nuclear targeting of gold nanoparticles in cancer cells induces DNA damage, causing cytokinesis arrest and apoptosis. J. Am. Chem. Soc. 2010, 132, 1517–1519. [Google Scholar] [CrossRef]
- Vigderman, L.; Zubarev, E.R. Therapeutic platforms based on gold nanoparticles and their covalent conjugates with drug molecules. Adv. Drug Deliv. Rev. 2013, 65, 663–676. [Google Scholar] [CrossRef]
- Vivero, L.; Sendra, J.; Parkkola, H.; Querol, J.; Ramis, M. Controlled-Release Nanoparticle System Comprising Hyaluronic Acid and Metal Nanoparticle Conjugate and Its Preparation. U.S. Patent Application No. WO 2011-EP74150, 11 December 2011. [Google Scholar]
- Mukhopadhyay, D.; Mukherjee, P.; Spaller, M. Peptides Targeted GAIP-Interacting Protein GIPC and Nanoparticles for Therapeutic and Diagnostic Applications. U.S. Patent Application No. US 14,060,932, 05 August 2010. [Google Scholar]
- Kim, J.H.; Choi, Y.J. Anti-Cancer Adjuvant Composition Containing Silver Nanoparticles. U.S. Patent Application No. KR 2016-181936, 9 July 2018. [Google Scholar]
- López, K.A.; Piña, M.N.; Alemany, R.; Vögler, O.; Barceló, F.; Morey, J. Antifolate modified iron oxide nanoparticles for targeted cancer therapy: Inclusion vs. covalent union. RSC Adv. 2014, 4, 19196–19204. [Google Scholar] [CrossRef]
- Li, Z.; Mu, Y.; Peng, C.; Lavin, M.F.; Shao, H.; Du, Z. Understanding the mechanisms of silica nanoparticles for nanomedicine. Wires Nanomed Nanobiotechol. 2020, 13, e1658. [Google Scholar]
- Kholer, N.; Sun, C.; Wang, J.; Zhang, M. Methotrexate-Modified Superparamagnetic nanoparticles and their intracellular uptake into human cancer cells. Langmuir 2005, 21, 8858–8864. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef] [PubMed]
- Jackman, A.L.; Taylor, G.A.; Gibson, W.; Kimbell, R.; Brown, M.; Calvert, A.H.; Judson, I.R.; Hughes, L.R. ICI D1694, a quinazoline antifolate thymidylate synthase inhibitor that is a potent inhibitor of L1210 tumor cell growth in vitro and in vivo: A new agent for clinical study. Cancer Res. 1991, 51, 5579–5586. [Google Scholar] [PubMed]
- Gonen, N.; Assaraf, Y.G. Antifolates in cancer therapy: Structure, activity and mechanisms of drug resistance. Drug Resist. Updates 2012, 15, 183–210. [Google Scholar] [CrossRef]
- Lederman, J.A.; Canevari, S.; Thigpen, T. Targeting the folate receptor: Diagnostic and therapeutic approaches to personalize cancer treatments. Ann. Oncol. 2015, 26, 2034–2043. [Google Scholar] [CrossRef]
- Shen, F.; Wu, M.; Ross, J.F.; Miller, D.; Ratnam, M. Folate Receptor Type. Gamma. Is Primarily a Secretory Protein Due to Lack of an Efficient Signal for Glycosylphosphatidylinositol Modification: Protein Characterization and Cell Type Specificity. Biochemistry 1995, 34, 5660–5665. [Google Scholar] [CrossRef]
- UniProt. Available online: https://www.uniprot.org/uniprot/P41439 (accessed on 30 December 2020).
- Zhao, R.; Diop-Bove, N.; Visentin, M.; Goldman, I.D. Mechanisms of membrane transport of folates into cells and across epithelia. Annu. Rev. Nutr. 2011, 31, 177–201. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.G.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [Green Version]
- Zwicke, G.L.; Mansoori, G.A.; Jeffery, C.J. Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Rev. 2012, 3, 18496. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Z.; Gao, Y.; Wientjes, M.G.; Au, J.L.-S. Improving delivery and efficacy of nanomedicines in solid tumors: Role of tumor priming. Nanomedicine (London) 2011, 6, 1605–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vander Heiden, M.G.; DeBerardinis, R.J. Understanding the Intersections between Metabolism and Cancer Biology. Cell 2017, 168, 657–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerqueira, B.B.; Lasham, A.; Shelling, A.N.; Al-Kassas, R. Nanoparticle therapeutics: Technologies and methods for overcoming cancer. Eur. J. Pharm. Biopharm. 2015, 97, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Palaia, I.; Giorgini, M.; De Medici, C.; Iadarola, R.; Vertechy, L.; Domenici, L.; Di Donato, V.; Tomao, F.; Muzii, L.; et al. Targeted drug delivery via folate receptors in recurrent ovarian cancer: A review. Onco Targets Ther. 2014, 7, 1223–1236. [Google Scholar] [CrossRef] [Green Version]
- Herizchi, R.; Abbasi, E.; Milani, M.; Akbarzadeh, A. Current methods for synthesis of gold nanoparticles. Artif. CellsNanomed. Biotechnol. 2014, 44, 596–602. [Google Scholar] [CrossRef]
- Gutiérrez, M.S.; Piña, M.N.; Morey, J. Fast microwave-assisted conjugation of magnetic nanoparticles with carboxylates of biological interest. RSC Adv. 2017, 7, 19385–19390. [Google Scholar] [CrossRef] [Green Version]
- Patil, V.; Malvankar, R.B.; Sastry, M. Role of particle size in individual and competitive diffusion of carboxylic acid derivatized colloid gold particles in thermally evaporated fatty amine films. Langmuir 1999, 15, 8197–8206. [Google Scholar] [CrossRef]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef]
- Zeng, S.; Baillargeat, D.; Ho, H.-P.; Yong, K.-T. Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chem. Soc. Rev. 2014, 43, 3426–3452. [Google Scholar] [CrossRef]
- O’Shannessy, D.J.; Davis, D.W.; Anderes, K.; Somers, E.B. Isolation of circulating tumor cells from multiple epithelial cancers with ApoStream(®) for detecting (or Monitoring) the expression of folate receptor alpha. Biomark. Insights 2016, 11, 7–18. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, I.; Sohn, K.-J.; Stempak, J.M.; Croxford, R.; Kim, Y.-I. Folate deficiency induces cell-specific changes in the steady-state transcript levels of genes involved in folate metabolism and 1-carbon transfer reactions in human colonic epithelial cells. J. Nutr. 2007, 137, 607–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, C.; Ribas, M.; Aiza, G.; Peinado, M.A. Genetic determinants of methotrexate responsiveness and resistance in colon cancer cells. Oncogene 2005, 24, 6842–6847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, W.; Tan, Q.; Qiao, J.; Shen, G.; Qi, L. D-Proline capped gold nanoclusters for turn-on detection of serum Raltitrexed. Chin. Chem. Lett. 2019, 30, 1627–1630. [Google Scholar] [CrossRef]
- Liao, J.; Jia, Y.; Wu, Y.; Shi, K.; Yang, D.; Li, P.; Qian, Z. Physical-, chemical-, and biological-responsive nanomedicine for cancer therapy. Wires Nanomed. Nanobiotechnol. 2020, 12, e1581. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morey, J.; Llinás, P.; Bueno-Costa, A.; León, A.J.; Piña, M.N. Raltitrexed-Modified Gold and Silver Nanoparticles for Targeted Cancer Therapy: Cytotoxicity Behavior In Vitro on A549 and HCT-116 Human Cancer Cells. Materials 2021, 14, 534. https://doi.org/10.3390/ma14030534
Morey J, Llinás P, Bueno-Costa A, León AJ, Piña MN. Raltitrexed-Modified Gold and Silver Nanoparticles for Targeted Cancer Therapy: Cytotoxicity Behavior In Vitro on A549 and HCT-116 Human Cancer Cells. Materials. 2021; 14(3):534. https://doi.org/10.3390/ma14030534
Chicago/Turabian StyleMorey, Jeroni, Pere Llinás, Alberto Bueno-Costa, Alberto J. León, and M. Nieves Piña. 2021. "Raltitrexed-Modified Gold and Silver Nanoparticles for Targeted Cancer Therapy: Cytotoxicity Behavior In Vitro on A549 and HCT-116 Human Cancer Cells" Materials 14, no. 3: 534. https://doi.org/10.3390/ma14030534
APA StyleMorey, J., Llinás, P., Bueno-Costa, A., León, A. J., & Piña, M. N. (2021). Raltitrexed-Modified Gold and Silver Nanoparticles for Targeted Cancer Therapy: Cytotoxicity Behavior In Vitro on A549 and HCT-116 Human Cancer Cells. Materials, 14(3), 534. https://doi.org/10.3390/ma14030534





