PROMETHEUS: A Copper-Based Polymetallic Catalyst for Automotive Applications. Part I: Synthesis and Characterization
Abstract
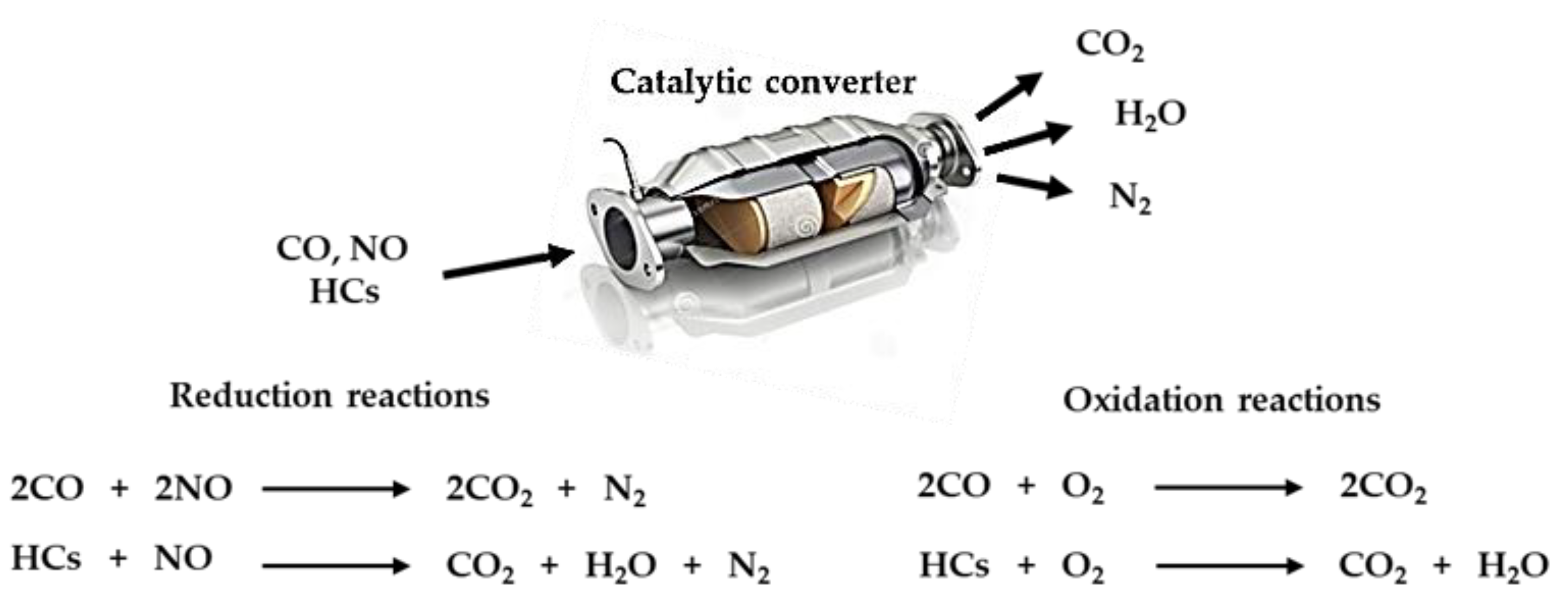
:1. Introduction
2. Materials and Methods
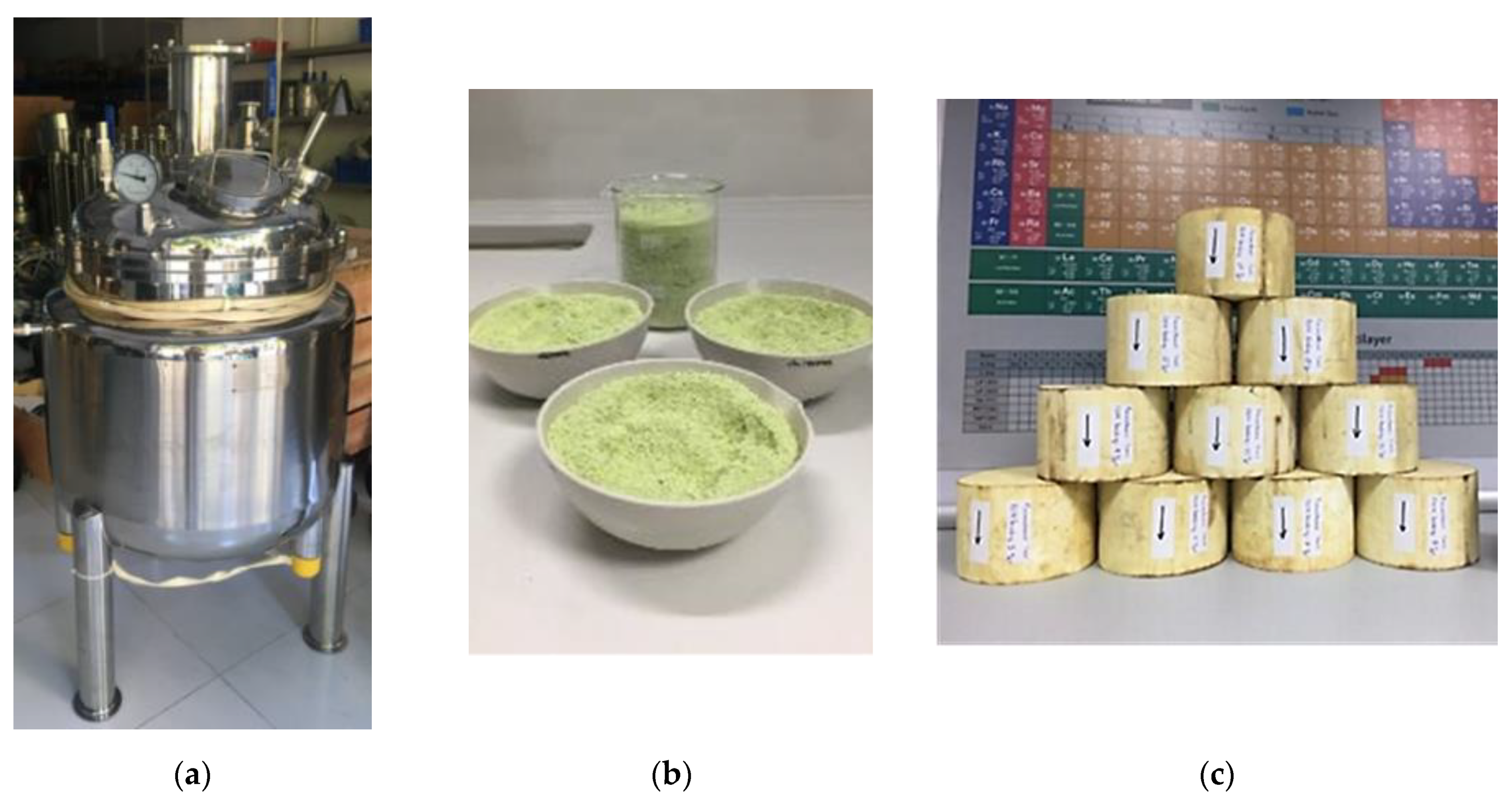
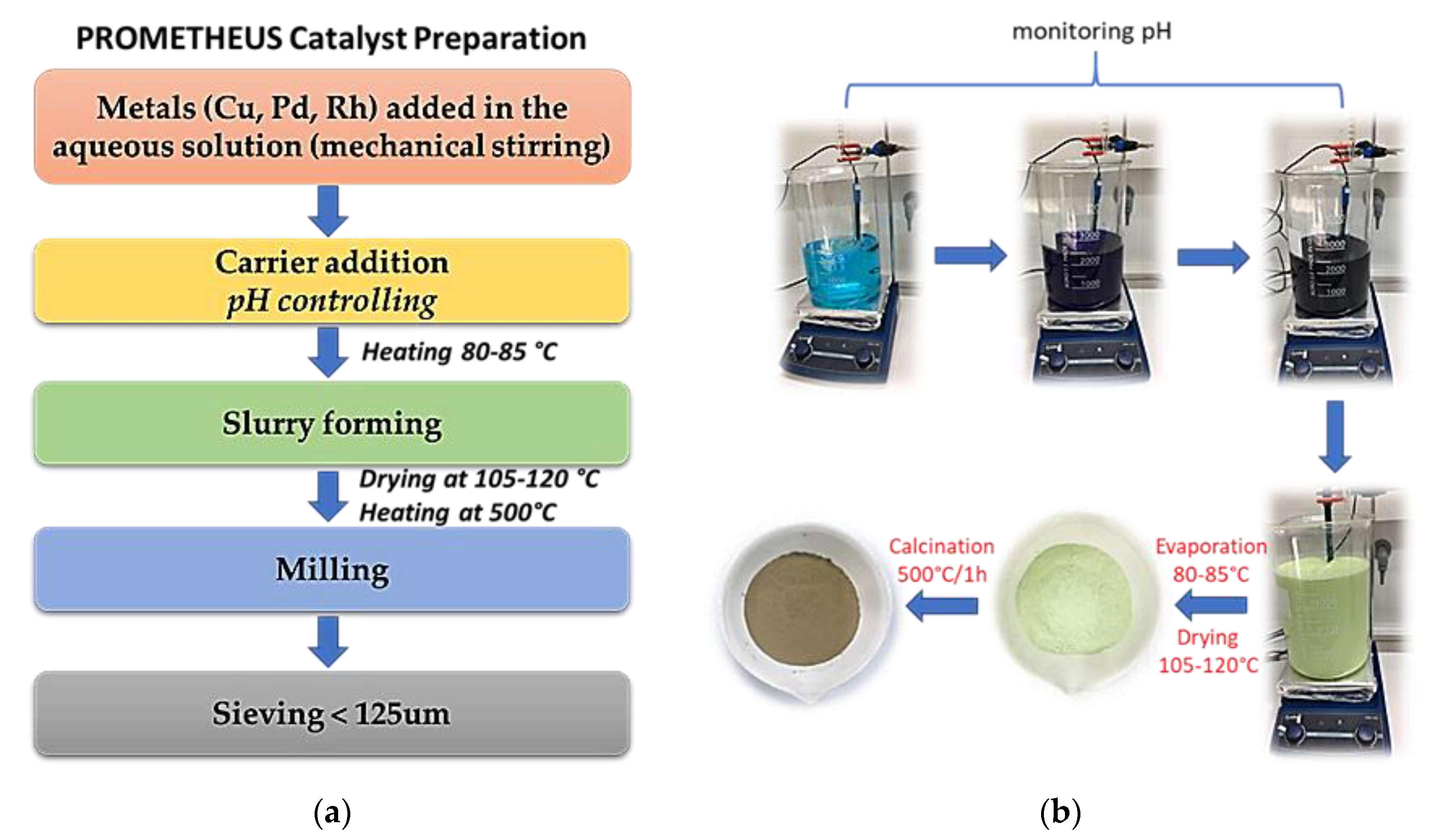
2.1. Prometheus Nano-Catalyst Powder Preparation
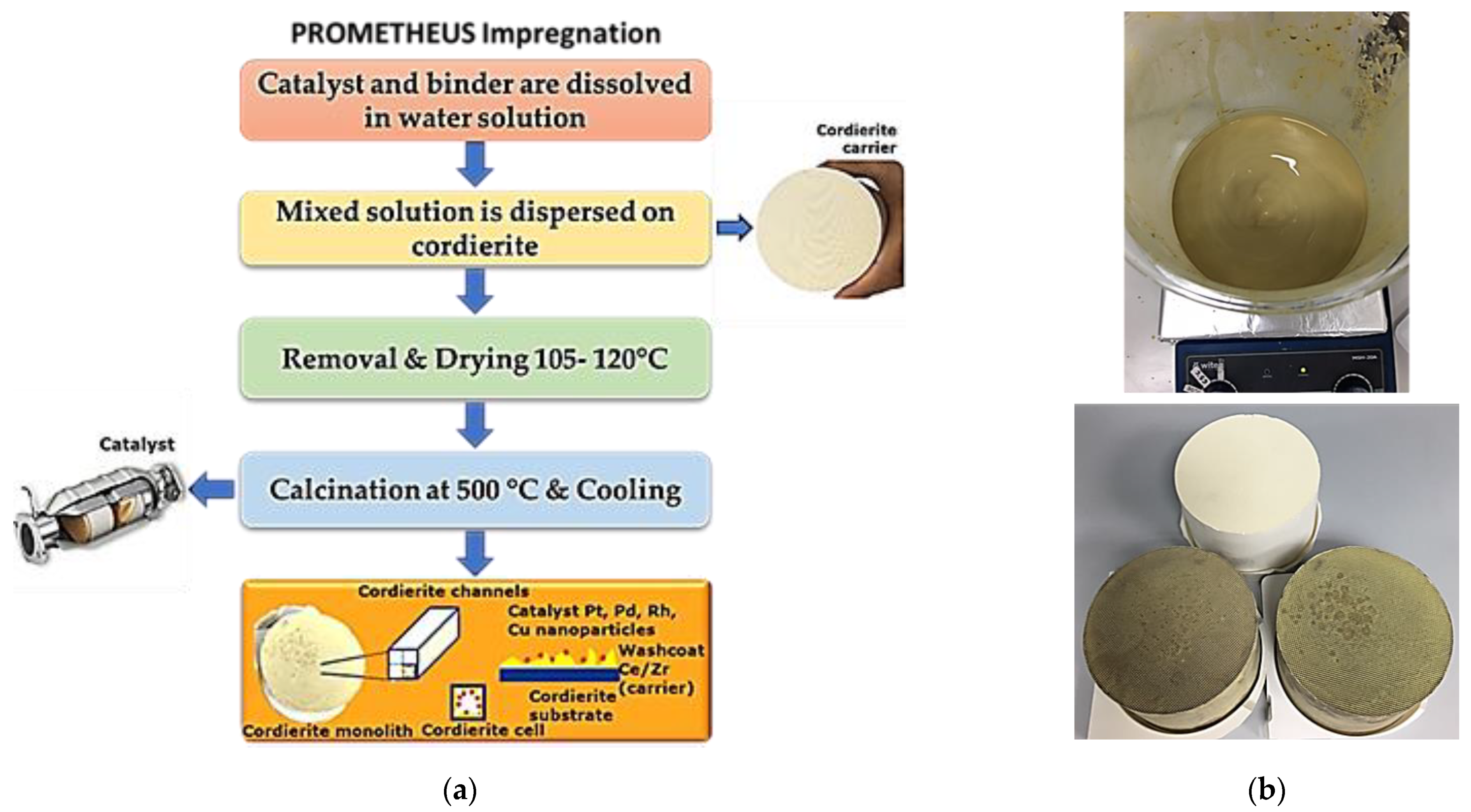
2.2. Prometheus Monolithic Catalysts
2.3. Materials’ Characterization
2.4. Catalytic Activity Measurement
3. Results
3.1. Morphology and Structure of the Catalyst
3.1.1. X-ray Diffraction Characterization
3.1.2. Raman Analysis
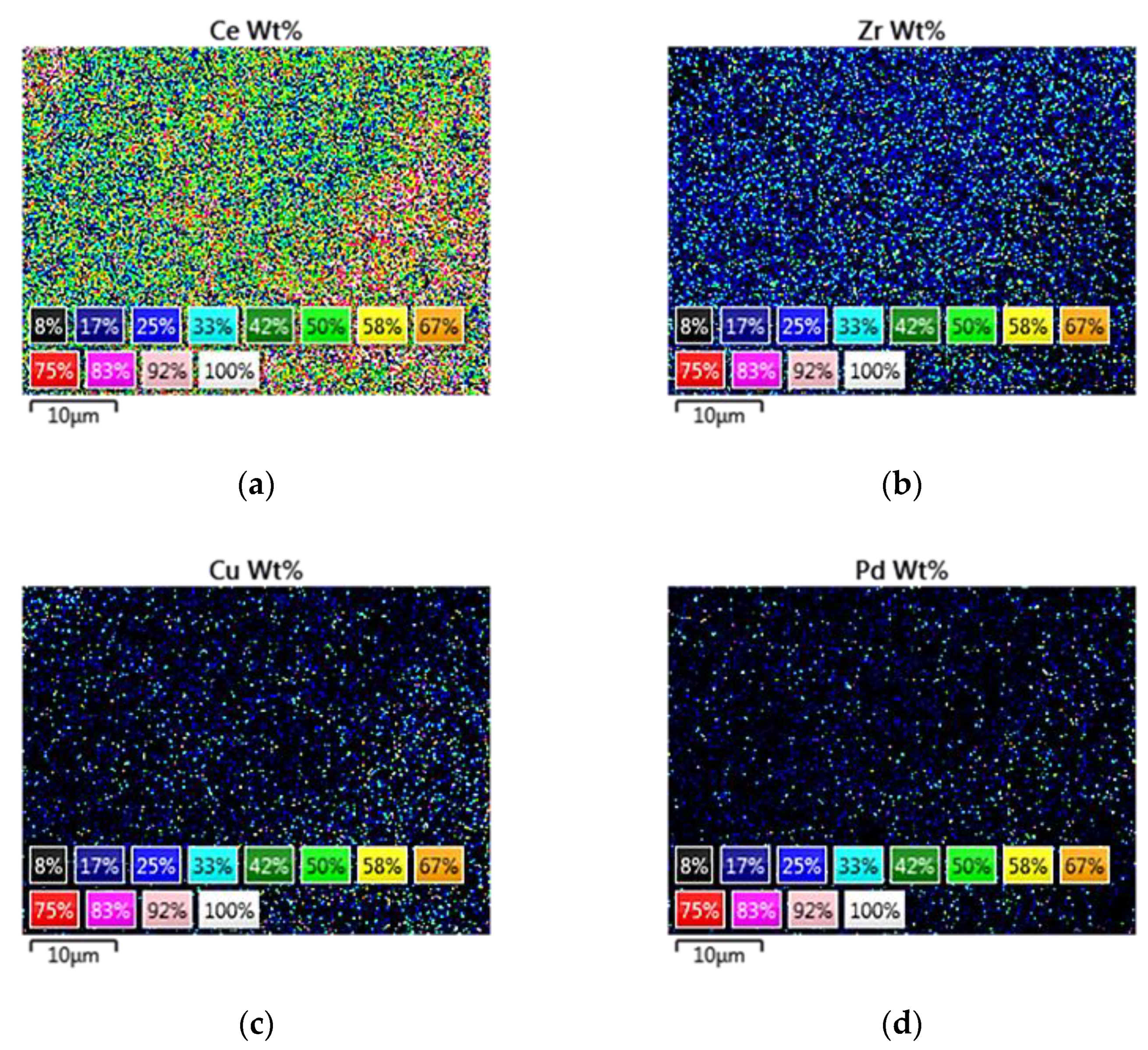
3.1.3. Scanning Electron Microscopy (SEM)—Energy-Dispersive X-ray Spectroscopy (EDS) Analysis
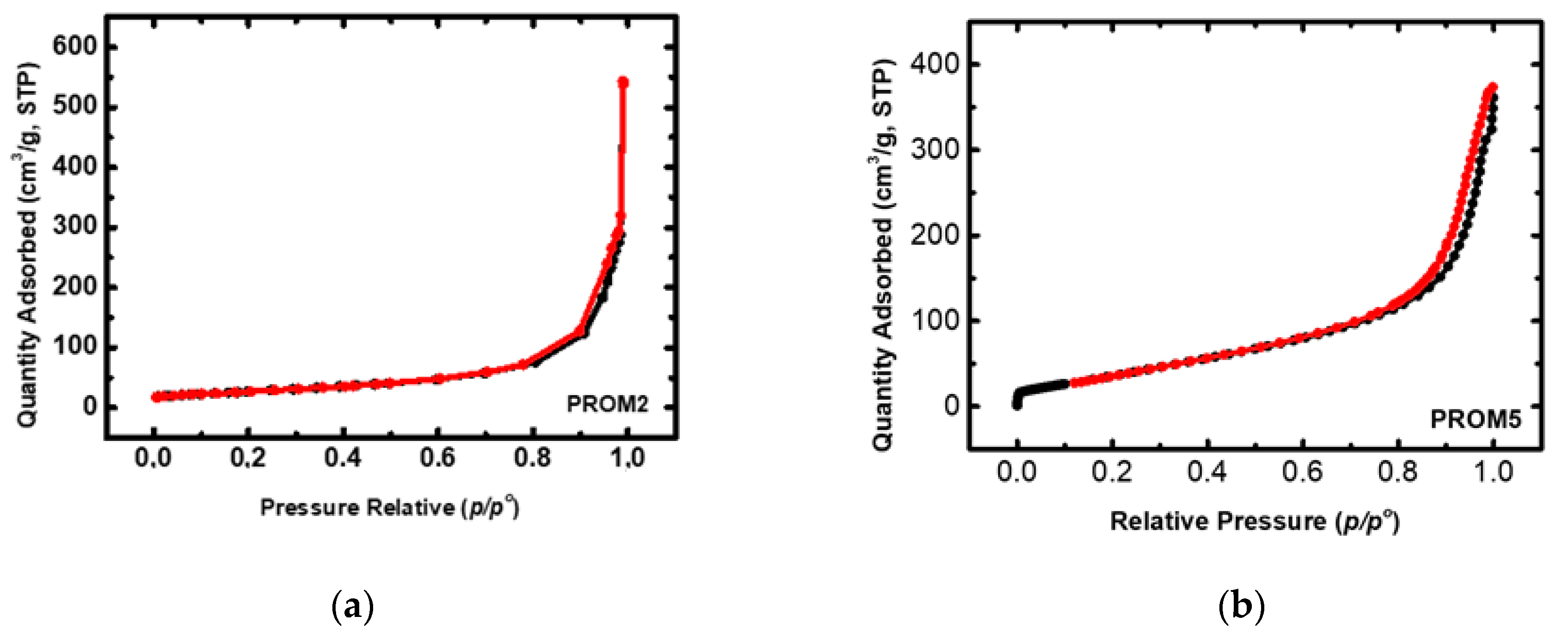
3.1.4. N2 Physisorption
3.2. Catalyst Loading
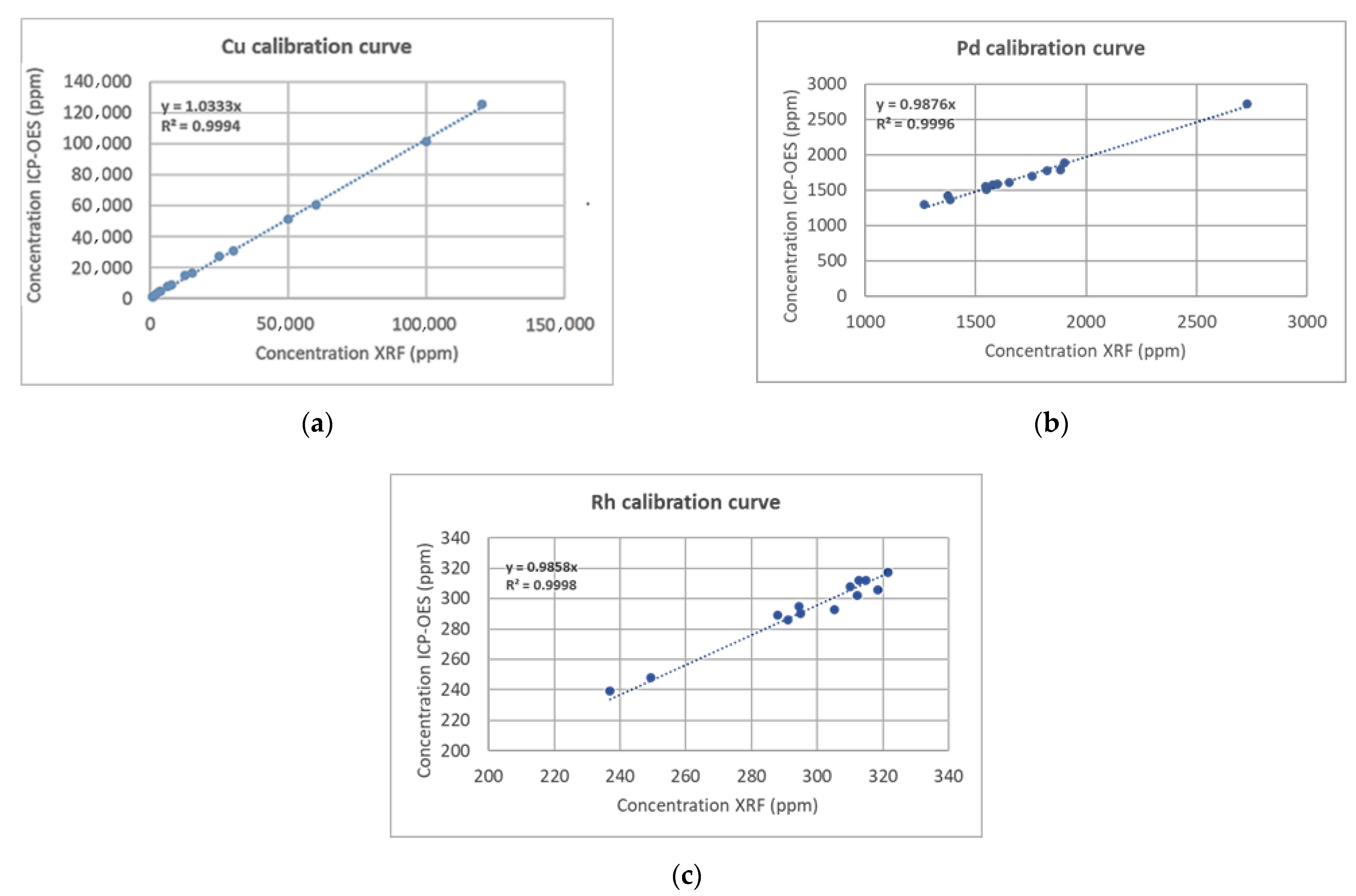
3.2.1. Inductively Coupled Plasma Mass Spectrometry (ICP-MS), X-ray Fluorescence (XRF) Analysis
3.2.2. Morphology of Full-Scale Prometheus Catalyst
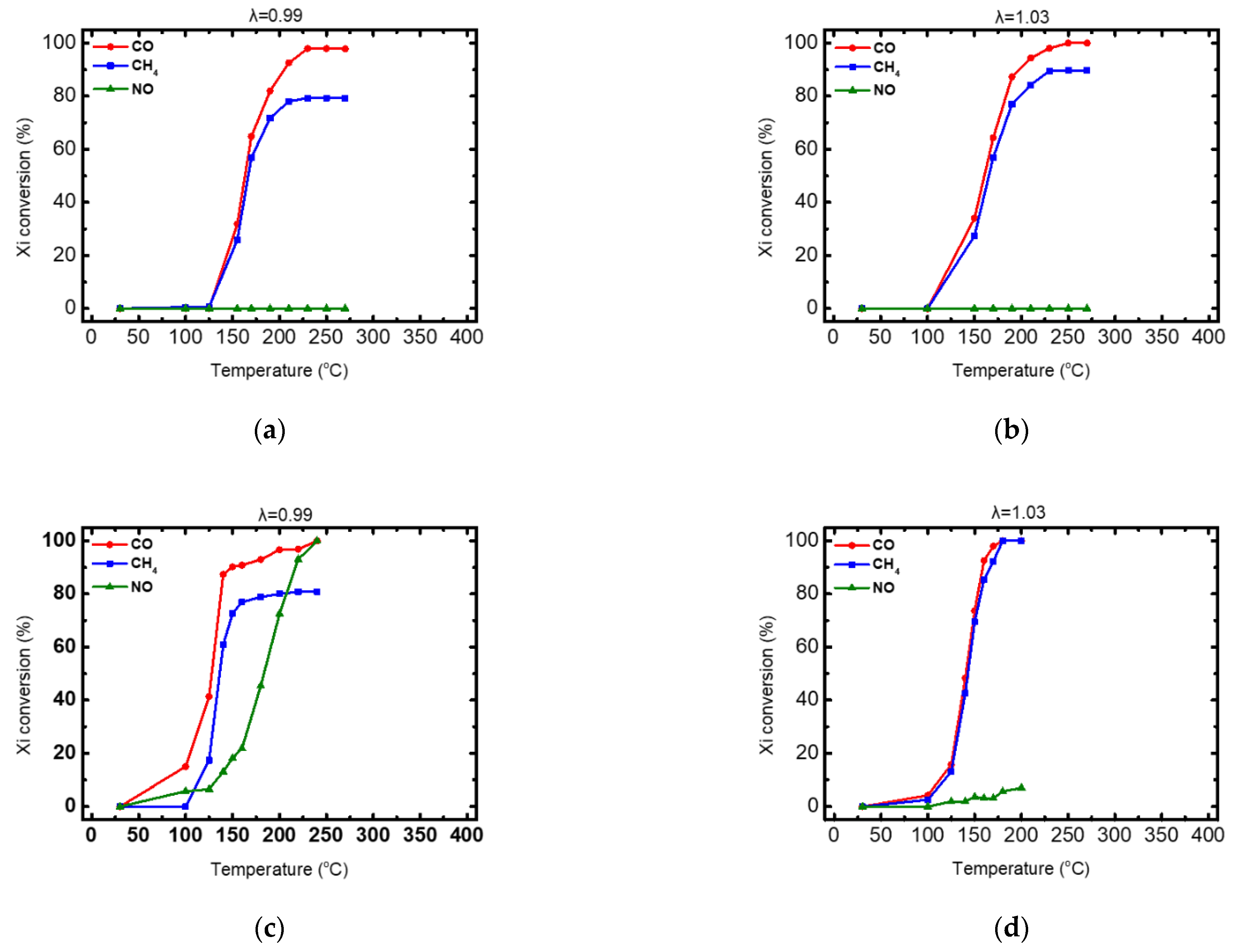
3.3. Catalytic Performance
3.3.1. Copper Monometallic and Prometheus Trimetallic Catalytic powders (WashCoats)
3.3.2. The 15 g/ft3 Trimetallic Prometheus Catalyst Powder
4. Conclusions
5. Patents
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gandhi, H.S.; Graham, G.W.; Mc Gabe, R.W. Automotive Exhaust Catalysis. J. Catal. 2003, 216, 433–442. [Google Scholar] [CrossRef]
- Li, G.; Wang, Q.; Zhao, B.; Zhou, R. A new insight into the role of transition metals doping with CeO2–ZrO2 and its application in Pd-only three-way catalysts for automotive emission control. Fuel 2012, 92, 360–368. [Google Scholar] [CrossRef]
- Wang, S.Y.; Li, N.; Luo, L.F.; Huang, W.X.; Pu, Z.Y.; Wang, Y.J.; Hu, G.S.; Luo, M.F.; Lu, J.Q. Probing Different Effects of Surface MOy and M+n Species (M=Cu, Ni, Co, Fe) for xMOy/Ce0.9M0.1−x O2−δ Catalysts in CO Oxidation. Appl. Catal. B 2014, 144, 325–332. [Google Scholar] [CrossRef]
- Martinez-Arias, A.; Fernadez-Garcia, M.; Galvez, O.; Coronado, J.M.; Anderson, J.A.; Conesa, J.C.; Soria, J.; Munuera, G. Comparative Study on Redox Properties and Catalytic Behavior for CO Oxidation of CuO/CeO2 and CuO/ZrCeO4 Catalysts. J. Catal. 2000, 195, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; He, B.; Lu, Z.; Hermansson, K. Physisorbed, Chemisorbed, and Oxidized CO on Highly Active Cu-CeO2 (111). J. Phys. Chem. C 2010, 114, 4486–4494. [Google Scholar] [CrossRef]
- Jia, A.; Jiang, S.; Lu, J.; Luo, M. Study of Catalytic Activity at the CuO-CeO2 Interface for CO Oxidation. J. Phys. Chem. C 2010, 114, 21605–21610. [Google Scholar] [CrossRef]
- Aneggi, E.; Llorca, J.; Boaro, M.; Trovarelli, A. Surface-structure Sensitivity of CO Oxidation over Polycrystalline Ceria Powders. J. Catal. 2005, 234, 88–95. [Google Scholar] [CrossRef]
- Morales, J.; Caballero, A.; Holgado, J.P.; Espinós, J.P.; González-Elipe, A.R. X-ray Photoelectron Spectroscopy and Infrared Study of the Nature of Cu Species in Cu/ZrO2 de-NOx Catalysts. Phys. Chem. B 2002, 106, 10185–10190. [Google Scholar] [CrossRef]
- Yao, X.; Yu, Q.; Ji, Z.; Lv, Y.; Cao, Y.; Tang, C.; Gao, F.; Dong, L.; Chen, Y. A Comparative Study of Different Doped Metal Cations on the Reduction, Adsorption and Activity of CuO/Ce0.67M0.33O2 (M=Zr4+, Sn4+, Ti4+) Catalysts for NO+CO Reaction. Appl. Catal. B 2013, 130−131, 293–304. [Google Scholar] [CrossRef]
- Yao, X.; Xiong, Y.; Zou, W.; Zhang, L.; Wu, S.; Dong, X.; Gao, F.; Deng, Y.; Tang, C.; Chen, Z.; et al. Correlation between the Physicochemical Properties and Catalytic Performances of CexSn1−xO2 Mixed Oxides for NO Reduction by CO. Appl. Catal. B 2014, 144, 152–165. [Google Scholar] [CrossRef]
- Kacimi, M.; Ziyad, M.; Liotta, L.F. Cu on Amorphous AlPO4: Preparation, Characterization and Catalytic Activity in NO Reduction by CO in Presence of Oxygen. Catal. Today 2015, 241, 151–158. [Google Scholar] [CrossRef]
- Ma, L.; Luo, M.-F.; Chen, S.-Y. Redox Behavior and Catalytic Properties of CuO/Ce0.8Zr0.2O2 Catalysts. Appl. Catal. A 2003, 242, 151–159. [Google Scholar] [CrossRef]
- Martínez-Arias, A.; Hungría, A.B.; Iglesias-Juez, A.; Fernández-García, M.; Anderson, J.A.; Conesa, J.C.; Munuera, G.; Soria, J. Redox and Catalytic Properties of CuO/CeO2 under CO + O2 + NO: Promoting Effect of NO on CO Oxidation. Catal. Today 2012, 180, 81–87. [Google Scholar] [CrossRef]
- Shore, L.; Ruettinger, W.F.; Farrauto, R.J. Platinum Group Metal Promoted Copper Oxidation Catalysts and Methods for Carbon Monoxide Remediation. U.S. Patent 2002/0131915 A1, 19 September 2002. [Google Scholar]
- Mrabet, D.; Abassi, A.; Cherizol, R.; Do, T.-O. One-pot Solvothermal Synthesis of Mixed Cu-Ce-Ox Nanocatalysts and their Catalytic Activity for Low Temperature CO oxidation. Appl. Catal. A 2012, 447–448, 60–66. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, Y.; Wang, Z.; Liu, W.; Wang, L. Catalytic oxidation of CO over mesoporous copper-doped ceria catalysts via a facile CTAB assisted synthesis. RSC Adv. 2018, 8, 14888–14897. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Dong, L.; Shen, M.; Liu, D.; Wang, J.; Ding, W.; Chen, Y. Influence of supports on the activities of copper oxide species in the low-temperature NO + CO reaction. Appl. Catal. B 2001, 31, 61–69. [Google Scholar] [CrossRef]
- Bedford, R.E.; LaBarge, W.J. Base Metal Automotive Exhaust Catalyst with Improved Activity and Stability and Method of Making the Catalysts. U.S. Patent 5,063,193, 5 November 1991. [Google Scholar]
- Hao, X.; Cai, J. Engine Exhaust Catalysts Containing Copper-Ceria. U.S. Patent 2011/0143921 A1, 29 September 2011. [Google Scholar]
- Park, S.-E.; Kim, G.-M.; Lee, Y.-J.; Chang, J.-S.; Han, S.-H. Method for Removing Nitrogen Oxides in Exhaust Gas by Selective Catalytic Reduction and Catalyst for Reduction of Nitrogen Oxides. U.S. Patent 5,879,645, 9 March 1999. [Google Scholar]
- Acres, G.J.K. Catalyst Comprising Platinum, Rhodium and a Base Metal. U.S. Patent 3,840,471, 8 October 1974. [Google Scholar]
- Kim, J.-R.; Myeong, W.-J.; Ihm, S.-K. Characteristics of CeO2-ZrO2 mixed oxide prepared by continuous hydrothermal synthesis in supercritical water as support of Rh catalyst for catalytic reduction of NO. J. Catal. 2009, 263, 123–133. [Google Scholar] [CrossRef]
- Li, C.; Gu, X.; Wang, Y.; Wang, Y.; Wnag, Y.; Liu, X.; Lu, G. Synthesis and Characterization of Mesostructured ceria-zirconia solid solution. J. Rare Earths 2009, 27, 211–215. [Google Scholar] [CrossRef]
- Lamas, D.G.; Lascalea, G.E.; Juarez, R.E.; Djurado, E.; Perez, L.; Walsoe de Reca, N.E. Metastable forms of the tetragonal phase in compositionally homogeneous, nanocrystalline zirconia-ceria powders synthesized by gel-combustion. J. Mater. Chem. 2003, 13, 904–910. [Google Scholar] [CrossRef]
- Enzo, S.; Delogu, F.; Frattini, R.; Primavera, A.; Trovarelli, A. Structural Characterization of ceria-zirconia powder catalysts prepared by high-energy mechanical milling: A neutron diffraction study. J. Mater. Res. 2000, 15, 1538–1545. [Google Scholar] [CrossRef]
- Escribano, V.S.; López, E.F.; Panizza, M.; Resini, C.; Amores, J.M.G.; Guido Busca, G. Characterization of cubic ceria–zirconia powders by X-ray diffraction and vibrational and electronic spectroscopy. Solid States Sci. 2003, 5, 1369–1376. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Rawski, M.; Vakros, J.; Avgouropoulos, G. A Novel Post-Synthesis Modification of CuO-CeO2 Catalysts: Effect on Their Activity for Selective CO Oxidation. ChemCatChem 2018, 10, 2096–2106. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Vakros, J.; Avgouropoulos, G. Impact of acid treatment of CuO-CeO2 catalysts on the preferential oxidation of CO reaction. Catal. Commun. 2018, 115, 68–72. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, C.; Hanson, J.C.; Robinson, R.; Herman, I.P.; Chan, S. Phases in Ceria-Zirconia Binary Oxide (1-x)CeO2-xZrO2. Nanoparticles J. Am. Ceram. Soc. 2006, 89, 1028–1036. [Google Scholar]
- Palma, V.; Pisano, D.; Martino, M.; Ricca, A.; Ciambelli, P. Comparative studies of low temperature water gas shift reaction over Plarinum based catalysts. Chem. Eng. Trans. 2014, 39, 31–36. [Google Scholar] [CrossRef]
- Yashima, M.; Arashi, H.; Kakihana, M.; Yoshimura, M. Raman Scattering Study of Cubic–Tetragonal Phase Transition in Zr1−xCexO2 Solid Solution. J. Am. Ceram. Soc. 1994, 77, 1067–1071. [Google Scholar] [CrossRef]
- Ozawaa, M.; Takahashi-Morita, M.; Kobayashi, K.; Haneda, M. Core-shell type ceria zirconia support for platinum and rhodium three-way catalysts. Catal. Today 2017, 281, 482–489. [Google Scholar] [CrossRef]
- Nicole, J.; Tsiplakides, D.; Pliangos, C.; Verykios, X.E.; Comninellis, C.; Vayenas, C.G. Electrochemical Promotion and Metal–Support Interactions. J. Catal. 2001, 204, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Vernoux, P.; Lizarraga, L.; Tsampas, M.N.; Sapountzi, F.M.; De Lucas-Consuegra, A.; Valverde, J.-L.; Souentie, S.; Vayenas, C.G.; Tsiplakides, D.; Balomenou, S.; et al. Ionically Conducting Ceramics as Active Catalyst Supports. Chem. Rev. 2013, 113, 8192–8260. [Google Scholar]
- Zafiris, G.S.; Gorte, R.J. Evidence for Low-Temperature Oxygen Migration from Ceria to Rh. J. Catal. 1993, 139, 561–567. [Google Scholar] [CrossRef]

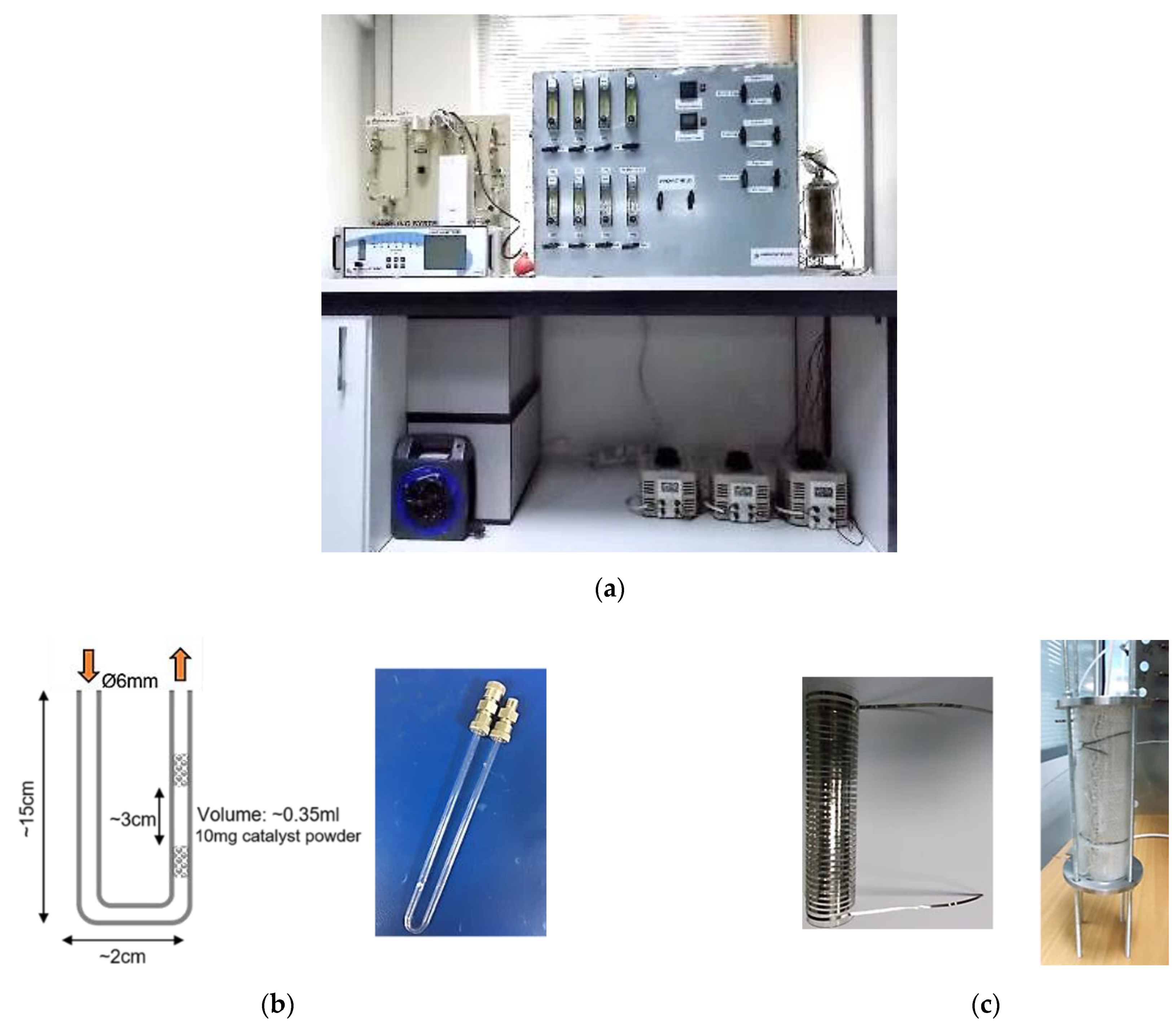

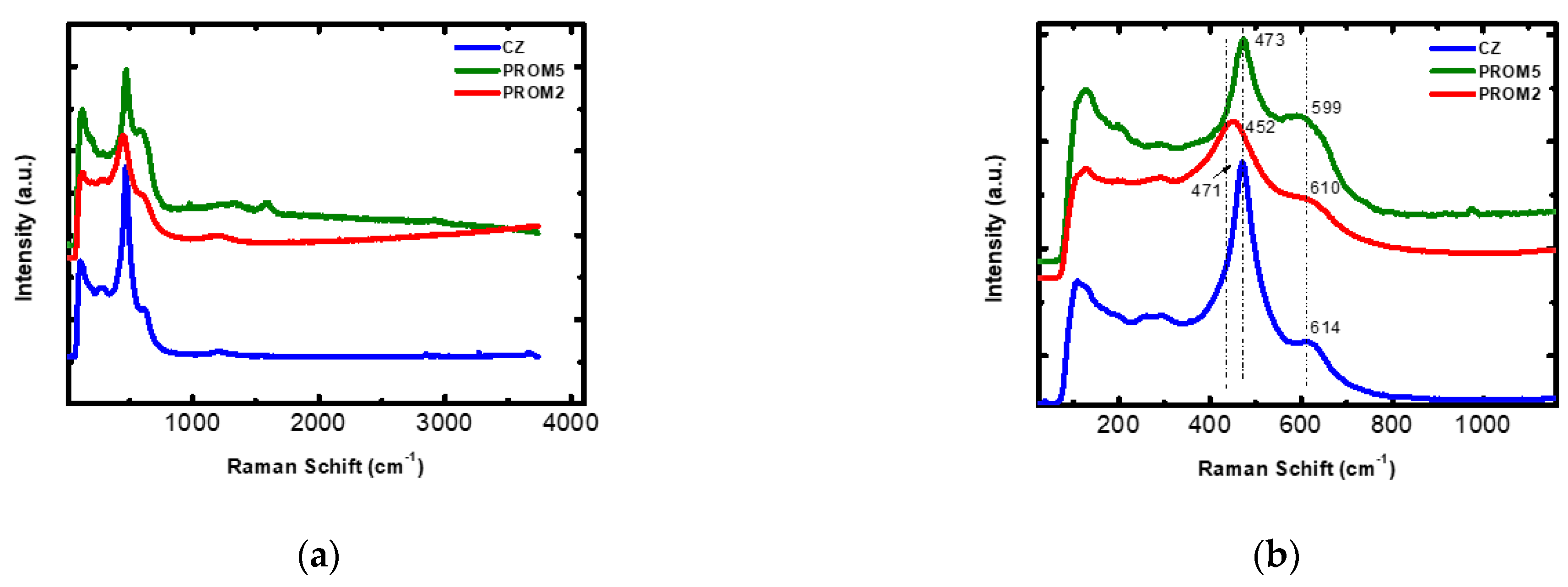
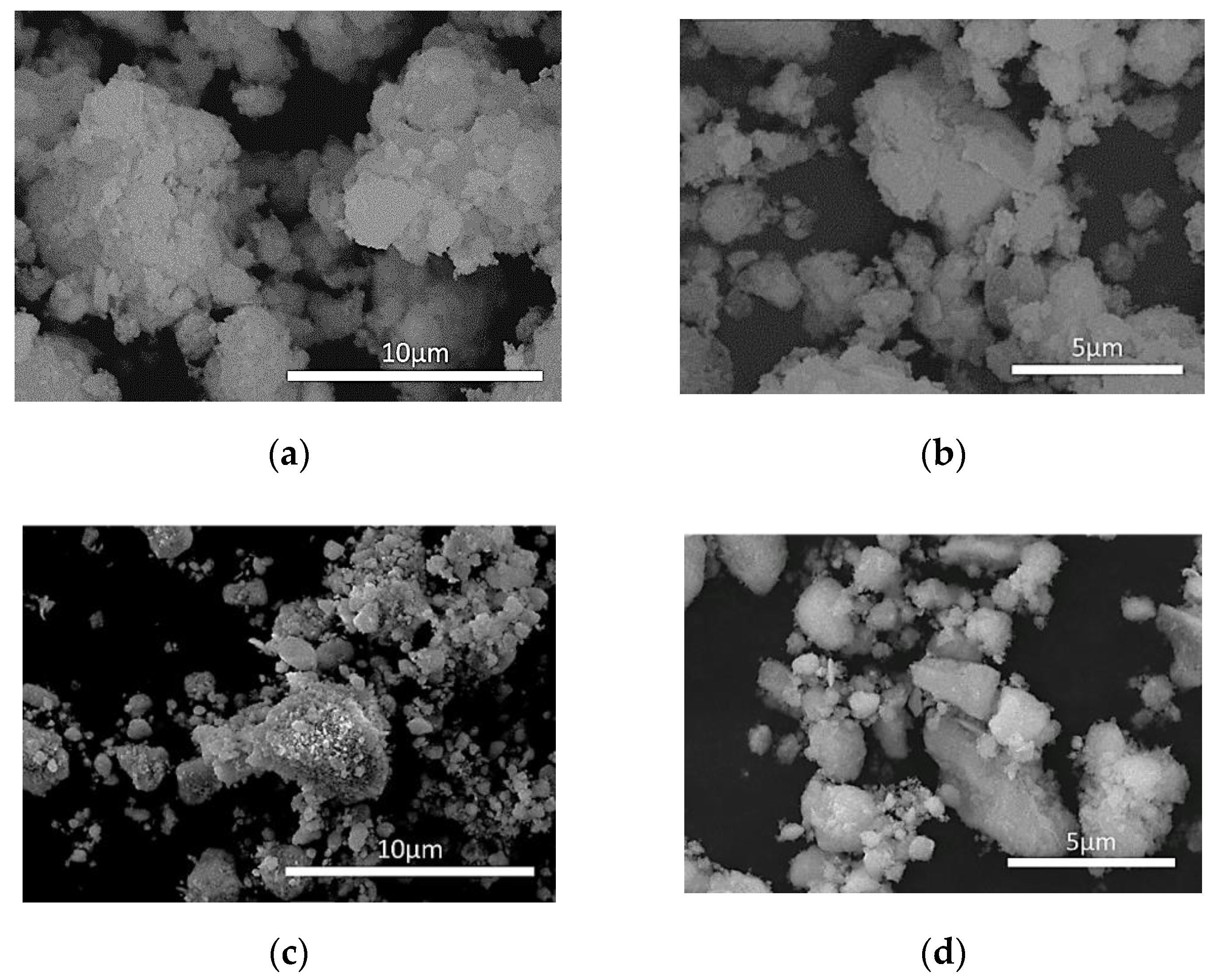

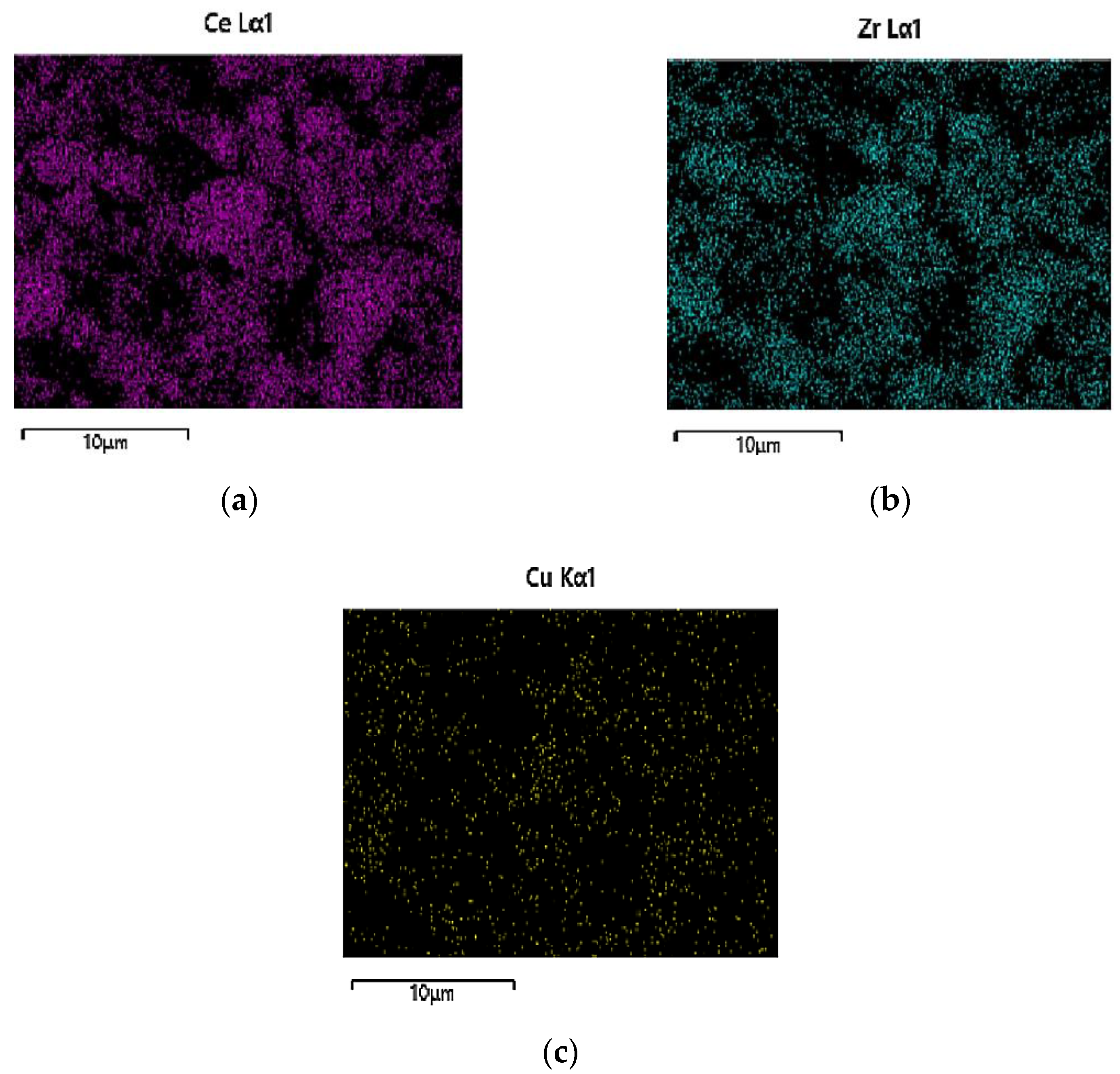

| Stage | Date | CO (g/Km) | ΤHC (g/Km) | HC + NOx (g/Km) | NOx (g/Km) | PM (g/Km) | PN/Km |
|---|---|---|---|---|---|---|---|
| Euro 1 | 1992 | 2.72 | - | 0.97 | - | - | - |
| Euro 2 | 1996 | 2.2 | - | 0.5 | - | - | - |
| Euro 3 | 2000 | 2.3 | 0.2 | - | 0.15 | - | - |
| Euro 4 | 2005 | 1.0 | 0.1 | - | 0.08 | - | - |
| Euro 5 | 2009 | 1.0 | 0.1 | - | 0.06 | 0.005 | - |
| Euro 6 | 2014 | 1.0 | 0.1 | - | 0.06 | 0.005 | 6.0 × 1011 |
| Gas Component | Rich-Burn Conditions λ ≈ 0.99 | Lean-Burn Conditions λ ≈ 1.03 |
|---|---|---|
| CO | 1% | 1% |
| CO2 | 12% | 12% |
| O2 | 0.91% | 0.95% |
| NO | 800 ppm | 800 ppm |
| CH4 | 2500 ppm | 2500 ppm |
| H2O | 10% | 10% |
| Pure N2 | balance | balance |
| Total vol. Flow rate | 300 sccm 1 | 300 sccm 1 |
| GHSV 2 | 50.000 h−1 | 50.000 h−1 |
| Catalyst | Element (wt%) | ||||
|---|---|---|---|---|---|
| Ce | Zr | Cu | Pd | Rh | |
| PROM2 | 69.0 | 27.0 | 2.5 | - | - |
| PROM5 | 53.4 | 15.0 | 3.0 | 0.6 | - |
| Sample | Technique | Elements (wt%) | ||
|---|---|---|---|---|
| Cu | Pd | Rh | ||
| PROM2 | ICP-MS | 1.47 | 0.504 | 0.097 |
| XRF | 1.51 | 0.494 | 0.094 | |
| PROM5 | ICP-MS | 2.53 | 0.823 | 0.335 |
| XRF | 2.65 | 1.124 | 0.481 | |
| Catalyst | CO Oxidation (%) | CH4 Oxidation (%) | NO Reduction (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T50 | T90 | T99 | Max. (%) | T50 | T90 | T99 | Max. (%) | T50 | T90 | T99 | Max. (%) | |
| CuCZ | 160 | 200 | - | 98 | 160 | - | - | 79 | - | - | - | 0 |
| PROM2 | 130 | 150 | 200 | 100 | 135 | - | - | 81 | 185 | 215 | 230 | 100 |
| Catalyst | CO Oxidation (%) | CH4 Oxidation (%) | NO Reduction (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T50 | T90 | T99 | Max. (%) | T50 | T90 | T99 | Max. (%) | T50 | T90 | T99 | Max. (%) | |
| CuCZ | 160 | 200 | 240- | 100 | 160 | 235 | - | 90 | - | - | - | 0 |
| PROM2 | 140 | 155 | 175 | 100 | 143 | 165 | 180 | 100 | - | - | - | 6 |
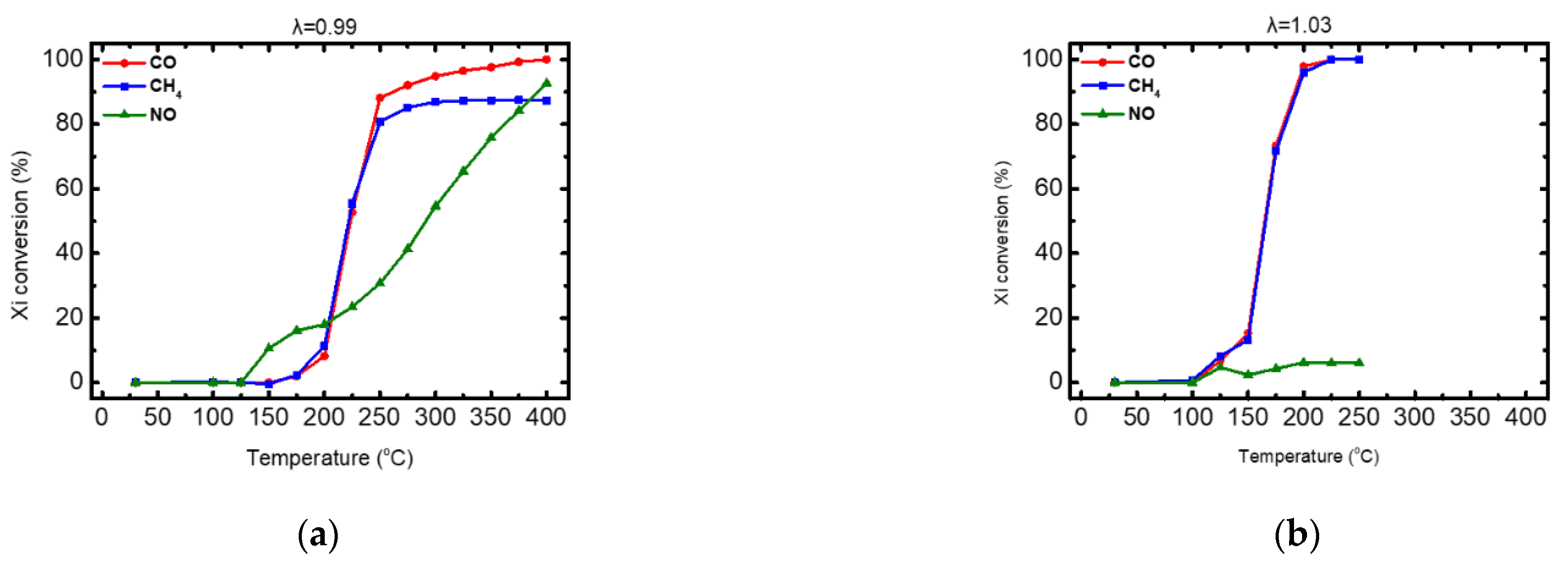
| CO Oxidation (%) | CH4 Oxidation (%) | NO Reduction (%) | ||||
|---|---|---|---|---|---|---|
| λ 0.99 | λ 1.03 | λ 0.99 | λ 1.03 | λ 0.99 | λ 1.03 | |
| T50 | 220 | 170 | 220 | 170 | 285 | - |
| T90 | 260 | 190 | - | 190 | 390 | - |
| T99 | 375 | 215 | - | 215 | - | - |
| efficiency | 100% | 100% | 87% | 100% | 96% | 6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakoumis, I. PROMETHEUS: A Copper-Based Polymetallic Catalyst for Automotive Applications. Part I: Synthesis and Characterization. Materials 2021, 14, 622. https://doi.org/10.3390/ma14030622
Yakoumis I. PROMETHEUS: A Copper-Based Polymetallic Catalyst for Automotive Applications. Part I: Synthesis and Characterization. Materials. 2021; 14(3):622. https://doi.org/10.3390/ma14030622
Chicago/Turabian StyleYakoumis, Iakovos. 2021. "PROMETHEUS: A Copper-Based Polymetallic Catalyst for Automotive Applications. Part I: Synthesis and Characterization" Materials 14, no. 3: 622. https://doi.org/10.3390/ma14030622







