Silk-Based Materials for Hard Tissue Engineering
Abstract
:1. Introduction
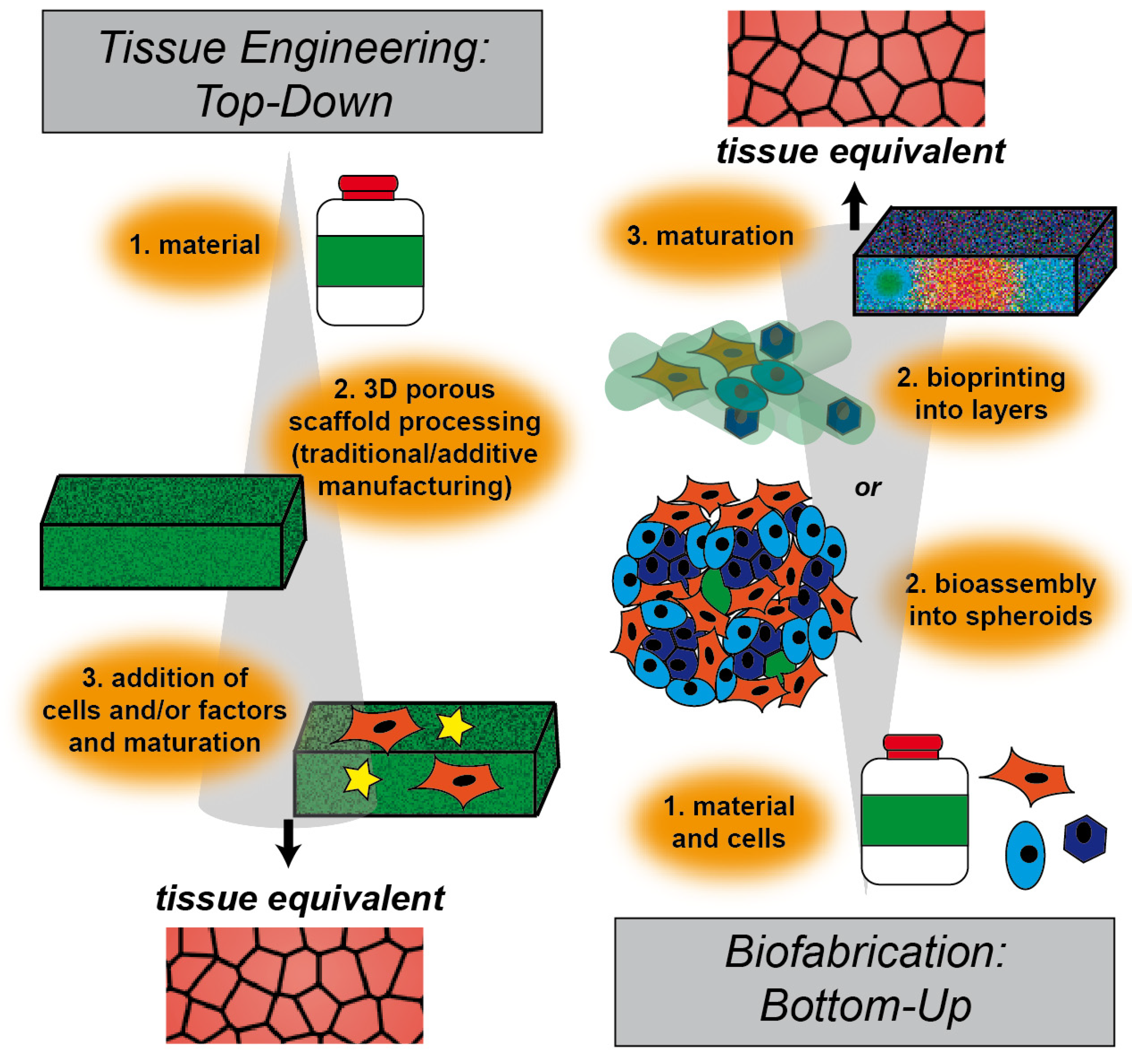
2. Tissue Engineering Approaches
3. Hard Tissue Engineering
3.1. State of the Art
3.2. Design Criteria and Challenges
4. Silk
4.1. Naturally Derived Silk
4.2. Bioengineered Silk
4.3. Silk-Based Morphologies
5. Silk-Based Hard Tissue Engineering
5.1. Bone Tissue Engineering
5.1.1. Non-Mineralized Scaffolds
5.1.2. Microcarriers for Bone Tissue Engineering
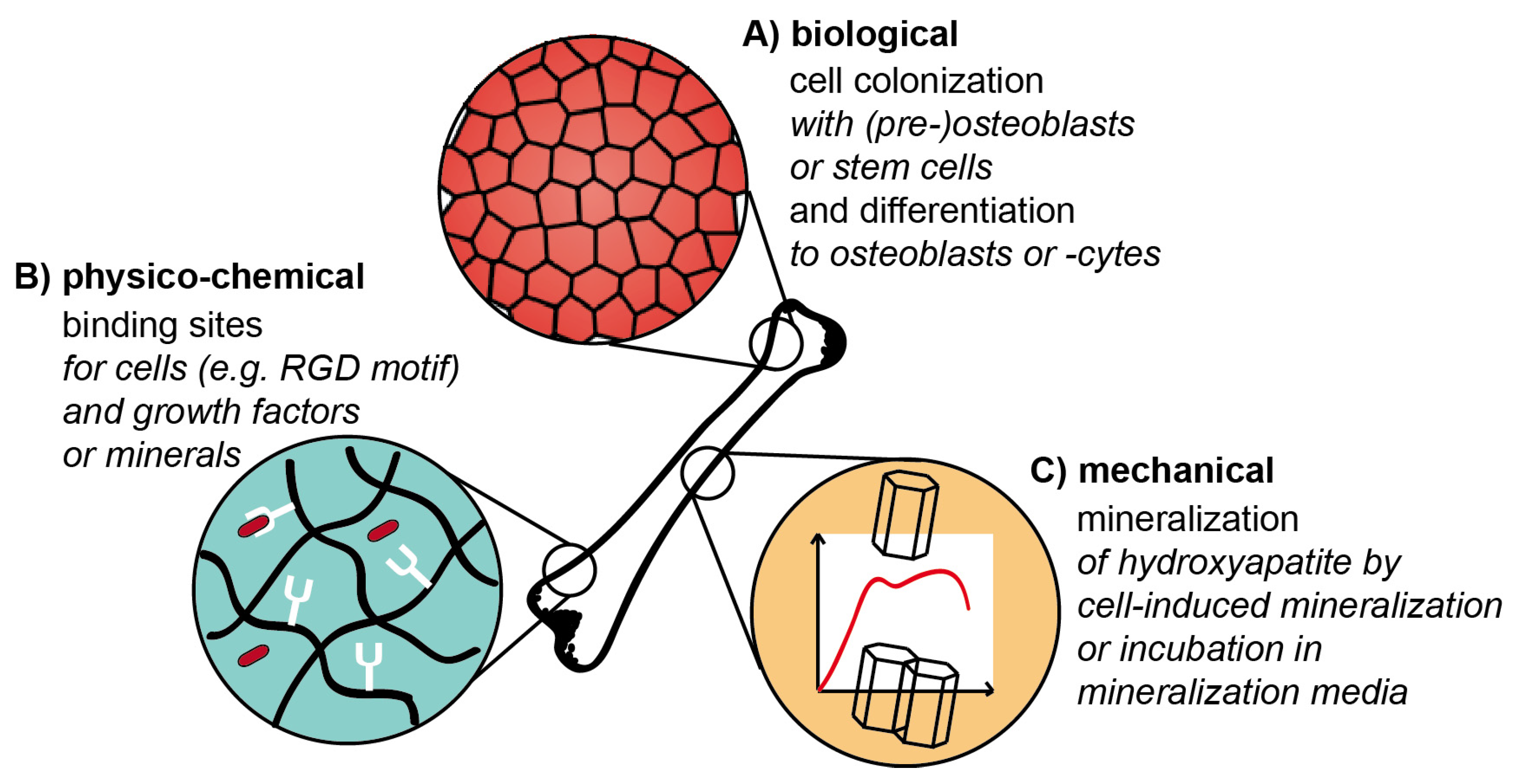
5.1.3. Biomineralized Scaffolds Using Specific Mineralization Tags
5.1.4. Biomineralization of Scaffolds Using Pre-Mineralization
5.2. Teeth and Mandible Tissue Engineering
5.3. Tissue Engineering of Bone Neighbouring Hard Tissues
6. Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic Systems for Hydroxyapatite Mineralization Inspired by Bone and Enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef] [Green Version]
- Beniash, E. Biominerals—hierarchical nanocomposites: The example of bone. WIRES Nanomed. Nanobi. 2011, 3, 47–69. [Google Scholar] [CrossRef] [Green Version]
- Gower, L.B. Biomimetic Model Systems for Investigating the Amorphous Precursor Pathway and Its Role in Biomineralization. Chem. Rev. 2008, 108, 4551–4627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiner, S.; Addadi, L. Crystallization Pathways in Biomineralization. In Annual Review of Materials Research; Clarke, D.R., Fratzl, P., Eds.; Annual Reviews: Palo Alto, CA, USA, 2011; Volume 41, pp. 21–40. [Google Scholar]
- George, A.; Veis, A. Phosphorylated Proteins and Control over Apatite Nucleation, Crystal Growth, and Inhibition. Chem. Rev. 2008, 108, 4670–4693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, H.C. Matrix vesicles and calcification. Curr. Rheumatol. Rep. 2003, 5, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lin, S.; Shao, X.; Zhang, Q.; Xue, C.; Zhang, S.; Lin, Y.; Zhu, B.; Cai, X. Effect of matrix stiffness on osteoblast functionalization. Cell Prolif. 2017, 50, e12338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zioupos, P.; Kirchner, H.O.; Peterlik, H. Ageing bone fractures: The case of a ductile to brittle transition that shifts with age. Bone 2020, 131, 115176. [Google Scholar] [CrossRef] [PubMed]
- Schmid, G.L.; Kühnast, B.; Heise, M.; Deutsch, T.; Frese, T. Ultrasonography in assessing suspected bone fractures: A cross-sectional survey amongst German general practitioners. BMC Fam. Prac. 2020, 21, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Qin, L.; Yang, K.; Ma, Z.; Wang, Y.; Cheng, L.; Zhao, D.W. Materials evolution of bone plates for internal fixation of bone fractures: A review. J. Mater. Sci. Technol. 2020, 36, 190–208. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, T.; Khademhosseini, A.; Langer, R. Chasing the Paradigm: Clinical Translation of 25 Years of Tissue Engineering. Tissue Eng. Part A 2019, 25, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, T.; Moroni, L. Tissue Engineering and Regenerative Medicine 2019: The Role of Biofabrication—A Year in Review. Tissue Eng. Part C Methods 2019, 26, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Khademhosseini, A. Modular tissue engineering: Engineering biological tissues from the bottom up. Soft Matter 2009, 5, 1312–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Zhou, Z.; Liu, W.; Zhao, Y.; Huang, T.; Li, X.; Duan, J.; Fang, J. Preparation and Characterization of Poly(L-lactide-co-glycolide-co-ε-caprolactone) Scaffolds by Thermally Induced Phase Separation. J. Macromol. Sci. Part B 2020, 59, 427–439. [Google Scholar] [CrossRef]
- Januariyasa, I.K.; Yusuf, Y. Porous carbonated hydroxyapatite-based scaffold using simple gas foaming method. J. Asian Ceram. Soc. 2020, 8, 634–641. [Google Scholar] [CrossRef]
- Judawisastra, H.; Nugraha, F.R.; Wibowo, U.A. Porous Architecture Evaluation of Silk Fibroin Scaffold from Direct Dissolution Salt Leaching Method. Macromol. Symp. 2020, 391, 1900187. [Google Scholar] [CrossRef]
- Khoramgah, M.S.; Ranjbari, J.; Abbaszadeh, H.-A.; Mirakabad, F.S.T.; Hatami, S.; Hosseinzadeh, S.; Ghanbarian, H. Freeze-dried multiscale porous nanofibrous three dimensional scaffolds for bone regenerations. BioImpacts 2020, 10, 73–85. [Google Scholar] [CrossRef]
- Madrid, A.P.M.; Vrech, S.M.; Sanchez, M.A.; Rodriguez, A.P. Advances in additive manufacturing for bone tissue engineering scaffolds. Mater. Sci. Eng. C 2019, 100, 631–644. [Google Scholar] [CrossRef]
- Gaspar, V.M.; Lavrador, P.; Borges, J.; Oliveira, M.B.; Mano, J.F. Advanced Bottom-Up Engineering of Living Architectures. Adv. Mater. 2020, 32, e1903975. [Google Scholar] [CrossRef]
- Elbert, D.L. Bottom-up tissue engineering. Curr. Opin. Biotechnol. 2011, 22, 674–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.S.; Santos, L.F.; Mendes, M.C.; Mano, J.F. Multi-layer pre-vascularized magnetic cell sheets for bone regeneration. Biomaterials 2020, 231, 119664. [Google Scholar] [CrossRef] [PubMed]
- Chuah, Y.J.; Tan, J.R.; Wu, Y.; Lim, C.S.; Hee, H.T.; Kang, Y.; Wang, D.-A. Scaffold-Free tissue engineering with aligned bone marrow stromal cell sheets to recapitulate the microstructural and biochemical composition of annulus fibrosus. Acta Biomater. 2020, 107, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Byun, H.; Lee, J.; Perikamana, S.K.M.; Shin, Y.M.; Kim, E.M.; Shin, H. Stem cell spheroids incorporating fibers coated with adenosine and polydopamine as a modular building blocks for bone tissue engineering. Biomaterials 2020, 230, 119652. [Google Scholar] [CrossRef]
- Kronemberger, G.S.; Matsui, R.A.M.; Miranda, G.D.A.S.D.C.E.; Granjeiro, J.M.; Baptista, L.S. Cartilage and bone tissue engineering using adipose stromal/stem cells spheroids as building blocks. World J. Stem Cells 2020, 12, 110–122. [Google Scholar] [CrossRef]
- Toda, S.; Frankel, N.W.; Lim, W.A. Engineering cell–cell communication networks: Programming multicellular behaviors. Curr. Opin. Chem. Biol. 2019, 52, 31–38. [Google Scholar] [CrossRef]
- Amaral, A.J.; Pasparakis, G. Cell membrane engineering with synthetic materials: Applications in cell spheroids, cellular glues and microtissue formation. Acta Biomater. 2019, 90, 21–36. [Google Scholar] [CrossRef]
- Qu, M.; Jiang, X.; Zhou, X.; Wang, C.; Wu, Q.; Ren, L.; Zhu, J.; Zhu, S.; Tebon, P.; Sun, W.; et al. Stimuli-Responsive Delivery of Growth Factors for Tissue Engineering. Adv. Health Mater. 2020, 9, e1901714. [Google Scholar] [CrossRef]
- Tang, J.D.; Mura, C.; Lampe, K.J. Stimuli-Responsive, Pentapeptide, Nanofiber Hydrogel for Tissue Engineering. J. Am. Chem. Soc. 2019, 141, 4886–4899. [Google Scholar] [CrossRef]
- Fathi-Achachelouei, M.; Keskin, D.; Bat, E.; Vrana, N.E.; Tezcaner, A. Dual growth factor delivery using PLGA nanoparticles in silk fibroin/PEGDMA hydrogels for articular cartilage tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 108, 2041–2062. [Google Scholar] [CrossRef]
- Groll, J.; Boland, T.; Blunk, T.; Burdick, J.A.; Kim, Y.K.; Dalton, P.D.; Derby, B.; Forgacs, G.; Li, Q.; Mironov, V.A.; et al. Biofabrication: Reappraising the definition of an evolving field. Biofabrication 2016, 8, 013001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moroni, L.; Boland, T.; Burdick, J.A.; De Maria, C.; Derby, B.; Forgacs, G.; Groll, J.; Li, Q.; Malda, J.; Mironov, V.A.; et al. Biofabrication: A Guide to Technology and Terminology. Trends Biotechnol. 2018, 36, 384–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Divakar, D.D.; Jastaniyah, N.T.; Altamimi, H.G.; Alnakhli, Y.O.; Alkheraif, A.A.; Haleem, S. Enhanced antimicrobial activity of naturally derived bioactive molecule chitosan conjugated silver nanoparticle against dental implant pathogens. Int. J. Biol. Macromol. 2018, 108, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.A.; Camci-Unal, G. Unconventional Tissue Engineering Materials in Disguise. Trends Biotechnol. 2020, 38, 178–190. [Google Scholar] [CrossRef]
- Levato, R.; Jungst, T.; Scheuring, R.G.; Blunk, T.; Groll, J.; Malda, J. From Shape to Function: The Next Step in Bioprinting. Adv. Mater. 2020, 32, e1906423. [Google Scholar] [CrossRef]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current Concepts in Scaffolding for Bone Tissue Engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar]
- Raquel Maia, F.; Correlo, V.M.; Oliveira, J.M.; Reis, R.L. Chapter 32—Natural Origin Materials for Bone Tissue Engineering: Properties, Processing, and Performance. In Principles of Regenerative Medicine, 3rd ed.; Atala, A., Lanza, R., Mikos, A.G., Nerem, R., Eds.; Academic Press: Boston, MA, USA, 2019; pp. 535–558. [Google Scholar]
- Wang, W.; Yeung, K.W. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Souza, A.T.P.; Lopes, H.B.; Freitas, G.P.; Ferraz, E.P.; Oliveira, F.S.; Almeida, A.L.G.; Weffort, D.; Beloti, M.M.; Rosa, A.L. Role of embryonic origin on osteogenic potential and bone repair capacity of rat calvarial osteoblasts. J. Bone Miner. Metab. 2020, 38, 481–490. [Google Scholar] [CrossRef]
- Vasquez-Sancho, F.; Abdollahi, A.; Damjanovic, D.; Catalan, G. Flexoelectricity in Bones. Adv. Mater. 2018, 30, 1705316. [Google Scholar] [CrossRef]
- Núñez-Toldrà, R.; Vasquez-Sancho, F.; Barroca, N.; Catalan, G. Investigation of The Cellular Response to Bone Fractures: Evidence for Flexoelectricity. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Lin, C.-C.; Chang, W.H.-S.; Cheng, T.-M.; Chiu, L.-H.; Wang, Y.-H.; Lin, C.-A.J.; Ho, Y.-S.; Zuo, C.S.; Wang, Y.-M.; Lai, W.-F.T. Two new, near-infrared, fluorescent probes as potential tools for imaging bone repair. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Pramanik, K.; Biswas, A. Silk fibroin coated TiO2 nanotubes for improved osteogenic property of Ti6Al4V bone implants. Mater. Sci. Eng. C 2019, 105, 109982. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Li, X.; Long, H.; Bin, S.; Xu, Y. Bioactive Tetracalcium Phosphate Scaffolds Fabricated by Selective Laser Sintering for Bone Regeneration Applications. Materials 2020, 13, 2268. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Gao, C.; Feng, P.; Xiao, T.; Shuai, C.; Peng, S. Calcium sulfate bone scaffolds with controllable porous structure by selective laser sintering. J. Porous Mater. 2015, 22, 1171–1178. [Google Scholar] [CrossRef]
- Shuai, C.; Gao, C.; Nie, Y.; Hu, H.; Zhou, Y.; Peng, S. Structure and properties of nano-hydroxypatite scaffolds for bone tissue engineering with a selective laser sintering system. Nanotechnology 2011, 22, 285703. [Google Scholar] [CrossRef]
- Duan, B.; Wang, M.; Zhou, W.Y.; Cheung, W.L.; Li, Z.Y.; Lu, W.W. Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. Acta Biomater. 2010, 6, 4495–4505. [Google Scholar] [CrossRef]
- Simpson, R.L.; Wiria, F.E.; Amis, A.A.; Chua, C.K.; Leong, K.F.; Hansen, U.N.; Chandrasekaran, M.; Lee, M.W. Development of a 95/5 poly(L-lactide-co-glycolide)/hydroxylapatite and β-tricalcium phosphate scaffold as bone replacement material via selective laser sintering. J. Biomed. Mater. Res. B 2008, 84B, 17–25. [Google Scholar] [CrossRef]
- Cox, S.C.; Thornby, J.A.; Gibbons, G.J.; Williams, M.A.; Mallick, K.K. 3D printing of porous hydroxyapatite scaffolds intended for use in bone tissue engineering applications. Mater. Sci. Eng. C 2015, 47, 237–247. [Google Scholar] [CrossRef]
- Ahn, M.-K.; Moon, Y.-W.; Maeng, W.-Y.; Koh, Y.-H.; Kim, H.-E. Design and Production of Continuously Gradient Macro/Microporous Calcium Phosphate (CaP) Scaffolds Using Ceramic/Camphene-Based 3D Extrusion. Materials 2017, 10, 719. [Google Scholar] [CrossRef] [Green Version]
- Chow, L.C.; Takagi, S. A natural bone cement—A laboratory novelty led to the development of revolutionary new biomaterials. J. Res. Natl. Inst. Stand. Technol. 2001, 106, 1029–1033. [Google Scholar] [CrossRef]
- Kretlow, J.D.; Mikos, A.G. Review: Mineralization of Synthetic Polymer Scaffolds for Bone Tissue Engineering. Tissue Eng. 2007, 13, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- Currey, J.; Butler, G. The mechanical properties of bone tissue in children. JBJS 1975, 57, 810–814. [Google Scholar] [CrossRef]
- Vogel, H.G. Influence of Maturation and Aging on Mechanical and Biochemical Parameters of Rat Bone. Gerontology 1979, 25, 16–23. [Google Scholar] [CrossRef]
- Dunlop, J.W.C.; Fratzl, P. Biological Composites. In Annual Review of Materials Research; Clarke, D.R., Ruhle, M., Zok, F., Eds.; Annual Reviews: Palo Alto, CA, USA, 2010; Volume 40, pp. 1–24. [Google Scholar]
- Hardy, J.G.; Torres-Rendon, J.G.; Leal-Egaña, A.; Walther, A.; Schlaad, H.; Cölfen, H.; Scheibel, T. Biomineralization of Engineered Spider Silk Protein-Based Composite Materials for Bone Tissue Engineering. Materials 2016, 9, 560. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choo, J.E.; Choi, Y.S.; Park, J.B.; Min, D.S.; Lee, S.J.; Rhyu, H.K.; Jo, I.H.; Chung, C.P.; Park, Y.J. Assembly of collagen-binding peptide with collagen as a bioactive scaffold for osteogenesis in vitro and in vivo. Biomaterials 2007, 28, 4257–4267. [Google Scholar] [CrossRef] [PubMed]
- Dinjaski, N.; Plowright, R.; Zhou, S.; Belton, D.J.; Perry, C.C.; Kaplan, D.L. Osteoinductive recombinant silk fusion proteins for bone regeneration. Acta Biomater. 2017, 49, 127–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Giachelli, C.M.; Steitz, S. Osteopontin: A versatile regulator of inflammation and biomineralization. Matrix Biol. 2000, 19, 615–622. [Google Scholar] [CrossRef]
- Jin, R.; Wang, Y.; Liu, Y.; Ding, C.; Xie, J.; Li, J. Biomineralization and osteogenic differentiation modulated by substrate stiffness. Eur. Polym. J. 2020, 122, 109395. [Google Scholar] [CrossRef]
- Midha, S.; Kumar, S.; Sharma, A.; Kaur, K.; Shi, X.; Naruphontjirakul, P.; Jones, J.R.; Ghosh, S. Silk fibroin-bioactive glass based advanced biomaterials: Towards patient-specific bone grafts. Biomed. Mater. 2018, 13, 055012. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Mertgen, A.-S.; Trossmann, V.T.; Guex, A.G.; Maniura-Weber, K.; Scheibel, T.; Rottmar, M. Multifunctional Biomaterials: Combining Material Modification Strategies for Engineering of Cell-Contacting Surfaces. ACS Appl. Mater. Interfaces 2020, 12, 21342–21367. [Google Scholar] [CrossRef] [PubMed]
- Le Saux, G.; Magenau, A.; Böcking, T.; Gaus, K.; Gooding, J.J. The Relative Importance of Topography and RGD Ligand Density for Endothelial Cell Adhesion. PLoS ONE 2011, 6, e21869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wohlrab, S.; Müller, S.; Schmidt, A.; Neubauer, S.; Kessler, H.; Leal-Egaña, A.; Scheibel, T. Cell adhesion and proliferation on RGD-modified recombinant spider silk proteins. Biomaterials 2012, 33, 6650–6659. [Google Scholar] [CrossRef] [PubMed]
- Bessa, P.C.; Balmayor, E.R.; Azevedo, H.S.; Nürnberger, S.; Casal, M.; Van Griensven, M.; Reis, R.L.; Redl, H. Silk fibroin microparticles as carriers for delivery of human recombinant BMPs. Physical characterization and drug release. J. Tissue Eng. Regen. Med. 2010, 4, 349–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bessa, P.C.; Casal, M.; Reis, R.L. Bone morphogenetic proteins in tissue engineering: The road from laboratory to clinic, part II (BMP delivery). J. Tissue Eng. Regen. Med. 2008, 2, 81–96. [Google Scholar] [CrossRef]
- Gomes, S.; Gallego-Llamas, J.; Leonor, I.B.; Mano, J.F.; Reis, R.L.; Kaplan, D.L. In Vivo Biological Responses to Silk Proteins Functionalized with Bone Sialoprotein. Macromol. Biosci. 2013, 13, 444–454. [Google Scholar] [CrossRef]
- Neubauer, V.J.; Scheibel, T. Spider Silk Fusion Proteins for Controlled Collagen Binding and Biomineralization. ACS Biomater. Sci. Eng. 2020, 6, 5599–5608. [Google Scholar] [CrossRef]
- Yousaf, S.S.; Houacine, C.; Khan, I.; Ahmed, W.; Jackson, M.J. Chapter 11—Importance of biomaterials in biomedical engineering. In Advances in Medical and Surgical Engineering; Ahmed, W., Phoenix, D.A., Jackson, M.J., Charalambous, C.P., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 149–176. [Google Scholar]
- Craig, C.L. Evolution of Arthopods Silks. Annu. Rev. Entomol. 1997, 42, 231–267. [Google Scholar] [CrossRef]
- Andersson, M.; Johansson, J.; Rising, A. Silk Spinning in Silkworms and Spiders. Int. J. Mol. Sci. 2016, 17, 1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardy, J.G.; Scheibel, T.R. Production and processing of spider silk proteins. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 3957–3963. [Google Scholar] [CrossRef] [Green Version]
- Eisoldt, L.; Smith, A.; Scheibel, T. Decoding the secrets of spider silk. Mater. Today 2011, 14, 80–86. [Google Scholar] [CrossRef]
- Salehi, S.; Koeck, K.; Scheibel, T. Spider Silk for Tissue Engineering Applications. Molecules 2020, 25, 737. [Google Scholar] [CrossRef] [Green Version]
- Pollini, M.; Paladini, F. Bioinspired Materials for Wound Healing Application: The Potential of Silk Fibroin. Materials 2020, 13, 3361. [Google Scholar] [CrossRef]
- Melke, J.J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Umuhoza, D.; Yang, F.; Long, D.; Hao, Z.; Dai, J.; Zhao, A. Strategies for Tuning the Biodegradation of Silk Fibroin-Based Materials for Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2020, 6, 1290–1310. [Google Scholar] [CrossRef]
- Nagano, A.; Tanioka, Y.; Sakurai, N.; Sezutsu, H.; Kuboyama, N.; Kiba, H.; Tanimoto, Y.; Ma, S.; Asakura, T. Regeneration of the femoral epicondyle on calcium-binding silk scaffolds developed using transgenic silk fibroin produced by transgenic silkworm. Acta Biomater. 2011, 7, 1192–1201. [Google Scholar] [CrossRef]
- Saotome, T.; Hayashi, H.; Tanaka, R.; Kinugasa, A.; Uesugi, S.; Tatematsu, K.-I.; Sezutsu, H.; Kuwabara, N.; Asakura, T. Introduction of VEGF or RGD sequences improves revascularization properties of Bombyx mori silk fibroin produced by transgenic silkworm. J. Mater. Chem. B 2015, 3, 7109–7116. [Google Scholar] [CrossRef]
- Gomes, S.C.; Leonor, I.B.; Mano, J.F.; Reis, R.; Kaplan, D.L. Natural and genetically engineered proteins for tissue engineering. Prog. Polym. Sci. 2012, 37, 1–17. [Google Scholar] [CrossRef]
- Huang, J.; Wong, C.; George, A.; Kaplan, D.L. The effect of genetically engineered spider silk-dentin matrix protein 1 chimeric protein on hydroxyapatite nucleation. Biomaterials 2007, 28, 2358–2367. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.; Wu, P.; Chen, X.; Zheng, G. The effect of water on the conformation transition of Bombyx mori silk fibroin. Vib. Spectrosc. 2009, 51, 105–109. [Google Scholar] [CrossRef]
- Arai, T.; Freddi, G.; Innocenti, R.; Tsukada, M. Biodegradation ofBombyx mori silk fibroin fibers and films. J. Appl. Polym. Sci. 2003, 91, 2383–2390. [Google Scholar] [CrossRef]
- Tucker, C.L.; Jones, J.A.; Bringhurst, H.N.; Copeland, C.G.; Addison, J.B.; Weber, W.S.; Mou, Q.; Yarger, J.L.; Lewis, R.V. Mechanical and Physical Properties of Recombinant Spider Silk Films Using Organic and Aqueous Solvents. Biomacromolecules 2014, 15, 3158–3170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spieß, K.; Wohlrab, S.; Scheibel, T. Structural characterization and functionalization of engineered spider silk films. Soft Matter 2010, 6, 4168–4174. [Google Scholar] [CrossRef]
- Ohgo, K.; Zhao, C.; Kobayashi, M.; Asakura, T. Preparation of non-woven nanofibers of Bombyx mori silk, Samia cynthia ricini silk and recombinant hybrid silk with electrospinning method. Polymer 2003, 44, 841–846. [Google Scholar] [CrossRef]
- Sukigara, S.; Gandhi, M.; Ayutsede, J.; Micklus, M.; Ko, F. Regeneration of Bombyx mori silk by electrospinning—part 1: Processing parameters and geometric properties. Polymer 2003, 44, 5721–5727. [Google Scholar] [CrossRef]
- Keirouz, A.; Fortunato, G.; Zhang, M.; Callanan, A.; Radacsi, N. Nozzle-free electrospinning of Polyvinylpyrrolidone/Poly(glycerol sebacate) fibrous scaffolds for skin tissue engineering applications. Med. Eng. Phys. 2019, 71, 56–67. [Google Scholar] [CrossRef]
- DeSimone, E.; Aigner, T.B.; Humenik, M.; Lang, G.; Scheibel, T. Aqueous electrospinning of recombinant spider silk proteins. Mater. Sci. Eng. C 2020, 106, 110145. [Google Scholar] [CrossRef]
- Keirouz, A.; Zakharova, M.; Kwon, J.; Robert, C.; Koutsos, V.; Callanan, A.; Chen, X.; Fortunato, G.; Radacsi, N. High-throughput production of silk fibroin-based electrospun fibers as biomaterial for skin tissue engineering applications. Mater. Sci. Eng. C 2020, 112, 110939. [Google Scholar] [CrossRef]
- Lammel, A.; Schwab, M.; Slotta, U.; Winter, G.; Scheibel, T. Processing Conditions for the Formation of Spider Silk Microspheres. ChemSusChem 2008, 1, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Jastrzebska, K.; Felcyn, E.; Kozak, M.; Szybowicz, M.; Buchwald, T.; Pietralik, Z.; Jesionowski, T.; Mackiewicz, A.; Dams-Kozlowska, H. The method of purifying bioengineered spider silk determines the silk sphere properties. Sci. Rep. 2016, 6, 28106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajkhowa, R.; Hu, X.; Tsuzuki, T.; Kaplan, D.L.; Wang, X. Structure and Biodegradation Mechanism of Milled Bombyx mori Silk Particles. Biomacromolecules 2012, 13, 2503–2512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Z.; Chen, X.; Yao, J.; Huang, L.; Shao, Z. The preparation of regenerated silk fibroin microspheres. Soft Matter 2007, 3, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Herold, H.M.; Döbl, A.; Wohlrab, S.; Humenik, M.; Scheibel, T. Designed Spider Silk-Based Drug Carrier for Redox- or pH-Triggered Drug Release. Biomacromolecules 2020, 21, 4904–4912. [Google Scholar] [CrossRef]
- Maniglio, D.; Bonani, W.; Migliaresi, C.; Motta, A. Silk fibroin porous scaffolds by N2O foaming. J. Biomater. Sci. Polym. Ed. 2018, 29, 491–506. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Feng, Q. Preparation of 3-D regenerated fibroin scaffolds with freeze drying method and freeze drying/foaming technique. J. Mater. Sci. Mater. Electron. 2006, 17, 1349–1356. [Google Scholar] [CrossRef]
- Schacht, K.; Vogt, J.; Scheibel, T. Foams Made of Engineered Recombinant Spider Silk Proteins as 3D Scaffolds for Cell Growth. ACS Biomater. Sci. Eng. 2016, 2, 517–525. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Schacht, K.; Jungst, T.; Schweinlin, M.; Ewald, A.; Groll, J.; Scheibel, T. Biofabrication of Cell-Loaded 3D Spider Silk Constructs. Angew. Chem. Int. Ed. 2015, 54, 2816–2820. [Google Scholar] [CrossRef]
- Chameettachal, S.; Midha, S.; Ghosh, S. Regulation of Chondrogenesis and Hypertrophy in Silk Fibroin-Gelatin-Based 3D Bioprinted Constructs. ACS Biomater. Sci. Eng. 2016, 2, 1450–1463. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, Z.; Cheng, Q.; Kaplan, D.L.; Li, G.; Wang, X. Ductility and Porosity of Silk Fibroin Films by Blending with Glycerol/Polyethylene Glycol and Adjusting the Drying Temperature. ACS Biomater. Sci. Eng. 2019, 6, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Sartika, D.; Hsin, W.C.; Wang, D.-H.; Cherng, J.-H.; Chang, S.-J.; Fan, G.-Y.; Wang, Y.-W.; Lee, C.-H.; Hong, P.-D.; Wang, C. Human Adipose-Derived Mesenchymal Stem Cells-Incorporated Silk Fibroin as a Potential Bio-Scaffold in Guiding Bone Regeneration. Polymers 2020, 12, 853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorishetty, P.; Balu, R.; Athukoralalage, S.S.; Greaves, T.L.; Mata, J.; De Campo, L.; Saha, N.; Zannettino, A.C.W.; Dutta, N.K.; Choudhury, N.R. Tunable Biomimetic Hydrogels from Silk Fibroin and Nanocellulose. ACS Sustain. Chem. Eng. 2020, 8, 2375–2389. [Google Scholar] [CrossRef]
- Qian, S.; Wang, Z.; Zheng, Z.; Ran, J.; Zhu, J.; Chen, W. A Collagen and Silk Scaffold for Improved Healing of the Tendon and Bone Interface in a Rabbit Model. Med. Sci. Monit. 2019, 25, 269–278. [Google Scholar] [CrossRef]
- Dellaquila, A.; Greco, G.; Campodoni, E.; Mazzocchi, M.; Mazzolai, B.; Tampieri, A.; Pugno, N.M.; Sandri, M. Optimized production of a high-performance hybrid biomaterial: Biomineralized spider silk for bone tissue engineering. J. Appl. Polym. Sci. 2019, 137, 48739. [Google Scholar] [CrossRef]
- Zafar, B.; Mottaghitalab, F.; Shahosseini, Z.; Negahdari, B.; Farokhi, M. Silk fibroin/alumina nanoparticle scaffold using for osteogenic differentiation of rabbit adipose-derived stem cells. Materials 2020, 9, 100518. [Google Scholar] [CrossRef]
- Liu, F.; Liu, C.; Zheng, B.; He, J.; Liu, J.; Chen, C.; Lee, I.-S.; Wang, X.; Liu, Y. Synergistic Effects on Incorporation of β-Tricalcium Phosphate and Graphene Oxide Nanoparticles to Silk Fibroin/Soy Protein Isolate Scaffolds for Bone Tissue Engineering. Polymer 2020, 12, 69. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Fang, M.; Xia, Y.; Hou, J.; Nan, X.; Zhao, B.; Wang, X. Preparation and biological properties of silk fibroin/nano-hydroxyapatite/graphene oxide scaffolds with an oriented channel-like structure. RSC Adv. 2020, 10, 10118–10128. [Google Scholar] [CrossRef]
- Ribeiro, V.P.; Pina, S.; Costa, J.B.; Cengiz, I.F.; García-Fernández, L.; Fernández-Gutiérrez, M.d.M.; Paiva, O.C.; Oliveira, A.L.; San-Román, J.; Oliveira, J.M.; et al. Enzymatically Cross-Linked Silk Fibroin-Based Hierarchical Scaffolds for Osteochondral Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 3781–3799. [Google Scholar] [CrossRef]
- Collado-González, M.; Pecci-Lloret, M.P.; García-Bernal, D.; Aznar-Cervantes, S.D.; Oñate-Sánchez, R.E.; Moraleda, J.M.; Cenis, J.L.; Rodríguez-Lozano, F.J. Biological effects of silk fibroin 3D scaffolds on stem cells from human exfoliated deciduous teeth (SHEDs). Odontology 2018, 106, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-S.; Han, G.-C.; Yan, S.-Q.; You, R.-C.; Zhang, Q. Facile Preparation of Silk Fibroin Scaffold Via Direct Solvent Exchange. J. Fiber Bioeng. Inform. 2020, 13, 13–21. [Google Scholar]
- Siavashani, A.Z.; Mohammadi, J.; Rottmar, M.; Senturk, B.; Nourmohammadi, J.; Sadeghi, B.; Huber, L.; Maniura-Weber, K. Silk fibroin/sericin 3D sponges: The effect of sericin on structural and biological properties of fibroin. Int. J. Biol. Macromol. 2020, 153, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Xingtong, L.; Zhentao, W.; Tingting, D.; Hongbo, G.; Juan, G. Hydrogels from silk fibroin and multiarmed hydrolyzed elastin peptide. Surf. Innov. 2020, 8, 216–223. [Google Scholar]
- Wu, N.; Yu, H.; Sun, M.; Li, Z.; Zhao, F.; Ao, Y.; Chen, H. Investigation on the Structure and Mechanical Properties of Highly Tunable Elastomeric Silk Fibroin Hydrogels Cross-Linked by γ-Ray Radiation. ACS Appl. Biomater. 2019, 3, 721–734. [Google Scholar] [CrossRef] [Green Version]
- Laomeephol, C.; Guedes, M.; Ferreira, H.; Reis, R.L.; Kanokpanont, S.; Laomeephol, C.; Neves, N.M. Phospholipid-induced silk fibroin hydrogels and their potential as cell carriers for tissue regeneration. J. Tissue Eng. Regen. Med. 2020, 14, 160–172. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.; Ratanavaraporn, J.; Yodmuang, S. Alginate-silk fibroin Bioink: A printable hydrogel for tissue engineering. In Proceedings of the 2019 12th Biomedical Engineering International Conference (BMEiCON), Ubon Ratchathani, Thailand, 19–22 November 2019; pp. 1–4. [Google Scholar]
- Sharma, A.; Desando, G.; Petretta, M.; Chawla, S.; Bartolotti, I.; Manferdini, C.; Paolella, F.; Gabusi, E.; Trucco, D.; Ghosh, S.; et al. Investigating the Role of Sustained Calcium Release in Silk-Gelatin-Based Three-Dimensional Bioprinted Constructs for Enhancing the Osteogenic Differentiation of Human Bone Marrow Derived Mesenchymal Stromal Cells. ACS Biomater. Sci. Eng. 2019, 5, 1518–1533. [Google Scholar] [CrossRef]
- Huemmerich, D.; Helsen, C.W.; Quedzuweit, S.; Oschmann, J.; Rudolph, A.R.; Scheibel, T. Primary Structure Elements of Spider Dragline Silks and Their Contribution to Protein Solubility. Biochemistry 2004, 43, 13604–13612. [Google Scholar] [CrossRef]
- Schacht, K.; Scheibel, T. Controlled Hydrogel Formation of a Recombinant Spider Silk Protein. Biomacromolecules 2011, 12, 2488–2495. [Google Scholar] [CrossRef]
- DeSimone, E.; Schacht, K.; Scheibel, T. Cations influence the cross-linking of hydrogels made of recombinant, polyanionic spider silk proteins. Mater. Lett. 2016, 183, 101–104. [Google Scholar] [CrossRef]
- DeSimone, E.; Schacht, K.; Jungst, T.; Groll, J.; Scheibel, T. Biofabrication of 3D constructs: Fabrication technologies and spider silk proteins as bioinks. Pure Appl. Chem. 2015, 87, 737–749. [Google Scholar] [CrossRef] [Green Version]
- DeSimone, E.; Schacht, K.; Pellert, A.; Scheibel, T. Recombinant spider silk-based bioinks. Biofabrication 2017, 9, 044104. [Google Scholar] [CrossRef] [PubMed]
- Thamm, C.; DeSimone, E.; Scheibel, T. Characterization of Hydrogels Made of a Novel Spider Silk Protein eMaSp1s and Evaluation for 3D Printing. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Bargel, H.; Scheibel, T. Recombinant Spider Silk-Silica Hybrid Scaffolds with Drug-Releasing Properties for Tissue Engineering Applications. Macromol. Rapid Commun. 2019, 41, e1900426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, S.; Bargel, H.; Anby, M.U.; LaFargue, D.; Scheibel, T.; LaFargue, D. Recombinant Spider Silk Hydrogels for Sustained Release of Biologicals. ACS Biomater. Sci. Eng. 2018, 4, 1750–1759. [Google Scholar] [CrossRef]
- Ding, Z.; Lu, G.; Cheng, W.; Xu, G.; Zuo, B.; Lu, Q.; Kaplan, D.L. Tough Anisotropic Silk Nanofiber Hydrogels with Osteoinductive Capacity. ACS Biomater. Sci. Eng. 2020, 6, 2357–2367. [Google Scholar] [CrossRef]
- Xu, G.; Ding, Z.; Lu, Q.; Zhang, X.; Zhou, X.; Xiao, L.; Lv, G.; Kaplan, D.L. Electric field-driven building blocks for introducing multiple gradients to hydrogels. Protein Cell 2020, 11, 267–285. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Chen, H.; Wang, Y.; Li, G.; Zheng, Z.; Kaplan, D.L.; Wang, X. Flexible Water-Absorbing Silk-Fibroin Biomaterial Sponges with Unique Pore Structure for Tissue Engineering. ACS Biomater. Sci. Eng. 2020, 6, 1641–1649. [Google Scholar] [CrossRef]
- Luetchford, K.A.; Chaudhuri, J.B.; De Bank, P.A. Silk fibroin/gelatin microcarriers as scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2020, 106, 110116. [Google Scholar] [CrossRef]
- Li, Z.; Chu, D.; Gao, Y.; Jin, L.; Zhang, X.; Cui, W.; Li, J. Biomimicry, biomineralization, and bioregeneration of bone using advanced three-dimensional fibrous hydroxyapatite scaffold. Mater. Today Adv. 2019, 3, 100014. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, K.; Ren, J.; Fan, Y.; Ling, S. Strong, ductile and lightweight bionanocomposites constructed by bioinspired hierarchical assembly. Compos. Commun. 2020, 17, 97–103. [Google Scholar] [CrossRef]
- Griffanti, G.; Jiang, W.; Nazhat, S.N. Bioinspired mineralization of a functionalized injectable dense collagen hydrogel through silk sericin incorporation. Biomater. Sci. 2019, 7, 1064–1077. [Google Scholar] [CrossRef] [PubMed]
- Johari, N.; Hosseini, H.R.M.; Samadikuchaksaraei, A. Mechanical modeling of silk fibroin/TiO2 and silk fibroin/fluoridated TiO2 nanocomposite scaffolds for bone tissue engineering. Iran. Polym. J. 2020, 29, 219–224. [Google Scholar] [CrossRef]
- Xing, X.; Cheng, G.; Yin, C.; Cheng, X.; Cheng, Y.; Ni, Y.; Zhou, X.; Deng, H.; Li, Z. Magnesium-containing silk fibroin/polycaprolactone electrospun nanofibrous scaffolds for accelerating bone regeneration. Arab. J. Chem. 2020, 13, 5526–5538. [Google Scholar] [CrossRef]
- Tanasa, E.; Zaharia, C.; Hudita, A.; Radu, I.-C.; Costache, M.; Galateanu, B. Impact of the magnetic field on 3T3-E1 preosteoblasts inside SMART silk fibroin-based scaffolds decorated with magnetic nanoparticles. Mater. Sci. Eng. C 2020, 110, 110714. [Google Scholar] [CrossRef]
- Narimani, M.; Teimouri, A.; Shahbazarab, Z. Synthesis, characterization and biocompatible properties of novel silk fibroin/graphene oxide nanocomposite scaffolds for bone tissue engineering application. Polym. Bull. 2019, 76, 725–745. [Google Scholar] [CrossRef]
- Nie, L.; Zhang, H.; Ren, A.; Li, Y.; Fu, G.; Cannon, R.D.; Ji, P.; Wu, X.-H.; Yang, S. Nano-hydroxyapatite mineralized silk fibroin porous scaffold for tooth extraction site preservation. Dent. Mater. 2019, 35, 1397–1407. [Google Scholar] [CrossRef]
- Wang, K.; Ma, Q.; Zhang, Y.; Han, G.-T.; Qu, C.-X.; Wang, S. Preparation of bacterial cellulose/silk fibroin double-network hydrogel with high mechanical strength and biocompatibility for artificial cartilage. Cellulose 2020, 27, 1845–1852. [Google Scholar] [CrossRef]
- Ding, X.; Zhu, M.; Xu, B.; Zhang, J.; Zhao, Y.; Ji, S.; Wang, L.; Wang, L.; Li, X.; Kong, D.; et al. Integrated Trilayered Silk Fibroin Scaffold for Osteochondral Differentiation of Adipose-Derived Stem Cells. ACS Appl. Mater. Interfaces 2014, 6, 16696–16705. [Google Scholar] [CrossRef]
- Zhao, Y.; Ding, X.; Dong, Y.; Sun, X.; Wang, L.; Ma, X.; Zhu, M.; Xu, B.; Yang, Q. Role of the Calcified Cartilage Layer of an Integrated Trilayered Silk Fibroin Scaffold Used to Regenerate Osteochondral Defects in Rabbit Knees. ACS Biomater. Sci. Eng. 2019, 6, 1208–1216. [Google Scholar] [CrossRef]
- Bradner, S.A.; McGill, M.; Golding, A.; Grudt, R.; Kaplan, D.L. Silk Hydrogel Microfibers for Biomimetic Fibrous Material Design. Macromol. Mater. Eng. 2019, 304, 1900045. [Google Scholar] [CrossRef]
- The Business Research Company. Orthopedic Biomaterials Market Global Report 2020-30: Covid 19 Growth and Challenges; The Business Research Company: London, UK, 2020; Available online: https://www.thebusinessresearchcompany.com/report/orthopedic-biomaterials-market-global-report-2020-30-covid-19-growth-and-change (accessed on 31 January 2021).
- Perrone, G.S.; Leisk, G.G.; Lo, T.J.; Moreau, J.E.; Haas, D.S.; Papenburg, B.J.; Golden, E.B.; Partlow, B.P.; Fox, S.E.; Ibrahim, A.M.S.; et al. The use of silk-based devices for fracture fixation. Nat. Commun. 2014, 5, 3385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Guo, C.; Fitzpatrick, V.; Ibrahim, A.; Zwierstra, M.J.; Hanna, P.; Lechtig, A.; Nazarian, A.; Lin, S.J.; Kaplan, D. Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater. 2019, 5, 61–81. [Google Scholar] [CrossRef]







| Mineralization | Silk Source | Filler Materials/Additives | Morphology/Fabrication Technique | Cell Types | Biocompatibility Study | Target Tissue |
|---|---|---|---|---|---|---|
| non-mineralized | Bombyx mori silk fibroin [106] | glycerol, PEG | 2D film casting | human dermal fibroblasts | in vitro | bone |
| – | Bombyx mori silk fibroin [107] | – | 3D porous scaffold/lyophilization | human adipose mesenchymal stem cells | in vitro and in vivo in rat calvarial bone model | bone |
| – | Bombyx mori silk fibroin [108] | bacterial nanocellulose; photo-crosslinker | 3D hydrogels/3D printing | mouse lung fibroblasts | in vitro | bone |
| – | Bombyx mori silk fibroin [109] | collagen I | 3D scaffold with aligned or knitted fibers/lyophilization | rabbit bone marrow stem cells | in vitro and in vivo in rotator cuff rabbit model | tendon-to-bone transition |
| biomineralized | recombinant spider silk [72] | – | 2D film casting | mouse pre-osteoblasts | in vitro | tendon-to-bone transition |
| – | Cupiennius salei spider silk fibers [110] | – | 2.5D fibers/naturally harvested | – | – | bone |
| pre-mineralized materials | Bombyx mori silk fibroin [111] | alumina nanoparticles | 3D porous scaffold/lyophilization | rabbit adipose-derived stem cells | in vitro | bone |
| – | Bombyx mori silk fibroin, soy protein [112] | graphene oxide, β-tricalcium phosphate | 3D porous scaffold/lyophilization | rat bone marrow stem cells | in vitro | bone |
| – | Bombyx mori silk fibroin [113] | graphene oxide, nano-hydroxyapatite | 3D porous scaffold/lyophilization | bone marrow stem cells, human umbilical vein endothelial cells (HUVECs) | in vitro | bone, vasculature |
| – | Bombyx mori silk fibroin [114] | doped β-tricalcium phosphate, crosslinker | 3D porous scaffold/lyophilization | human osteoblasts, human articular chondrocytes | in vitro | bone, cartilage |
| – | Bombyx mori silk fibroin [115] | – | 3D porous sponges/salt leaching | stem cells from human exfoliated deciduous teeth | in vitro | teeth |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neubauer, V.J.; Döbl, A.; Scheibel, T. Silk-Based Materials for Hard Tissue Engineering. Materials 2021, 14, 674. https://doi.org/10.3390/ma14030674
Neubauer VJ, Döbl A, Scheibel T. Silk-Based Materials for Hard Tissue Engineering. Materials. 2021; 14(3):674. https://doi.org/10.3390/ma14030674
Chicago/Turabian StyleNeubauer, Vanessa J., Annika Döbl, and Thomas Scheibel. 2021. "Silk-Based Materials for Hard Tissue Engineering" Materials 14, no. 3: 674. https://doi.org/10.3390/ma14030674
APA StyleNeubauer, V. J., Döbl, A., & Scheibel, T. (2021). Silk-Based Materials for Hard Tissue Engineering. Materials, 14(3), 674. https://doi.org/10.3390/ma14030674