Calorimetric Studies of Magnesium-Rich Mg-Pd Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schlapbach, L.; Zuttel, A. Hydrogen-Storage Materials for Mobile Applications. Nature 2002, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Moradi, R.; Groth, K.M. Hydrogen Storage and Delivery: Review of the State of the Art Technologies and Risk and Reliability Analysis. Int. J. Hydrog. Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadjixenophontos, E.; Dematteis, E.M.; Berti, N.; Wołczyk, A.R.; Huen, P.; Brighi, M.; Le, T.T.; Santoru, A.; Payandeh, S.; Peru, F. A Review of the MSCA ITN ECOSTORE—Novel Complex Metal Hydrides for Efficient and Compact Storage of Renewable Energy as Hydrogen and Electricity. Inorganics 2020, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Crivello, J.-C.; Dam, B.; Denys, R.; Dornheim, M.; Grant, D.; Huot, J.; Jensen, T.R.; De Jongh, P.; Latroche, M.; Milanese, C. Review of Magnesium Hydride-Based Materials: Development and Optimisation. Appl. Phys. A 2016, 122, 97. [Google Scholar] [CrossRef] [Green Version]
- Prabhukhot, P.R.; Wagh Mahesh, M.; Gangal Aneesh, C. A Review on Solid State Hydrogen Storage Material. Adv. Energy Power 2016, 11–22. [Google Scholar] [CrossRef]
- Baran, A.; Polański, M. Magnesium-Based Materials for Hydrogen Storage—A Scope Review. Materials 2020, 13, 3993. [Google Scholar] [CrossRef]
- Webb, C.J. A Review of Catalyst-Enhanced Magnesium Hydride as a Hydrogen Storage Material. J. Phys. Chem. Solids 2015, 84, 96–106. [Google Scholar] [CrossRef]
- Reilly, J.J., Jr.; Wiswall, R.H., Jr. The Reaction of Hydrogen with Alloys of Magnesium and Copper1. Inorg. Chem. 1967, 6, 2220–2223. [Google Scholar] [CrossRef]
- Reilly, J.J., Jr.; Wiswall, R.H., Jr. Reaction of Hydrogen with Alloys of Magnesium and Nickel and the Formation of Mg2NiH4. Inorg. Chem. 1968, 7, 2254–2256. [Google Scholar] [CrossRef]
- Fadonougbo, J.O.; Jung, J.-Y.; Suh, J.-Y.; Lee, Y.-S.; Shim, J.-H.; Cho, Y.W. Low Temperature Formation of Mg2FeH6 by Hydrogenation of Ball-Milled Nano-Crystalline Powder Mixture of Mg and Fe. Mater. Des. 2017, 135, 239–245. [Google Scholar] [CrossRef]
- Fadonougbo, J.O.; Jung, J.Y.; Suh, J.-Y.; Lee, Y.-S.; Shim, J.-H.; Fleury, E.; Cho, Y.W. The Role of Fe Particle Size and Oxide Distribution on the Hydrogenation Properties of Ball-Milled Nano-Crystalline Powder Mixtures of Fe and Mg. J. Alloys Compd. 2019, 806, 1039–1046. [Google Scholar] [CrossRef]
- Sun, Z.; Lu, X.; Nyahuma, F.M.; Yan, N.; Xiao, J.; Su, S.; Zhang, L. Enhancing Hydrogen Storage Properties of MgH2 by Transition Metals and Carbon Materials: A Brief Review. Front. Chem. 2020, 8, 552. [Google Scholar] [CrossRef]
- Dufour, J.; Huot, J. Rapid Activation, Enhanced Hydrogen Sorption Kinetics and Air Resistance in Laminated Mg–Pd 2.5 at.%. J. Alloys Compd. 2007, 439, 5–7. [Google Scholar] [CrossRef]
- Huot, J.; Enoki, H.; Akiba, E. Synthesis, Phase Transformation, and Hydrogen Storage Properties of Ball-Milled TiV0.9Mn1.1. J. Alloys Compd. 2008, 453, 203–209. [Google Scholar] [CrossRef]
- Huot, J.; Yonkeub, A.; Dufour, J. Rietveld Analysis of Neutron Powder Diffraction of Mg6Pd Alloy at Various Hydriding Stages. J. Alloys Compd. 2009, 475, 168–172. [Google Scholar] [CrossRef]
- Fadonougbo, J.O.; Kim, H.-J.; Suh, B.-C.; Suh, J.-Y.; Lee, Y.-S.; Shim, J.-H.; Yim, C.D.; Cho, Y.W. Kinetics and Thermodynamics of Near Eutectic Mg-Mg2Ni Composites Produced by Casting Process. Int. J. Hydrogen Energy 2020, 45, 29009–29022. [Google Scholar] [CrossRef]
- Ouyang, L.; Liu, F.; Wang, H.; Liu, J.; Yang, X.-S.; Sun, L.; Zhu, M. Magnesium-Based Hydrogen Storage Compounds: A Review. J. Alloys Compd. 2020, 832. [Google Scholar] [CrossRef]
- Crivello, J.C.; Denys, R.V.; Dornheim, M.; Felderhoff, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; de Jongh, P.; Latroche, M.; Walker, G.S.; et al. Mg-Based Compounds for Hydrogen and Energy Storage. Appl. Phys. A 2016, 122. [Google Scholar] [CrossRef] [Green Version]
- Pistidda, C. Metals in Hydrogen Technology. Metals 2020, 10, 456. [Google Scholar] [CrossRef] [Green Version]
- Floriano, R.; Leiva, D.R.; Melo, G.C.; Ishikawa, T.T.; Huot, J.; Kaufman, M.; Figueroa, S.J.A.; Mendoza-Zélis, L.A.; Damonte, L.C.; Botta, W.J. Low Temperature Rolling of AZ91 Alloy for Hydrogen Storage. Int. J. Hydrogen Energy 2017, 42, 29394–29405. [Google Scholar] [CrossRef]
- Skryabina, N.; Aptukov, V.; Romanov, P.; Fruchart, D.; de Rango, P.; Girard, G.; Grandini, C.; Sandim, H.; Huot, J.; Lang, J.; et al. Microstructure Optimization of Mg-Alloys by the ECAP Process Including Numerical Simulation, SPD Treatments, Characterization, and Hydrogen Sorption Properties. Molecules 2018, 24, 89. [Google Scholar] [CrossRef] [Green Version]
- Huot, J.; Tousignant, M. Effect of Cold Rolling on Metal Hydrides. Mater. Trans. 2019, 60, 1571–1576. [Google Scholar] [CrossRef] [Green Version]
- Xin, G.; Yang, J.; Fu, H.; Li, W.; Zheng, J.; Li, X. Excellent Hydrogen Sorption Kinetics of Thick Mg–Pd Films under Mild Conditions by Tailoring Their Structures. R. Soc. Chem. 2013, 3, 4167–4170. [Google Scholar] [CrossRef]
- Urretavizcaya, G.; Sarmiento Chávez, A.C.; Castro, F.J. Hydrogen Absorption and Desorption in the Mg-Ag System. J. Alloys Compd. 2014, 611, 202–209. [Google Scholar] [CrossRef]
- Si, T.Z.; Zhang, J.B.; Liu, D.M.; Zhang, Q.A. A New Reversible Mg3Ag-H2 System for Hydrogen Storage. J. Alloys Compd. 2013, 581, 246–249. [Google Scholar] [CrossRef]
- Dufour, J.; Huot, J. Study of Mg6Pd Alloy Synthesized by Cold Rolling. J. Alloys Compd. 2007, 446–447, 147–151. [Google Scholar] [CrossRef]
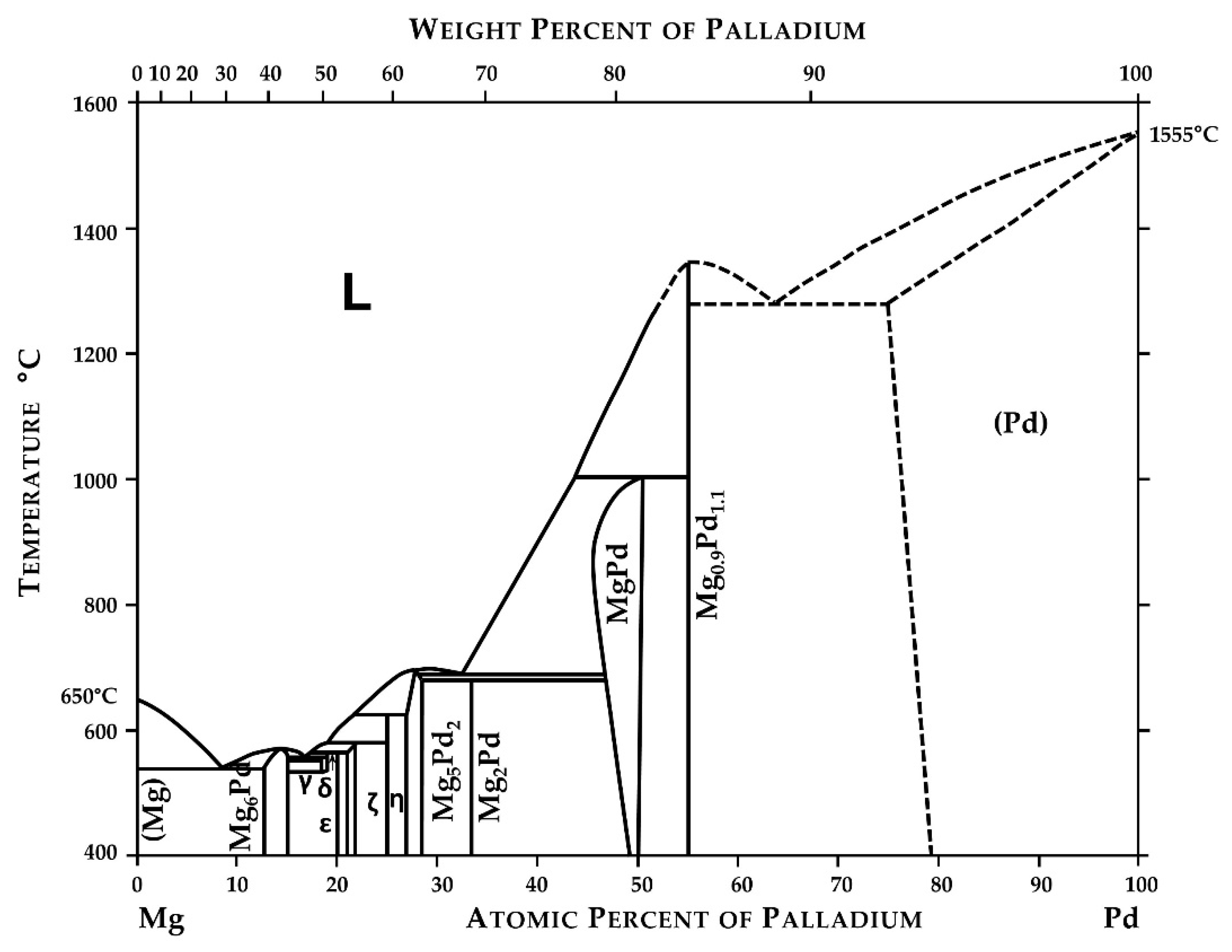
- Nayeb-Hashemi, A.A.; Clark, J.B. The Mg-Pd (Magnesium-Palladium) System. Bull. Alloy Phase Diagr. 1985, 6, 164–167. [Google Scholar] [CrossRef]
- Savitsky, E.M.; Terekhova, V.F.; Birun, N.A. Equilibrium Diagram of the Mg−Pd System. Russ. J. Inorg. Chem. 1962, 7, 1228–1231. [Google Scholar]
- Ferro, R. Research on the Alloys of Noble Metals with the More Electropositive Elements: III. Micrographic and X-ray Examination of Some Magnesium-Platinum Alloys. J. Less Common Met. 1959, 1, 424–438. [Google Scholar] [CrossRef]
- Kripyakevich, P.I.; Gladyshevskii, E.I. Crystal Structures of Some Compounds of Palladium with Magnesium. Sov. Phys. Crystallogr. 1960, 5, 552–554. [Google Scholar]
- Makongo, J.P.A.; Prots, Y.; Burkhardt, U.; Niewa, R.; Kudla, C.; Kreiner, G. A Case Study of Complex Metallic Alloy Phases: Structure and Disorder Phenomena of Mg–Pd Compounds. Philos. Mag. 2006, 86, 427–433. [Google Scholar] [CrossRef]
- Okamoto, H. Mg-Pd (Magnesium-Palladium). J. Phase Equilibria Diffus. 2010, 31, 407–408. [Google Scholar] [CrossRef]
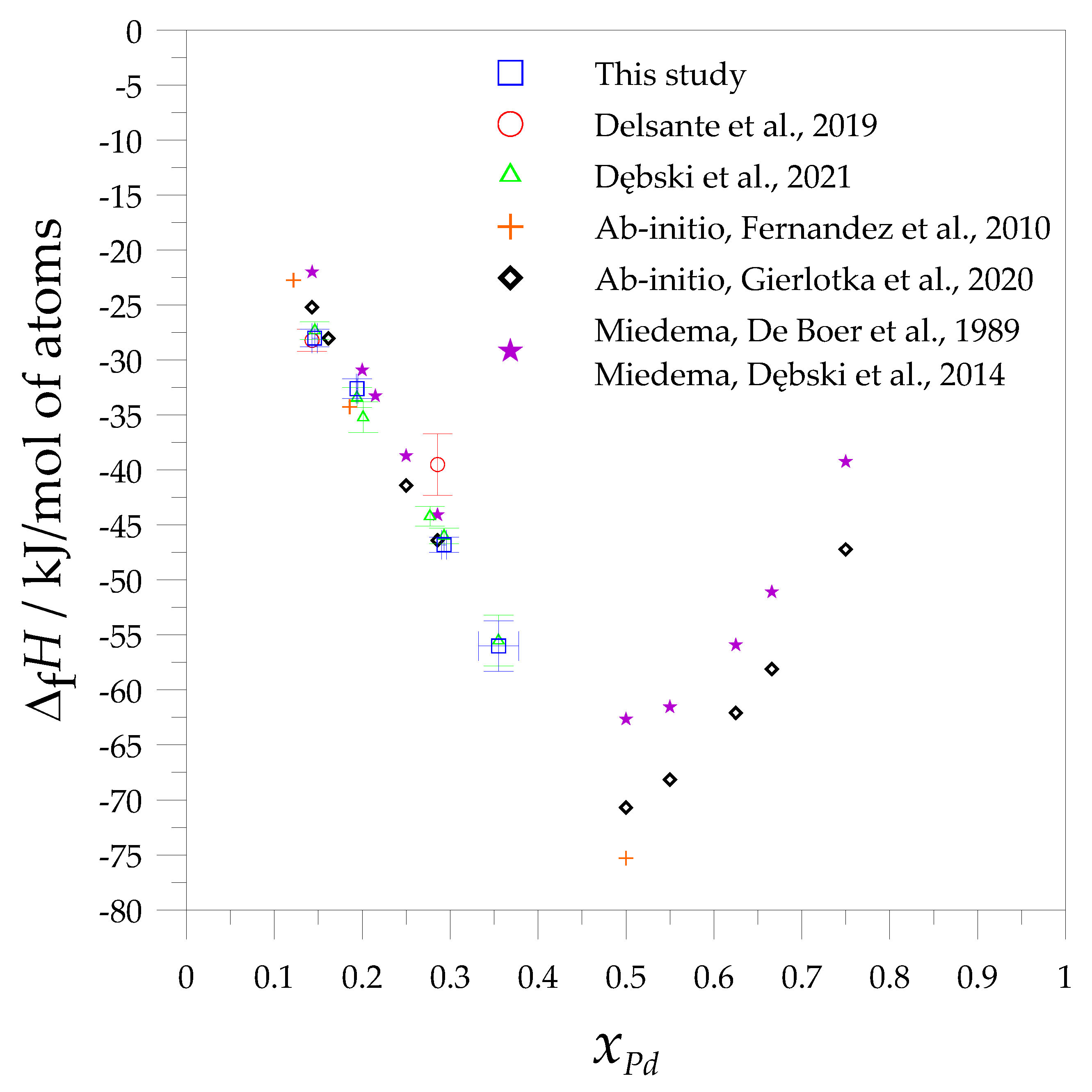
- Fernandez, J.F.; Ares, J.R.; Cuevas, F.; Bodega, J.; Leardini, F.; Sanchez, C. A Thermodynamic Study of the Hydrogenation of the Pseudo-Binary Mg6Pd0.5Ni0.5 Intermetallic Compound. Intermetallics 2010, 18, 233–241. [Google Scholar] [CrossRef]
- Fernandez, J.F.; Widomb, M.; Cuevas, F.; Ares, J.R.; Bodega, J.; Leardini, F.; Mihalkovi, M.; Sánchez, C. First-Principles Phase Stability Calculations and Estimation of Finite Temperature Effects on Pseudo-Binary Mg6(PdxNi1−x) Compounds. Intermetallics 2011, 19, 502–510. [Google Scholar] [CrossRef]
- Delsante, S.; Novakovic, R.; Gagliolo, A.; Borzone, G. Thermodynamic Investigation on the Mg–Pd Intermetallic Phases. J. Chem. Thermodyn. 2019, 139, 1–8. [Google Scholar] [CrossRef]
- Dębski, A.; Pęska, M.; Dworecka-Wójcik, J.; Terlicka, S.; Gąsior, W.; Gierlotka, W.; Polański, M. Structural and Calorimetric Studies of Magnesium-Rich Mg-PD alloys. J. Alloys Compd. 2021, 858. [Google Scholar] [CrossRef]
- Gierlotka, W.; Dębski, A.; Terlicka, S.; Gąsior, W.; Pęska, M.; Polański, M. Insight into Phase Stability in the Mg-Pd System: The Ab Initio Calculations. J. Phase Equilibria Diffus. 2020, 41, 681–686. [Google Scholar] [CrossRef]
- Colinet, C. High Temperature Calorimetry: Recent Developments. J. Alloys Compd. 1995, 220, 76–87. [Google Scholar] [CrossRef]
- Dȩbski, A.; Dȩbski, R.; Ga̧sior, W.; Góral, A. Formation Enthalpy of Intermetallic Phases from Ag-Ca System. Experiment vs. Modeling. J. Alloys Compd. 2014, 610, 701–705. [Google Scholar] [CrossRef]
- Dębski, A.; Terlicka, S.; Budziak, A.; Gąsior, W. Calorimetric and XRD Studies of Ag-Rich Alloys from Ag-Li System. J. Alloys Compd. 2018, 732, 210–217. [Google Scholar] [CrossRef]
- Dębski, A.; Braga, M.H.; Terlicka, S.; Gąsior, W.; Góral, A. Formation Enthalpy of Ga-Li Intermetallic Phases. Experiment vs. Calculations. J. Chem. Thermodyn. 2018, 124, 101–106. [Google Scholar] [CrossRef]
- Chen, S.L.; Daniel, S.; Zhang, F.; Chang, Y.A.; Yan, X.-Y.; Xie, F.-Y.; Schmid-Fetzer, R.; Oates, W.A. The PANDAT Software Package and Its Applications. Calphad Comput. Coupling Phase Diagr. Thermochem. 2002, 26, 175–188. [Google Scholar] [CrossRef]
- Dinsdale, A.T. SGTE Data for Pure Elements. Calphad 1991, 15, 317–425. [Google Scholar] [CrossRef]
- De Boer, F.R.; Boom, R.; Mattens, W.C.M.; Miedema, A.R.; Niessen, A.K. Cohesion in Metals: Transition Metal Alloys (Cohesion and Structure); Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Dębski, A.; Dębski, R.; Gąsior, W. New Features of ENTALL Database: Comparison of Experimental and Model Formation Enthalpies. Arch. Metall. Mater. 2014, 59, 1337–1343. [Google Scholar] [CrossRef]
- Rzyman, K.; Moser, Z.; Gachon, J.C. Calorimetric Studies of the Enthalpies of Formation of Al3Ti, AlTi, AlTi3 and Al2Ti Compounds. Arch. Metall. Materials 2004, 49, 545–563. [Google Scholar]
| Chemical Name | Source | Purity (Mass %) | Analysis Method |
|---|---|---|---|
| Magnesium | Sigma Aldrich | 99.9 | Certified purity |
| Palladium | Safina a.s. | 99.95 | Certified purity |
| Argon | Air Products | 99.9999 | Certified purity |
| No. | Alloys (Phases) | Annealing Temperature | Annealing Time (h) |
|---|---|---|---|
| 1 | 14.6 at.% Pd | 663 | 84 |
| 2 | 19.4 at.% Pd | 663 | 84 |
| 3 | 29.3 at.% Pd | 663 | 72 |
| 4 | 35.5 at.% Pd | 663 | 72 |
| Measurement No. | Dropped Mass of Samples (g) | At.% of Mg in Al Bath | Heat Effects ΔHef (kJ/mol) | |
|---|---|---|---|---|
| 1 | 0.0225 | 0.16 | 21.4 | −8.6 |
| 2 | 0.0397 | 0.45 | 21.2 | −8.8 |
| 3 | 0.0276 | 0.65 | 21.6 | −8.5 |
| 4 | 0.0414 | 0.95 | 21.7 | −8.3 |
| Average | - | - | 21.5 | −8.6 |
| Standard error | - | - | 1.1 | 1.1 |
| Measurement No. | Dropped Amount of Samples (g) | At.% of Pd in Al Bath | Heat Effects ΔHef (kJ/mol) | |
|---|---|---|---|---|
| 1 | 0.0822 | 0.14 | −154.6 | −186.9 |
| 2 | 0.0850 | 0.28 | −154.1 | −186.4 |
| 3 | 0.0860 | 0.42 | −154.4 | −186.7 |
| 4 | 0.0894 | 0.57 | −154.7 | −187.0 |
| 5 | 0.0861 | 0.71 | −154.6 | −186.9 |
| Average | - | - | −154.5 | −186.8 |
| Standard deviation | - | - | 1.1 | 1.1 |
| Alloys | T (K) | Sample No. | ΔHef (kJ/mol of atoms) | ΔfH (kJ/mol of atoms) |
|---|---|---|---|---|
| 14.6 at.% Pd (Mg6Pd) | 298 | 1 | 24.8 | −29.0 |
| 2 | 23.4 | −27.6 | ||
| 3 | 22.7 | −27.0 | ||
| 4 | 23.4 | −27.7 | ||
| 5 | 25.3 | −29.5 | ||
| 6 | 22.9 | −27.2 | ||
| Average | 23.8 | −28.0 | ||
| Standard error | 1.2 | 1.2 | ||
| 19.4 at.% Pd ~(ε) | 298 | 1 | 19.3 | −31.9 |
| 2 | 21.4 | −34.1 | ||
| 3 | 19.2 | −31.9 | ||
| 4 | 20.0 | −32.7 | ||
| Average | 20.0 | −32.6 | ||
| Standard error | 1.6 | 1.6 | ||
| 29.3 at.% Pd ~(Mg5Pd2) | 298 | 1 | 17.6 | −47.7 |
| 2 | 16.4 | −46.5 | ||
| 3 | 16.7 | −46.8 | ||
| 4 | 16.3 | −46.4 | ||
| Average | 16.7 | −46.8 | ||
| Standard error | 1.4 | 1.4 | ||
| 35.5 at.% Pd ~(Mg2Pd) | 298 | 1 | 15.5 | −56.5 |
| 2 | 16.1 | −57.1 | ||
| 3 | 14.6 | −55.6 | ||
| 4 | 13.8 | −54.9 | ||
| Average | 15.0 | −56.0 | ||
| Standard error | 1.6 | 1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dębski, A.; Terlicka, S.; Gąsior, W.; Gierlotka, W.; Pęska, M.; Dworecka-Wójcik, J.; Polański, M. Calorimetric Studies of Magnesium-Rich Mg-Pd Alloys. Materials 2021, 14, 680. https://doi.org/10.3390/ma14030680
Dębski A, Terlicka S, Gąsior W, Gierlotka W, Pęska M, Dworecka-Wójcik J, Polański M. Calorimetric Studies of Magnesium-Rich Mg-Pd Alloys. Materials. 2021; 14(3):680. https://doi.org/10.3390/ma14030680
Chicago/Turabian StyleDębski, Adam, Sylwia Terlicka, Władysław Gąsior, Wojciech Gierlotka, Magda Pęska, Julita Dworecka-Wójcik, and Marek Polański. 2021. "Calorimetric Studies of Magnesium-Rich Mg-Pd Alloys" Materials 14, no. 3: 680. https://doi.org/10.3390/ma14030680







