Phase Formation and Thermal Stability of Reactively Sputtered YTaO4–ZrO2 Coatings
Abstract
:1. Introduction
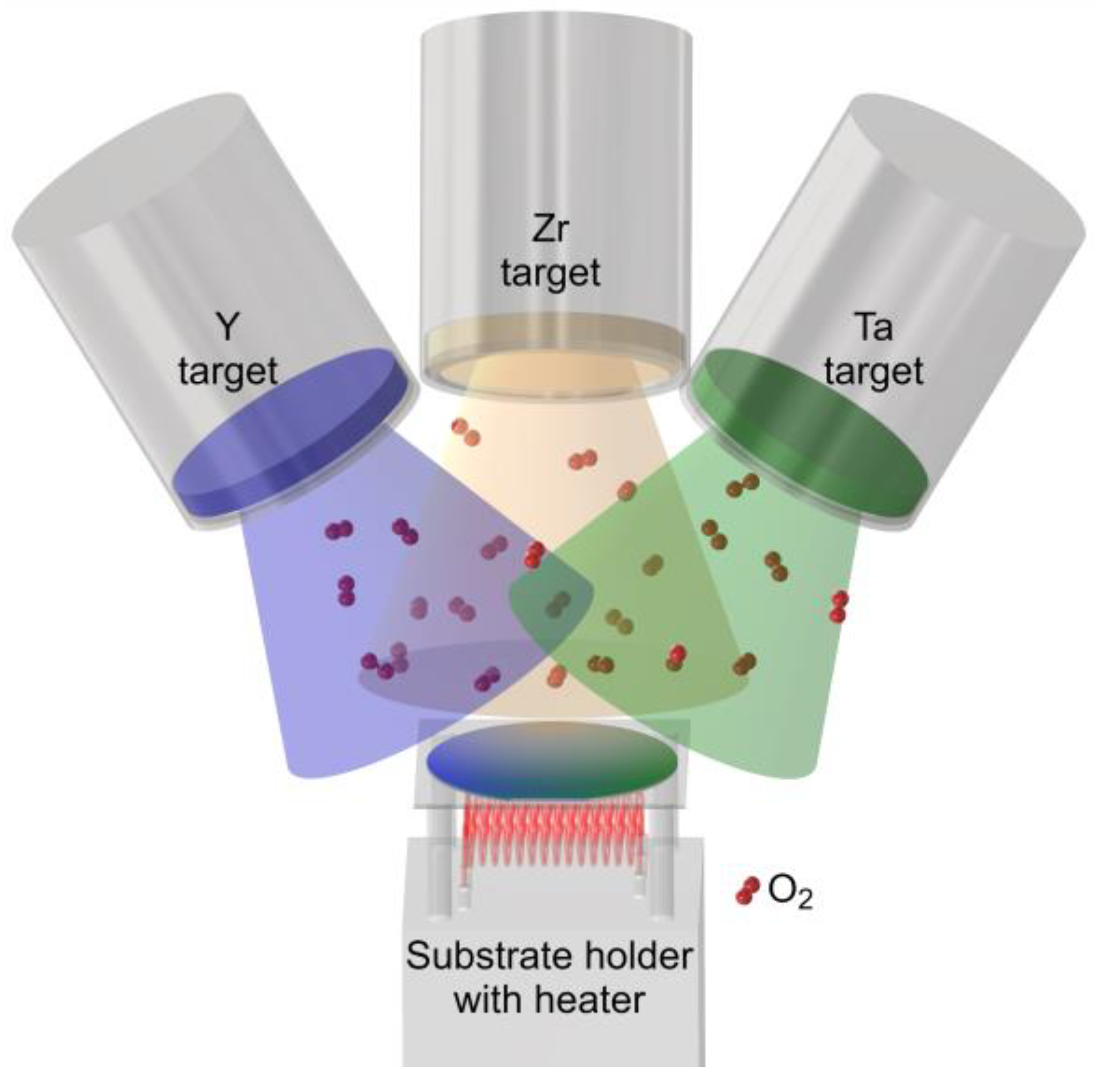
2. Materials and Methods
3. Results and Discussion
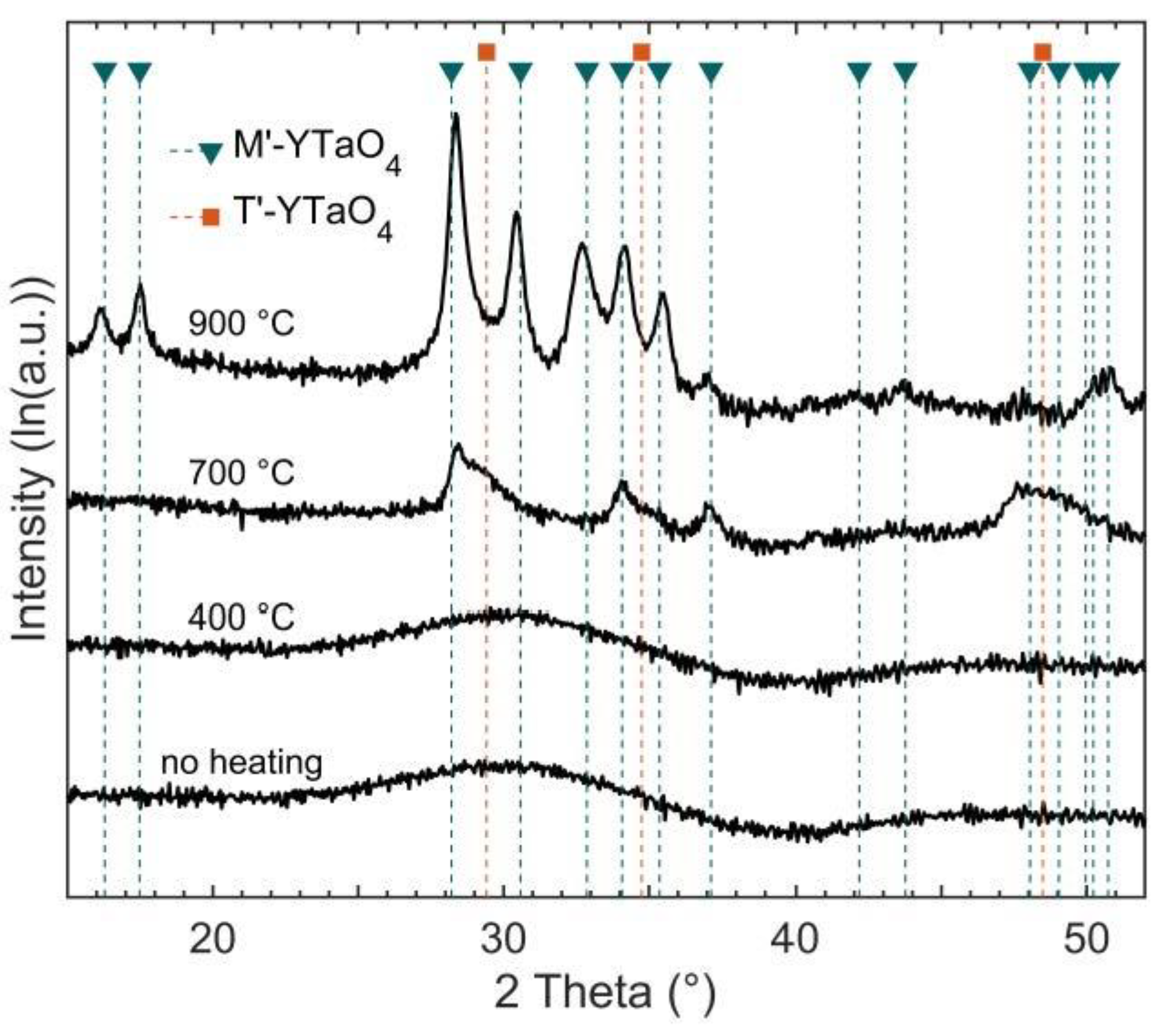
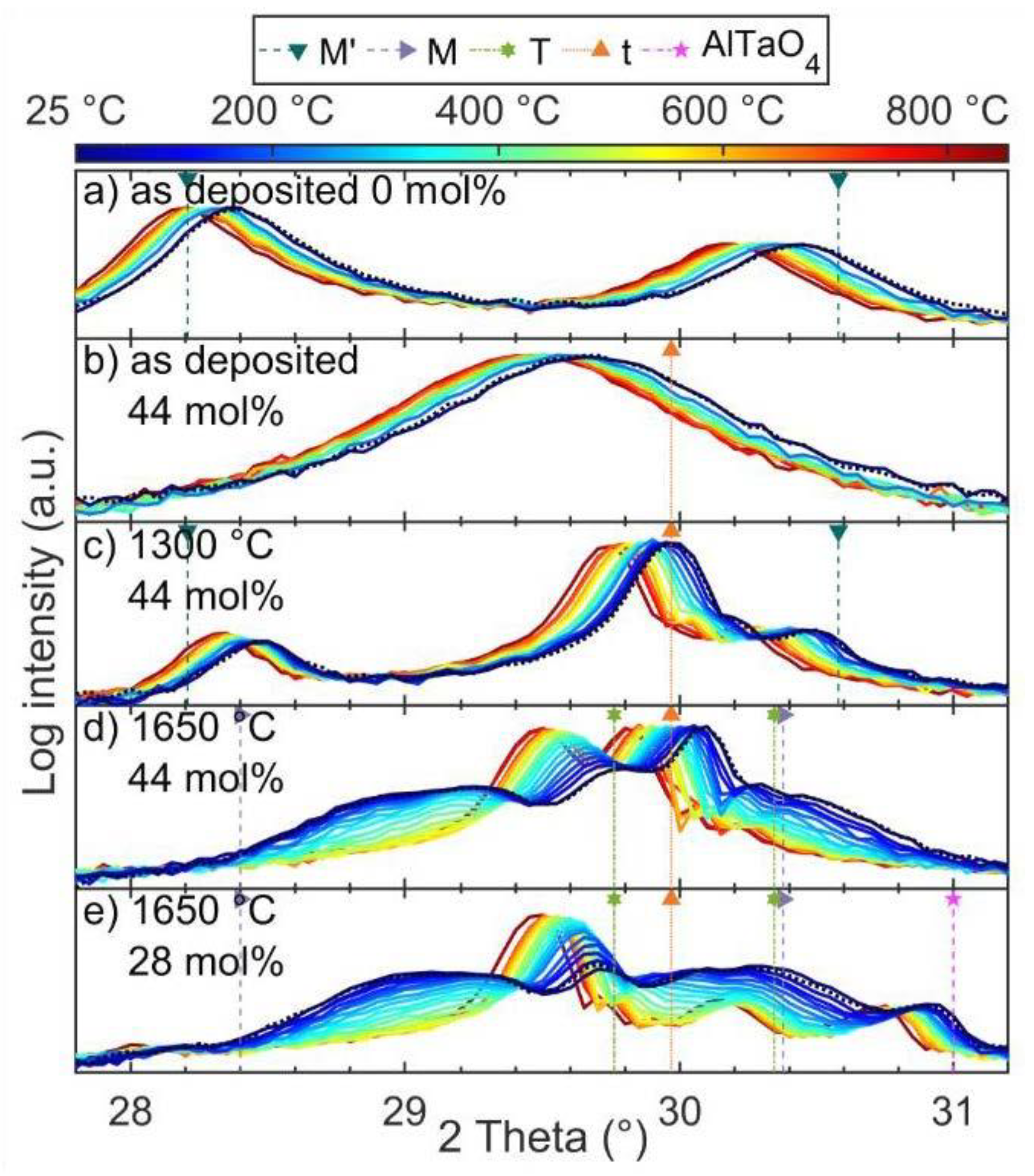
3.1. Effect of Substrate Temperature on YTaO4 Depositions
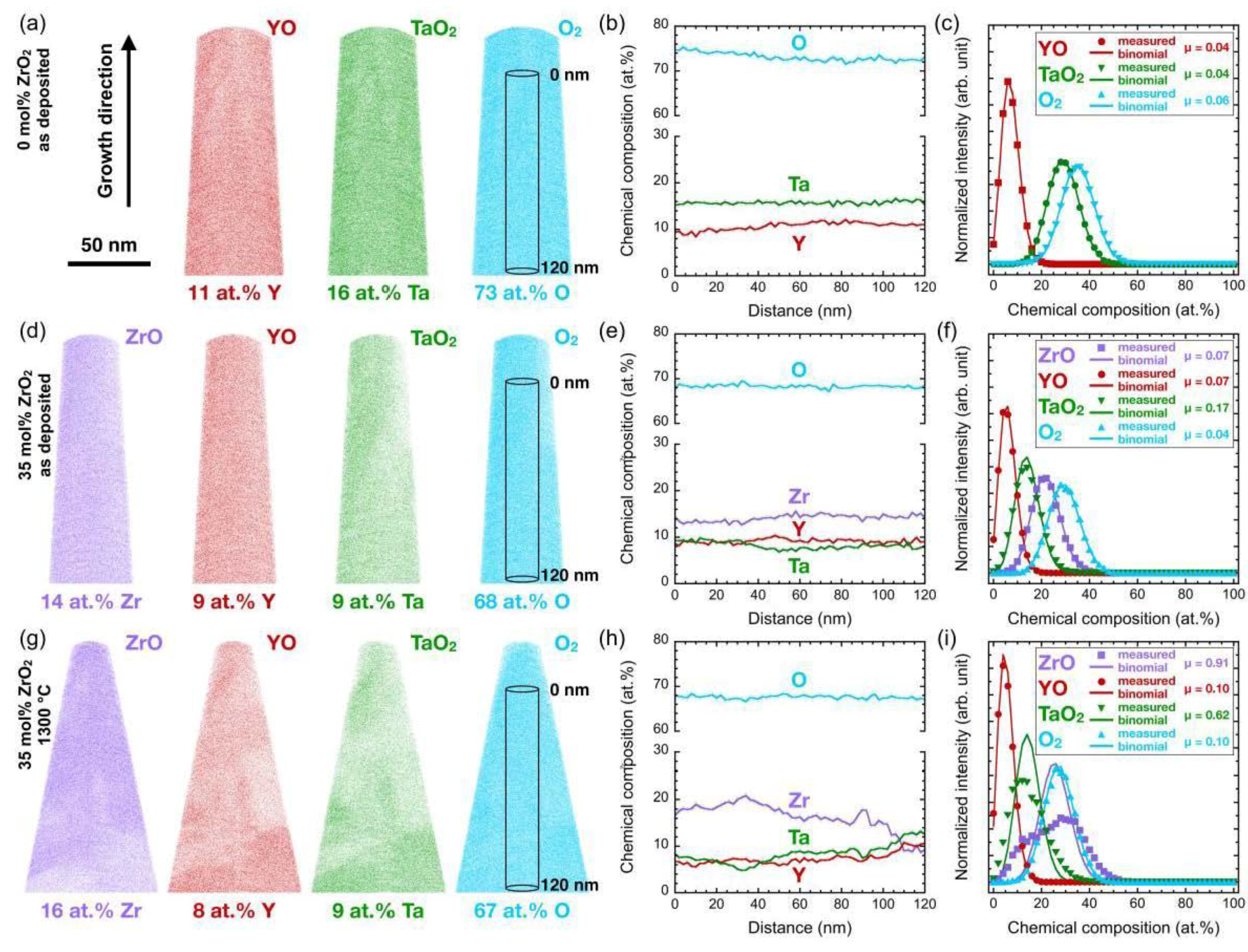
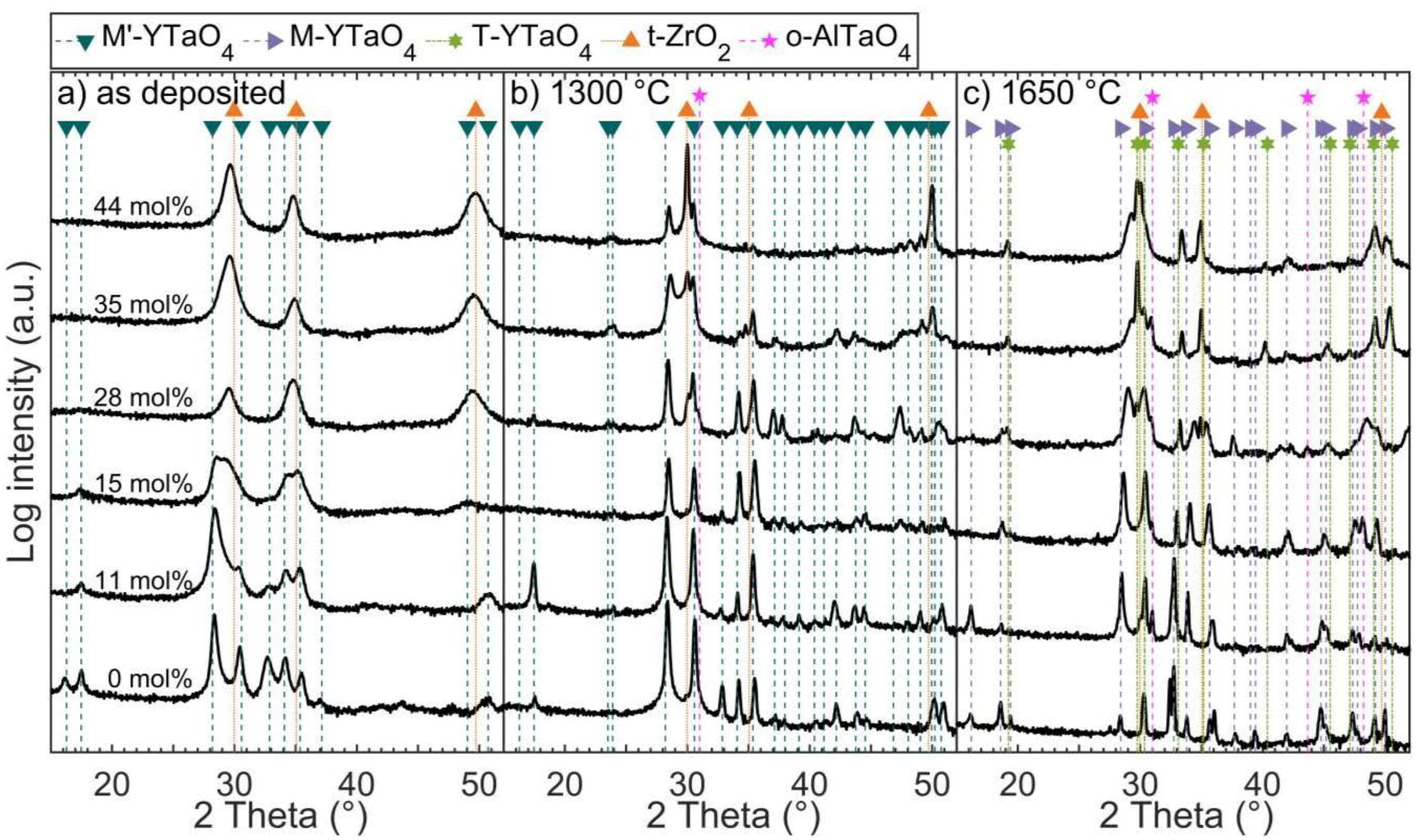
3.2. Effect of Zirconia Alloying on Phase Formation of As-Deposited Coatings
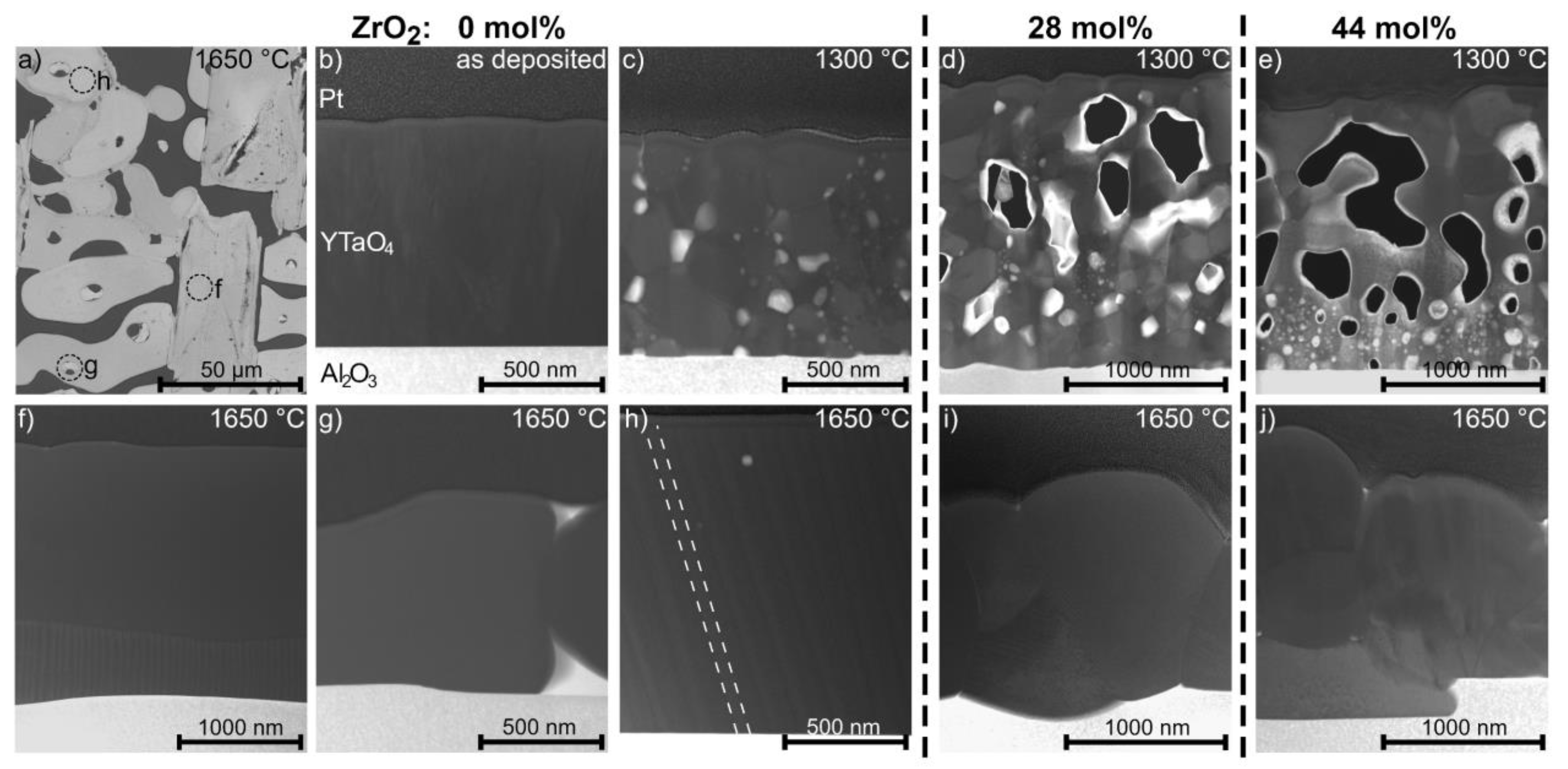
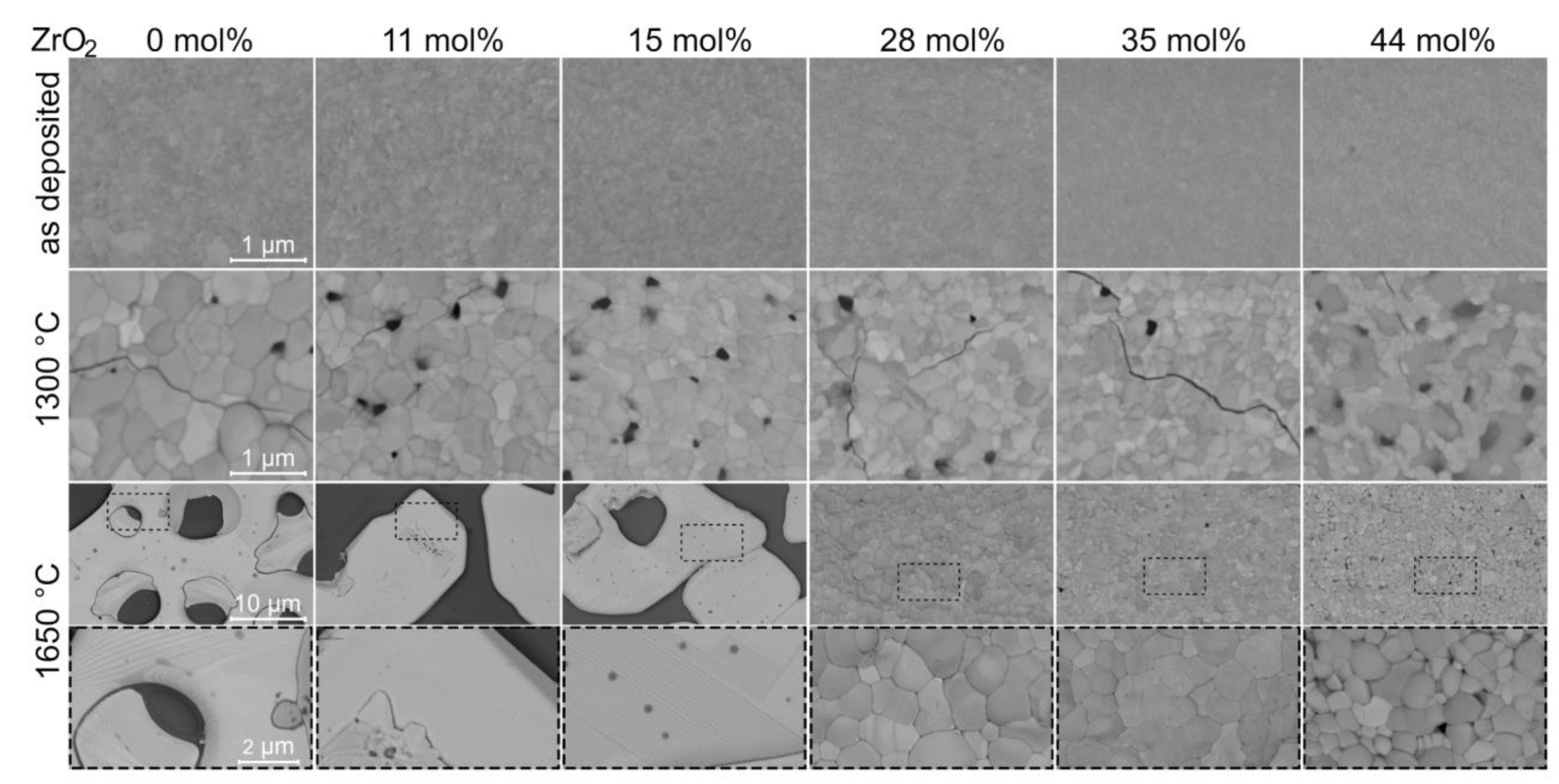
3.3. Effect of Annealing on Zirconia Alloyed YTaO4 Coatings
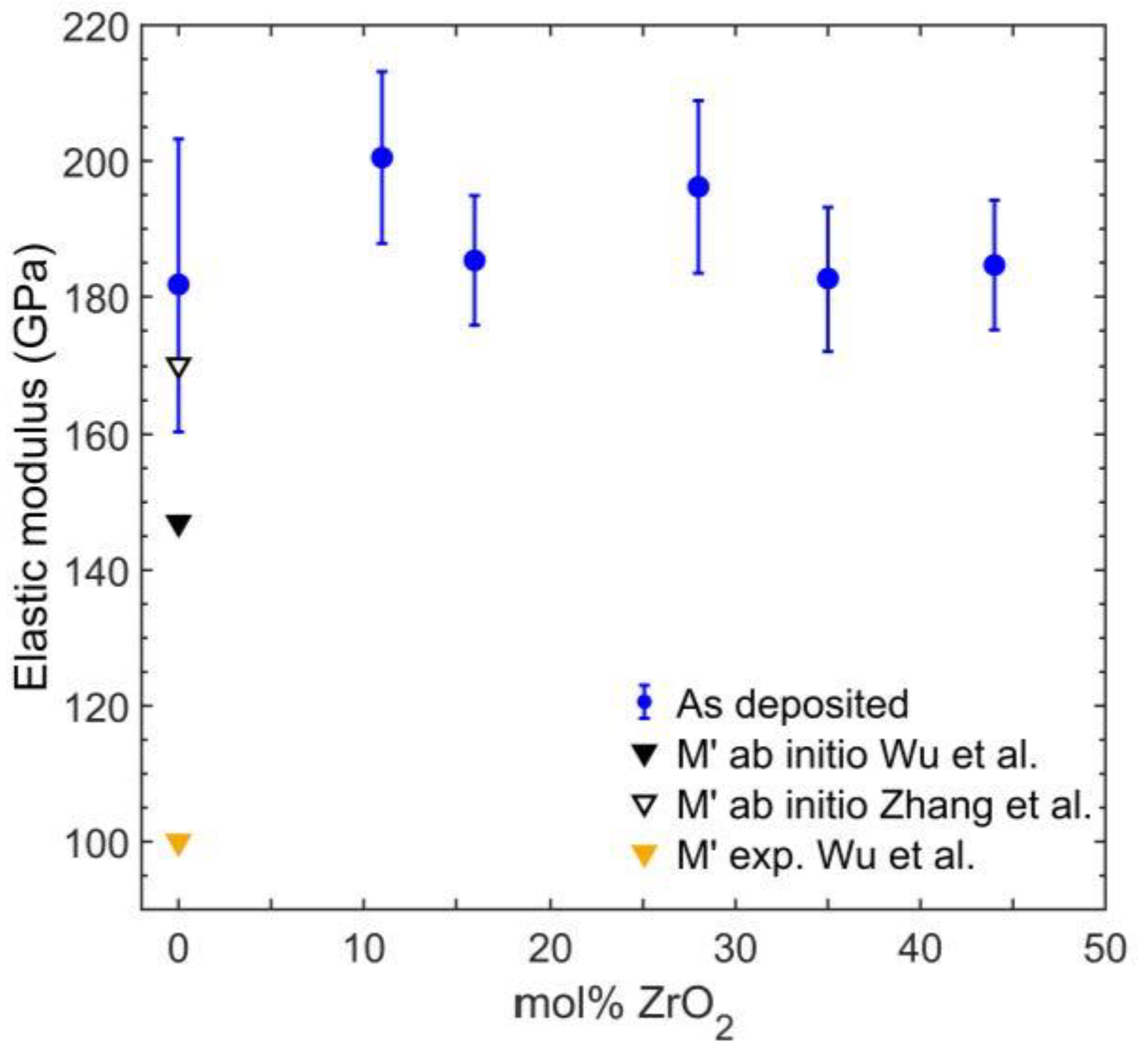
3.4. Elastic Properties of As-Deposited Coatings
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Padture, N.P.; Gell, M.; Jordan, E.H. Materials science-Thermal barrier coatings for gas-turbine engine applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef]
- Clarke, D.R.; Oechsner, M.; Padture, N.P. Thermal-barrier coatings for more efficient gas-turbine engines. MRS Bull. 2012, 37, 891–898. [Google Scholar] [CrossRef] [Green Version]
- Padture, N.P. Advanced structural ceramics in aerospace propulsion. Nat. Mater. 2016, 15, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Pitek, F.M.; Levi, C.G. Opportunities for TBCs in the ZrO2–YO1.5–TaO2.5 system. Surf. Coat. Technol. 2007, 201, 6044–6050. [Google Scholar] [CrossRef]
- Limarga, A.M.; Shian, S.; Leckie, R.M.; Levi, C.G.; Clarke, D.R. Thermal conductivity of single- and multi-phase compositions in the ZrO2–Y2O3–Ta2O5 system. J. Eur. Ceram. Soc. 2014, 34, 3085–3094. [Google Scholar] [CrossRef]
- Feng, J.; Shian, S.; Xiao, B.; Clarke, D.R. First-principles calculations of the high-temperature phase transformation in yttrium tantalate. Phys. Rev. B 2014, 90, 094102. [Google Scholar] [CrossRef]
- Zong, R.; Wu, F.; Song, P.; Feng, J. Influence of zirconia alloying on the thermophysical and mechanical properties of YTaO4 ceramics. Ceram. Int. 2019, 45, 24894–24899. [Google Scholar] [CrossRef]
- Shian, S.; Sarin, P.; Gurak, M.; Baram, M.; Kriven, W.M.; Clarke, D.R. The tetragonal–monoclinic, ferroelastic transformation in yttrium tantalate and effect of zirconia alloying. Acta Mater. 2014, 69, 196–202. [Google Scholar] [CrossRef]
- Yang, W.; Ye, F.; Yan, S.; Guo, L. The corrosion behaviors of thermal barrier material of M-YTaO4 attacked by CMAS at 1250 °C. Ceram. Int. 2020, 46, 9311–9318. [Google Scholar] [CrossRef]
- Wolten, G.M.; Chase, A.B. Synthetic Fergusonites and a New Polymorph of Yttrium Tantalate. Am. Miner. 1967, 52, 1536–1541. [Google Scholar]
- Wolten, G.M. The structure of the M’-phase of YTaO4, a third Fergusonite polymorph. Acta Crystallogr. 1967, 23, 939–944. [Google Scholar] [CrossRef]
- Mather, S.A.; Davies, P.K. Nonequilibrium Phase-Formation in Oxides Prepared at Low-Temperature-Fergusonite-Related Phases. J. Am. Ceram. Soc. 1995, 78, 2737–2745. [Google Scholar] [CrossRef]
- Heinze, S.G.; Natarajan, A.R.; Levi, C.G.; Van der Ven, A. Crystallography and substitution patterns in the ZrO2−YTaO4 system. Phys. Rev. Mater. 2018, 2, 073607. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, G.; Yang, L.; Zhou, Y.; Du, Y. Thermodynamic modeling of YO1.5-TaO2.5 system and the effects of elastic strain energy and diffusion on phase transformation of YTaO4. J. Eur. Ceram. Soc. 2019, 39, 5036–5047. [Google Scholar] [CrossRef]
- Flamant, Q.; Gurak, M.; Clarke, D.R. The effect of zirconia substitution on the high-temperature transformation of the monoclinic-prime phase in yttrium tantalate. J. Eur. Ceram. Soc. 2018, 38, 3925–3931. [Google Scholar] [CrossRef]
- Yokogawa, Y.; Yoshimura, M. High-Temperature Phase-Relations in the System Y2O3-Ta2O5. J. Am. Ceram. Soc. 1991, 74, 2077–2081. [Google Scholar] [CrossRef]
- Ryumin, M.A.; Sazonov, E.G.; Guskov, V.N.; Nikiforova, G.E.; Gagarin, P.G.; Guskov, A.V.; Gavrichev, K.S.; Baldaev, L.K.; Mazilin, I.V.; Golushina, L.N. Low-Temperature heat capacity of yttrium orthotantalate. Inorg. Mater. 2016, 52, 1149–1154. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Chong, X.; Zhou, R.; Feng, J. Microstructure and thermal properties of a promising thermal barrier coating: YTaO4. Ceram. Int. 2016, 42, 13876–13881. [Google Scholar] [CrossRef]
- Brixner, L.H.; Chen, H.Y. On the Structural and Luminescent Properties of the M’ LnTaO4 Rare Earth Tantalates. J. Electrochem. Soc. 1983, 130, 2435–2443. [Google Scholar]
- Yildirim, S.; Demirci, S.; Ertekin, K.; Celik, E.; Alicikus, Z.A. Production, characterization, and luminescent properties of Eu3+ doped yttrium niobate–tantalate films. J. Adv. Ceram. 2017, 6, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Gurak, M.; Flamant, Q.; Laversenne, L.; Clarke, D.R. On the Yttrium Tantalate–Zirconia phase diagram. J. Eur. Ceram. Soc. 2018, 38, 3317–3324. [Google Scholar] [CrossRef]
- Kim, D.J.; Tien, T.Y. Phase-Stability and Physical-Properties Of Cubic and Tetragonal ZrO2 in the System ZrO2-Y2O3-Ta2O5. J. Am. Ceram. Soc. 1991, 74, 3061–3065. [Google Scholar] [CrossRef]
- Shukla, S.; Seal, S. Mechanisms of room temperature metastable tetragonal phase stabilisation in zirconia. Int. Mater. Rev. 2005, 50, 45–64. [Google Scholar] [CrossRef]
- Macauley, C.A.; Fernandez, A.N.; Van Sluytman, J.S.; Levi, C.G. Phase equilibria in the ZrO2-YO1.5-TaO2.5 system at 1250 °C. J. Eur. Ceram. Soc. 2018, 38, 4523–4532. [Google Scholar] [CrossRef]
- Macauley, C.A.; Fernandez, A.N.; Levi, C.G. Phase equilibria in the ZrO2-YO1.5-TaO2.5 system at 1500 °C. J. Eur. Ceram. Soc. 2017, 37, 4888–4901. [Google Scholar] [CrossRef]
- Fernandez, A.N.; Macauley, C.A.; Park, D.; Levi, C.G. Sub-solidus phase equilibria in the YO1.5-TaO2.5 system. J. Eur. Ceram. Soc. 2018, 38, 4786–4798. [Google Scholar] [CrossRef]
- Van Sluytman, J.S.; Krämer, S.; Tolpygo, V.K.; Levi, C.G. Microstructure evolution of ZrO2–YbTaO4 thermal barrier coatings. Acta Mater. 2015, 96, 133–142. [Google Scholar] [CrossRef]
- Heinze, S.G.; Van Sluytman, J.S.; Levi, C.G. Microstructure evolution and physical properties of ZrO2-(Y + Yb)O1.5-TaO2.5 thermal barrier coatings. Surf. Coat. Technol. 2020, 389, 125648. [Google Scholar] [CrossRef]
- Thompson, K.; Lawrence, D.; Larson, D.J.; Olson, J.D.; Kelly, T.F.; Gorman, B. In situ site-specific specimen preparation for atom probe tomography. Ultramicroscopy 2007, 107, 131–139. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An Improved Technique for Determining Hardness and Elastic-Modulus Using Load and Displacement Sensing Indentation Experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Bolvardi, H.; Emmerlich, J.; Mráz, S.; Arndt, M.; Rudigier, H.; Schneider, J.M. Low temperature synthesis of Mo2BC thin films. Thin Solid Film. 2013, 542, 5–7. [Google Scholar] [CrossRef]
- Walter, C.; Sigumonrong, D.P.; El-Raghy, T.; Schneider, J.M. Towards large area deposition of Cr2AlC on steel. Thin Solid Film. 2006, 515, 389–393. [Google Scholar] [CrossRef]
- Abdulkadhim, A.; to Baben, M.; Takahashi, T.; Schnabel, V.; Hans, M.; Polzer, C.; Polcik, P.; Schneider, J.M. Crystallization kinetics of amorphous Cr2AlC thin films. Surf. Coat. Technol. 2011, 206, 599–603. [Google Scholar] [CrossRef]
- Moody, M.P.; Stephenson, L.T.; Ceguerra, A.V.; Ringer, S.P. Quantitative binomial distribution analyses of nanoscale like-solute atom clustering and segregation in atom probe tomography data. Microsc. Res. Tech. 2008, 71, 542–550. [Google Scholar] [CrossRef]
- Shannon, R. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Bhattachaya, A.; Shklover, V.; Kunze, K.; Steurer, W. Effect of 7YSZ on the long-term stability of YTaO4 doped ZrO2 system. J. Eur. Ceram. Soc. 2011, 31, 2897–2901. [Google Scholar]
- McHale, J.M.; Auroux, A.; Perrotta, A.J.; Navrotsky, A. Surface energies and thermodynamic phase stability in nanocrystalline aluminas. Science 1997, 277, 788–791. [Google Scholar] [CrossRef] [Green Version]
- Hans, M.; Music, D.; Chen, Y.T.; Patterer, L.; Eriksson, A.O.; Kurapov, D.; Ramm, J.; Arndt, M.; Rudigier, H.; Schneider, J.M. Crystallite size-dependent metastable phase formation of TiAlN coatings. Sci. Rep. 2017, 7, 16096. [Google Scholar] [CrossRef] [Green Version]
- Scherrer, P. Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nachr. Ges. Wiss. Gött. Math.-Phys. Kl. 1918, 98–100. [Google Scholar]
- Nahif, F.; Music, D.; Mráz, S.; Bolvardi, H.; Conrads, L.; Schneider, J.M. The effect of Si alloying on the thermal stability of Al2O3 films deposited by filtered cathodic arc. Surf. Coat. Technol. 2013, 235, 250–258. [Google Scholar] [CrossRef]
- Thompson, C.V. Solid-State Dewetting of Thin Films. Annu. Rev. Mater. Res. 2012, 42, 399–434. [Google Scholar] [CrossRef]
- Clarke, D.R.; Levi, C.G. Materials Design for the Next Generation Thermal Barrier Coatings. Annu. Rev. Mater. Res. 2003, 33, 383–417. [Google Scholar] [CrossRef]
- Aizu, K. Possible Species of “Ferroelastic” Crystals and of Simultaneously Ferroelectric and Ferroelastic Crystals. J. Phys. Soc. Japan 1969, 27, 387–396. [Google Scholar] [CrossRef]
- Wu, P.; Zhou, Y.; Wu, F.; Hu, M.; Chong, X.; Feng, J. Theoretical and experimental investigations of mechanical properties for polymorphous YTaO4 ceramics. J. Am. Ceram. Soc. 2019, 102, 7656–7664. [Google Scholar] [CrossRef]
- Ruess, H.; Music, D.; Bahr, A.; Schneider, J.M. Effect of chemical composition, defect structure, and stress state on the elastic properties of (V1−x Al x )1-y N y. J. Phys. Condens. Matter 2019, 32, 025901. [Google Scholar] [CrossRef]


| Abbreviation | Compound | Space Group | PDF Number |
|---|---|---|---|
| M’ | YTaO4 | P2/a | 00-024-1425 |
| M | YTaO4 | I2 | 00-024-1415 |
| T’ | YTaO4 | P42/nmc | 00-050-0846 |
| T | YTaO4 | I41/a | Feng et al. [6] |
| T | ZrO2 | P42/nmc | 00-043-0308 |
| O | AlTaO4 | Pbcn | 01-079-2410 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stelzer, B.; Pingen, K.; Hans, M.; Holzapfel, D.M.; Richter, S.; Mayer, J.; Pradeep, K.G.; Schneider, J.M. Phase Formation and Thermal Stability of Reactively Sputtered YTaO4–ZrO2 Coatings. Materials 2021, 14, 692. https://doi.org/10.3390/ma14030692
Stelzer B, Pingen K, Hans M, Holzapfel DM, Richter S, Mayer J, Pradeep KG, Schneider JM. Phase Formation and Thermal Stability of Reactively Sputtered YTaO4–ZrO2 Coatings. Materials. 2021; 14(3):692. https://doi.org/10.3390/ma14030692
Chicago/Turabian StyleStelzer, Bastian, Katrin Pingen, Marcus Hans, Damian M. Holzapfel, Silvia Richter, Joachim Mayer, Konda Gokuldoss Pradeep, and Jochen M. Schneider. 2021. "Phase Formation and Thermal Stability of Reactively Sputtered YTaO4–ZrO2 Coatings" Materials 14, no. 3: 692. https://doi.org/10.3390/ma14030692







