Local Induction Heating Capabilities of Zeolites Charged with Metal and Oxide MNPs for Application in HDPE Hydrocracking: A Proof of Concept
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. MNPs Synthesis
2.3. MNP-Based Catalysts Preparation
2.4. HDPE Films Preparation
2.5. MNP-Based Cataysts Characterization
2.6. Thermogravimetric Experiments
2.7. Induction Heating Assays
3. Results and Discussion
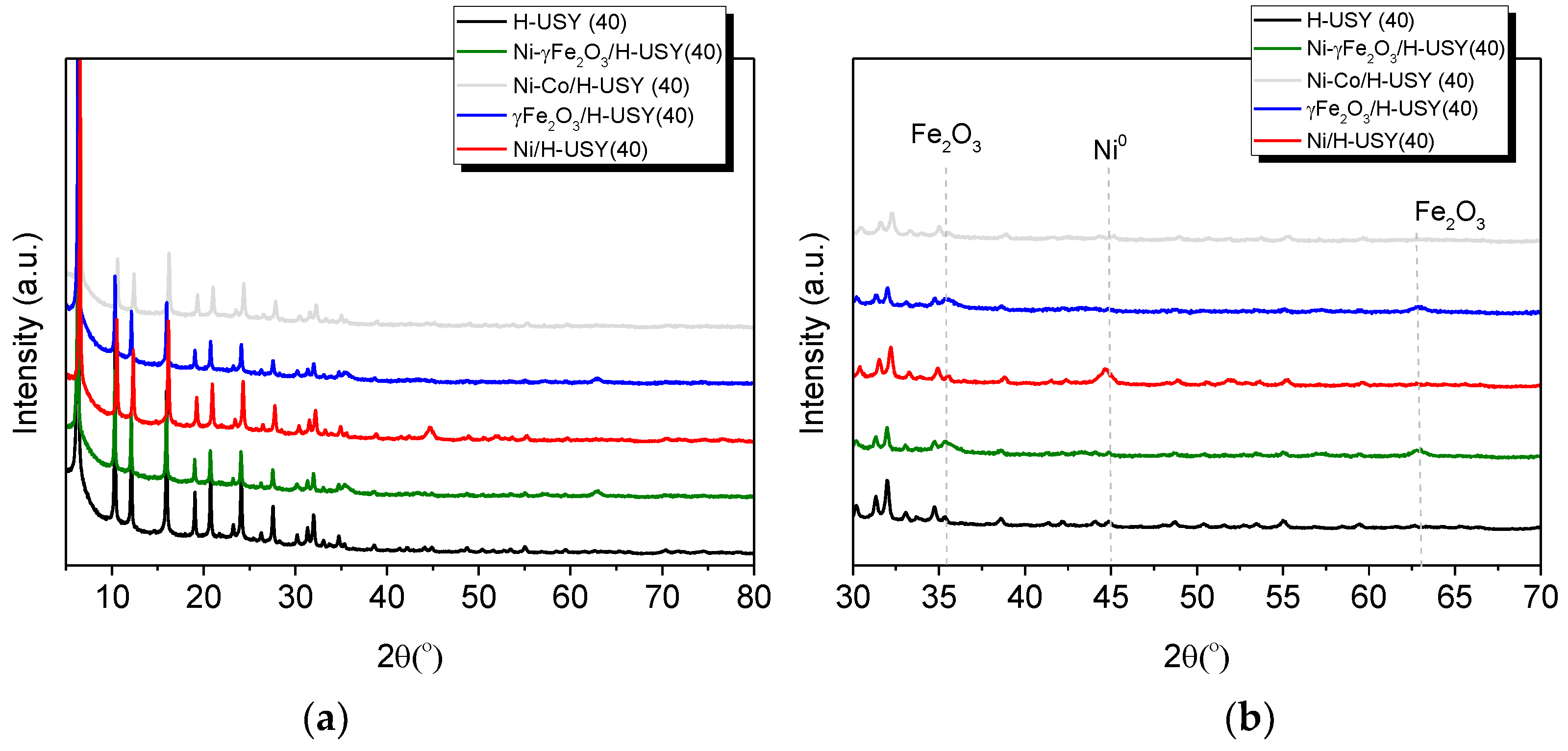
3.1. Catalyst Characterization
3.2. Degradation Experiments
3.3. Induction Heating Assays
3.3.1. Induction Heating on Impregnated H-USY(40)
3.3.2. Heating Induction on HDPE and Reinforced Catalysts Films
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Cz | Heat capacity |
| d | Diameter |
| D | Molecular weight dispersion |
| FTIR | Fourier transform infrared spectroscopy |
| FCC | Fluid catalytic cracking |
| HDPE | High density polyethylene |
| IMA | Institute of Magnetism Applied |
| IST | Institute Superior Technique |
| MNC | Magnetic nanoparticle concentration |
| MNP | Magnetic nanoparticle |
| Ms | Saturation magnetization |
| MW | Molecular weight |
| MWD | Molecular weight distribution |
| SAR | Specific absorption rate |
| SEM | Scanning electron microscopy |
| Sext | External surface area |
| TEM | Transmission electron microscopy |
| TGA | Thermogravimetric analysis |
| Tm | Melting temperature |
| UCM | University Complutense of Madrid |
| Vmeso | Mesoporous volume |
| Vmicro | Microporous volume |
| Vtotal | Total pore volume |
| W | Weight |
| PXRD | Powder X-Ray diffraction |
References
- PlasticsEurope. Plastics—The Facts 2019; PlasticsEurope: Brussels, Belgium, 2019. [Google Scholar]
- Siddiqui, M.N.; Redhwi, H.H. Catalytic coprocessing of waste plastics and petroleum residue into liquid fuel oils. J. Anal. Appl. Pyrolysis 2009, 86, 141–147. [Google Scholar] [CrossRef]
- Schmidt, C.; Krauth, T.; Wagner, S. Correction to export of plastic debris by rivers into the sea. Environ. Sci. Technol. 2018, 52, 927. [Google Scholar] [CrossRef]
- Munir, D.; Abdullah; Piepenbreier, F.; Usman, M.R. Hydrocracking of a plastic mixture over various micro-mesoporous composite zeolites. Powder Technol. 2017, 316, 542–550. [Google Scholar] [CrossRef]
- Munir, D.; Irfan, M.F.; Usman, M.R. Hydrocracking of virgin and waste plastics: A detailed review. Renew. Sustain. Energy Rev. 2018, 90, 490–515. [Google Scholar] [CrossRef]
- Mendes, P.S.F.; Silva, J.M.; Ribeiro, M.F.; Bouchy, C.; Daudin, A. Quantification of the available acid sites in the hydrocracking of nitrogen-containing feedstocks over USY shaped NiMo-catalysts. J. Ind. Eng. Chem. 2019, 71, 167–176. [Google Scholar] [CrossRef]
- Ding, W.; Liang, J.; Anderson, L.L. Hydrocracking and hydroisomerization of high-density polyethylene and waste plastic over zeolite and silica—Alumina-supported Ni and Ni-Mo sulfides. Energy Fuels 1997, 11, 1219–1223. [Google Scholar] [CrossRef]
- Sriningsih, W.; Saerodji, M.G.; Trisunaryanti, W.; Triyono; Armunanto, R.; Falah, I.I. Fuel Production from LDPE Plastic Waste over Natural Zeolite Supported Ni, Ni-Mo, Co and Co-Mo Metals. Procedia Environ. Sci. 2014, 20, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Galadima, A.; Muraza, O. Hydrocracking catalysts based on hierarchical zeolites: A recent progress. J. Ind. Eng. Chem. 2018, 61, 265–280. [Google Scholar] [CrossRef]
- Ali, M.A.; Tatsumi, T.; Masuda, T. Development of heavy oil hydrocracking catalysts using amorphous silica-alumina and zeolites as catalyst supports. Appl. Catal. A Gen. 2002, 233, 77–90. [Google Scholar] [CrossRef]
- Ochoa, R.; Van Woert, H.; Lee, W.H.; Subramanian, R.; Kugler, E.; Eklund, P.C. Catalytic degradation of medium density polyethylene over silica-alumina supports. Fuel Process. Technol. 1996, 49, 119–136. [Google Scholar] [CrossRef]
- Mosio-Mosiewski, J.; Warzala, M.; Morawski, I.; Dobrzanski, T. High-pressure catalytic and thermal cracking of polyethylene. Fuel Process. Technol. 2007, 88, 359–364. [Google Scholar] [CrossRef]
- Escola, J.M.; Aguado, J.; Serrano, D.P.; García, A.; Peral, A.; Briones, L.; Calvo, R.; Fernandez, E. Catalytic hydroreforming of the polyethylene thermal cracking oil over Ni supported hierarchical zeolites and mesostructured aluminosilicates. Appl. Catal. B Environ. 2011, 106, 405–415. [Google Scholar] [CrossRef]
- Metecan, I.H.; Ozkan, A.R.; Isler, R.; Yanik, J.; Saglam, M.; Yuksel, M. Naphtha derived from polyolefins. Fuel 2005, 84, 619–628. [Google Scholar] [CrossRef]
- Utami, S.; Wijaya, K.; Trisunaryanti, W. Pt-promoted sulfated zirconia as catalyst for hydrocracking of LDPE plastic waste into liquid fuels. Mater. Chem. Phys. 2018, 213, 548–555. [Google Scholar] [CrossRef]
- Pan, Z.; Xue, X.; Zhang, C.; Wang, D.; Xie, Y.; Zhang, R. Production of aromatic hydrocarbons by hydro-liquefaction of high-density polyethylene (HDPE) over Ni/HZSM-5. J. Anal. Appl. Pyrolysis 2018, 136, 208–214. [Google Scholar] [CrossRef]
- Hauli, L.; Wijaya, K.; Syoufian, A. Hydrocracking of LDPE Plastic Waste into Liquid Fuel over Sulfated Zirconia from a Commercial Zirconia Nanopowder. Orient. J. Chem. 2019, 35, 128–133. [Google Scholar] [CrossRef]
- Samperio, J.A. Alternative Catalytic Processes for the Valorization of Plastic Waste to Fuels; Universidad del País Vasco: Basque Country, Spain, 2016. [Google Scholar]
- Niether, C.; Faure, S.; Bordet, A.; Deseure, J.; Chatenet, M.; Carrey, J.; Chaudret, B.; Rouet, A. Improved water electrolysis using magnetic heating of FeC–Ni core–shell nanoparticles. Nat. Energy 2018, 1–8. [Google Scholar] [CrossRef]
- Zyuzin, M.V.; Cassani, M.; Barthel, M.J.; Gavilan, H.; Silvestri, N.; Escudero, A.; Scarpellini, A.; Lucchesi, F.; Teran, F.J.; Parak, W.J.; et al. Confining Iron Oxide Nanocubes inside Submicrometric Cavities as a Key Strategy to Preserve Magnetic Heat Losses in an Intracellular Environment. ACS Appl. Mater. Interfaces 2019, 11, 41957–41971. [Google Scholar] [CrossRef]
- Rubia-Rodríguez, I.; Santana-Otero, A.; Spassov, S.; Tombácz, E.; Johansson, C.; De La Presa, P.; Francisco, J.T.; del Puerto, M.M.; Veintemillas-Verdaguer, S.; Nguyen, T.K.T.; et al. Whither Magnetic Hyperthermia? A Tentative Roadmap. Materials 2021, 14, 706. [Google Scholar] [CrossRef]
- Rivas-Murias, B.; Asensio, J.M.; Mille, N.; Rodríguez-González, B.; Fazzini, P.F.; Carrey, J.; Chaudret, B.; Salgueiriño, V. Magnetically Induced CO2 Methanation Using Exchange-Coupled Spinel Ferrites in Cuboctahedron-Shaped Nanocrystals. Angew. Chem. Int. Ed. 2020, 59, 15537–15542. [Google Scholar] [CrossRef]
- Bordet, A.; Lacroix, L.M.; Fazzini, P.F.; Carrey, J.; Soulantica, K.; Chaudret, B. Magnetically Induced Continuous CO2Hydrogenation Using Composite Iron Carbide Nanoparticles of Exceptionally High Heating Power. Angew. Chem. Int. Ed. 2016, 55, 15894–15898. [Google Scholar] [CrossRef]
- Urraca, J.L.; Cortés-Llanos, B.; Aroca, C.; de la Presa, P.; Pérez, L.; Moreno-Bondi, M.C. Magnetic Field-Induced Polymerization of Molecularly Imprinted Polymers. J. Phys. Chem. C 2018. [Google Scholar] [CrossRef]
- Beck, M.M.; Lammel, C.; Gleich, B. Improving heat generation of magnetic nanoparticles by pre-orientation of particles in a static three tesla magnetic field. J. Magn. Magn. Mater. 2017, 427, 195–199. [Google Scholar] [CrossRef]
- Bayerl, T.; Duhovic, M.; Mitschang, P.; Bhattacharyya, D. The heating of polymer composites by electromagnetic induction—A review. Compos. Part A 2014, 57, 27–40. [Google Scholar] [CrossRef]
- Apostolidis, P.; Liu, X.; van de Ven, M.; Erkens, S.; Scarpas, T. Control the crosslinking of epoxy-asphalt via induction heating. Int. J. Pavement Eng. 2019, 8436. [Google Scholar] [CrossRef] [Green Version]
- Meffre, A.; Mehdaoui, B.; Kelsen, V.; Fazzini, P.F.; Carrey, J.; Lachaize, S.; Respaud, M.; Chaudret, B. A simple chemical route toward monodisperse iron carbide nanoparticles displaying tunable magnetic and unprecedented hyperthermia properties. Nano Lett. 2012, 12, 4722–4728. [Google Scholar] [CrossRef]
- Caetano, P.M.A.; Albuquerque, A.S.; Fernandez-Outon, L.E.; Macedo, W.A.A.; Ardisson, J.D. Structure, magnetism and magnetic induction heating of NixCo(1-x)Fe2O4 nanoparticles. J. Alloys Compd. 2018, 758, 247–255. [Google Scholar] [CrossRef]
- Bordet, A.; Lacroix, L.M.; Soulantica, K.; Chaudret, B. A New Approach to the Mechanism of Fischer-Tropsch Syntheses Arising from Gas Phase NMR and Mass Spectrometry. ChemCatChem 2016, 8, 1727–1731. [Google Scholar] [CrossRef]
- Verde, E.L.; Landi, G.T.; Gomes, J.A.; Sousa, M.H.; Bakuzis, A.F. Magnetic hyperthermia investigation of cobalt ferrite nanoparticles: Comparison between experiment, linear response theory, and dynamic hysteresis simulations. J. Appl. Phys. 2012, 111. [Google Scholar] [CrossRef]
- Vinum, M.G.; Almind, M.R.; Engbæk, J.S.; Vendelbo, S.B.; Hansen, M.F.; Frandsen, C.; Bendix, J.; Mortensen, P.M. Dual-Function Cobalt–Nickel Nanoparticles Tailored for High-Temperature Induction-Heated Steam Methane Reforming. Angew. Chem. Int. Ed. 2018, 57, 10569–10573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verde, E.L.; Landi, G.T.; Carrião, M.S.; Drummond, A.L.; Gomes, J.A.; Vieira, E.D.; Sousa, M.H.; Bakuzis, A.F. Field dependent transition to the non-linear regime in magnetic hyperthermia experiments: Comparison between maghemite, copper, zinc, nickel and cobalt ferrite nanoparticles of similar sizes. AIP Adv. 2012, 2. [Google Scholar] [CrossRef]
- Wang, W.; Tuci, G.; Duong-Viet, C.; Liu, Y.; Rossin, A.; Luconi, L.; Nhut, J.M.; Nguyen-Dinh, L.; Pham-Huu, C.; Giambastiani, G. Induction Heating: An Enabling Technology for the Heat Management in Catalytic Processes. ACS Catal. 2019, 9, 7921–7935. [Google Scholar] [CrossRef]
- Cullity, B.D.; Graham, C.D.G. Introduction to Magnetic Materials; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Morales, I.; Muñoz, M.; Costa, C.S.; Alonso, J.M.; Silva, J.M.; Multigner, M.; Quijorna, M.; Ribeiro, M.R.; de la Presa, P. Induction heating in nanoparticle impregnated zeolite. Materials 2020, 13, 4013. [Google Scholar] [CrossRef]
- De La Presa, P.; Luengo, Y.; Multigner, M.; Costo, R.; Morales, M.P.; Rivero, G.; Hernando, A. Study of heating efficiency as a function of concentration, size, and applied field in γ-Fe2O3 nanoparticles. J. Phys. Chem. C 2012, 116, 25602–25610. [Google Scholar] [CrossRef]
- Costa, C.S.; Muñoz, M.; Ribeiro, M.R.; Silva, J.M. A thermogravimetric study of HDPE conversion under a reductive atmosphere. Catal. Today 2020. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.M.; Faria, J. Catalysis from Theory to Application; Coimbra University Press: Coimbra, Portugal, 2008. [Google Scholar] [CrossRef] [Green Version]
- Etim, U.J.; Xu, B.; Zhang, Z.; Zhong, Z.; Bai, P.; Qiao, K.; Yan, Z. Improved catalytic cracking performance of USY in the presence of metal contaminants by post-synthesis modification. Fuel 2016, 178, 243–252. [Google Scholar] [CrossRef]
- Levecque, P.; Gammon, D.W.; Jacobs, P.; Vos, D.; Sels, B. The use of ultrastable Y zeolites in the Ferrier rearrangement of acetylated and benzylated glycals. Green Chem. 2010, 828–835. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Graça, I.; Lopes, J.M.; Henriques, C. Enhanced activity of CO2 hydrogenation to CH4 over Ni based zeolites through the optimization of the Si/Al ratio. Microporous Mesoporous Mater. 2018, 267, 9–19. [Google Scholar] [CrossRef]
- Wong, S.; Ngadi, N.; Abdullah, T.A.T.; Inuwa, I.M. Catalytic Cracking of LDPE Dissolved in Benzene Using Nickel-Impregnated Zeolites. Ind. Eng. Chem. Res. 2016, 55, 2543–2555. [Google Scholar] [CrossRef]
- Caldeira, V.P.S.; Santos, A.G.D.; Oliveira, D.S.; Lima, R.B.; Souza, L.D.; Pergher, S.B.C. Polyethylene catalytic cracking by thermogravimetric analysis: Effects of zeolitic properties and homogenization process. J. Therm. Anal. Calorim. 2017, 130, 1939–1951. [Google Scholar] [CrossRef]
- Coelho, A.; Costa, L.; Marques, M.M.; Fonseca, I.M.; Lemos, M.A.N.D.A.; Lemos, F. Applied Catalysis A: General The effect of ZSM-5 zeolite acidity on the catalytic degradation of high-density polyethylene using simultaneous DSC / TG analysis. Appl. Catal. A Gen. 2012, 413–414, 183–191. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, M.A.; Torres, T.E.; Andrés-Vergés, M.; Costo, R.; de la Presa, P.; Serna, C.J.; Morales, M.P.; Marquina, C.; Ibarra, M.R.; Goya, G.F. Magnetic nanoparticles for power absorption: Optimizing size, shape and magnetic properties. J. Solid State Chem. 2009, 182, 2779–2784. [Google Scholar] [CrossRef]





| Material | Ms (emu/g) | Curie Temperature (°C) | |
|---|---|---|---|
| 0 K | 293 K | ||
| Fe | 221.9 | 218.0 | 770 |
| Co | 162.5 | 161 | 1131 |
| Ni | 57.5 | 54.4 | 358 |
| γ-Fe2O3 | 83.5 | 76 | Unstable |
| Fe3O4 | 98.0 | 92 | 585 |
| Catalyst Type | Sext (m2/g) | Vmicro (cm3/g) | Vmeso (cm3/g) | Vtotal (cm3/g) |
|---|---|---|---|---|
| H-USY(40) | 251 | 0.210 | 0.250 | 0.460 |
| Ni/H-USY(40) | 255 | 0.147 | 0.250 | 0.397 |
| γ-Fe2O3/H-USY(40) | 168 | 0.185 | 0.235 | 0.420 |
| Ni–γ-Fe2O3/H-USY(40) | 155 | 0.063 | 0.165 | 0.228 |
| Sample | T5% (°C) | T50% (°C) | T95% (°C) |
|---|---|---|---|
| HDPE | 433 | 478 | 488 |
| HDPE + H-USY(40) | 270 | 357 | 399 |
| HDPE+ γ-Fe2O3/H-USY(40) | 384 | 409 | 425 |
| HDPE+ Ni/γ-Fe2O3/H-USY(40) | 335 | 401 | 418 |
| Frequency (kHz) | Field (Oe) | γ-Fe2O3 (∆T/∆t)/[Fe] (K/s) | Ni–γ-Fe2O3 (∆T/∆t)/[Fe] (K/s) |
|---|---|---|---|
| 112 | 172 | 14.57 | 38.29 |
| 165 | 133 | 8.57 | 22.86 |
| 177 | 124 | 12.57 | 21.14 |
| 263 | 73 | 18.29 | 28.00 |
| 331 | 60 | 22.29 | 34.86 |
| 468 | 73 | 11.71 | 20.00 |
| 526 | 94 | 23.43 | 35.43 |
| 625 | 50 | 6.00 | 12.00 |
| 740 | 35 | 6.57 | 9.14 |
| 990 | 32 | 4.00 | 6.86 |
| Frequency (kHz) | Field (Oe) | γ-Fe2O3 (∆T/∆t)/[Fe] (K/s) | Ni-γ-Fe2O3 (∆T/∆t)/[Fe] (K/s) |
|---|---|---|---|
| 112 | 172 | 12.57 | 16.00 |
| 165 | 133 | 5.71 | 9.14 |
| 177 | 124 | 8.00 | 9.14 |
| 263 | 73 | 10.29 | 18.29 |
| 331 | 60 | 13.71 | 20.57 |
| 468 | 73 | 5.71 | 9.14 |
| 526 | 94 | 14.86 | 29.71 |
| 625 | 50 | 2.29 | 2.29 |
| 740 | 35 | 1.14 | 4.57 |
| 990 | 32 | 1.14 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz, M.; Morales, I.; Costa, C.S.; Multigner, M.; de la Presa, P.; Alonso, J.M.; Silva, J.M.; Ribeiro, M.d.R.; Torres, B.; Rams, J. Local Induction Heating Capabilities of Zeolites Charged with Metal and Oxide MNPs for Application in HDPE Hydrocracking: A Proof of Concept. Materials 2021, 14, 1029. https://doi.org/10.3390/ma14041029
Muñoz M, Morales I, Costa CS, Multigner M, de la Presa P, Alonso JM, Silva JM, Ribeiro MdR, Torres B, Rams J. Local Induction Heating Capabilities of Zeolites Charged with Metal and Oxide MNPs for Application in HDPE Hydrocracking: A Proof of Concept. Materials. 2021; 14(4):1029. https://doi.org/10.3390/ma14041029
Chicago/Turabian StyleMuñoz, Marta, Irene Morales, Cátia S. Costa, Marta Multigner, Patricia de la Presa, Jose M. Alonso, João M. Silva, Maria do Rosário Ribeiro, Belén Torres, and Joaquín Rams. 2021. "Local Induction Heating Capabilities of Zeolites Charged with Metal and Oxide MNPs for Application in HDPE Hydrocracking: A Proof of Concept" Materials 14, no. 4: 1029. https://doi.org/10.3390/ma14041029










