1. Introduction
In dentistry, the development of computer-aided design/computer-aided manufacturing (CAD/CAM) systems in the 1980s brought a significant change to the field [
1]. Continuous automatization and computerization finally led to the formation of a so-called digital dentistry, where the whole workflow—from assessment of the dentition situation by modern scan methods, via modelling of the restoration by adapted software, to the automated production of the restoration by CAM—is completed digitally. All necessary data is recorded virtually, so that, ideally, the manual preparation of impressions or models can be discarded.
The CAM method that has been developed primarily for this case is milling. This technique is now state of the art. In recent years, additive manufacturing (AM) has gained increasing attention as a complementary approach and, for certain cases, has found its way into dental laboratories and practices [
2]. To enable a successful restoration, like a crown or bridge, high precision and good surface quality are necessary. Vat photo-polymerization is an AM method that excels in these categories. By combining this technology with the colloidal processing route, the production of full ceramic parts is possible [
3].
This work focuses on lithography-based ceramic manufacturing (LCM) with regards to the technology and lithium disilicate with regards to the material. Lithium disilicate is one of the materials used predominantly for full ceramic restorations (the other one being zirconia) [
4]. In LCM, suspensions or pastes are used, whose formulation can be rather complex. Changing one constituent or the fractions of the composition can easily result in a negative influence on other properties of the formulation. Here an approach was chosen that includes a change of the solvent composition. The aim was to change certain properties of the suspension, such as refractive index, rheology, and debinding behavior, without interfering too much with the polymerization mechanism.
Table 1 lists typical components for such a suspension. In the following, these components and considerations of importance regarding their selection are discussed.
The starting point for the formulation is the filler. Amount of solid loading, particle size, particle size distribution, shape of the powder particles, and chemical constitution are factors that must be evaluated when developing a formulation. Amount of solid loading, particle size, and particle shape mainly determine the rheological behavior of the suspension. The viscosity, for example, increases with increasing solid loading and deviation from spherical particle shape [
5,
6].
The chemical constitution of the powder is pivotal for the strategy of stabilizing the suspension against agglomeration and sedimentation. Colloidal suspensions are divided into lyophilic and lyophobic colloids. In lyophilic colloids, the solvent has a strong affinity to the dispersed particles. In this case, the liquid is strongly adsorbed on the surface, and the system is stable by itself, because the free Gibbs energy is decreased during the process of dispersing. On the other hand, lyophobic colloids show no affinity between liquid and particle surface. The free Gibbs energy increases during the process of dispersing and attractive forces between the particles are active, which is the reason for quick agglomeration. In most cases, ceramic suspensions are lyophobic colloids [
7]. Therefore, it is useful to acquire knowledge about what kind of attractive forces appear during dispersing and how they can be overcome by purposefully introducing repulsive forces to create suspensions with the desired stability.
A meaningful tool with regards to these considerations is the dispersing agent. By binding to the particle surface, it allows one to specifically introduce repulsive forces. In general, the following strategies are used for this purpose: electrostatic stabilization, steric stabilization, or electrosteric stabilization. In the case of suspensions in organic liquids, as they come into use in, for example, slip casting or tape casting, mostly steric stabilization is applied, where unloaded polymer chains are adsorbed on the particle surface [
8]. Stable suspensions have the advantage that, in LCM, they enable higher homogeneity with regards to the solid loading of green bodies. In the case of unstable suspensions, over the course of the printing process, segregation and sedimentation take place, which leads to a different solid loading of the green bodies, depending on the height of the printed object [
9].
Thixotropy is an effective instrument to prevent sedimentation and, at the same time, enables a good processability. It is defined as: “A time dependency of the flowing properties of non-Newtonian fluids, whose viscosity is decreased by continuous external influences and returns to starting viscosity only after the ceasing of load [
10]”. It is important to stress that in addition to shear-thinning behavior, thixotropic materials exhibit a time dependency of the viscosity recovery (i.e., after a mechanical load has been removed for a certain time period, the viscosity returns to its initial state).
In LCM, this is extensively practical. As long as no shearing forces are applied to the suspension (e.g., during storage), the suspension is ideally highly viscous and stable. When shearing forces are applied by the doctor blade during coating, the structural strength of the suspension is decreased. This way, the suspension yields to the load and enables easy processing. After the coating process, thixotropy leads to an increase of structural strength and therefore to the stability of the suspension after a given time period. This way, introducing a thixotropic additive enables the stabilization during storage and processing of an otherwise unstable suspension [
11].
Since the basic mechanism for layer generation in LCM is based on photo-polymerization, optical properties like the refractive index are especially relevant for this method. When the difference in refractive index between dispersing phase and dispersed phase is high, the penetration depth of light is low and the widening, due to scattering, is high. When the difference in refractive index is low, penetration depth is high and widening low. To enable a high precision in LCM, it is necessary to target a small difference in refractive index (refractive index matching). The penetration depth can afterwards be decreased by utilizing an appropriate concentration of absorber [
12].
Absorbers are chemical substances, which, ideally, are inert with regards to the polymerization and, by absorbing photons, reduce the penetration depth of light into the material. In vat photo-polymerization, the penetration depth determines the z-resolution. This is significant, for example, in the case of overhanging features of the part to be built. As the currently built part is always surrounded by redundant photo-reactive suspension, overhangs will inevitably be filled with cured material if the penetration (cure depth) is considerably higher than the layer thickness. Therefore, the cure depth should be of the same magnitude as the layer height. By varying the concentration of absorber, the cure depth can be adjusted for a given exposure. Therefore, it is essential that an absorbing agent efficiently absorbs at the emission wavelength of the light source. If the substance absorbs in the visible wavelength spectrum, it is called a dye [
13]. Using an absorber leads to a higher conversion of double bonds at the same cure depth [
14]. Understanding this is essential for the additive manufacturing process. The cure depth can also be decreased by reducing the exposure, but layers generated in this manner possess only insufficient mechanical stability and easily rupture (e.g., when detached from the vat or, in the worst case, when only gelled).
Being another main constituent of typical photo-reactive suspensions, the solvent has several functions. Firstly, it dissolves the solid organic constituents (photo-initiator and absorber). Secondly, it reduces the viscosity of the suspension. Thirdly, it assists the debinding process. Thermal debinding is by far the most implemented method. In this case, the binder is removed by heating, typically at atmospheric pressure and in oxidizing or non-oxidizing atmosphere. The mechanisms and physico-chemical factors that are fundamental to this process have been examined intensively in the literature. The debinding is controlled by heat transfer into the green body and mass transport of volatile substances and waste products, through the filled and unfilled pores, to the outside of the green body. It is important to mention that ceramic particles can influence the degradation process of the pure polymer [
15].
As mentioned, debinding often takes place in oxidizing atmosphere (in many cases ambient air). It was shown that the oxidizing atmosphere at first hinders the chemical degradation of poly(methyl methacrylate) at low temperatures (200–300 °C), but accelerates it at higher temperatures later. In any case, it is a core property of the oxidizing atmosphere to reduce the amount of residual carbon in ceramic bodies [
16].
In general, the debinding process can be divided into three phases. In phase one, warming takes place until the softening of the binder starts (lower than 200 °C). The chemical degradation is limited in this phase, but volatile lower-molecular-weight species (e.g., solvents) must diffuse through binder-filled pores and convert into the gas phase. The rapid generation of these species and the presence of dissolved air or captured air bubbles can lead to defects in the ceramic body [
17]. In phase two (200–300 °C), higher-molecular-weight products are quickly degraded, and the diffusion and degassing of the resulting degradation products takes place. If the softened/molten binder possesses enough mobility, a redistribution of binder can happen, and therefore a non-planar debinding front can appear. This can ease and accelerate the exit of volatile species. Lower-molecular-weight species, like plasticizers or waxes, can leave a concentration gradient behind [
18]. In phase three (300–400 °C), the rest of the binder is slowly removed by degradation and degassing. This process is now alleviated by the high porosity of the brown body, but a residual amount of carbon is still present. Often, temperatures up to 600 °C or higher are necessary to significantly reduce this residual amount of carbon.
4. Discussion
4.1. Refractive Index
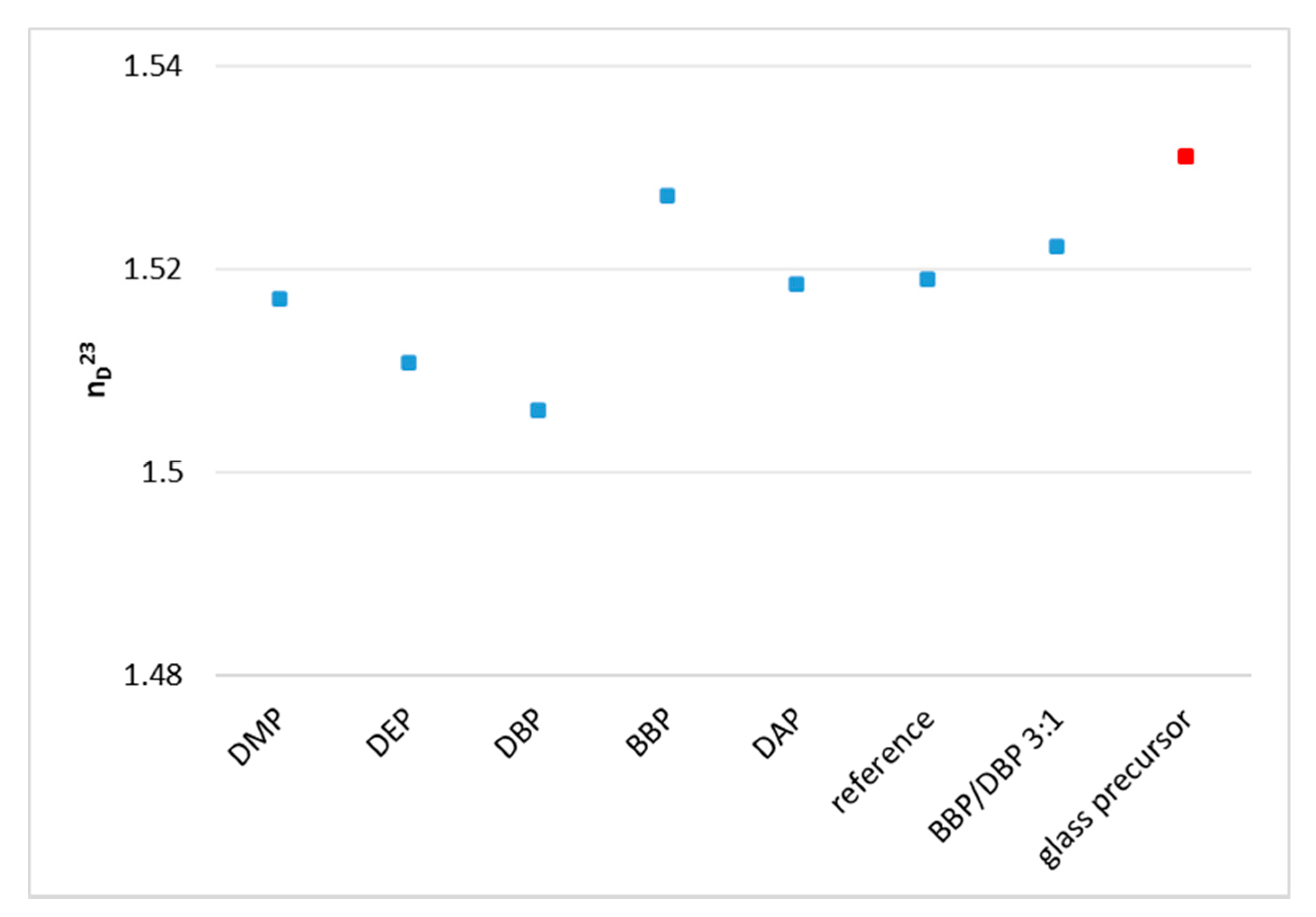
Using phthalates, the refractive index of the organic fraction can be increased significantly and therefore matched closely to the refractive index of the inorganic phase. By choosing a 3:1 mixture of BBP/DBP, the difference between organic and inorganic phase is Δn = 0.0089.
Some issues persist with regards to a perfect refractive index matching. Firstly, the target value is a literature value. The refractive index of the filler varies according to the producer, with a relatively big margin (n = 1.531 ± 0.003). The experimental determination of the refractive index of the glass powder batch at hand should give a more exact and reliable value. Secondly, the refractive indices of all the components of the formulation should be determined at processing temperature and processing wavelength to reach the target value precisely. The presented solvents are an appropriate tool for that.
4.2. Debinding
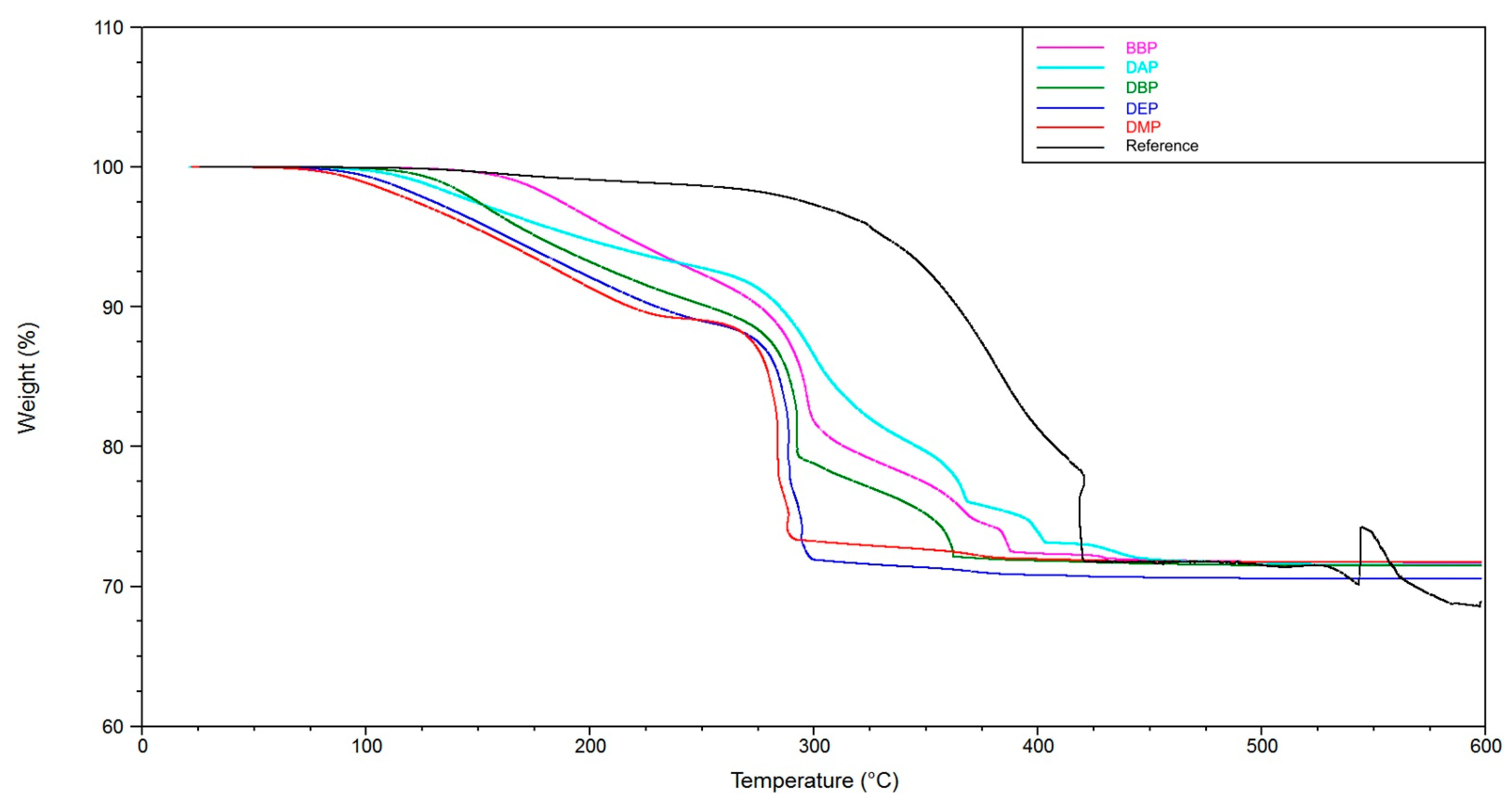
The TGA and TMA experiments clearly show two things. On the one hand, the utilization of a solvent enables a debinding procedure without crack extension (see reference without solvent). On the other hand, the choice of solvent is a deciding factor for the speed that can be applied to this procedure. At a heating rate of 1 K/min, the application of BBP and DAP leads to crack extension. In the case of DMP, DEP, and DBP, this heating rate could be used without crack extension. An additional observation is that by adding a component that is easily debindable (in this case DBP), the crack extension can effectively be avoided. Accordingly, a 3:1 mixture of BBP/DBP leads to a successful debinding procedure at the same heating rate.
A possible explanation is that the DBP molecules, due to their chemical constitution, possess a weaker intermolecular interaction with the other components inside the green body, for example BBP, and exit the green body at lower temperatures (TGA,
Figure 12). An interesting subject for future work would be to determine the intermolecular interactions by quantum mechanical calculations. We estimate that, consequently, more open porosity is present in the green body that facilitates the exit of polymer degradation products at a later stage of the procedure. If both mechanisms happen chronologically separated, a successful debinding is to be expected. In the case of BBP, the exit of the solvent molecules happens at a higher temperature/later in time, which is the reason why, presumably, not enough open porosity is present when the degradation of the polymer network starts. Consequently, a higher pressure builds up inside the green body, which eventually leads to fracture of the sample, as shown in the TMA measurements (
Figure 13 and
Figure 14). Therefore, it is necessary to choose an easily debindable component that establishes enough open porosity early on, before the degradation of the polymer network begins.
4.3. Rheology
Likewise, regarding rheology, the choice of the solvent is of major significance. Accordingly, the viscosity of a suspension prepared without a solvent is at 100–75 Pa·s for a shear rate range of 15–45 s−1. Opposingly, the viscosity of the suspension prepared with the 3:1 mixture of BBP/DBP is at 30 Pa·s for the same shear rate range.
An explanation for the different rheological behavior of the DBP suspension could be the already mentioned weaker intermolecular forces. Due to the lipophile character of DBP (compared to, for example, DMP), the non-covalent network structure of the suspension is not as pronounced. This network structure seems to be significant for the buildup of stability.
4.4. Absorbing Agent
The results of the exposure tests could identify a deciding disadvantage of DO3: at a concentration that is necessary to reduce the penetration depth to an appropriate extent, the critical polymerization energy is increased by a factor of four. This shows an inhibition of the polymerization by the mono azo dye. We assume that the reason for this is the nitro function on the phenyl ring, which acts as a radical quencher by mesomeric effect. With Sudan IV, an alternative option was presented that can reduce Dp effectively without increasing Ec. With this alternative, the exposure time could be reduced from 10 s to 1 s.
Conclusively, it was shown that by changing the solvent, many important properties of a photo-reactive suspension for the additive manufacturing of glass ceramics can be influenced, and by combination, those properties can be adapted according to requirements.
Future investigations should focus on other groups of solvents to improve the refractive index matching even further, and to see if shown benefits can be reproduced with other chemical substances. Additionally, it should be investigated if these considerations regarding the formulation of a photo-reactive suspension are also valid for other types of glasses or glass ceramics, and how these changes affect the final properties of the ceramics, like translucency/transparency or mechanical strength.