Design of Sodium Alginate/Gelatin-Based Emulsion Film Fused with Polylactide Microparticles Charged with Plant Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Polylactide Microparticles
2.3. Production of Emulsion Films
2.4. Characterization of Materials
2.4.1. Depiction of PLA Microparticles and Materials
2.4.2. Emulsion Particles Size Distribution
2.4.3. Loading Capacity of Materials with PLA Microparticles
2.4.4. Contact Angle and Surface Free Energy Measurements
2.4.5. Film Color and Opacity
2.4.6. Moisture Content Measurements
2.4.7. Mechanical Properties
2.4.8. Biophysical Skin Parameters Assays
2.4.9. Statistical Analysis
3. Results and Discussion
3.1. Appearance and Structure of Materials
3.2. Contact Angle and Surface Free Energy Measurements
3.3. Film Color and Opacity
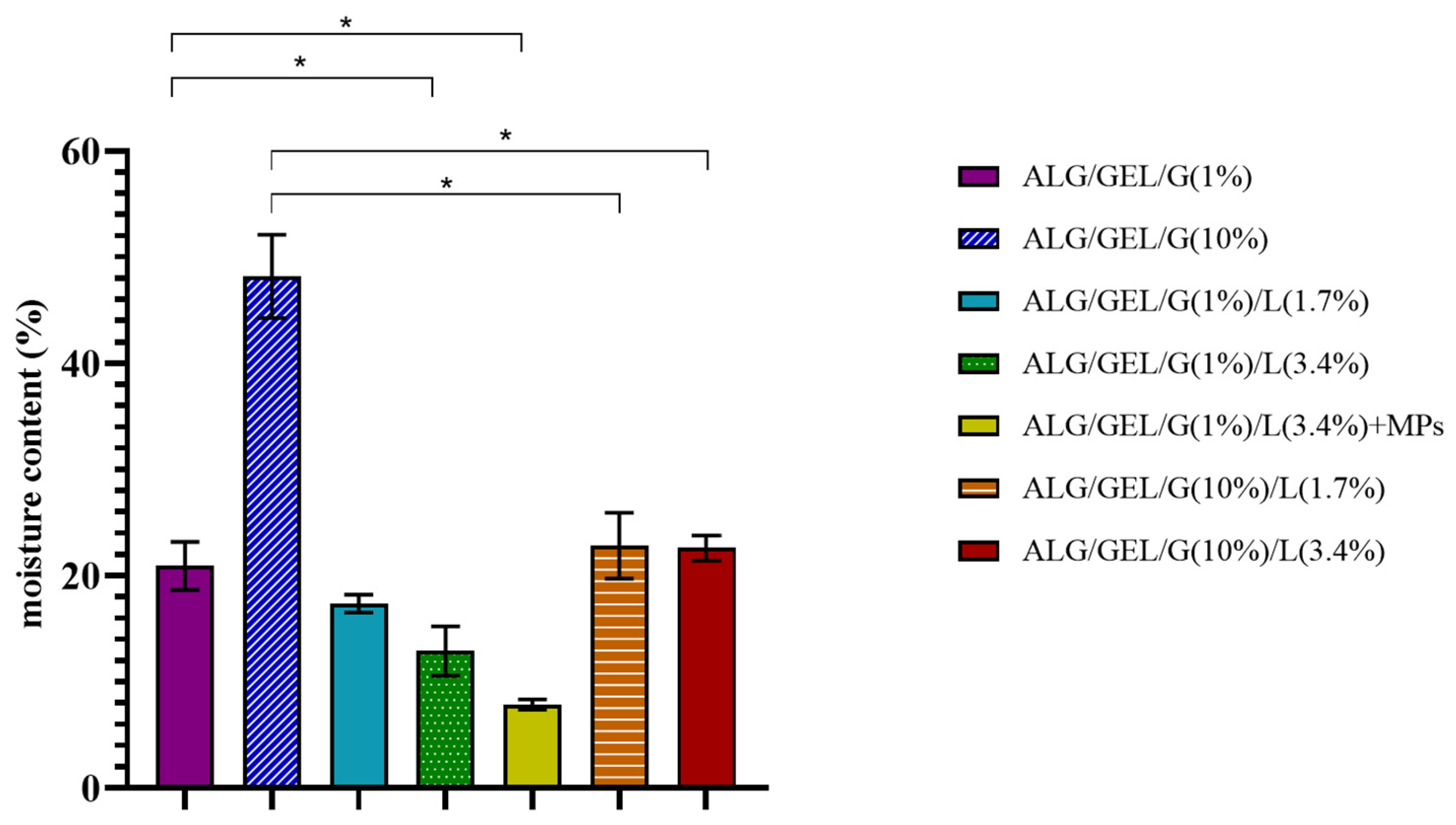
3.4. Moisture Content Measurements
3.5. Mechanical Properties
3.6. Biophysical Skin Parameters Assays
3.6.1. Skin Color
3.6.2. Skin Hydration
3.6.3. Transepidermal Water Loss (TEWL)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dou, L.; Li, B.; Zhang, K.; Chu, X.; Hou, H. Physical properties and antioxidant activity of gelatin-sodium alginate edible films with tea polyphenols. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozlowska, E.; Trocer, P.; Kostek, M.; Śliwiński, M.; Henriques, M.H.F.; Bartkowiak, A.; Sobolewski, P. Whey protein concentrate/isolate biofunctional films modified with melanin from watermelon (Citrullus lanatus) seeds. Materials 2020, 13, 3876. [Google Scholar] [CrossRef]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Bekhit, M.; Arab-Tehrany, E.; Kahn, C.J.F.; Cleymand, F.; Fleutot, S.; Desobry, S.; Sánchez-González, L. Bioactive films containing alginate-pectin composite microbeads with lactococcus lactis subsp. Lactis: Physicochemical characterization and antilisterial activity. Int. J. Mol. Sci. 2018, 19, 574. [Google Scholar] [CrossRef] [Green Version]
- Gordon, P.W.; Brooker, A.D.M.; Chew, Y.M.J.; Wilson, D.I.; York, D.W. Studies into the swelling of gelatine films using a scanning fluid dynamic gauge. Food Bioprod. Process. 2010, 88, 357–364. [Google Scholar] [CrossRef]
- Mackie, W.; Noy, R.; Sellen, D.B. Solution properties of sodium alginate. Biopolymers 1980, 19, 1839–1860. [Google Scholar] [CrossRef]
- Fu, S.; Thacker, A.; Sperger, D.M.; Boni, R.L.; Buckner, I.S.; Velankar, S.; Munson, E.J.; Block, L.H. Relevance of rheological properties of sodium alginate in solution to calcium alginate gel properties. AAPS Pharmscitech 2011, 12, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Tønnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef]
- Pereira, L.; Sousa, A.; Coelho, H.; Amado, A.M.; Ribeiro-Claro, P.J.A. Use of FTIR, FT-Raman and 13C-NMR spectroscopy for identification of some seaweed phycocolloids. Biomol. Eng. 2003, 20, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Harris, P. Food Gels; Springer Netherlands: Dordrecht, The Netherlands, 1990; ISBN 9789400907553. [Google Scholar]
- Siewert, C.D.; Haas, H.; Cornet, V.; Nogueira, S.S.; Nawroth, T.; Uebbing, L.; Ziller, A.; Al-Gousous, J.; Radulescu, A.; Schroer, M.A.; et al. Hybrid Biopolymer and Lipid Nanoparticles with Improved Transfection Efficacy for mRNA. Cells 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Mashiah, R.; Seror, J.; Kadar, A.; Dolkart, O.; Pritsch, T.; Goldberg, R.; Klein, J. Lipid-hyaluronan synergy strongly reduces intrasynovial tissue boundary friction. Acta Biomater. 2019, 83, 314–321. [Google Scholar] [CrossRef]
- Petrin, T.H.C.; Fadel, V.; Martins, D.B.; Dias, S.A.; Cruz, A.; Sergio, L.M.; Arcisio-Miranda, M.; Castanho, M.A.R.B.; Dos Santos Cabrera, M.P. Synthesis and Characterization of Peptide-Chitosan Conjugates (PepChis) with Lipid Bilayer Affinity and Antibacterial Activity. Biomacromolecules 2019, 20, 2743–2753. [Google Scholar] [CrossRef]
- Gilbert, L.; Picard, C.; Savary, G.; Grisel, M. Rheological and textural characterization of cosmetic emulsions containing natural and synthetic polymers: Relationships between both data. Colloids Surf. A Physicochem. Eng. Asp. 2013, 421, 150–163. [Google Scholar] [CrossRef]
- Muschiolik, G. Multiple emulsions for food use. Curr. Opin. Colloid Interface Sci. 2007, 12, 213–220. [Google Scholar] [CrossRef]
- Schijns, V.E.J.C.; Strioga, M.; Ascarateil, S. Oil-based emulsion vaccine adjuvants. Curr. Protoc. Immunol. 2014, 106, 2.18.1–2.18.7. [Google Scholar] [CrossRef] [PubMed]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T.; Nilsuwan, K. Emulsion film based on fish skin gelatin and palm oil: Physical, structural and thermal properties. Food Hydrocoll. 2015, 48, 248–259. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, W.; Wang, K.; Liu, Y.; Liu, A.; Zhang, S.; Zhao, Y. Impact of melting point of palm oil on mechanical and water barrier properties of gelatin-palm oil emulsion film. Food Hydrocoll. 2016, 60, 243–251. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Spotti, M.L.; Cecchini, J.P.; Spotti, M.J.; Carrara, C.R. Brea Gum (from Cercidium praecox) as a structural support for emulsion-based edible films. LWT Food Sci. Technol. 2016, 68, 127–134. [Google Scholar] [CrossRef]
- Jahaniaval, F.; Kakuda, Y.; Marcone, M.F. Fatty acid and triacylglycerol compositions of seed oils of five Amaranthus accessions and their comparison to other oils. J. Am. Oil Chem. Soc. 2000, 77, 847–852. [Google Scholar] [CrossRef]
- Pazzoti, G.; Souza, C.; Veronezi, C.; Luzia, D.; Jorge, N. Evaluation of Oxidative Stability of Compound Oils under Accelerated Storage Conditions. Braz. Arch. Biol. Technol. 2018, 61. [Google Scholar] [CrossRef] [Green Version]
- Taghvaei, M.; Jafari, S.M.; Assadpoor, E.; Nowrouzieh, S.; Alishah, O. Optimization of microwave-assisted extraction of cottonseed oil and evaluation of its oxidative stability and physicochemical properties. Food Chem. 2014, 160, 90–97. [Google Scholar] [CrossRef] [PubMed]
- El-Mallah, M.H.; El-Shami, S.M.; Hassanien, M.M.M.; Abdel-Razek, A.G. Effect of chemical refining steps on the minor and major components of cottonseed oil. Agric. Biol. J. N. Am. 2011, 2, 341–349. [Google Scholar] [CrossRef]
- Tulloch, A.P. Beeswax—Composition and Analysis. Bee World 1980, 61, 47–62. [Google Scholar] [CrossRef]
- Esfanjani, A.F.; Jafari, S.M.; Assadpoor, E.; Mohammadi, A. Nano-encapsulation of saffron extract through double-layered multiple emulsions of pectin and whey protein concentrate. J. Food Eng. 2015, 165, 149–155. [Google Scholar] [CrossRef]
- Oh, J.K. Polylactide (PLA)-based amphiphilic block copolymers: Synthesis, self-assembly, and biomedical applications. Soft Matter 2011, 7, 5096–5108. [Google Scholar] [CrossRef] [Green Version]
- Kizilbey, K. Optimization of Rutin-Loaded PLGA Nanoparticles Synthesized by Single-Emulsion Solvent Evaporation Method. ACS Omega 2019, 4, 555–562. [Google Scholar] [CrossRef]
- Bamidele, O.P.; Emmambux, M.N. Encapsulation of bioactive compounds by “extrusion” technologies: A review. Crit. Rev. Food Sci. Nutr. 2020, 1–19. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of antioxidant phenolic compounds extracted from spent coffee grounds by freeze-drying and spray-drying using different coating materials. Food Chem. 2017, 237, 623–631. [Google Scholar] [CrossRef] [Green Version]
- Tavares, L.; Noreña, C.P.Z. Encapsulation of Ginger Essential Oil Using Complex Coacervation Method: Coacervate Formation, Rheological Property, and Physicochemical Characterization. Food Bioprocess Technol. 2020, 13, 1405–1420. [Google Scholar] [CrossRef]
- Pan, H.M.; Yu, H.; Guigas, G.; Fery, A.; Weiss, M.; Patzel, V.; Trau, D. Engineering and Design of Polymeric Shells: Inwards Interweaving Polymers as Multilayer Nanofilm, Immobilization Matrix, or Chromatography Resins. ACS Appl. Mater. Interfaces 2017, 9, 5447–5456. [Google Scholar] [CrossRef]
- Boudou, T.; Crouzier, T.; Ren, K.; Blin, G.; Picart, C. Multiple functionalities of polyelectrolyte multilayer films: New biomedical applications. Adv. Mater. 2010, 22, 441–467. [Google Scholar] [CrossRef]
- Li, L.; Zhang, W.; Peng, J.; Xue, B.; Liu, Z.; Luo, Z.; Lu, D.; Zhao, X. A novel shell material-highland barley starch for microencapsulation of cinnamon essential oil with different preparation methods. Materials 2020, 13, 1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hategekimana, J.; Masamba, K.G.; Ma, J.; Zhong, F. Encapsulation of vitamin E: Effect of physicochemical properties of wall material on retention and stability. Carbohydr. Polym. 2015, 124, 172–179. [Google Scholar] [CrossRef]
- Cortial, A.; Vocanson, M.; Loubry, E.; Briançon, S. Hot homogenization process optimization for fragrance encapsulation in solid lipid nanoparticles. Flavour Fragr. J. 2015, 30, 467–477. [Google Scholar] [CrossRef]
- Mohan, A.; Rajendran, S.R.C.K.; He, Q.S.; Bazinet, L.; Udenigwe, C.C. Encapsulation of food protein hydrolysates and peptides: A review. RSC Adv. 2015, 5, 79270–79278. [Google Scholar] [CrossRef]
- Lv, Y.; Pan, Z.; Song, C.; Chen, Y.; Qian, X. Locust bean gum/gellan gum double-network hydrogels with superior self-healing and pH-driven shape-memory properties. Soft Matter 2019, 15, 6171–6179. [Google Scholar] [CrossRef] [PubMed]
- Pavli, F.; Argyri, A.A.; Skandamis, P.; Nychas, G.J.; Tassou, C.; Chorianopoulos, N. Antimicrobial activity of oregano essential oil incorporated in sodium alginate edible films: Control of Listeria monocytogenes and spoilage in ham slices treated with high pressure processing. Materials 2019, 12, 3726. [Google Scholar] [CrossRef] [Green Version]
- Khalid, K.; Teixeira da Silva, J. Biology of Calendula officinalis Linn.: Focus on pharmacology, biological activities and agronomic practices. Med. Aromat. Plant Sci. Biotechnol. 2012, 6, 12–27. [Google Scholar]
- John, R.; Jan, N. Calendula Officinalis-An Important Medicinal Plant with Potential Biological Properties. Proc. Indian Natl. Sci. Acad. 2017, 93, 769–787. [Google Scholar] [CrossRef]
- Preethi, K.C.; Kuttan, G.; Kuttan, R. Antioxidant potential of an extract of Calendula officinalis flowers in vitro and in vivo. Pharm. Biol. 2006, 44, 691–697. [Google Scholar] [CrossRef] [Green Version]
- Ukiya, M.; Akihisa, T.; Yasukawa, K.; Tokuda, H.; Suzuki, T.; Kimura, Y. Anti-inflammatory, anti-tumor-promoting, and cytotoxic activities of constituents of marigold (Calendula officinalis) flowers. J. Nat. Prod. 2006, 69, 1692–1696. [Google Scholar] [CrossRef] [PubMed]
- Efstratiou, E.; Hussain, A.I.; Nigam, P.S.; Moore, J.E.; Ayub, M.A.; Rao, J.R. Antimicrobial activity of Calendula officinalis petal extracts against fungi, as well as Gram-negative and Gram-positive clinical pathogens. Complement. Ther. Clin. Pract. 2012, 18, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Medina, E.; Garcia-Lora, A.; Paco, L.; Algarra, I.; Collado, A.; Garrido, F. A new extract of the plant calendula officinalis produces a dual in vitro effect: Cytotoxic anti-tumor activity and lymphocyte activation. BMC Cancer 2006, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, Y.M.; Catini, C.D.; Vicentini, F.T.M.C.; Nomizo, A.; Gerlach, R.F.; Fonseca, M.J.V. Protective effect of Calendula officinalis extract against UVB-induced oxidative stress in skin: Evaluation of reduced glutathione levels and matrix metalloproteinase secretion. J. Ethnopharmacol. 2010, 127, 596–601. [Google Scholar] [CrossRef]
- Chandran, P.K.; Kuttan, R. Effect of Calendula officinalis flower extract on acute phase proteins, antioxidant defense mechanism and granuloma formation during thermal burns. J. Clin. Biochem. Nutr. 2008, 43, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Vargas, E.A.T.; Do Vale Baracho, N.C.; De Brito, J.; De Queiroz, A.A.A. Hyperbranched polyglycerol electrospun nanofibers for wound dressing applications. Acta Biomater. 2010, 6, 1069–1078. [Google Scholar] [CrossRef]
- Okuma, C.H.; Andrade, T.A.M.; Caetano, G.F.; Finci, L.I.; Maciel, N.R.; Topan, J.F.; Cefali, L.C.; Polizello, A.C.M.; Carlo, T.; Rogerio, A.P.; et al. Development of lamellar gel phase emulsion containing marigold oil (Calendula officinalis) as a potential modern wound dressing. Eur. J. Pharm. Sci. 2015, 71, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Pedram Rad, Z.; Mokhtari, J.; Abbasi, M. Calendula officinalis extract/PCL/Zein/Gum arabic nanofibrous bio-composite scaffolds via suspension, two-nozzle and multilayer electrospinning for skin tissue engineering. Int. J. Biol. Macromol. 2019, 135, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Aro, A.A.; Perez, M.O.; Vieira, C.P.; Esquisatto, M.A.M.; Rodrigues, R.A.F.; Gomes, L.; Pimentel, E.R. Effect of Calendula officinalis cream on achilles tendon healing. Anat. Rec. 2015, 298, 428–435. [Google Scholar] [CrossRef]
- Akhtar, N.; Khan, B.A.; Haji, M.; Khan, S.; Ahmad, M.; Rasool, F.; Mahmood, T.; Rasul, A. Evaluation of various functional skin parameters using a topical cream of calendula officinalis extract. Afr. J. Pharm. Pharmacol. 2011, 5, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Bernatoniene, J.; Masteikova, R.; Davalgiene, J.; Peciura, R.; Gauryliene, R.; Bernatoniene, R.; Majiene, D.; Lazauskas, R.; Civinskiene, G.; Velziene, S.; et al. Topical application of Calendula officinalis (L.): Formulation and evaluation of hydrophilic cream with antioxidant activity. J. Med. Plants Res. 2011, 5, 868–877. [Google Scholar]
- Varka, E.-M.; Tsatsaroni, E.; Xristoforidou, N.; Darda, A.-M. Stability Study of O/W Cosmetic Emulsions Using Rosmarinus officinalis and Calendula officinalis Extracts. Open J. Appl. Sci. 2012, 2, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Rossi, J.A.J. Colorimetry to total phenolics with phosphomolybdic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Boun, H.R.; Huxsoll, C.C. Control of Minimally Processed Carrot (Daucus carota) Surface Discoloration Caused by Abrasion Peeling. J. Food Sci. 1991, 56, 416–418. [Google Scholar] [CrossRef]
- Han, J.H.; Floros, J.D. Casting antimicrobial packaging films and measuring their physical properties and antimicrobial activity. J. Plast. Film Sheeting 1997, 13, 287–298. [Google Scholar] [CrossRef]
- Aguirre-Loredo, R.Y.; Rodríguez-Hernández, A.I.; Morales-Sánchez, E.; Gómez-Aldapa, C.A.; Velazquez, G. Effect of equilibrium moisture content on barrier, mechanical and thermal properties of chitosan films. Food Chem. 2016, 196, 560–566. [Google Scholar] [CrossRef]
- Kozlowska, J.; Tylkowski, B.; Stachowiak, N.; Prus-Walendziak, W. Controlling the skin barrier quality through the application of polymeric films containing microspheres with encapsulated plant extract. Processes 2020, 8, 530. [Google Scholar] [CrossRef]
- Stachowiak, N.; Kowalonek, J.; Kozlowska, J. Effect of plasticizer and surfactant on the properties of poly (vinyl alcohol)/chitosan films. Int. J. Biol. Macromol. 2020, 164, 2100–2107. [Google Scholar] [CrossRef]
- Aydogdu, A.; Radke, C.J.; Bezci, S.; Kirtil, E. Characterization of curcumin incorporated guar gum/orange oil antimicrobial emulsion films. Int. J. Biol. Macromol. 2020, 148, 110–120. [Google Scholar] [CrossRef]
- Shaw, N.B.; Monahan, F.J.; O’Riordan, E.D.; O’Sullivan, M. Physical properties of WPI films plasticized with glycerol, xylitol, or sorbitol. J. Food Sci. 2002, 67, 164–167. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, Y.; Shi, Q. Characterization of a novel edible film based on gum ghatti: Effect of plasticizer type and concentration. Carbohydr. Polym. 2016, 153, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of plasticizer type and concentration on physical properties of biodegradable films based on sugar palm (Arenga pinnata) starch for food packaging. J. Food Sci. Technol. 2016, 53, 326–336. [Google Scholar] [CrossRef] [Green Version]
- Fabra, M.J.; Talens, P.; Chiralt, A. Tensile properties and water vapor permeability of sodium caseinate films containing oleic acid-beeswax mixtures. J. Food Eng. 2008, 85, 393–400. [Google Scholar] [CrossRef]
- Han, J.H.; Seo, G.H.; Park, I.M.; Kim, G.N.; Lee, D.S. Physical and mechanical properties of pea starch edible films containing beeswax emulsions. J. Food Sci. 2006, 71. [Google Scholar] [CrossRef]
- Limpisophon, K.; Tanaka, M.; Osako, K. Characterisation of gelatin-fatty acid emulsion films based on blue shark (Prionace glauca) skin gelatin. Food Chem. 2010, 122, 1095–1101. [Google Scholar] [CrossRef]
- Heinrich, U.; Koop, U.; Leneveu-Duchemin, M.C.; Osterrieder, K.; Bielfeldt, S.; Chkarnat, C.; Degwert, J.; Häntschel, D.; Jaspers, S.; Nissen, H.P.; et al. Multicentre comparison of skin hydration in terms of physical-, physiological- and product-dependent parameters by the capacitive method (Corneometer CM 825). Int. J. Cosmet. Sci. 2003, 25, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Velasco, M.V.R.; Vieira, R.P.; Fernandes, A.R.; Dario, M.F.; Pinto, C.A.S.O.; Pedriali, C.A.; Kaneko, T.M.; Baby, A.R. Short-term clinical of peel-off facial mask moisturizers. Int. J. Cosmet. Sci. 2014, 36, 355–360. [Google Scholar] [CrossRef]

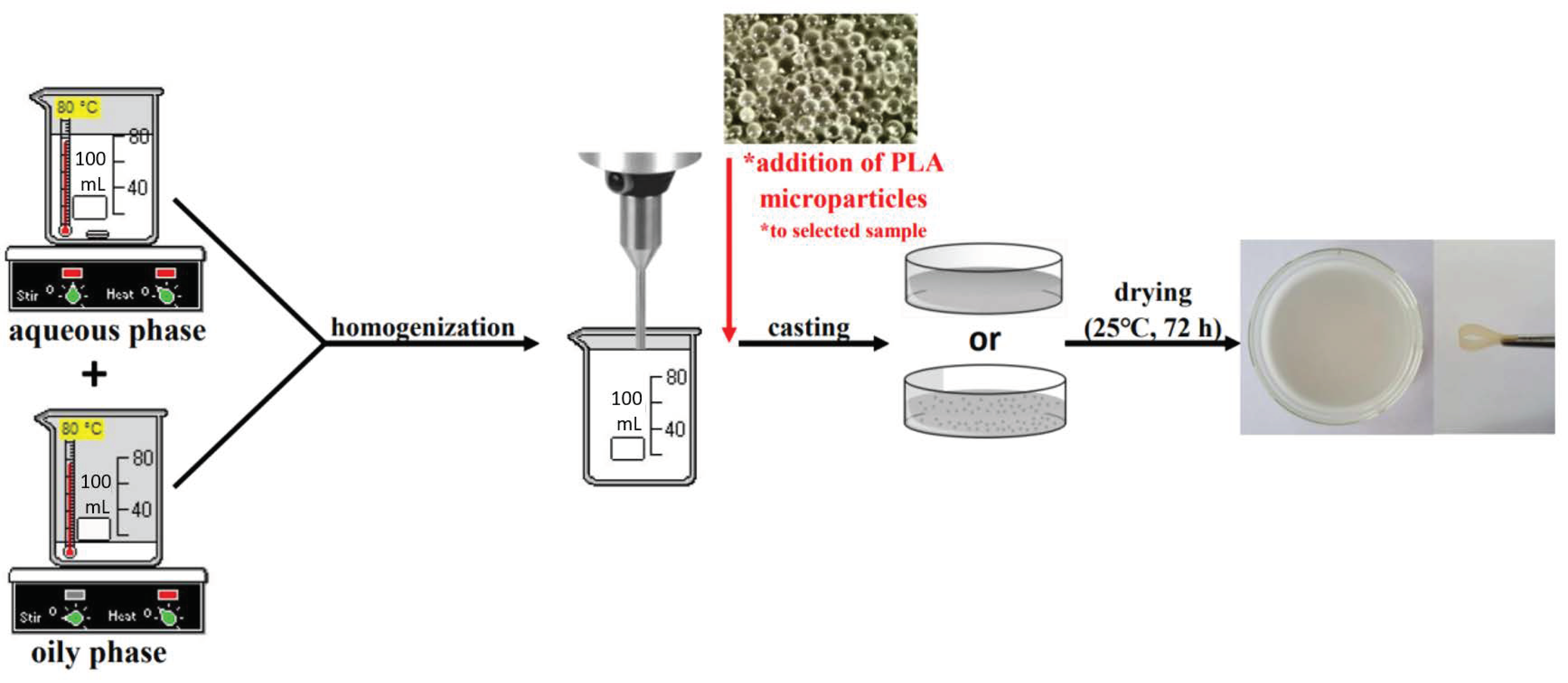







| Sample | Composition of Materials (% w/w) | Addition (% w/w) | |||||
|---|---|---|---|---|---|---|---|
| Aqueous Phase | Oily Phase | PLA MPs | |||||
| ALG | GEL | G | Cottonseed Oil | Beeswax | Span-80 | ||
| ALG/GEL/G (1%) | 2 | 1 | 1 | - | - | - | - |
| ALG/GEL/G (10%) | 2 | 1 | 10 | - | - | - | - |
| ALG/GEL/G (1%)/L (1.7%) | 2 | 1 | 1 | 1.2 | 0.5 | 0.35 | - |
| ALG/GEL/G (1%)/L (3.4%) | 2 | 1 | 1 | 2.4 | 1 | 0.7 | - |
| ALG/GEL/G (1%)/L (3.4%) + MPs | 2 | 1 | 1 | 2.4 | 1 | 0.7 | 6 |
| ALG/GEL/G (10%)/L (1.7%) | 2 | 1 | 10 | 1.2 | 0.5 | 0.35 | - |
| ALG/GEL/G (10%)/L (3.4%) | 2 | 1 | 10 | 2.4 | 1 | 0.7 | - |
| Sample | Contact Angle (°) | Surface Free Energy (γs) (mJ/m2) | Dispersive (γsd) and Polar (γsp) Components (mJ/m2) | ||
|---|---|---|---|---|---|
| D | G | γsd | γsp | ||
| ALG/GEL/G (1%) | 54.2 | 61.5 | 37.56 | 23.92 | 13.64 |
| ALG/GEL/G (10%) | 69.3 | 99.9 | 23.12 | 22.75 | 0.39 |
| ALG/GEL/G (1%)/L (1.7%) | 38.2 | 39.0 | 51.92 | 28.69 | 23.24 |
| ALG/GEL/G (1%)/L (3.4%) | 48.8 | 51.8 | 43.58 | 25.19 | 18.40 |
| ALG/GEL/G (10%)/L (1.7%) | 34.6 | 22.5 | 59.21 | 28.28 | 30.93 |
| ALG/GEL/G (10%)/L (3.4%) | 39.5 | 45.2 | 48.81 | 29.08 | 19.72 |
| Sample | L* | a* | b* | ΔE | WI | Op (A600/mm) |
|---|---|---|---|---|---|---|
| ALG/GEL/G (1%) | 89.49 ± 0.04 | −1.19 ± 0.07 | 5.78 ± 0.06 | 6.61 ± 0.05 | 87.95 ± 0.05 | 1.10 ± 0.03 |
| ALG/GEL/G (10%) | 85.39 ± 0.07 | −1.45 ± 0.08 | 8.75 ± 0.10 | 10.00 ± 0.12 | 82.91 ± 0.11 | 1.30 ± 0.05 |
| ALG/GEL/G (1%)/L (1.7%) | 82.33 ± 0.14 | −0.37 ± 0.03 | 14.07 ± 0.11 | 16.04 ± 0.15 | 77.41 ± 0.16 | 7.35 ± 0.11 |
| ALG/GEL/G (1%)/L (3.4%) | 82.70 ± 0.09 | −0.85 ± 0.04 | 13.79 ± 0.07 | 15.64 ± 0.03 | 77.86 ± 0.03 | 7.05 ± 0.09 |
| ALG/GEL/G (1%)/L (3.4%) + MPs | 80.84 ± 0.03 | −0.46 ± 0.02 | 20.44 ± 0.04 | 22.52 ± 0.03 | 71.98 ± 0.02 | 6.26 ± 0.15 |
| ALG/GEL/G (10%)/L (1.7%) | 86.58 ± 0.06 | −2.33 ± 0.04 | 12.19 ± 0.10 | 13.16 ± 0.09 | 81.73 ± 0.05 | 2.84 ± 0.04 |
| ALG/GEL/G (10%)/L (3.4%) | 84.66 ± 0.03 | −2.68 ± 0.02 | 14.62 ± 0.14 | 15.94 ± 0.13 | 78.64 ± 0.09 | 4.46 ± 0.06 |
| Sample | Emod (MPa) | Fmax (N) | Elongation at Fmax (mm) | Fbreak (N) | Elongation at Break (%) |
|---|---|---|---|---|---|
| ALG/GEL/G (1%) | 1350.0 ± 91.4 | 20.4 ± 1.8 | 1.2 ± 0.2 | 20.3 ± 2.0 | 4.8 ± 0.8 |
| ALG/GEL/G (10%) | 2.2 ± 0.7 | 0.5 ± 0 | 37.9 ± 3.0 | 0.3 ± 0 | 173.0 ± 7.4 |
| ALG/GEL/G (1%)/L (1.7%) | 547.8 ± 19.8 | 19.2 ± 1.5 | 2.0 ± 0.2 | 19.1 ± 1.6 | 8.3 ± 0.7 |
| ALG/GEL/G (1%)/L (3.4%) | 266.4 ± 15.9 | 13.3 ± 0.9 | 2.4 ± 0.2 | 13.2 ± 1.0 | 9.9 ± 0.8 |
| ALG/GEL/G (1%)/L (3.4%) + MPs | 27.8 ± 4.6 | 1.7 ± 0.2 | 3.3 ± 0.7 | 1.6 ± 0.2 | 13.5 ± 2.8 |
| ALG/GEL/G (10%)/L (1.7%) | 0.8 ± 0.2 | 0.4 ± 0 | 30.2 ± 0.8 | 0.2 ± 0 | 135.6 ± 6.2 |
| ALG/GEL/G (10%)/L (3.4%) | 1.4 ± 0.1 | 0.3 ± 0 | 20.7 ± 3.2 | 0.2 ± 0 | 114.6 ± 4.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prus-Walendziak, W.; Kozlowska, J. Design of Sodium Alginate/Gelatin-Based Emulsion Film Fused with Polylactide Microparticles Charged with Plant Extract. Materials 2021, 14, 745. https://doi.org/10.3390/ma14040745
Prus-Walendziak W, Kozlowska J. Design of Sodium Alginate/Gelatin-Based Emulsion Film Fused with Polylactide Microparticles Charged with Plant Extract. Materials. 2021; 14(4):745. https://doi.org/10.3390/ma14040745
Chicago/Turabian StylePrus-Walendziak, Weronika, and Justyna Kozlowska. 2021. "Design of Sodium Alginate/Gelatin-Based Emulsion Film Fused with Polylactide Microparticles Charged with Plant Extract" Materials 14, no. 4: 745. https://doi.org/10.3390/ma14040745
APA StylePrus-Walendziak, W., & Kozlowska, J. (2021). Design of Sodium Alginate/Gelatin-Based Emulsion Film Fused with Polylactide Microparticles Charged with Plant Extract. Materials, 14(4), 745. https://doi.org/10.3390/ma14040745







