Modelling ATR-FTIR Spectra of Dental Bonding Systems to Investigate Composition and Polymerisation Kinetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Filler Separation and Weight Percentage (Filler Load)
2.3. FTIR Spectra of Components, Primers, Adhesives and Fillers
2.4. Model for Estimation of Component Ratios
2.5. Polymerization Kinetics
2.6. Statistical Analysis
3. Results
3.1. Chemical Composition: OBFL and CFSE
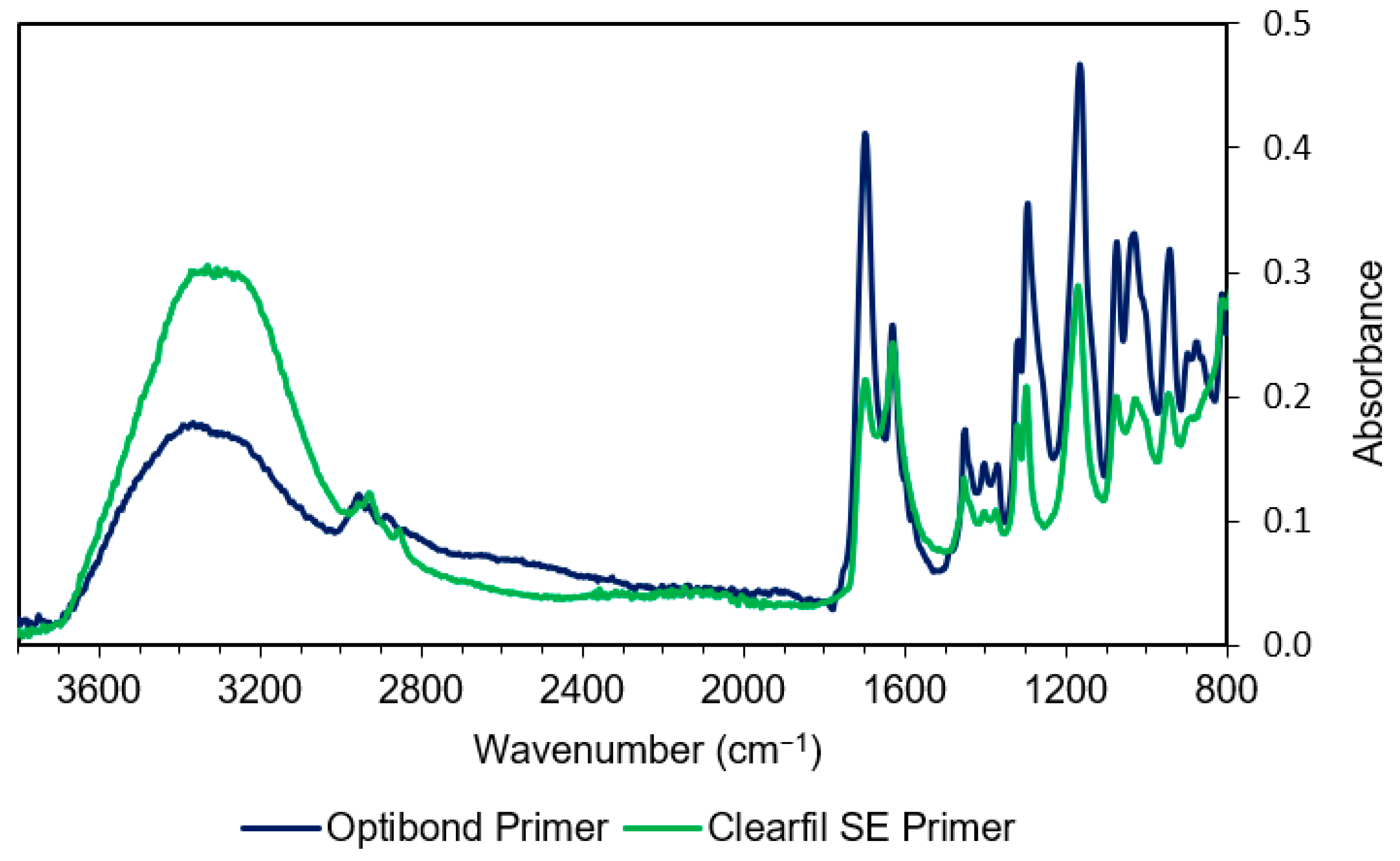
3.1.1. Primer Spectra
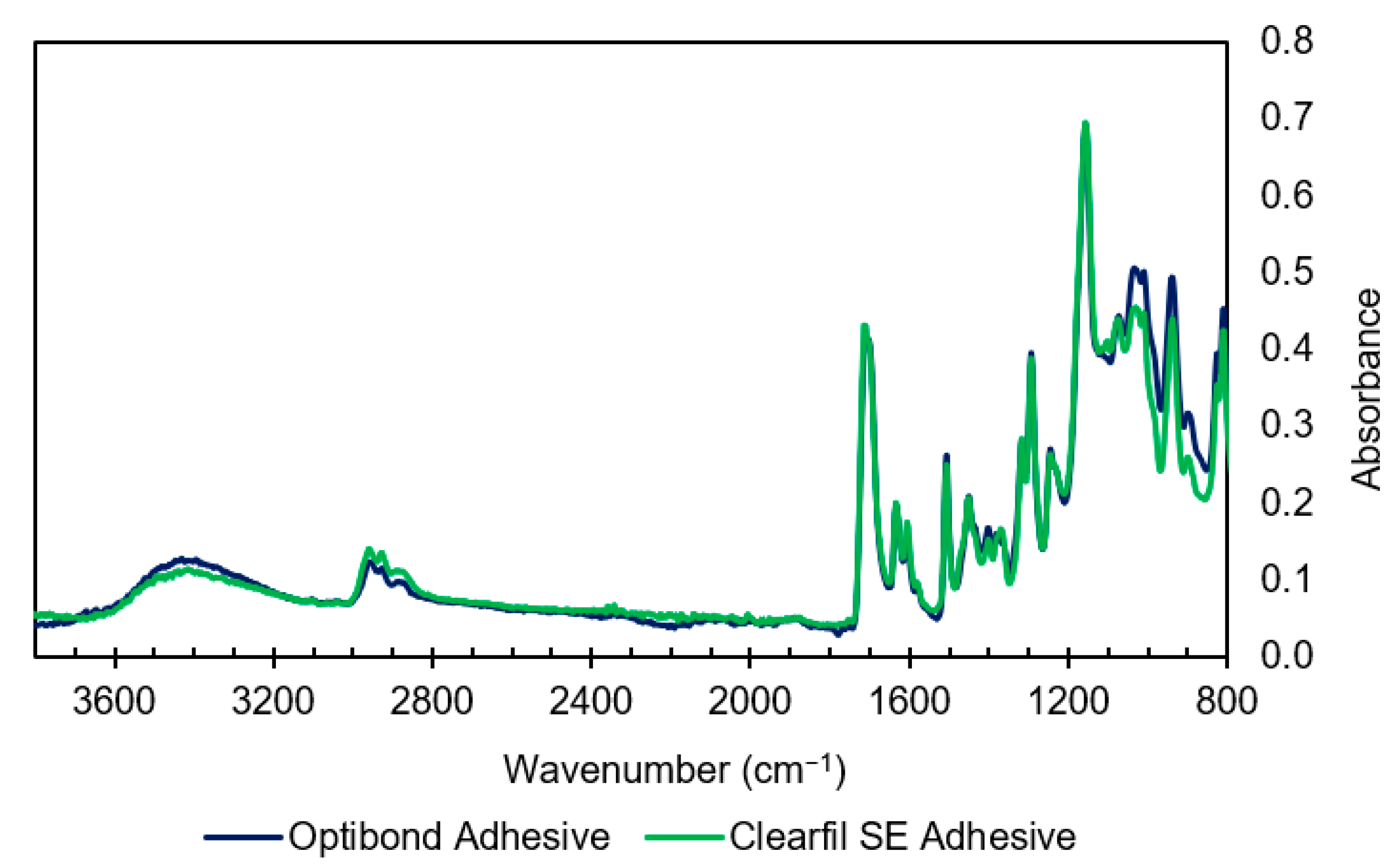
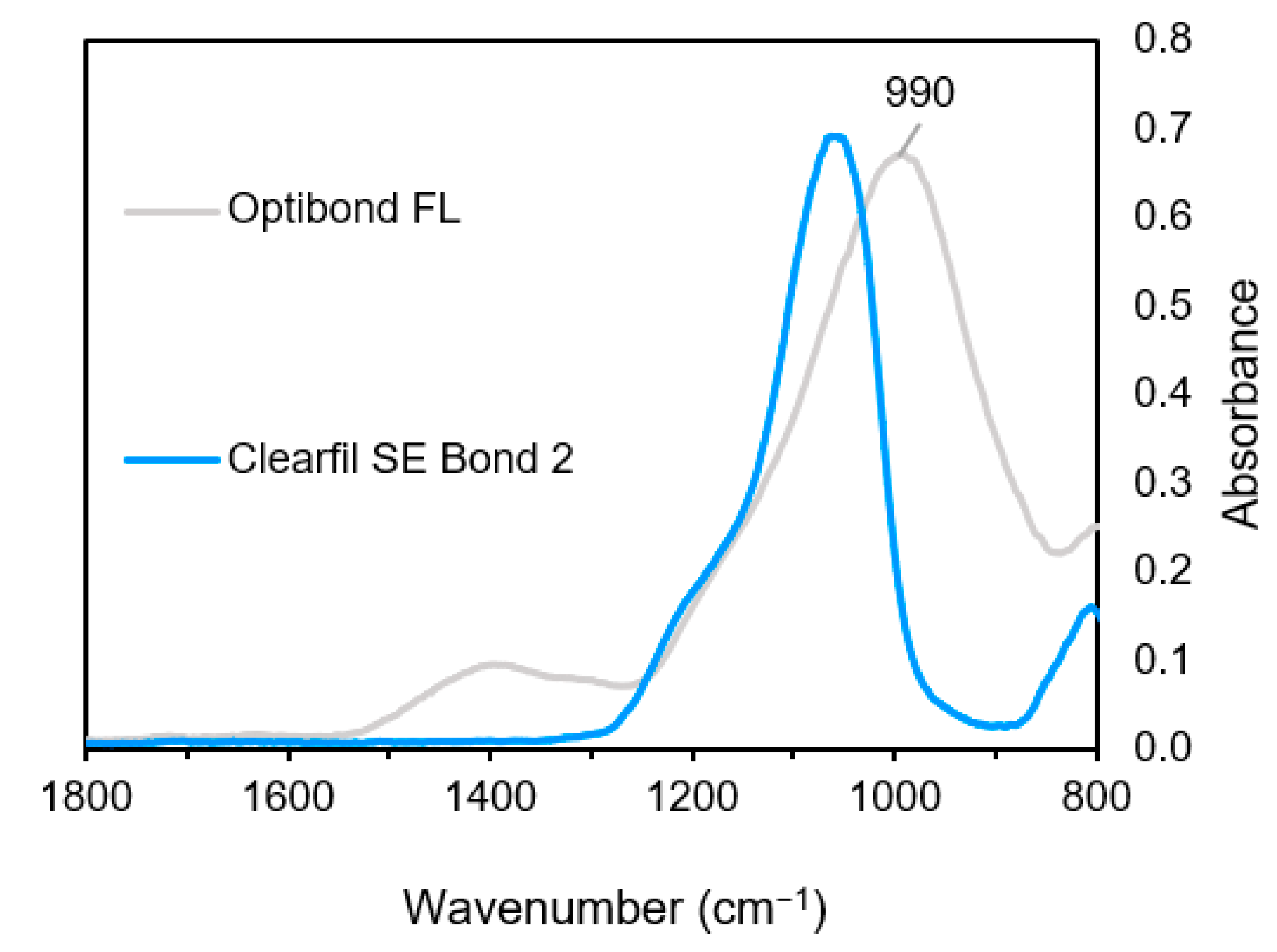
3.1.2. Adhesive Spectra
3.1.3. Filler Spectra
3.1.4. Model Spectra
3.2. Polymerisation Kinetics and Filler Load
3.3. Agreement with the Information Supplied by the Manufacturers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moszner, N.; Hirt, T. New polymer-chemical developments in clinical dental polymer materials: Enamel-dentin adhesives and restorative composites. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 4369–4402. [Google Scholar] [CrossRef]
- Van Landuyt, K.L.; Snauwaert, J.; De Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28, 3757–3785. [Google Scholar] [CrossRef]
- Brignardello-Petersen, R. Three universal adhesive systems may perform similarly when used to restore noncarious cervical lesions. J. Am. Dent. Assoc. 2019, 150, e27. [Google Scholar] [CrossRef]
- Spencer, P.; Ye, Q.; Park, J.; Topp, E.M.; Misra, A.; Marangos, O.; Wang, Y.; Bohaty, B.S.; Singh, V.; Sene, F.; et al. Adhesive/Dentin Interface: The Weak Link in the Composite Restoration. Ann. Biomed. Eng. 2010, 38, 1989–2003. [Google Scholar] [CrossRef] [Green Version]
- Ye, Q.; Spencer, P.; Yuca, E.; Tamerler, C. Engineered Peptide Repairs Defective Adhesive-Dentin Interface. Macromol. Mater. Eng. 2017, 302. [Google Scholar] [CrossRef] [PubMed]
- Lampitelli, N. The Romance plural isogloss and linguistic change: A comparative study of Romance nouns. Lingua 2014, 140, 158–179. [Google Scholar] [CrossRef] [Green Version]
- Marshall, S.J.; Bayne, S.C.; Baier, R.; Tomsia, A.P.; Marshall, G.W. A review of adhesion science. Dent. Mater. 2010, 26, e11–e16. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; Yoshihara, K.; Van Landuyt, K.; Yoshida, Y.; Peumans, M. From Buonocore’s Pioneering Acid-Etch Technique to Self-Adhering Restoratives. A Status Perspective of Rapidly Advancing Dental Adhesive Technology. J. Adhes. Dent 2020, 22, 7–34. [Google Scholar] [PubMed]
- Sezinando, A. Looking for the ideal adhesive—A review. Rev. Port. Estomatol. Med. Dentária Cir. Maxilofac. 2014, 55, 194–206. [Google Scholar] [CrossRef] [Green Version]
- Peumans, M.; De Munck, J.; Van Landuyt, K.; Van Meerbeek, B. Thirteen-year randomized controlled clinical trial of a two-step self-etch adhesive in non-carious cervical lesions. Dent. Mater. 2015, 31, 308–314. [Google Scholar] [CrossRef]
- Van Landuyt, K.; Yoshida, Y.; Hirata, I.; Snauwaert, J.; De Munck, J.; Okazaki, M.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Influence of the Chemical Structure of Functional Monomers on Their Adhesive Performance. J. Dent. Res. 2008, 87, 757–761. [Google Scholar] [CrossRef]
- Navarra, C.O.; Cadenaro, M.; Frassetto, A.; Fontanive, L.; Di Lenarda, R.; Breschi, L. Degree of Conversion of Self-etch Adhesives: In Situ Micro-Raman Analysis. Oper. Dent. 2016, 41, 501–510. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I.M. A guide through the dental dimethacrylate polymer network structural characterization and interpretation of physico-mechanical properties. Materials 2019, 12, 4057. [Google Scholar] [CrossRef] [Green Version]
- Leprince, J.G.; Palin, W.M.; Hadis, M.A.; Devaux, J.; Leloup, G. Progress in dimethacrylate-based dental composite technology and curing efficiency. Dent. Mater. 2013, 29, 139–156. [Google Scholar] [CrossRef]
- Cadenaro, M.; Maravic, T.; Comba, A.; Mazzoni, A.; Fanfoni, L.; Hilton, T.; Ferracane, J.; Breschi, L. The role of polymerization in adhesive dentistry. Dent. Mater. 2018, 35, e1–e22. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.N.; Lanza, M.D.S.; Dourado, L.G.; Carvalho, E.M.; Bauer, J. Impact of Solvent Evaporation and Curing Protocol on Degree of Conversion of Etch-and-Rinse and Multimode Adhesives Systems. Int. J. Dent. 2019, 2019, 5496784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- French, J.; Kuczaj, E. Working Through Loss and Change With People With Learning Difficulties. J. Br. Inst. Ment. Handicap. (APEX) 2009, 20, 108–111. [Google Scholar] [CrossRef]
- Khan, A.S.; Khalid, H.; Sarfraz, Z.; Khan, M.; Iqbal, J.; Muhammad, N.; Fareed, M.A.; Rehman, I.U. Vibrational spectroscopy of selective dental restorative materials. Appl. Spectrosc. Rev. 2016, 52, 507–540. [Google Scholar] [CrossRef]
- Ferracane, J.; Hilton, T.; Stansbury, J.; Watts, D.; Silikas, N.; Ilie, N.; Heintze, S.; Cadenaro, M.; Hickel, R. Academy of Dental Materials guidance—Resin composites: Part II—Technique sensitivity (handling, polymerization, dimensional changes). Dent. Mater. 2017, 33, 1171–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, P.; Lapkowski, M.; LeGeros, R.Z.; Bouler, J.-M.; Jean, A.; Daculsi, G. Fourier-transform infrared spectroscopy study of an organic–mineral composite for bone and dental substitute materials. J. Mater. Sci. Mater. Electron. 1997, 8, 621–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Q.; Parthasarathy, R.; Abedin, F.; Laurence, J.S.; Misra, A.; Spencer, P. Multivariate analysis of attenuated total reflection Fourier transform infrared (ATR FT-IR) spectroscopic data to confirm phase partitioning in methacrylate-based dentin adhesive. Appl. Spectrosc. 2013, 67, 1473–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadogiannis, D.; Dimitriadi, M.; Zafiropoulou, M.; Gaintantzopoulou, M.-D.; Eliades, G. Universal Adhesives: Setting Characteristics and Reactivity with Dentin. Materials 2019, 12, 1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, A.M. FTIR investigation of polymerisation and polyacid neutralisation kinetics in resin-modified glass-ionomer dental cements. Biomaterials 2002, 23, 3289–3295. [Google Scholar] [CrossRef]
- Young, A.M.; Rafeeka, S.; Howlett, J. FTIR investigation of monomer polymerisation and polyacid neutralisation kinetics and mechanisms in various aesthetic dental restorative materials. Biomaterials 2004, 25, 823–833. [Google Scholar] [CrossRef]
- Dressano, D.; Salvador, M.V.O.; Oliveira, M.T.; Marchi, G.M.; Fronza, B.M.; Hadis, M.; Palin, W.; Lima, A.F. Chemistry of novel and contemporary resin-based dental adhesives. J. Mech. Behav. Biomed. Mater. 2020, 110, 103875. [Google Scholar] [CrossRef]
- Peumans, M.; De Munck, J.; Van Landuyt, K.L.; Poitevin, A.; Lambrechts, P.; Van Meerbeek, B. A 13-year clinical evaluation of two three-step etch-and-rinse adhesives in non-carious class-V lesions. Clin. Oral Investig. 2010, 16, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Sarr, M.; Kane, A.W.; Vreven, J.; Mine, A.; Van Landuyt, K.L.; Peumans, M.; Lambrechts, P.; Van Meerbeek, B.; De Munck, J. Microtensile Bond Strength and Interfacial Characterization of 11 Contemporary Adhesives Bonded to Bur-cut Dentin. Oper. Dent. 2010, 35, 94–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stavridakis, M.M.; Krejci, I.; Magne, P. Immediate dentin sealing of onlay preparations: Thickness of pre-cured Dentin Bonding Agent and effect of surface cleaning. Oper. Dent. 2005, 30, 747–757. [Google Scholar] [PubMed]
- Özcan, M.; Dündar, M.; Çömlekoğlu, M.E. Adhesion concepts in dentistry: Tooth and material aspects. J. Adhes. Sci. Technol. 2012, 26, 2661–2681. [Google Scholar] [CrossRef] [Green Version]
- Mobarak, E.H.; Seyam, R. Interfacial Nanoleakage and Bonding of Self-Adhesive Systems Cured with a Modified-Layering Technique to Dentin of Weakened Roots. Oper. Dent. 2013, 38, E154–E165. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Spencer, P. Hybridization Efficiency of the Adhesive/Dentin Interface with Wet Bonding. J. Dent. Res. 2003, 82, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Tauscher, S.; Angermann, J.; Catel, Y.; Moszner, N. Evaluation of alternative monomers to HEMA for dental applications. Dent. Mater. 2017, 33, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.; Ulbaldini, A.; Sato, F.; Baesso, M.; Bento, A.C.; Andrade, L.; Lima, S.; Pascotto, R.C. Chemical Interaction Analysis of an Adhesive Containing 10-Methacryloyloxydecyl Dihydrogen Phosphate (10-MDP) With the Dentin in Noncarious Cervical Lesions. Oper. Dent. 2017, 42, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Zhou, L.; Zhang, Z.; Niu, L.; Zhang, L.; Chen, C.; Zhou, J.; Yang, H.; Wang, X.; Fu, B.; et al. Paucity of Nanolayering in Resin-Dentin Interfaces of MDP-based Adhesives. J. Dent. Res. 2016, 95, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Yoshihara, K.; Hayakawa, S.; Nagaoka, N.; Okihara, T.; Matsumoto, T.; Minagi, S.; Osaka, A.; Van Landuyt, K.; Van Meerbeek, B. HEMA Inhibits Interfacial Nano-layering of the Functional Monomer MDP. J. Dent. Res. 2012, 91, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, K.; Nagaoka, N.; Hayakawa, S.; Okihara, T.; Yoshida, Y.; Van Meerbeek, B. Chemical interaction of glycero-phosphate dimethacrylate (GPDM) with hydroxyapatite and dentin. Dent. Mater. 2018, 34, 1072–1081. [Google Scholar] [CrossRef]
- Zorzin, J.; Petschelt, A.; Ebert, J.; Lohbauer, U. pH neutralization and influence on mechanical strength in self-adhesive resin luting agents. Dent. Mater. 2012, 28, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Cadenaro, M.; Breschi, L.; Rueggeberg, F.A.; Suchko, M.; Grodin, E.; Agee, K.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H. Effects of residual ethanol on the rate and degree of conversion of five experimental resins. Dent. Mater. 2009, 25, 621–628. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Spencer, P.; Yao, X.; Brenda, B. Effect of solvent content on resin hybridization in wet dentin bonding. J. Biomed. Mater. Res. Part A 2007, 82, 975–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdigão, J. Current perspectives on dental adhesion: (1) Dentin adhesion – not there yet. Jpn. Dent. Sci. Rev. 2020, 56, 190–207. [Google Scholar] [CrossRef]
- Güler, A.U.; Sarikaya, I.B.; Güler, E.; Yücel, A.C. Effect of Filler Ratio in Adhesive Systems on the Shear Bond Strength of Resin Composite to Porcelains. Oper. Dent. 2009, 34, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, E.-J.; Kim, M. Effect of different immediate dentin sealing techniques on the microtensile bond strength. Oral Biol. Res. 2017, 41, 63–68. [Google Scholar] [CrossRef]
- De Souza, G.M.; Braga, R.R.; Cesar, P.F.; Lopes, G.C. Correlation between clinical performance and degree of conversion of resin cements: A literature review. J. Appl. Oral Sci. 2015, 23, 358–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stansbury, J.W. Dimethacrylate network formation and polymer property evolution as determined by the selection of monomers and curing conditions. Dent. Mater. 2012, 28, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charton, C.; Falk, V.; Marchal, P.; Pla, F.; Colon, P. Influence of Tg, viscosity and chemical structure of monomers on shrinkage stress in light-cured dimethacrylate-based dental resins. Dent. Mater. 2007, 23, 1447–1459. [Google Scholar] [CrossRef] [PubMed]
- Walters, N.J.; Xia, W.; Salih, V.; Ashley, P.F.; Young, A.M. Poly(propylene glycol) and urethane dimethacrylates improve conversion of dental composites and reveal complexity of cytocompatibility testing. Dent. Mater. 2016, 32, 264–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanabusa, M.; Yoshihara, K.; Yoshida, Y.; Okihara, T.; Yamamoto, T.; Momoi, Y.; Van Meerbeek, B. Interference of functional monomers with polymerization efficiency of adhesives. Eur. J. Oral Sci. 2016, 124, 204–209. [Google Scholar] [CrossRef]
- Sato, K.; Hosaka, K.; Takahashi, M.; Ikeda, M.; Tian, F.; Komada, W.; Nakajima, M.; Foxton, R.; Nishitani, Y.; Pashley, D.H.; et al. Dentin Bonding Durability of Two-step Self-etch Adhesives with Improved of Degree of Conversion of Adhesive Resins. J Adhes Dent 2017, 19, 31–37. [Google Scholar]
- Halvorson, R.H.; Erickson, R.L.; Davidson, C.L. The effect of filler and silane content on conversion of resin-based composite. Dent. Mater. 2003, 19, 327–333. [Google Scholar] [CrossRef]
- Tichy, A.; Hosaka, K.; Bradna, P.; Ikeda, M.; Abdou, A.; Nakajima, M.; Tagami, J. Subsequent application of bonding agents to a one-step self-etch adhesive — Its effect with/without previous light-curing. Dent. Mater. 2019, 35, e299–e309. [Google Scholar] [CrossRef]
- Aljabo, A.; Xia, W.; Liaqat, S.; Khan, M.; Knowles, J.; Ashley, P.; Young, A. Conversion, shrinkage, water sorption, flexural strength and modulus of re-mineralizing dental composites. Dent. Mater. 2015, 31, 1279–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekambaram, M.; Yiu, C.K.Y.; Matinlinna, J.P. An overview of solvents in resin–dentin bonding. Int. J. Adhes. Adhes. 2015, 57, 22–33. [Google Scholar] [CrossRef]
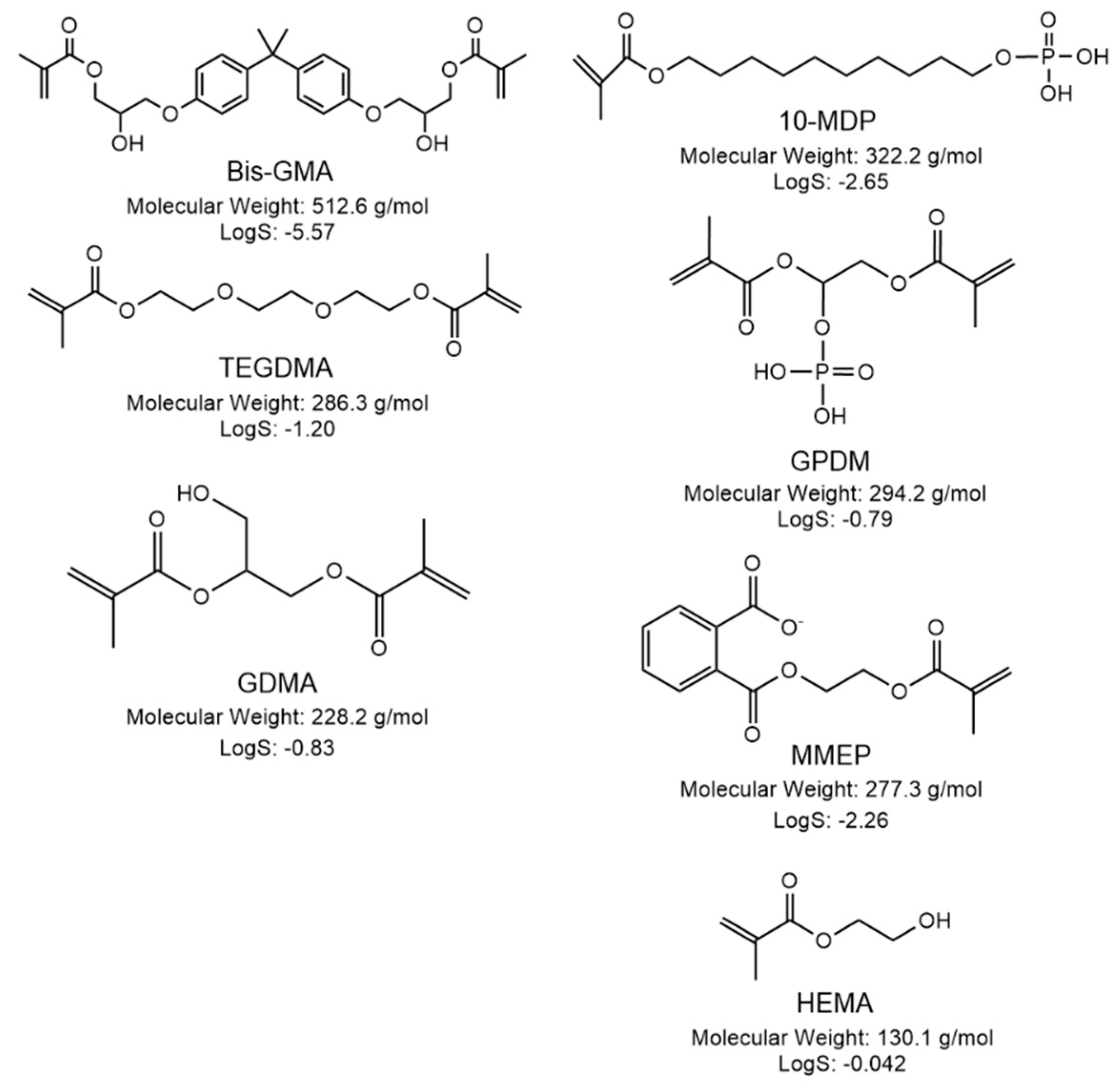



| Material | Type | Primer | Adhesive |
|---|---|---|---|
| Optibond™ FL (Kerr, USA) OBFL | 3-step ER bonding system | HEMA (10–30%) MMEP (10–30%) GPDM (5–10%) Solvent: Water/Ethanol | HEMA (10–30%) Bis-GMA (N/A%) GDMA * Filler: Barium aluminosilicate Sodium hexafluorosilicate and fumed silica with silane (48 wt.%) |
| Clearfil SE 2 (Kuraray, JP) CFSE | 2-step SE bonding system | HEMA (20–40%) 10-MDP * Hydrophilic aliphatic Dimethacrylate * Solvent: Water | HEMA (20–40%) Bis-GMA (25–45%) 10-MDP * Hydrophobic aliphatic Dimethacrylate * Filler: Colloidal silica (N/A%) |
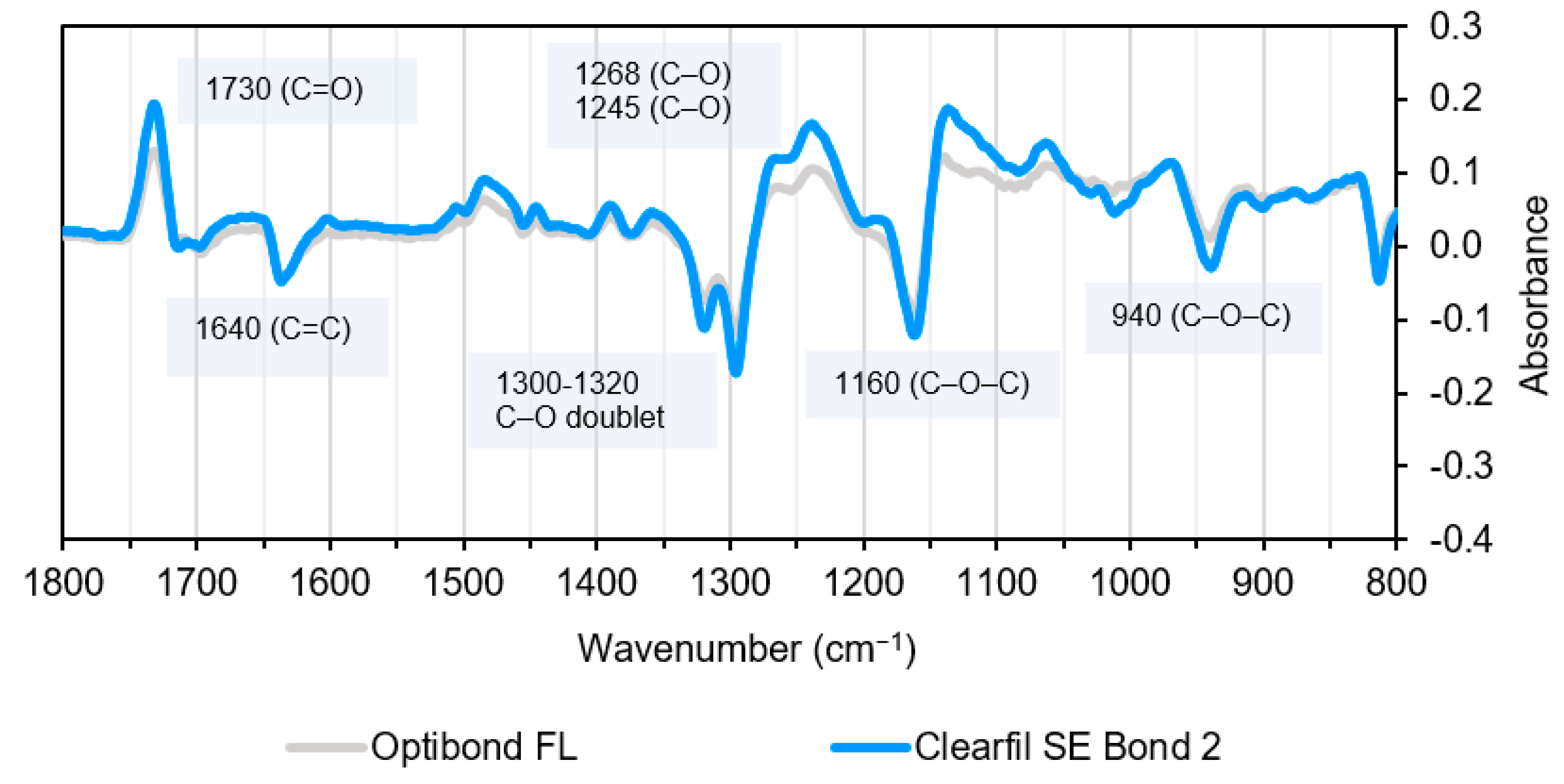
| Wavenumber (cm−1) | Methacrylate Assignment | Wavenumber (cm−1) | Assignment | Compound |
|---|---|---|---|---|
| 2940 | C–H stretch | 3400 | O–H stretch | HEMA, Bis-GMA |
| 1700–1720 | C=O stretch | 3300 | O–H stretch | Water, Ethanol |
| 1640 | C=C stretch | 1635 | O-H bend | Water |
| 1350–1450 | C–H bending | 1610 | Aromatic C=C | Bis-GMA |
| 1320, 1300 | C–O stretch doublet | 1510 | Aromatic C=C | Bis-GMA |
| 1240 | Aromatic C–O | Bis-GMA | ||
| 1120 | C–O–C stretch | TEGDMA | ||
| 1080 | C–OH stretch | HEMA | ||
| 1050/1090 | C–OH stretch | Ethanol | ||
| 1000 | P–O stretch | 10-MDP | ||
| 900 | C–C–O stretch | HEMA | ||
| 880 830 | C–C–O stretch C–C–O stretch | Ethanol Bis-GMA | ||
| 650–900 | Water hindered rotation | Water |
| Wavenumber (cm−1) | Assignment | Compound | Bonding System |
|---|---|---|---|
| 1635 | O–H stretch, Aliphatic C=C | Water, Monomers | OBFL, CFSE |
| 1604–1610 | Aromatic C=C | Bis-GMA, MEPP | OBFL, CFSE |
| 1510 | Aromatic C=C | Bis-GMA, MEPP | OBFL, CFSE |
| 1240 | Aromatic C–O | Bis-GMA | OBFL, CFSE |
| 1080 | C–OH stretch | HEMA | OBFL, CFSE |
| 1050/1090 | C–OH stretch | Ethanol | OBFL |
| 1000–1010 | P–O stretch/Si–O | 10-MDP GPDM | CFSE OBFL |
| 900 | C–C–O stretch | HEMA | OBFL, CFSE |
| Components | Pure Spectrum Fraction (X) Primer | Adhesive | ||
|---|---|---|---|---|
| OBFL | CFSE | OBFL | CFSE | |
| HEMA | 0.55 | 0.30 | 0.50 | 0.45 |
| Bis-GMA | - | - | 0.50 | 0.45 |
| 10-MDP | 0.15 | 0.10 | - | 0.10 |
| Water | 0.15 | 0.60 | - | - |
| Ethanol | 0.15 | - | - | - |
| Filler | - | - | 0.32 | 0.30 |
| Background absorbance | 0.04 | 0.02 | 0.02 | 0.03 |
| Sum of mod (difference) | 12.9 | 8.1 | 7.3 | 7.8 |
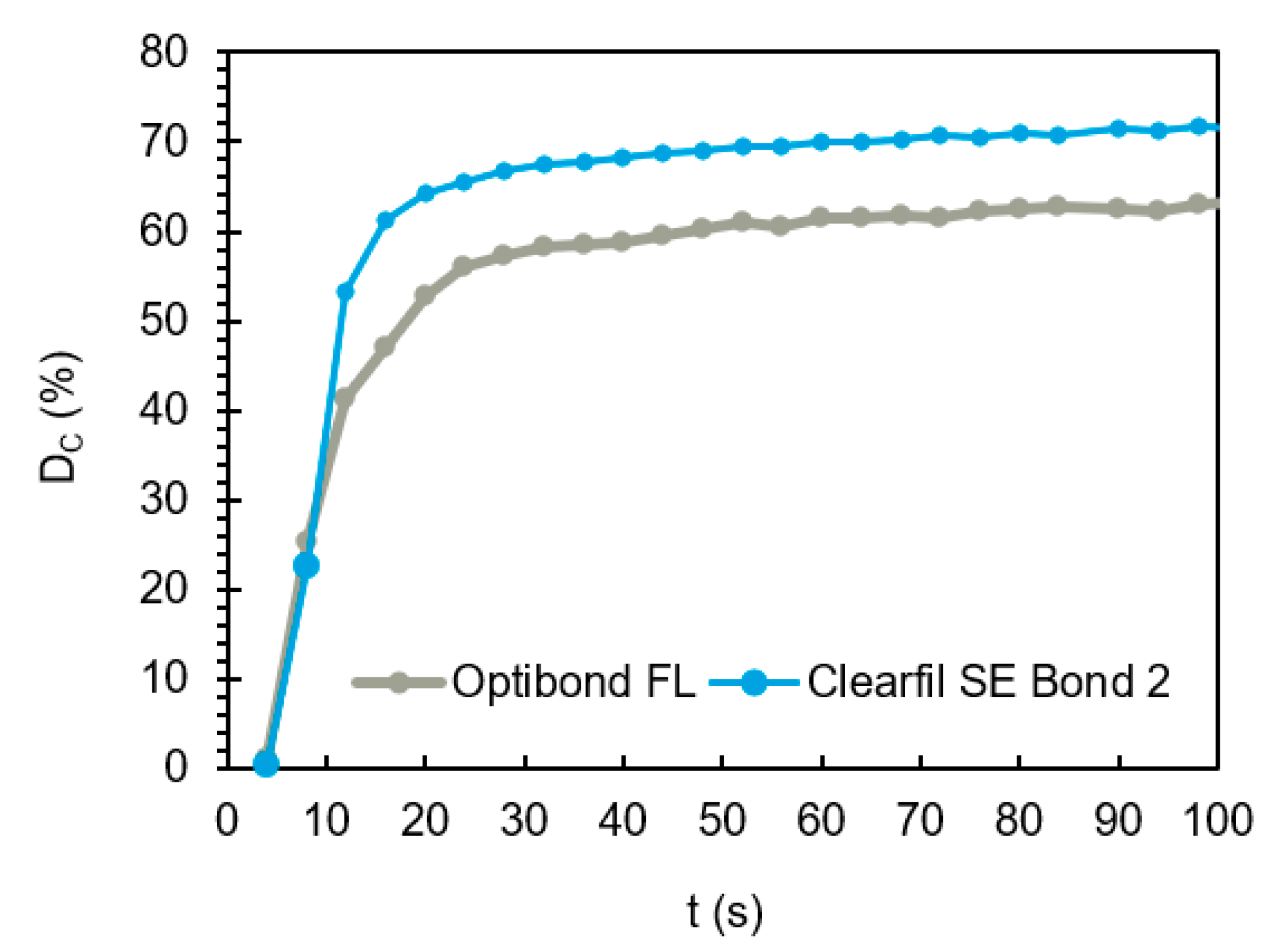
| Material | Filler (wt.%) | DC (%) | Rp,max (%s−1) |
|---|---|---|---|
| OBFL | 48 ± 3 | 74 ± 1 A | 4.0 ± 1.6 A |
| CFSE | 5 ± 1 | 79 ± 2 B | 5.0 ± 0.1 A |
| Components | Component Fractions (FTIR and Filler Load Determination) | |||||||
|---|---|---|---|---|---|---|---|---|
| OBFL P | Kerr | OBFL A | Kerr | CFSE P | Kuraray | CFSE A | Kuraray | |
| HEMA | 0.55 | N 1 | 0.50 | N | 0.30 | Y | 0.45 | N |
| Bis-GMA | - | - | 0.50 | N/A | - | - | 0.45 | Y |
| GPDM/10-MDP | 0.15 | N 1 | - | - | 0.10 | N/A | 0.10 | N |
| Water | 0.15 | N/A | - | - | 0.60 | N/A | - | - |
| Ethanol | 0.15 | N/A | - | - | - | - | - | - |
| Filler | - | - | 0.48 | Y | - | - | 0.05 | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado, A.H.; Young, A.M. Modelling ATR-FTIR Spectra of Dental Bonding Systems to Investigate Composition and Polymerisation Kinetics. Materials 2021, 14, 760. https://doi.org/10.3390/ma14040760
Delgado AH, Young AM. Modelling ATR-FTIR Spectra of Dental Bonding Systems to Investigate Composition and Polymerisation Kinetics. Materials. 2021; 14(4):760. https://doi.org/10.3390/ma14040760
Chicago/Turabian StyleDelgado, António HS, and Anne M. Young. 2021. "Modelling ATR-FTIR Spectra of Dental Bonding Systems to Investigate Composition and Polymerisation Kinetics" Materials 14, no. 4: 760. https://doi.org/10.3390/ma14040760







