Facile Synthesis of Ag Nanowire/TiO2 and Ag Nanowire/TiO2/GO Nanocomposites for Photocatalytic Degradation of Rhodamine B
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Chemicals
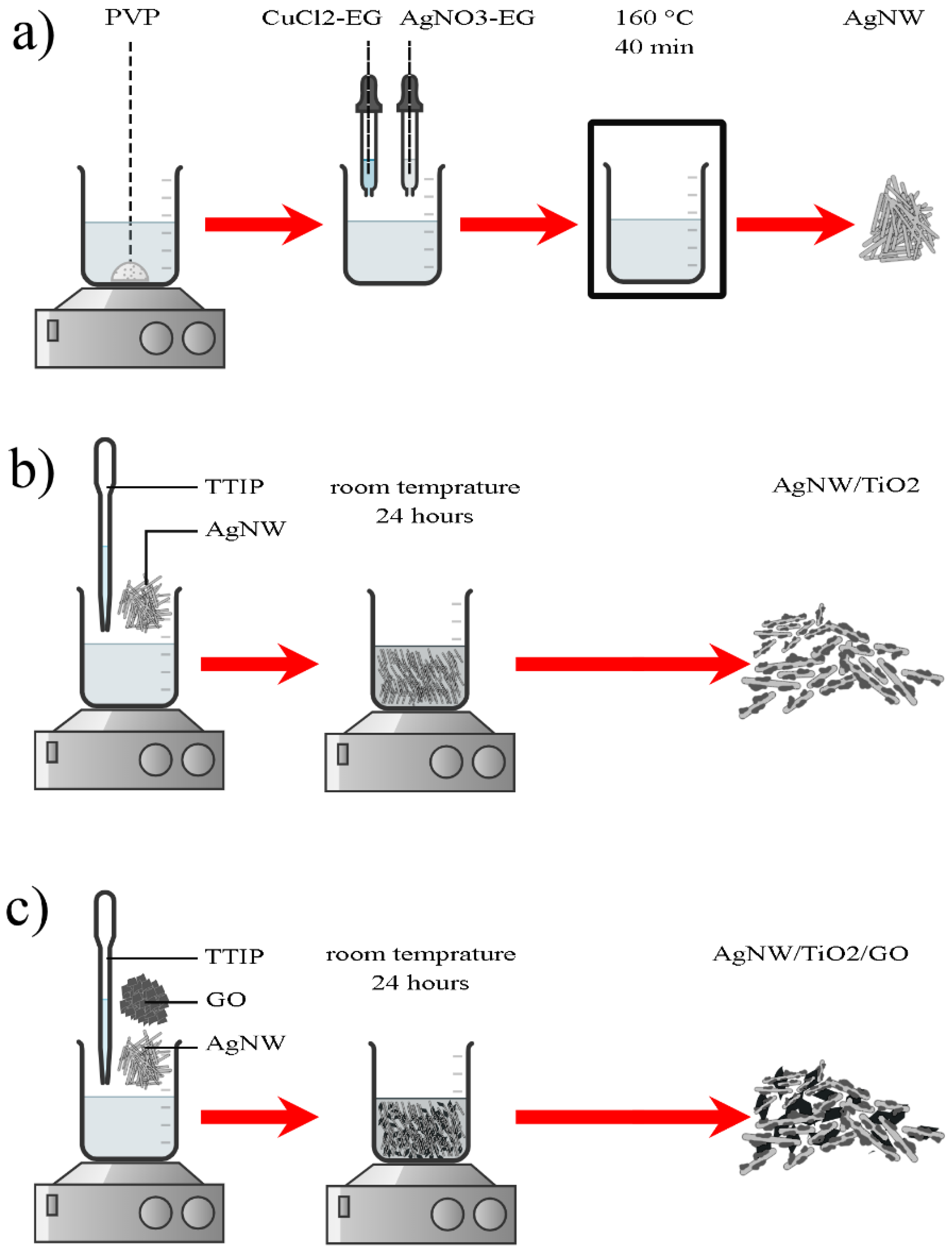
2.2. Synthesis of AgNW/TiO2 and AgNW/TiO2/GO Nanocomposites
2.3. Characterization
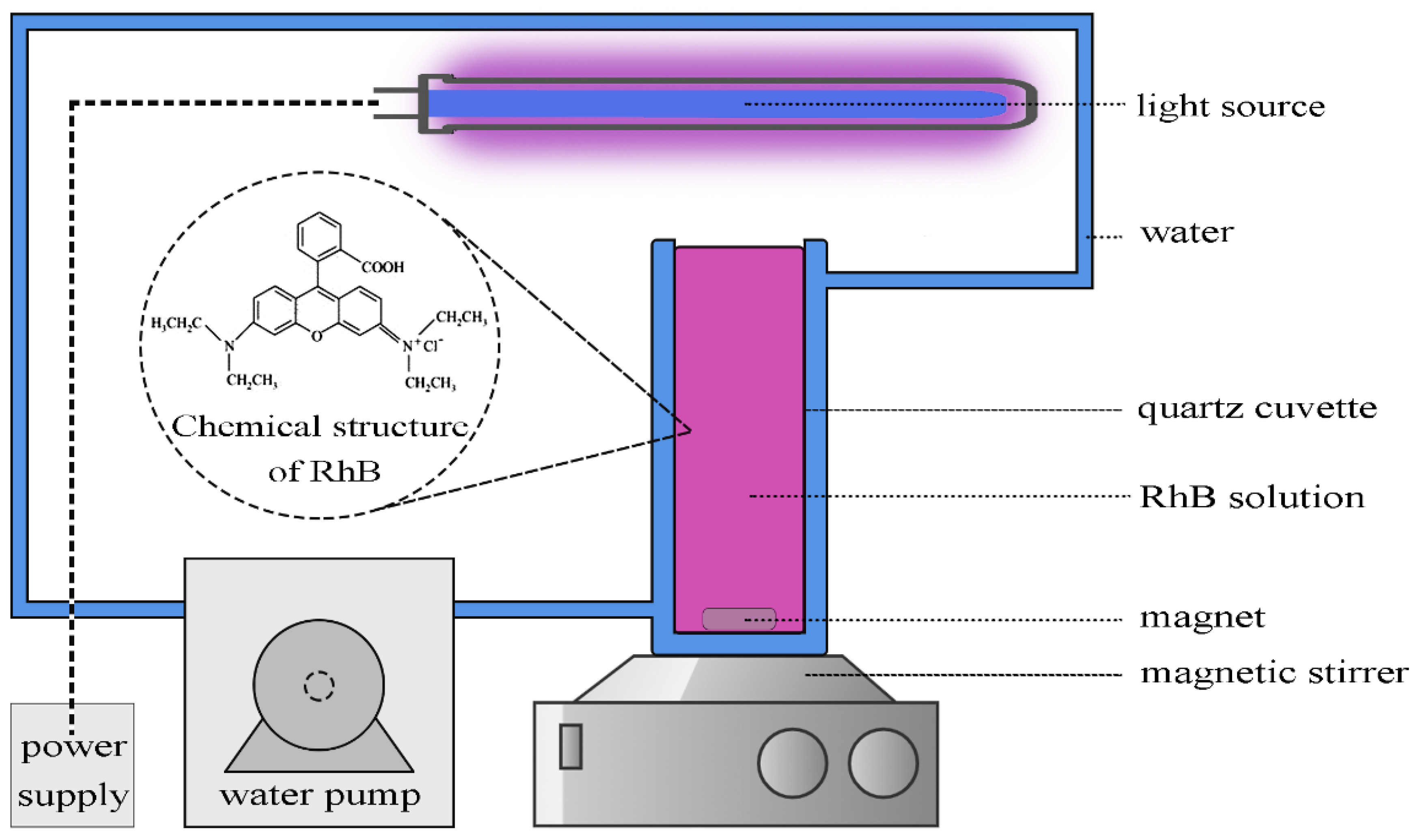
2.4. Photocatalytic Experiments
3. Results and Discussion
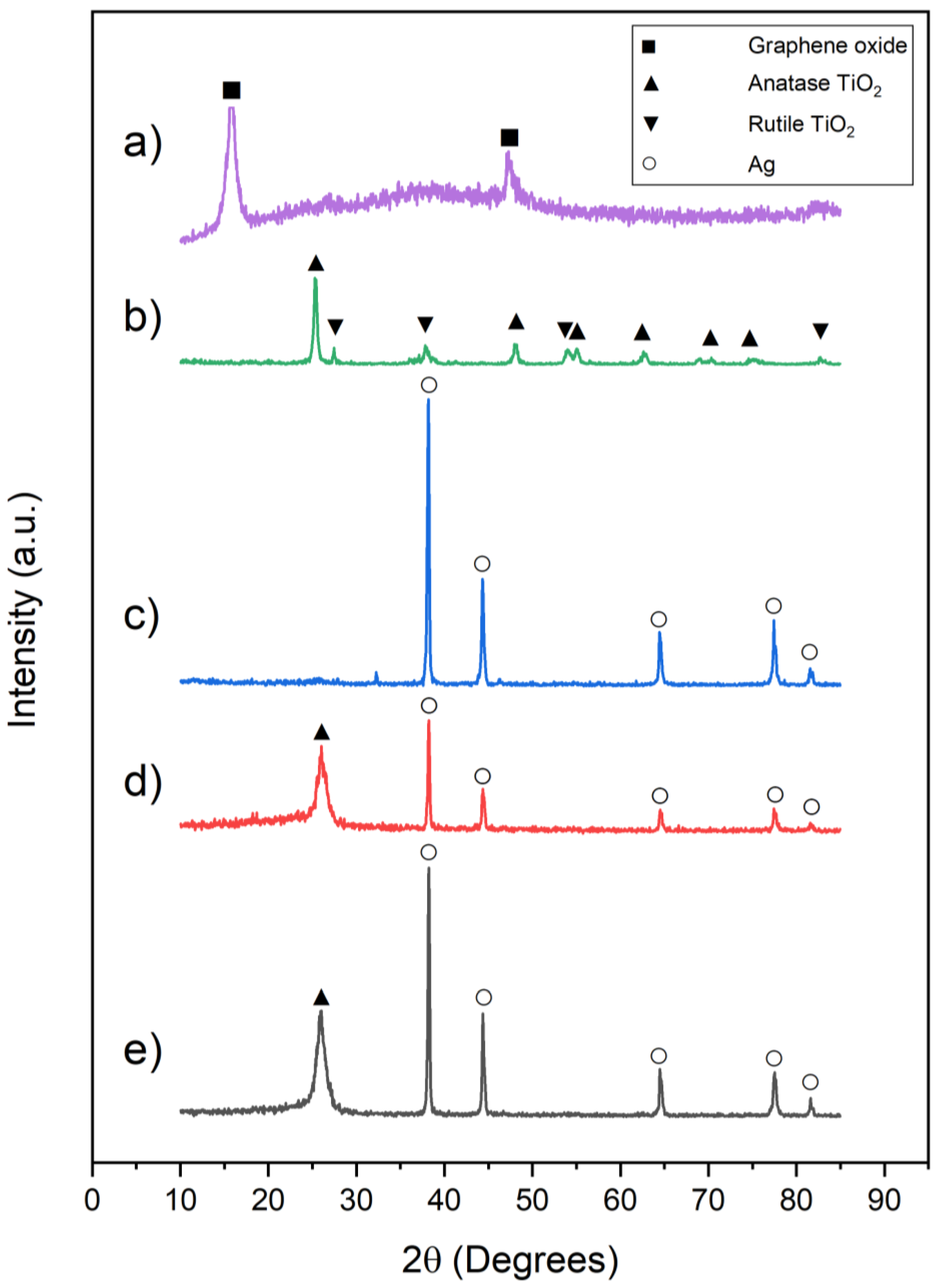
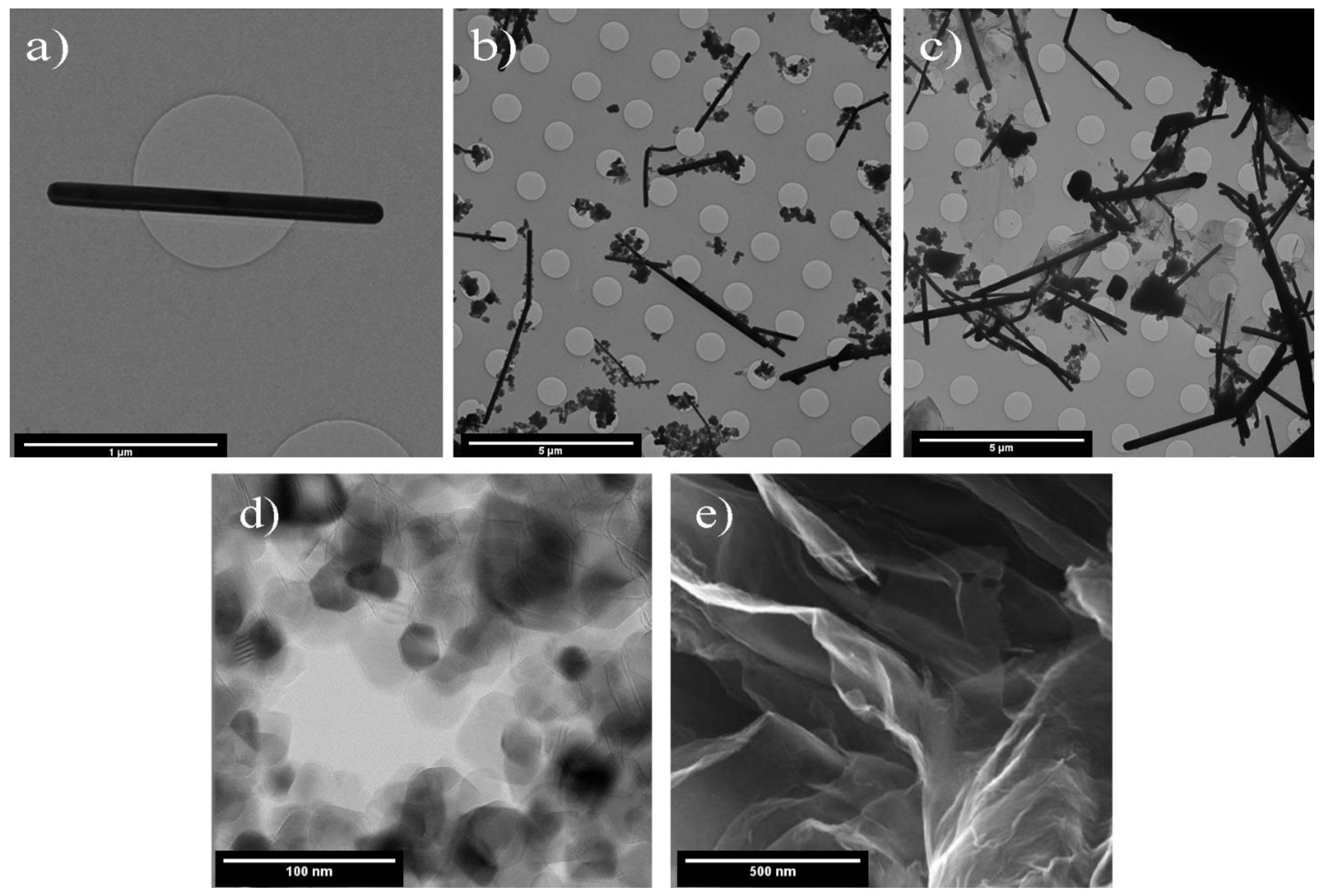
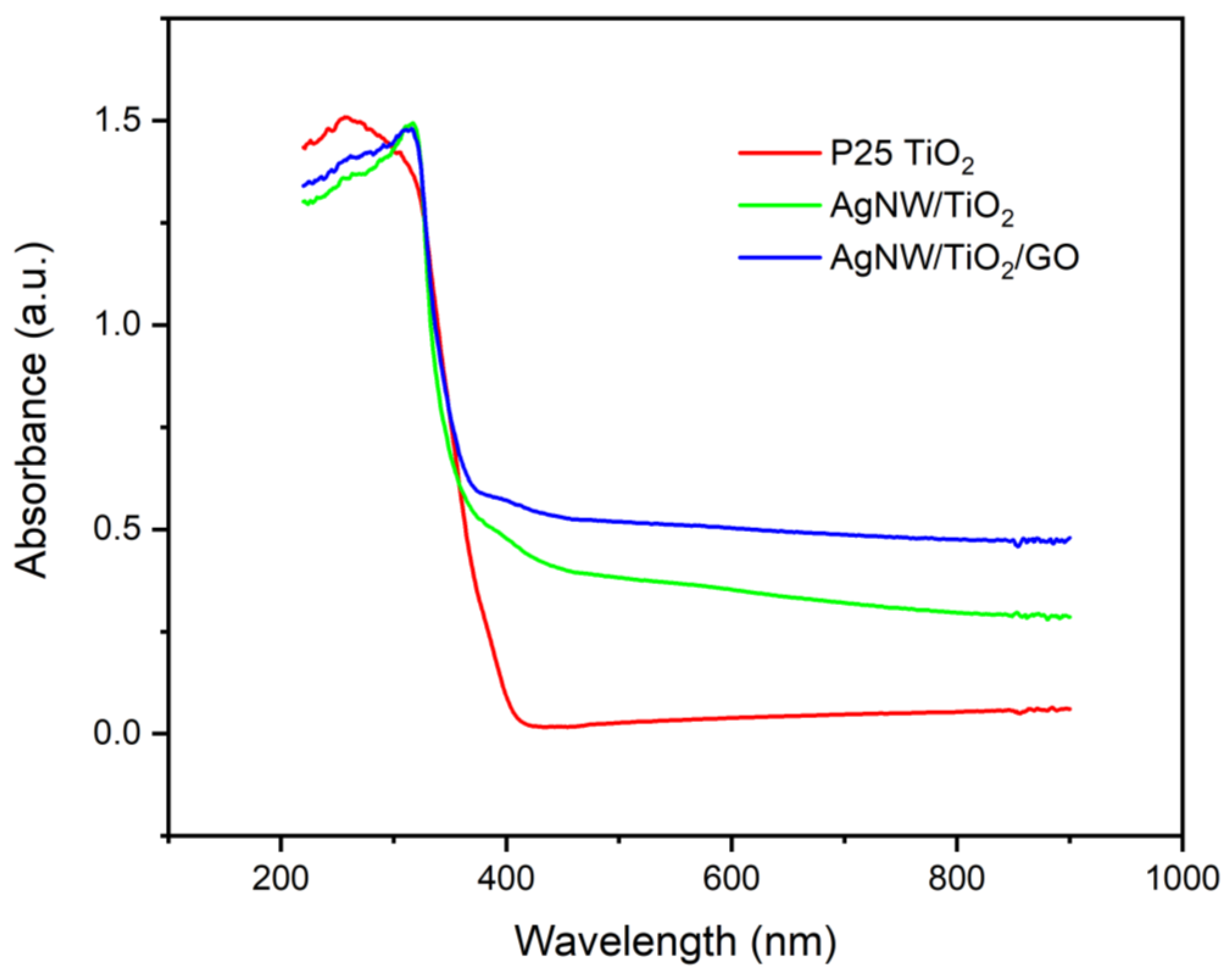
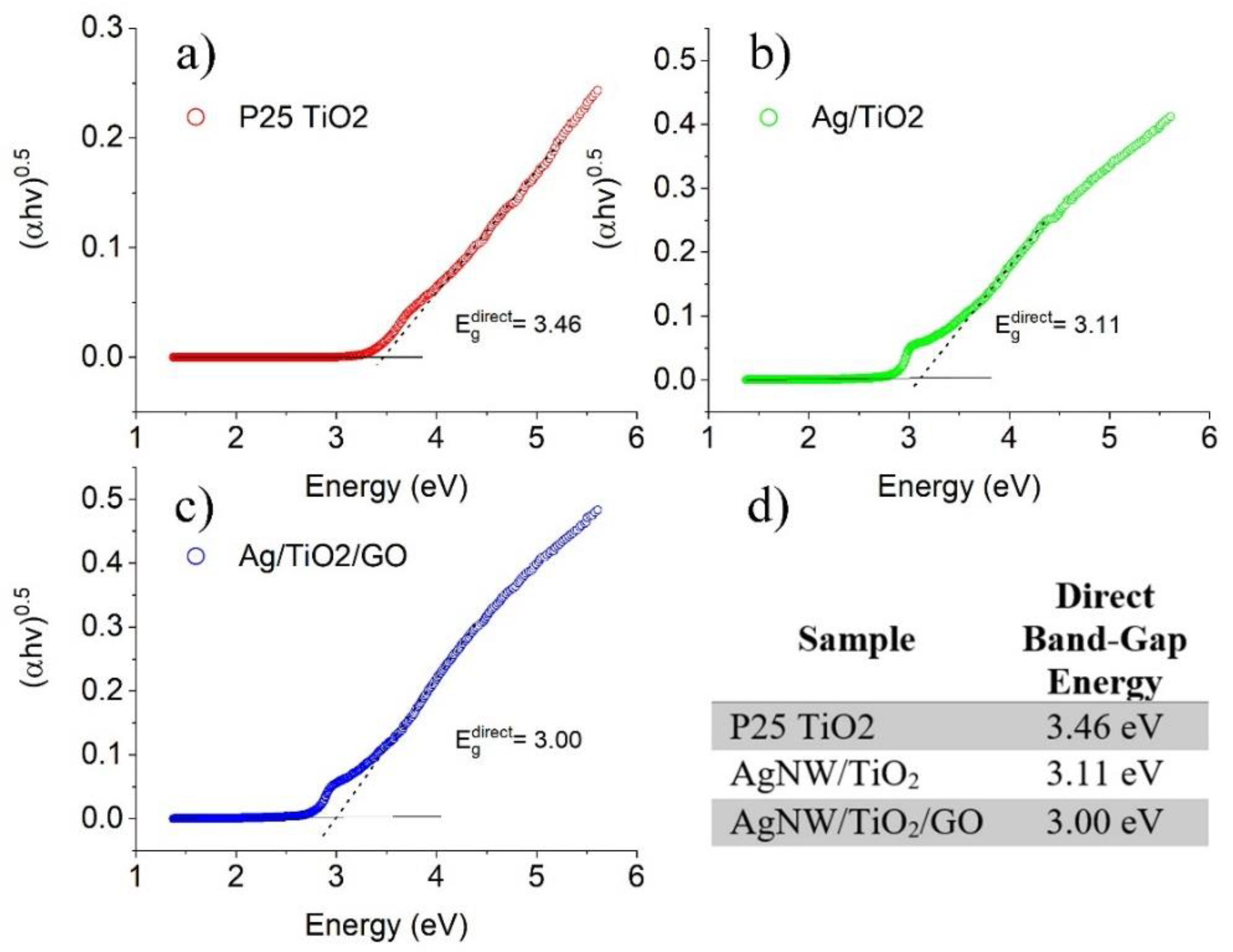
3.1. Characterization of Synthesized Nanocomposites
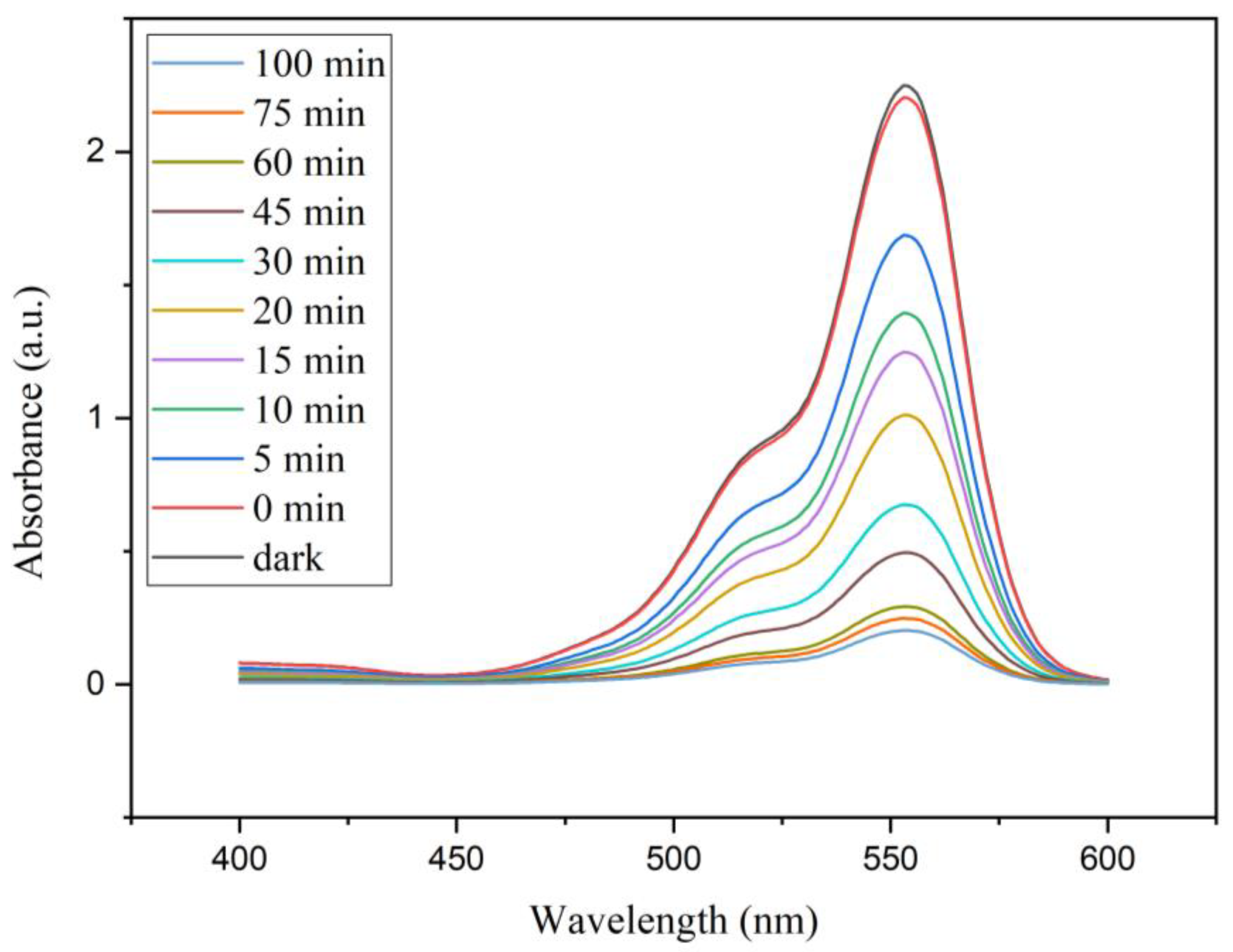
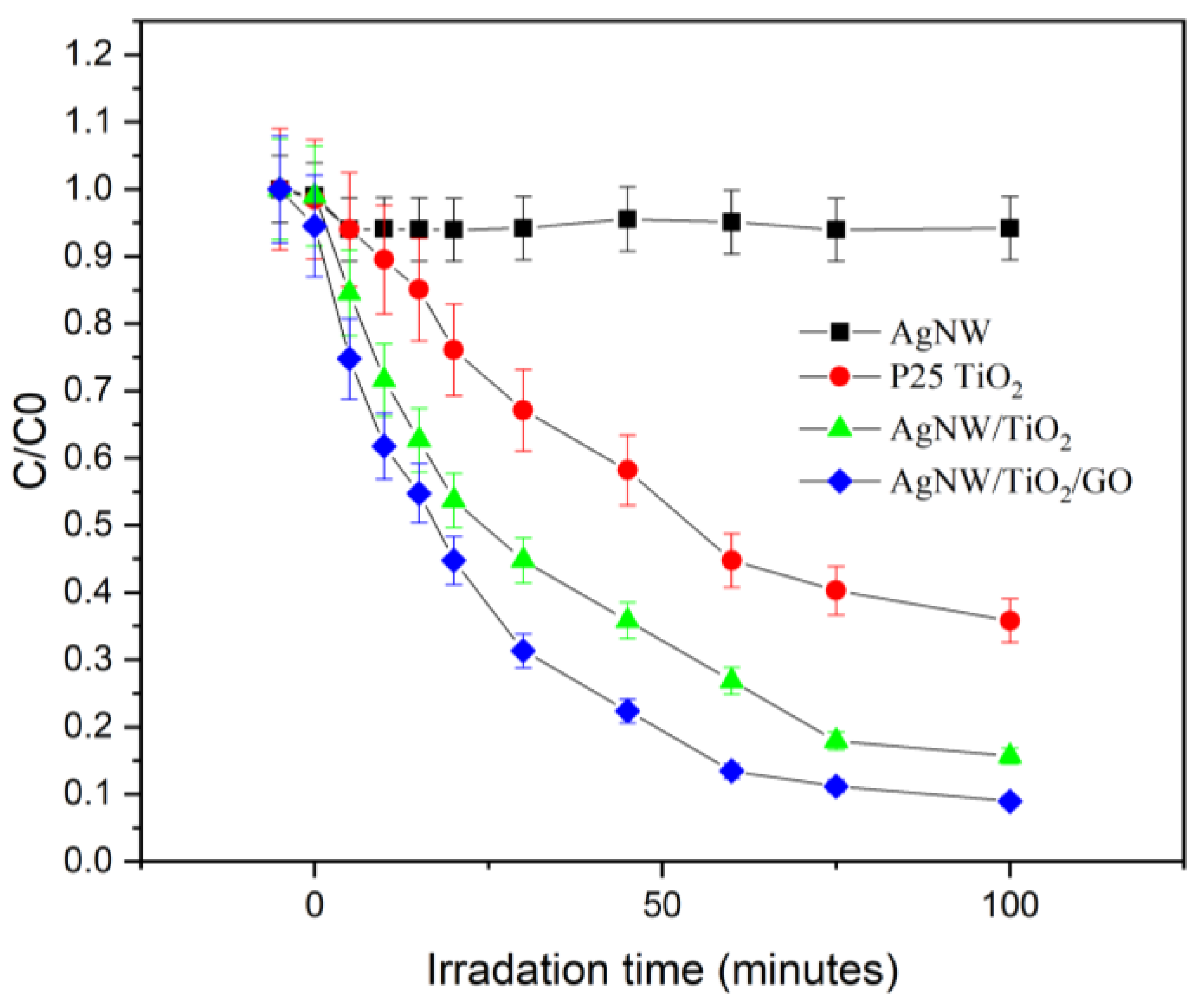
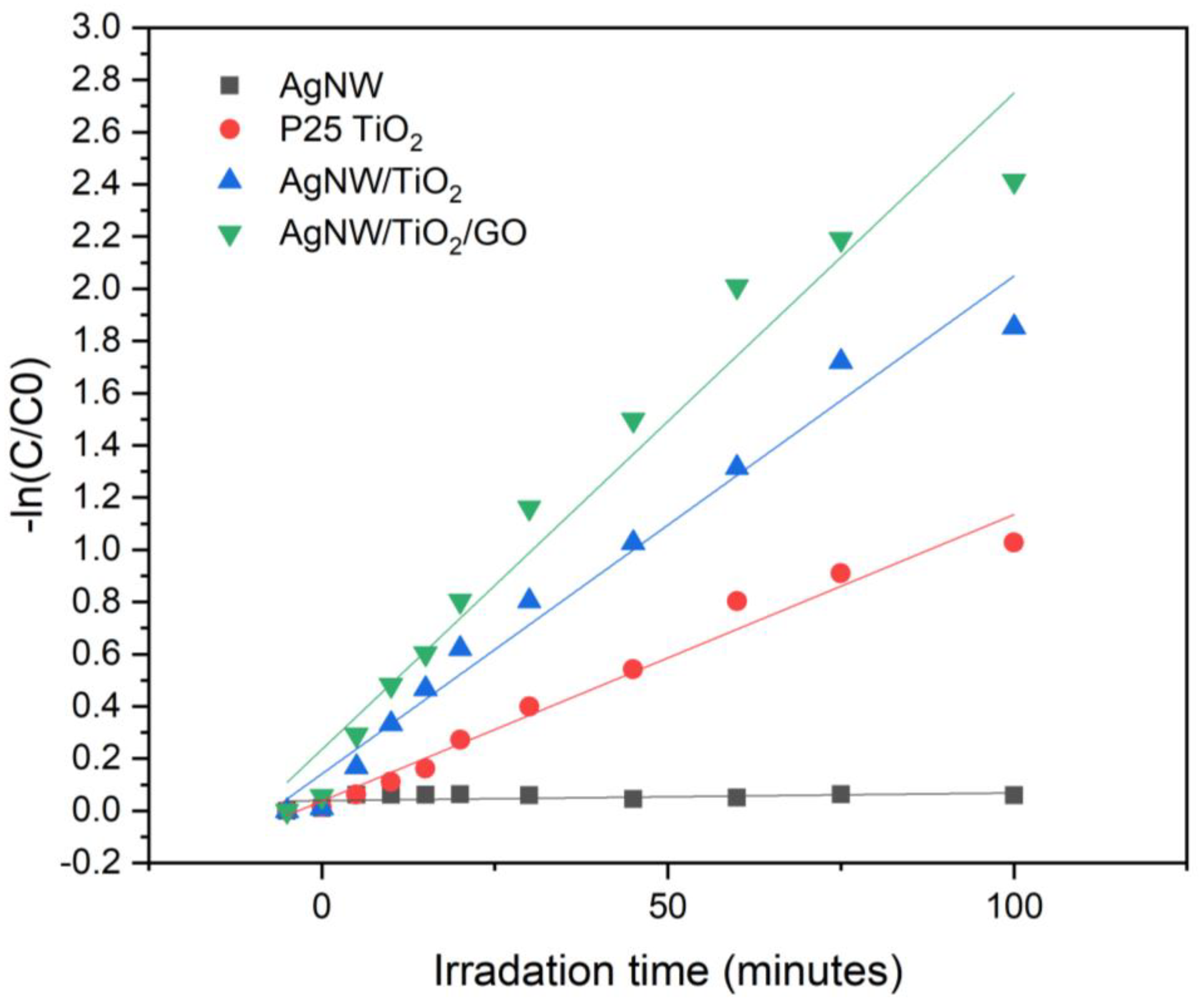
3.2. Characterization of Photocatalytic Behaviour of Nanocomposites
4. Conclusions
- -
- The proposed straightforward synthesis method resulted in the formation of Ag nanowires with an average length of 3 µm and an average diameter of 50 nm. XRD results also confirmed that TiO2 was successfully synthesized. In the AgNW/TiO2 sample, colonies of TiO2 nanoparticles were very well attached to Ag nanowires.
- -
- In AgNW/TiO2/GO nanocomposite samples, GO sheets were homogeneously dispersed within the composite mixture. Clusters of TiO2 nanoparticles were also found to be attached to GO sheets. FTIR results also confirmed the formation of atomic bonding at AgNW/TiO2 and GO/TiO2 junctions.
- -
- Results showed that synthesized AgNW/TiO2/GO nanocomposite has superior photocatalytic activity, compared to AgNW and AgNW/TiO2 samples. This has to do with higher light-harvesting due to the localized surface plasmon resonance (SPR) effect of Ag nanowires, lower e−–h+ pair diffusion distance, more effective photo-generated charge transfer, and more enhanced charge separation.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cohen-Tanugi, D.; Grossman, J.C. Water Desalination across Nanoporous Graphene. Nano Lett. 2012, 12, 3602–3608. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Guo, Y.; Guo, R.; Liu, X. A Review of Visible Light-Active Photocatalysts for Water Disinfection: Features and Prospects. Chem. Eng. J. 2019, 373, 624–641. [Google Scholar] [CrossRef]
- Yahya, N.; Aziz, F.; Jamaludin, N.A.; Mutalib, M.A.; Ismail, A.F.; Salleh, W.N.W.; Jaafar, J.; Yusof, N.; Ludin, N.A. A Review of Integrated Photocatalyst Adsorbents for Wastewater Treatment. J. Environ. Chem. Eng. 2018, 6, 7411–7425. [Google Scholar] [CrossRef]
- Khan, M.M.; Adil, S.F.; Al-Mayouf, A. Metal Oxides as Photocatalysts. J. Saudi Chem. Soc. 2015, 19, 462–464. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.; Kronawitter, C.; Kiriakidis, G. An Overview of Photocatalytic Materials. J. Mater. 2017, 3, 1–2. [Google Scholar] [CrossRef]
- Yazdan Mehr, M.; Bahrami, A.; van Driel, W.D.; Fan, X.J.; Davis, J.L.; Zhang, G.Q. Degradation of Optical Materials in Solid-State Lighting Systems. Int. Mater. Rev. 2020, 65, 102–128. [Google Scholar] [CrossRef] [Green Version]
- Khouzani, M.K.; Bahrami, A.; Mehr, M.Y.; van Driel, W.D.; Zhang, G. Towards Multi-Functional SiO2 @YAG:Ce Core–Shell Optical Nanoparticles for Solid State Lighting Applications. Nanomaterials 2020, 10, 153. [Google Scholar] [CrossRef] [Green Version]
- Isari, A.A.; Payan, A.; Fattahi, M.; Jorfi, S.; Kakavandi, B. Photocatalytic Degradation of Rhodamine B and Real Textile Wastewater Using Fe-Doped TiO2 Anchored on Reduced Graphene Oxide (Fe-TiO2/RGO): Characterization and Feasibility, Mechanism and Pathway Studies. Appl. Surf. Sci. 2018, 462, 549–564. [Google Scholar] [CrossRef]
- Bai, H.; Liu, Z.; Liu, L.; Sun, D.D. Large-Scale Production of Hierarchical TiO2 Nanorod Spheres for Photocatalytic Elimination of Contaminants and Killing Bacteria. Chem. A Eur. J. 2013, 19, 3061–3070. [Google Scholar] [CrossRef]
- Hajipour, P.; Bahrami, A.; Eslami, A.; Hosseini-Abari, A.; Hagh Ranjbar, H.R. Chemical Bath Synthesis of CuO-GO-Ag Nanocomposites with Enhanced Antibacterial Properties. J. Alloys Compd. 2020, 821. [Google Scholar] [CrossRef]
- Umrao, S.; Abraham, S.; Theil, F.; Pandey, S.; Ciobota, V.; Shukla, P.K.; Rupp, C.J.; Chakraborty, S.; Ahuja, R.; Popp, J. A Possible Mechanism for the Emergence of an Additional Band Gap Due to a Ti–O–C Bond in the TiO2–Graphene Hybrid System for Enhanced Photodegradation of Methylene Blue under Visible Light. RSC Adv. 2014, 4, 59890–59901. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, C. TiO2/Graphene Composite from Thermal Reaction of Graphene Oxide and Its Photocatalytic Activity in Visible Light. J. Mater. Sci. 2011, 46, 2622–2626. [Google Scholar] [CrossRef]
- Chaudhary, D.; Singh, S.; Vankar, V.D.; Khare, N. A Ternary Ag/TiO2/CNT Photoanode for Efficient Photoelectrochemical Water Splitting under Visible Light Irradiation. Int. J. Hydrog. Energy 2017, 42, 7826–7835. [Google Scholar] [CrossRef]
- Li, J.-S.; Wang, Y.; Liu, C.-H.; Li, S.-L.; Wang, Y.-G.; Dong, L.-Z.; Dai, Z.-H.; Li, Y.-F.; Lan, Y.-Q. Coupled Molybdenum Carbide and Reduced Graphene Oxide Electrocatalysts for Efficient Hydrogen Evolution. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Yeh, T.-F.; Cihlář, J.; Chang, C.-Y.; Cheng, C.; Teng, H. Roles of Graphene Oxide in Photocatalytic Water Splitting. Mater. Today 2013, 16, 78–84. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Y.; Xu, Y.-J. Recent Progress on Graphene-Based Photocatalysts: Current Status and Future Perspectives. Nanoscale 2012, 4, 5792–5813. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-Based Semiconductor Photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef]
- Tunc, I.; Bruns, M.; Gliemann, H.; Grunze, M.; Koelsch, P. Bandgap Determination and Charge Separation in Ag@ TiO2 Core Shell Nanoparticle Films. Surf. Interface Anal. 2010, 42, 835–841. [Google Scholar] [CrossRef]
- Alakhras, F.; Alhajri, E.; Haounati, R.; Ouachtak, H.; Addi, A.A.; Saleh, T.A. A Comparative Study of Photocatalytic Degradation of Rhodamine B Using Natural-Based Zeolite Composites. Surf. Interfaces 2020, 20, 100611. [Google Scholar] [CrossRef]
- Zhao, T.; Sun, R.; Yu, S.; Zhang, Z.; Zhou, L.; Huang, H.; Du, R. Size-Controlled Preparation of Silver Nanoparticles by a Modified Polyol Method. Colloids Surf. A Physicochem. Eng. Asp. 2010, 366, 197–202. [Google Scholar] [CrossRef]
- Salehi, T.; Taherizadeh, A.; Bahrami, A.; Allafchian, A.; Ghafarinia, V. Toward a Highly Functional Hybrid ZnO Nanofiber–RGO Gas Sensor. Adv. Eng. Mater. 2020, 22, 2000005. [Google Scholar] [CrossRef]
- Standridge, S.D.; Schatz, G.C.; Hupp, J.T. Toward Plasmonic Solar Cells: Protection of Silver Nanoparticles via Atomic Layer Deposition of TiO2. Langmuir 2009, 25, 2596–2600. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Duan, X. Progress, Challenge and Perspective of Heterogeneous Photocatalysts. Chem. Soc. Rev. 2013, 42, 2568–2580. [Google Scholar] [CrossRef] [PubMed]
- Kochuveedu, S.T.; Jang, Y.H.; Kim, D.H. A Study on the Mechanism for the Interaction of Light with Noble Metal-Metal Oxide Semiconductor Nanostructures for Various Photophysical Applications. Chem. Soc. Rev. 2013, 42, 8467–8493. [Google Scholar] [CrossRef]
- Gerislioglu, B.; Dong, L.; Ahmadivand, A.; Hu, H.; Nordlander, P.; Halas, N.J. Monolithic Metal Dimer-on-Film Structure: New Plasmonic Properties Introduced by the Underlying Metal. Nano Lett. 2020, 20, 2087–2093. [Google Scholar] [CrossRef]
- Bumajdad, A.; Madkour, M. Understanding the Superior Photocatalytic Activity of Noble Metals Modified Titania under UV and Visible Light Irradiation. Phys. Chem. Chem. Phys. 2014, 16, 7146–7158. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Song, J.; Lee, S.; Lee, Y.W.; Wi, D.H.; Goo, B.S.; Han, S.W. Hierarchical Metal–Semiconductor–Graphene Ternary Heteronanostructures for Plasmon-Enhanced Wide-Range Visible-Light Photocatalysis. J. Mater. Chem. A 2019, 7, 15831–15840. [Google Scholar] [CrossRef]
- Adly, M.S.; El-Dafrawy, S.M.; El-Hakam, S.A. Application of Nanostructured Graphene Oxide/Titanium Dioxide Composites for Photocatalytic Degradation of Rhodamine B and Acid Green 25 Dyes. J. Mater. Res. Technol. 2019, 8, 5610–5622. [Google Scholar] [CrossRef]
- Lv, N.; Li, Y.; Huang, Z.; Li, T.; Ye, S.; Dionysiou, D.D.; Song, X. Synthesis of GO/TiO2/Bi2WO6 Nanocomposites with Enhanced Visible Light Photocatalytic Degradation of Ethylene. Appl. Catal. B Environ. 2019, 246, 303–311. [Google Scholar] [CrossRef]


| Parameter | Values |
|---|---|
| Thickness | 3.4–7 nm |
| Carbon Purity | ~99% |
| Number of Layers | The average number of layers: 6–10 |
| Surface Area (BET) | 100–300 m2/g |
| Bulk Density | 1 g/cc |
| Lateral dimension | 10–50 μm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajipour, P.; Bahrami, A.; Mehr, M.Y.; van Driel, W.D.; Zhang, K. Facile Synthesis of Ag Nanowire/TiO2 and Ag Nanowire/TiO2/GO Nanocomposites for Photocatalytic Degradation of Rhodamine B. Materials 2021, 14, 763. https://doi.org/10.3390/ma14040763
Hajipour P, Bahrami A, Mehr MY, van Driel WD, Zhang K. Facile Synthesis of Ag Nanowire/TiO2 and Ag Nanowire/TiO2/GO Nanocomposites for Photocatalytic Degradation of Rhodamine B. Materials. 2021; 14(4):763. https://doi.org/10.3390/ma14040763
Chicago/Turabian StyleHajipour, Pejman, Abbas Bahrami, Maryam Yazdan Mehr, Willem Dirk van Driel, and Kouchi Zhang. 2021. "Facile Synthesis of Ag Nanowire/TiO2 and Ag Nanowire/TiO2/GO Nanocomposites for Photocatalytic Degradation of Rhodamine B" Materials 14, no. 4: 763. https://doi.org/10.3390/ma14040763
APA StyleHajipour, P., Bahrami, A., Mehr, M. Y., van Driel, W. D., & Zhang, K. (2021). Facile Synthesis of Ag Nanowire/TiO2 and Ag Nanowire/TiO2/GO Nanocomposites for Photocatalytic Degradation of Rhodamine B. Materials, 14(4), 763. https://doi.org/10.3390/ma14040763






