Resistance of Soda Residue–Fly Ash Based Geopolymer Mortar to Acid and Sulfate Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of Geopolymer Mortars
2.3. Testing Methods
2.3.1. Determination of Fresh and Physical Properties
2.3.2. Determination of Long-Term Mechanical Properties
2.3.3. Characterization of Acid and Sulfate Attack
2.3.4. Investigation into Attack Mechanisms through SEM–EDS and FTIR
3. Results and Discussion
3.1. Long-Term Physical Properties of SR–FFA–GEO Mortars
3.2. Long-Term Mechanical Properties of SR–FFA–GEO Mortars
3.3. Strength and Mass Changes of SR–FFA–GEO Mortar under Water Environment
3.4. Strength and Mass Losses of SR–FFA–GEO Mortar under HCl and Na2SO4 Attack
3.5. Microstructures and Gel Products under HCl and Na2SO4 Attack through SEM–EDS Analysis
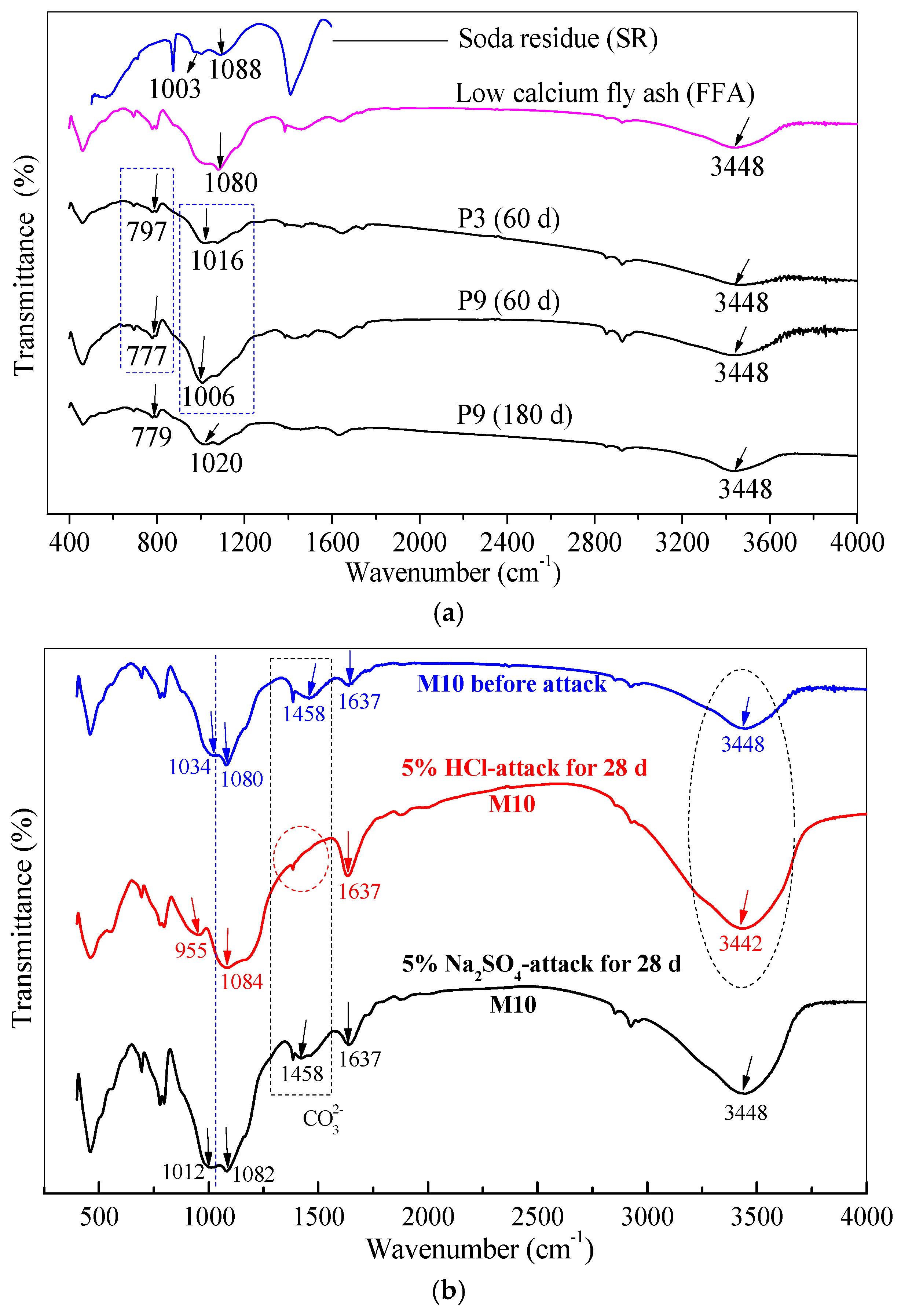
3.6. Chemical Bonds of Gel Products under HCl and Na2SO4 Attack through FTIR Analysis
4. Conclusions
- (1)
- The compressive strengths (19.3 MPa) of soda residue/low-calcium-fly-ash-based geopolymer mortar with 20% soda residue are stable at 360 days old, when cured at room temperature. In particular, the compressive strength, porosity and shrinkage are influenced more by the addition percentage of soda residue. Moreover, the geopolymer mortar with 20% soda residue (with low water absorption of 1.8%) keeps stable in compressive strength and mass loss under water environment for 360 d.
- (2)
- The soda residue/low-calcium-fly-ash-based geopolymer mortar with 20% soda residue cured for 360 d at room temperature is recommended to investigate the resistance to acid and sulfate attack owing to the better stability with the exclusion of internal hydration.
- (3)
- Under 5% HCl solution attack for 28 d, the mass loss of the geopolymer mortar with 20% soda residue reaches 5.82%, and the compressive strength loss is 47.2%. However, under 5% Na2SO4 solution attack for 28 d, there is no compressive strength and mass losses for the geopolymer mortar with 20% soda residue. Therefore, the geopolymer mortar with 20% soda residue possesses the superior resistance to Na2SO4 attack, as well as the better resistance to HCl attack than that of ordinary Portland cement material.
- (4)
- From the SEM–EDS and FTIR analysis, the calcites from soda residue cause the chemical reaction with the environmental HCl to produce some CO2 gas, which leads to the losses in compressive strength and mass under HCl attack. Thus, the attack mechanisms of HCl solution are derived from the addition of soda residue. Moreover, more Na+ cations entering the Si-O-Al structure make soda residue/low-calcium-fly-ash-based geopolymer mortar obtain the superior resistance to Na2SO4 attack without compressive strength loss and mass loss.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tho-In, T.; Sata, V.; Boonserm, K.; Chindaprasirt, P. Compressive strength and microstructure analysis of geopolymer paste using waste glass powder and fly ash. J. Clean. Prod. 2018, 172, 2892–2898. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly ash-based geopolymer: Clean production, properties and applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Hu, W.; Nie, Q.; Huang, B.; Shu, X.; He, Q. Mechanical and microstructural characterization of geopolymers derived from red mud and fly ashes. J. Clean. Prod. 2018, 186, 799–806. [Google Scholar] [CrossRef]
- Assi, L.; Carter, K.; Deaver, E.; Anay, R.; Ziehl, P. Sustainable concrete: Building a greener future. J. Clean. Prod. 2018, 198, 1641–1651. [Google Scholar] [CrossRef]
- Nematollahi, B.; Sanjayan, J.; Shaikh, F.U.A. Synthesis of heat and ambient cured one-part geopolymer mixes with different grades of sodium silicate. Ceram. Int. 2015, 41, 5696–5704. [Google Scholar] [CrossRef]
- Roviello, G.; Ricciotti, L.; Tarallo, O.; Ferone, C.; Colangelo, F.; Roviello, V.; Cioffi, R. Innovative Fly Ash Geopolymer-Epoxy Composites: Preparation, Microstructure and Mechanical Properties. Materials 2016, 9, 461. [Google Scholar] [CrossRef] [PubMed]
- Castel, A.; Foster, S.J. Bond strength between blended slag and Class F fly ash geopolymer concrete with steel reinforcement. Cem. Concr. Res. 2015, 72, 48–53. [Google Scholar] [CrossRef]
- Sarker, P.K.; McBeath, S. Fire endurance of steel reinforced fly ash geopolymer concrete elements. Constr. Build. Mater. 2015, 90, 91–98. [Google Scholar] [CrossRef]
- Hashimoto, S.; Machino, T.; Ando, K.; Daiko, Y.; Honda, S.; Iwamoto, Y. Hot sulfuric acid-resistance of fly-ash-based geopolymer paste product due to the precipitation of natroalunite crystals. Constr. Build. Mater. 2017, 151, 714–719. [Google Scholar] [CrossRef]
- Colangelo, F.; Cioffi, R.; Roviello, G.; Capasso, I.; Caputo, D.; Aprea, P.; Liguori, B.; Ferone, C. Thermal cycling stability of fly ash based geopolymer mortars. Compos. Part. B-Eng. 2017, 129, 11–17. [Google Scholar] [CrossRef]
- Pasupathy, K.; Berndt, M.; Sanjayan, J.; Rajeev, P.; Cheema, D.S. Durability of low-calcium fly ash based geopolymer concrete culvert in a saline environment. Cem. Concr. Res. 2017, 100, 297–310. [Google Scholar] [CrossRef]
- Saha, S.; Rajasekaran, C. Enhancement of the properties of fly ash based geopolymer paste by incorporating ground granulated blast furnace slag. Constr. Build. Mater. 2017, 146, 615–620. [Google Scholar] [CrossRef]
- Gorhan, G.; Kurklu, G. The influence of the NaOH solution on the properties of the fly ash-based geopolymer mortar cured at different temperatures. Compos. Part. B Eng. 2014, 58, 371–377. [Google Scholar] [CrossRef]
- Xu, F.; Deng, X.; Peng, C.; Zhu, J.; Chen, J. Mix design and flexural toughness of PVA fiber reinforced fly ash-geopolymer composites. Constr. Build. Mater. 2017, 150, 179–189. [Google Scholar] [CrossRef]
- Swanepoel, J.C.; Strydom, C.A. Utilisation of fly ash in a geopolymeric material. Appl. Geochem. 2002, 17, 1143–1148. [Google Scholar] [CrossRef]
- Pavithra, P.; Reddy, M.S.; Dinakar, P.; Rao, B.H.; Satpathy, B.K.; Mohanty, A.N. A mix design procedure for geopolymer concrete with fly ash. J. Clean. Prod. 2016, 133, 117–125. [Google Scholar] [CrossRef]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P.; Chindaprasirt, P. NaOH-activated ground fly ash geopolymer cured at ambient temperature. Fuel 2011, 90, 2118–2124. [Google Scholar] [CrossRef]
- Fahim Huseien, G.; Mirza, J.; Ismail, M.; Ghoshal, S.K.; Abdulameer Hussein, A. Geopolymer mortars as sustainable repair material: A comprehensive review. Renew. Sust. Energ. Rev. 2017, 80, 54–74. [Google Scholar] [CrossRef]
- Bakharev, T. Geopolymeric materials prepared using Class F fly ash and elevated temperature curing. Cem. Concre. Res. 2005, 35, 1224–1232. [Google Scholar] [CrossRef]
- Hadi, M.N.S.; Al-Azzawi, M.; Yu, T. Effects of fly ash characteristics and alkaline activator components on compressive strength of fly ash-based geopolymer mortar. Constr. Build. Mater. 2018, 175, 41–54. [Google Scholar] [CrossRef]
- Li, B.J.; Li, G.Z. Study on mechanical properties of soda residue/fly ash composite cementitious material. Adv. Mater. Res.-Switz. 2011, 194–196, 1026–1029. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C.; Zuo, L.; Zhu, Q.; Ma, W.; Liu, Y. Preparation and characterization of press-formed fly ash cement incorporating soda residue. Mater. Lett. 2020, 259, 126852. [Google Scholar] [CrossRef]
- Liu, Y.L.; Wang, Y.S.; Fang, G.H.; Alrefaei, Y.; Dong, B.Q.; Xing, F. A preliminary study on capsule-based self-healing grouting materials for grouted splice sleeve connection. Constr. Build. Mater. 2018, 170, 418–423. [Google Scholar] [CrossRef]
- Nie, Q.; Hu, W.; Huang, B.; Shu, X.; He, Q. Synergistic utilization of red mud for flue-gas desulfurization and fly ash-based geopolymer preparation. J. Hazard. Mater. 2019, 369, 503–511. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C.; Zuo, L.; Wang, L.; Zhu, Q.; Liu, Y.; Zhou, B. Synthesis and characterization of fly ash geopolymer paste for goaf backfill: Reuse of soda residue. J. Clean. Prod. 2020, 260, 121045. [Google Scholar] [CrossRef]
- Zhao, X.H.; Liu, C.Y.; Zuo, L.M.; Wang, L.; Zhu, Q.; Wang, M.K. Investigation into the effect of calcium on the existence form of geopolymerized gel product of fly ash based geopolymers. Cem. Concr. Comp. 2019, 103, 279–292. [Google Scholar] [CrossRef]
- Zhao, X.H.; Liu, C.Y.; Wang, L.; Zuo, L.M.; Zhu, Q.; Ma, W. Physical and mechanical properties and micro characteristics of fly ash-based geopolymers incorporating soda residue. Cem. Concr. Comp. 2019, 98, 125–136. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, L.; Ma, G.; Zhao, X.; Zhao, X. Preparation and properties of bio-geopolymer composites with waste cotton stalk materials. J. Clean. Prod. 2020, 245, 118842. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; Provis, J.L.; van Deventer, J.S.J. Effect of calcium silicate sources on geopolymerisation. Cem. Concr. Res. 2008, 38, 554–564. [Google Scholar] [CrossRef]
- Temuujin, J.; Williams, R.P.; van Riessen, A. Effect of mechanical activation of fly ash on the properties of geopolymer cured at ambient temperature. J. Mater. Process. Tech. 2009, 209, 5276–5280. [Google Scholar] [CrossRef]
- Temuujin, J.; van Riessen, A.; Williams, R. Influence of calcium compounds on the mechanical properties of fly ash geopolymer pastes. J. Hazard. Mater. 2009, 167, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Song, R.J.; Zhao, Q.X.; Zhang, J.R.; Liu, J.Z. Microstructure and composition of hardened paste of soda residue-slag-cement binding material system. Front. Mater. 2019, 6, 211. [Google Scholar] [CrossRef]
- Yu, S.J.; Bi, W.Y. Utilization of industrial soda residue in highway construction. Prog. Environ. Sci. Tech. 2007, 1, 1332–1336. [Google Scholar]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- GB/T 17671-1999. Method of Testing Cements—Determinationof Strength (Idt ISO 679: 1989). 1999. Available online: http://www.cssn.net.cn/cssn/front/4311243.html (accessed on 4 February 2021). (In Chinese).
- Chen, C.; Gong, W.L.; Lutze, W.; Pegg, I.L.; Zhai, J.P. Kinetics of fly ash leaching in strongly alkaline solutions. J. Mater. Sci. 2011, 46, 590–597. [Google Scholar] [CrossRef]
- Nie, Y.; Ma, H.; Yang, J.; Su, Y.; Li, R.; Gao, F. Mechanism of polymerization during the solidification of fly ash-based geopolymers. Geoscience 2006, 20, 340–346. (In Chinese) [Google Scholar]
- Mehta, A.; Siddique, R. Sulfuric acid resistance of fly ash based geopolymer concrete. Constr. Build. Mater. 2017, 146, 136–143. [Google Scholar] [CrossRef]
- GB/T 2419-2005. Test Method for Fluidity of Cement Mortar. 2005. Available online: http://www.cssn.net.cn/cssn/front/6824280.html (accessed on 4 February 2021). (In Chinese).
- Wongsa, A.; Boonserm, K.; Waisurasingha, C.; Sata, V.; Chindaprasirt, P. Use of municipal solid waste incinerator (MSWI) bottom ash in high calcium fly ash geopolymer matrix. J. Clean. Prod. 2017, 148, 49–59. [Google Scholar] [CrossRef]
- Graytee, A.; Sanjayan, J.G.; Nazari, A. Development of a high strength fly ash-based geopolymer in short time by using microwave curing. Ceram. Int. 2018, 44, 8216–8222. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, X.; Liu, Y.; Zhao, Y.; Chen, Z.; Zhang, Y.; Guo, L.; Shu, X.; Liu, J. Hydration kinetics, pore structure, 3D network calcium silicate hydrate, and mechanical behavior of graphene oxide reinforced cement composites. Constr. Build. Mater. 2018, 190, 150–163. [Google Scholar] [CrossRef]
- Cwirzen, A.; Provis, J.L.; Penttala, V.; Habermehl-Cwirzen, K. The effect of limestone on sodium hydroxide-activated metakaolin-based geopolymers. Constr. Build. Mater. 2014, 66, 53–62. [Google Scholar] [CrossRef]
- Vafaei, M.; Allahverdi, A.; Dong, P. Acid attack on geopolymer cement mortar based on waste-glass powderand calcium aluminate cement at mild concentration. Constr. Build. Mater. 2018, 193, 363–372. [Google Scholar] [CrossRef]
- Bakharev, T. Durability of geopolymer materials in sodium and magnesium sulfate solutions. Cem. Concr. Res. 2005, 35, 1233–1246. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, D.; Zhou, W.; Lu, H.; Wang, L. Study on sulfate-resistance of fly ash-based geopolymers. New Build. Mater. 2008, 7, 41–44. (In Chinese) [Google Scholar]
- Garcia-Lodeiro, I.; Palomo, A.; Fernandez-Jimenez, A.; Macphee, D.E. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O-CaO-Al2O3-SiO2-H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Tchakoute, H.K.; Ruscher, C.H.; Djobo, J.N.Y.; Kenne, B.B.D.; Njopwouo, D. Influence of gibbsite and quartz in kaolin on the properties of metakaolin-based geopolymer cements. Appl. Clay Sci. 2015, 107, 188–194. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, X.; Zhou, B.; Lin, Y.; Gao, H. Performance Optimization and Characterization of Soda Residue-Fly Ash Geopolymer Paste for Goaf Backfill: Beta-Hemihydrate Gypsum Alternative to Sodium Silicate. Materials 2020, 13, 5604. [Google Scholar] [CrossRef]
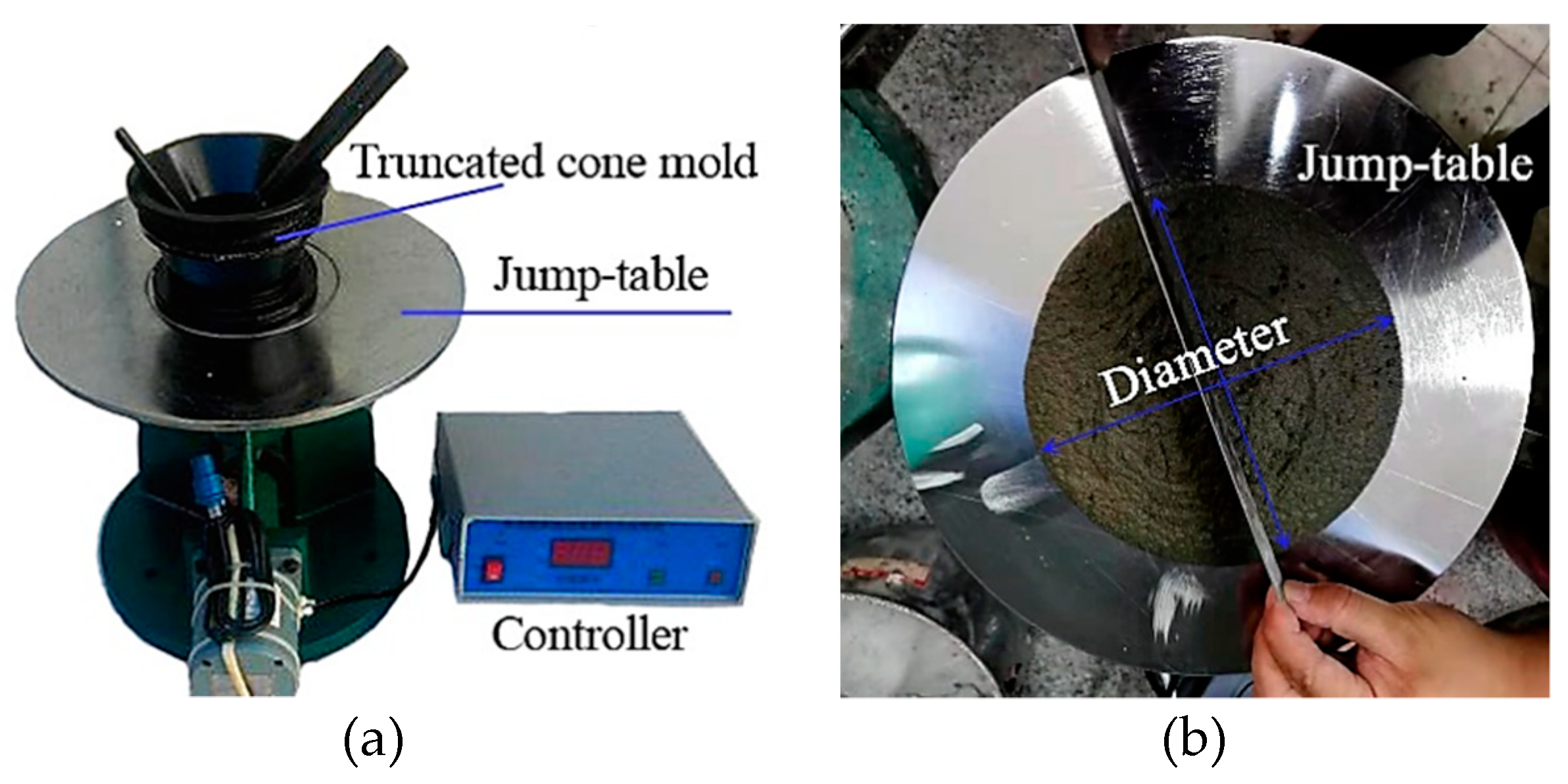
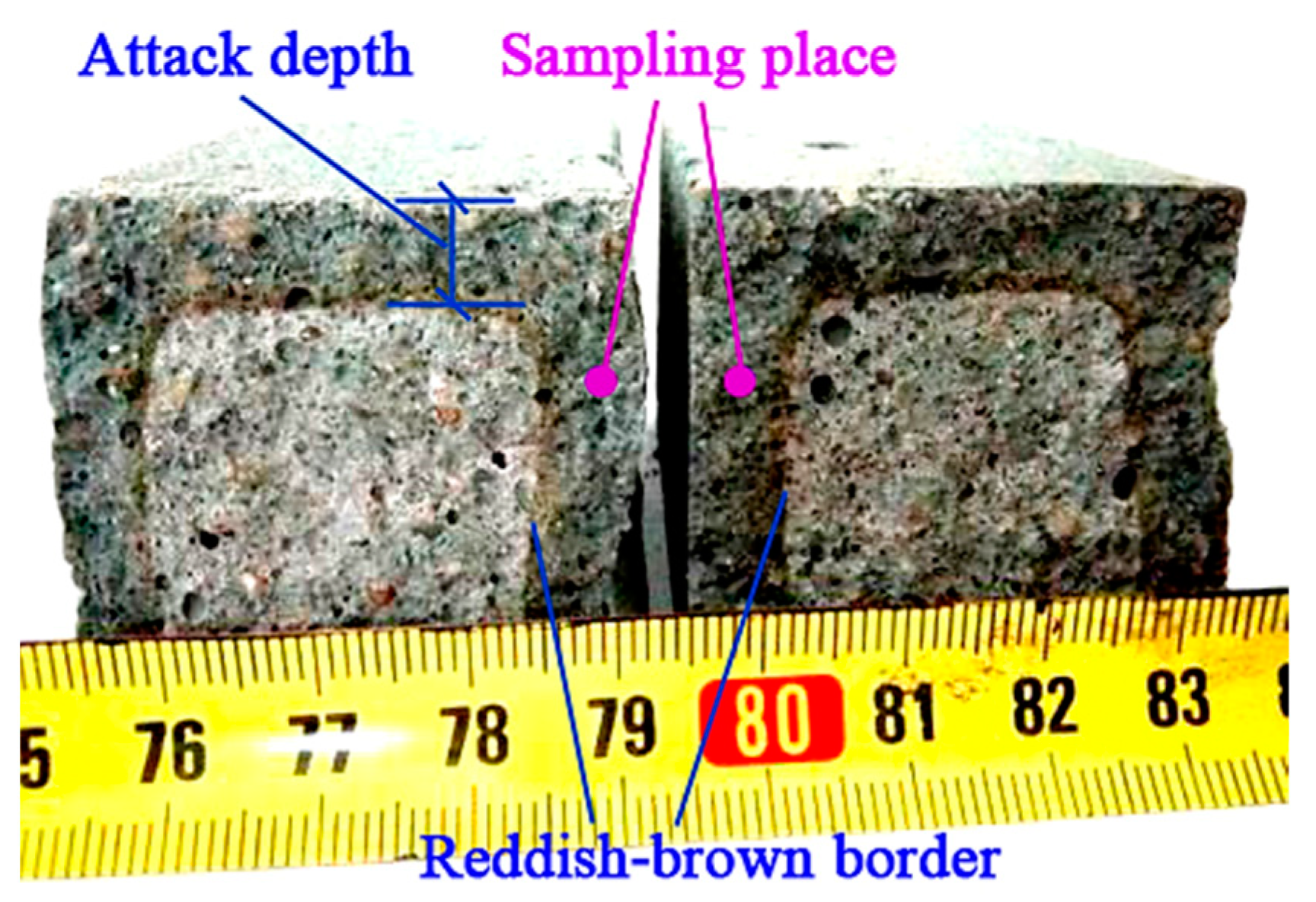
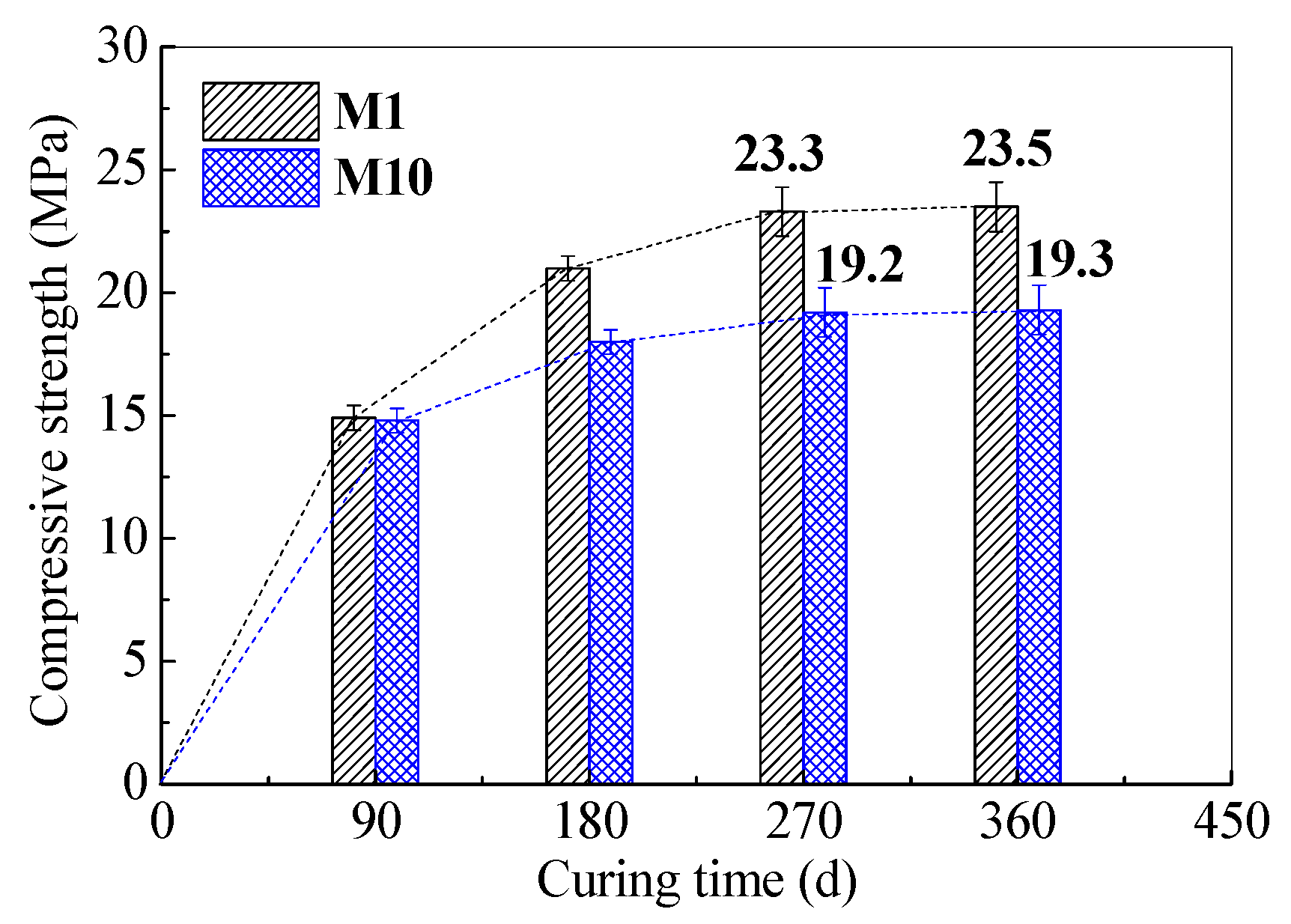



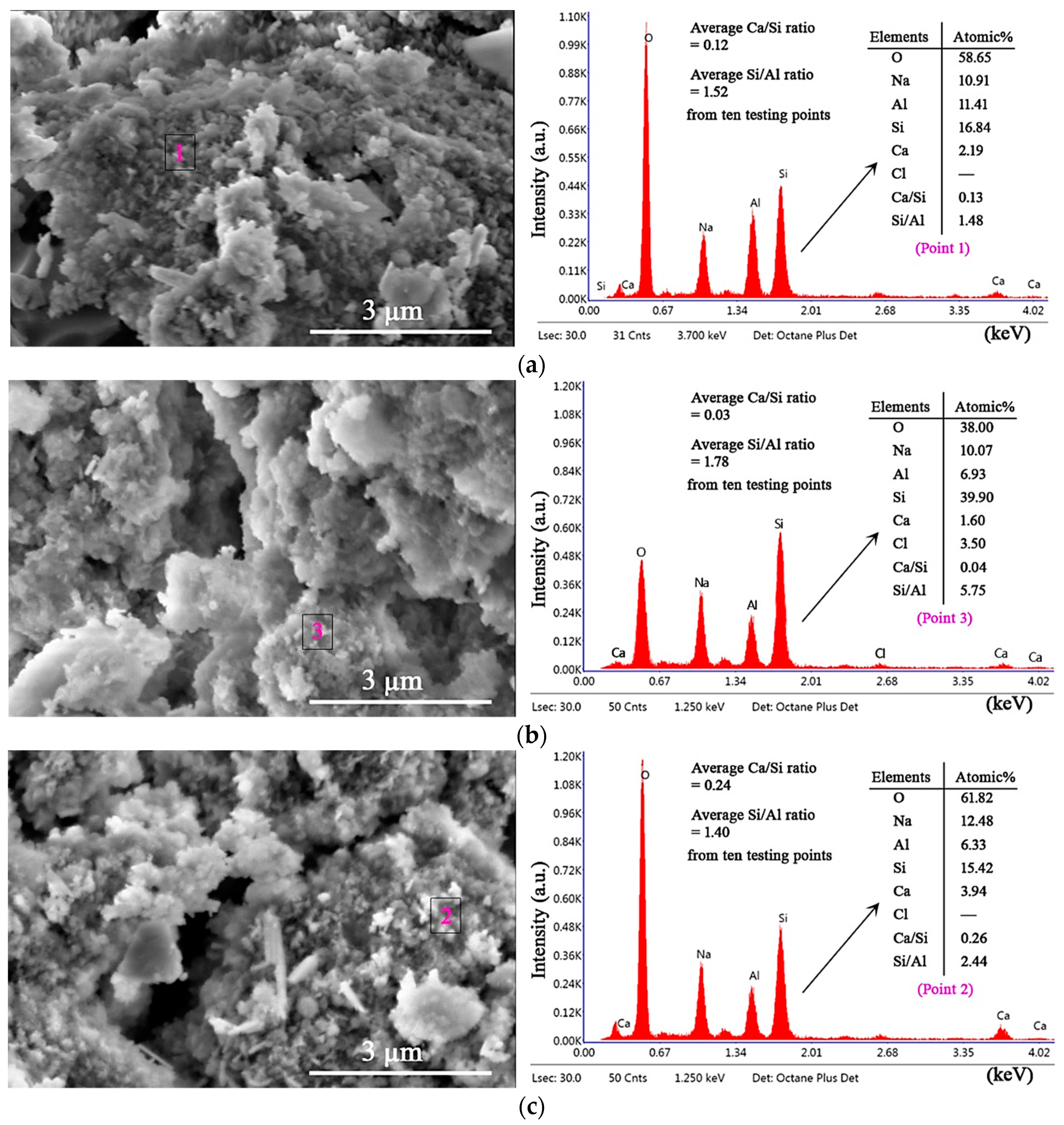
| Materials | Chemical Composites and Mass Percentages 1 | |||||||
|---|---|---|---|---|---|---|---|---|
| Soda residue (SR) | CaCO3 | Ca(OH)2 | CaCl2 | CaSO4 | NaCl | SiO2 | Al2O3 | Acid Insoluble |
| Percentage (%) | 64.0 | 10.0 | 6.0 | 3.0 | 4.0 | 3.0 | 2.0 | 8.0 |
| Low-calcium fly ash (FFA) | SiO2 | Al2O3 | Fe2O3 | CaO | FeO | MgO | LOI 2 | Others |
| Percentage (%) | 51.20 | 25.32 | 7.80 | 5.32 | 2.20 | 1.80 | 3.05 | 3.31 |
| Solid Powders | Specific Surface Area (m2/kg) | pH Value at w100 1 | Amount Passing #325 Sieve | Mean Particle Size (mm) | Specific Gravity 2 |
|---|---|---|---|---|---|
| SR | — | 8.35 | 24% | 0.25 | 2.35 |
| FFA | 510 | 5.90 | 76% | — | 2.44 |
| No. | FFA (g) | SR (g) | SR Content | Standard Stand (g) | NaOH Solution (mol/L) | Na/Si Ratio | Al/Si Ratio | Ca/Si Ratio |
|---|---|---|---|---|---|---|---|---|
| M 1 | 450 | 0 | 0.0% | 1350 | 8 | 0.54 | 0.57 | 0.11 |
| M 2 | 440 | 10 | 2.2% | 1350 | 8 | 0.55 | 0.57 | 0.12 |
| M 3 | 430 | 20 | 4.4% | 1350 | 8 | 0.57 | 0.57 | 0.12 |
| M 4 | 420 | 30 | 6.7% | 1350 | 8 | 0.58 | 0.57 | 0.13 |
| M 5 | 410 | 40 | 8.9% | 1350 | 8 | 0.59 | 0.57 | 0.13 |
| M 6 | 400 | 50 | 11.1% | 1350 | 8 | 0.61 | 0.57 | 0.14 |
| M 7 | 390 | 60 | 13.3% | 1350 | 8 | 0.63 | 0.57 | 0.15 |
| M 8 | 380 | 70 | 15.6% | 1350 | 8 | 0.64 | 0.57 | 0.15 |
| M 9 | 370 | 80 | 17.8% | 1350 | 8 | 0.66 | 0.57 | 0.16 |
| M10 | 360 | 90 | 20.0% | 1350 | 8 | 0.68 | 0.57 | 0.17 |
| No. | SR Content | Fluidity (mm) | Bulk Density (g/cm3) | Difference Percentage for 150~360 d | |||
|---|---|---|---|---|---|---|---|
| 150 d 1 | Difference | 360 d | Difference | ||||
| M 1 | 0.0% | 184 | 2.145 | ±0.017 | 2.143 | ±0.015 | 0.09% |
| M 2 | 2.2% | 181 | 2.135 | ±0.015 | 2.134 | ±0.013 | 0.05% |
| M 3 | 4.4% | 176 | 2.123 | ±0.023 | 2.12 | ±0.020 | 0.14% |
| M 4 | 6.7% | 171 | 2.117 | ±0.016 | 2.115 | ±0.014 | 0.09% |
| M 5 | 8.9% | 164 | 2.113 | ±0.020 | 2.111 | ±0.022 | 0.09% |
| M 6 | 11.1% | 158 | 2.107 | ±0.015 | 2.104 | ±0.017 | 0.14% |
| M 7 | 13.3% | 153 | 2.104 | ±0.025 | 2.101 | ±0.023 | 0.14% |
| M 8 | 15.6% | 152 | 2.101 | ±0.012 | 2.097 | ±0.012 | 0.19% |
| M 9 | 17.8% | 149 | 2.097 | ±0.014 | 2.093 | ±0.013 | 0.19% |
| M10 | 20.0% | 147 | 2.093 | ±0.010 | 2.091 | ±0.012 | 0.10% |
| No. | SR Content | 360 d Porosity | Initial Casting Height (mm) | Final Casting Height (mm) | Average Shrinkage for 90 d | Initial Casting Height (mm) | Final Casting Height (mm) | Average Shrinkage for 360 d |
|---|---|---|---|---|---|---|---|---|
| M 1 | 0.0% | 6.23% | 40.00 | 37.92 | −5.20% | 40.00 | 37.92 | −5.19% |
| M 3 | 4.4% | 8.34% | 40.00 | 38.42 | −3.96% | 40.00 | 38.42 | −3.97% |
| M 5 | 8.9% | 10.34% | 40.00 | 38.98 | −2.56% | 40.00 | 38.98 | −2.56% |
| M 7 | 13.3% | 11.67% | 40.00 | 39.54 | −1.15% | 40.00 | 39.56 | −1.09% |
| M 9 | 17.8% | 13.14% | 40.00 | 40.00 | 0.00% | 40.00 | 40.00 | 0.00% |
| M10 | 20.0% | 14.36% | 40.00 | 40.00 | 0.00% | 40.00 | 40.00 | 0.00% |
| No. | SR Content | Maximal Water Absorption at 7 h | Environmental pH Value | Immersion Age | Compressive Strength (MPa) | Mortar Mass (g) |
|---|---|---|---|---|---|---|
| M 1 | 0.0% | 1.5% | 11.345 | 0 d | 23.5 | 543 |
| 7 d | 23.6 | 541 | ||||
| 14 d | 23.6 | 540 | ||||
| 21 d | 23.7 | 540 | ||||
| 28 d | 23.7 | 540 | ||||
| M10 | 20.0% | 1.8% | 11.248 | 0 d | 19.3 | 540 |
| 7 d | 19.3 | 542 | ||||
| 14 d | 19.4 | 541 | ||||
| 21 d | 19.5 | 541 | ||||
| 28 d | 19.5 | 541 |
| Samples | SR Content | 5% HCl-Attack | 5% Na2SO4-Attack | ||
|---|---|---|---|---|---|
| Strength Loss | Mass Loss | Strength Loss | Mass Loss | ||
| M10 | 20% | 47.2% | 5.82% | 0.0% | 0.00% |
| OPC 1 | — | 85.2% | 40.97% | 5.8% | 6.83% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Wang, H.; Zhou, B.; Gao, H.; Lin, Y. Resistance of Soda Residue–Fly Ash Based Geopolymer Mortar to Acid and Sulfate Environments. Materials 2021, 14, 785. https://doi.org/10.3390/ma14040785
Zhao X, Wang H, Zhou B, Gao H, Lin Y. Resistance of Soda Residue–Fly Ash Based Geopolymer Mortar to Acid and Sulfate Environments. Materials. 2021; 14(4):785. https://doi.org/10.3390/ma14040785
Chicago/Turabian StyleZhao, Xianhui, Haoyu Wang, Boyu Zhou, Han Gao, and Yonghui Lin. 2021. "Resistance of Soda Residue–Fly Ash Based Geopolymer Mortar to Acid and Sulfate Environments" Materials 14, no. 4: 785. https://doi.org/10.3390/ma14040785
APA StyleZhao, X., Wang, H., Zhou, B., Gao, H., & Lin, Y. (2021). Resistance of Soda Residue–Fly Ash Based Geopolymer Mortar to Acid and Sulfate Environments. Materials, 14(4), 785. https://doi.org/10.3390/ma14040785









