Assessment of SnFe2O4 Nanoparticles for Potential Application in Theranostics: Synthesis, Characterization, In Vitro, and In Vivo Toxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SnFe2O4 NPs
2.3. Characterization
2.4. Cells and Culture Condition
2.5. In Vitro Cytotoxicity Evaluation
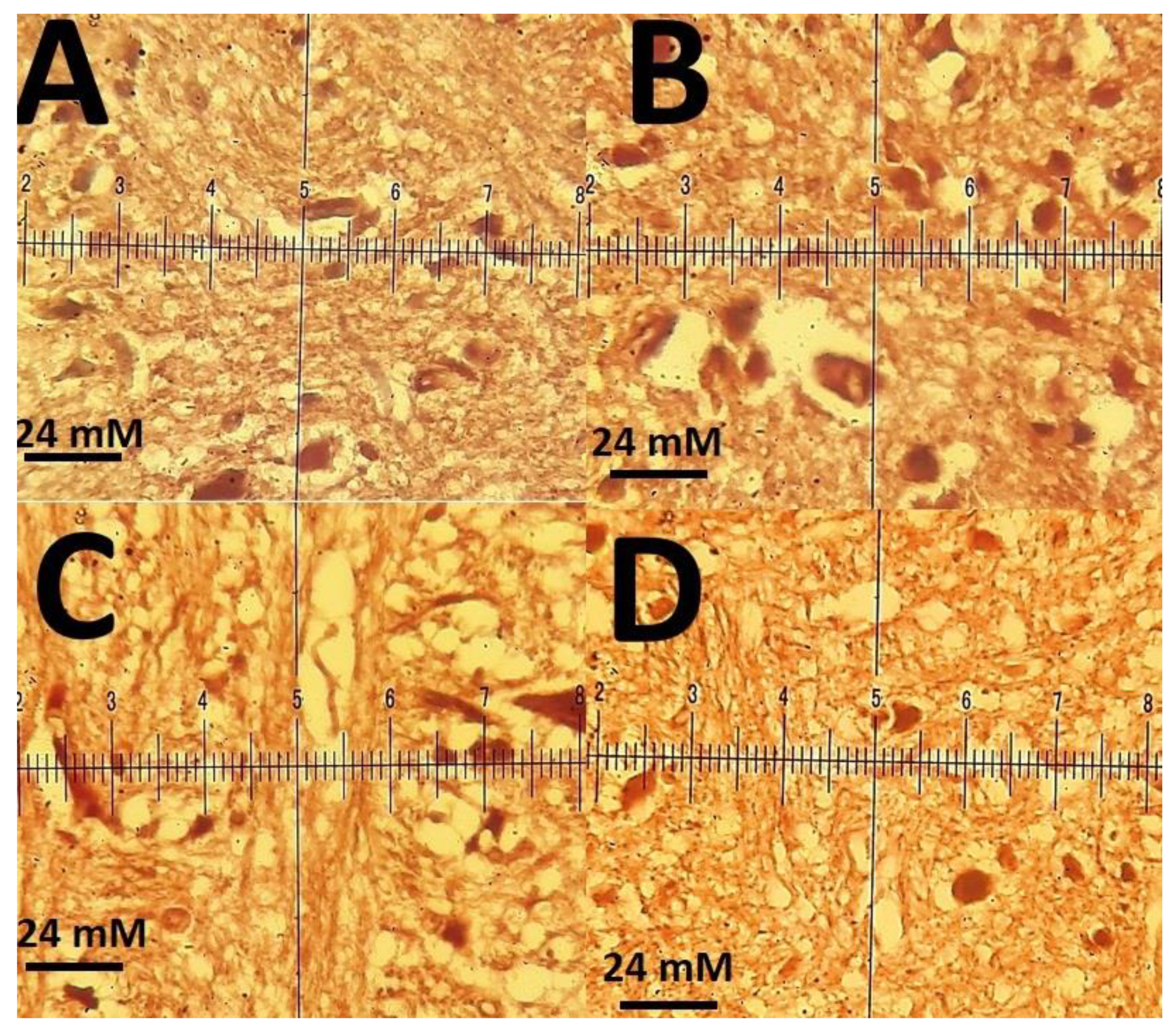
2.6. Animal Treatments and Experimental Design
2.7. Serum Biochemical Parameters
2.8. Statistical analysis
3. Results
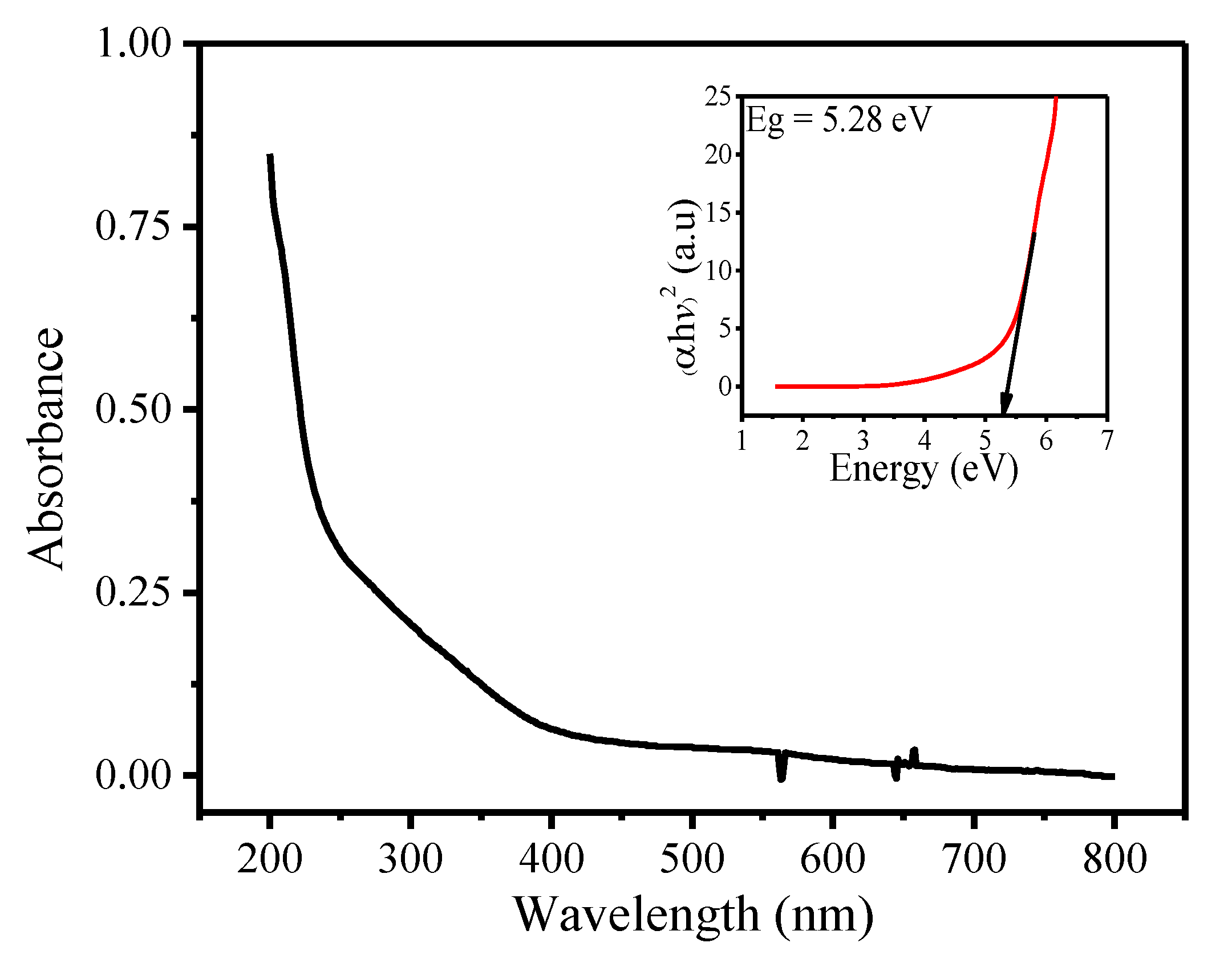
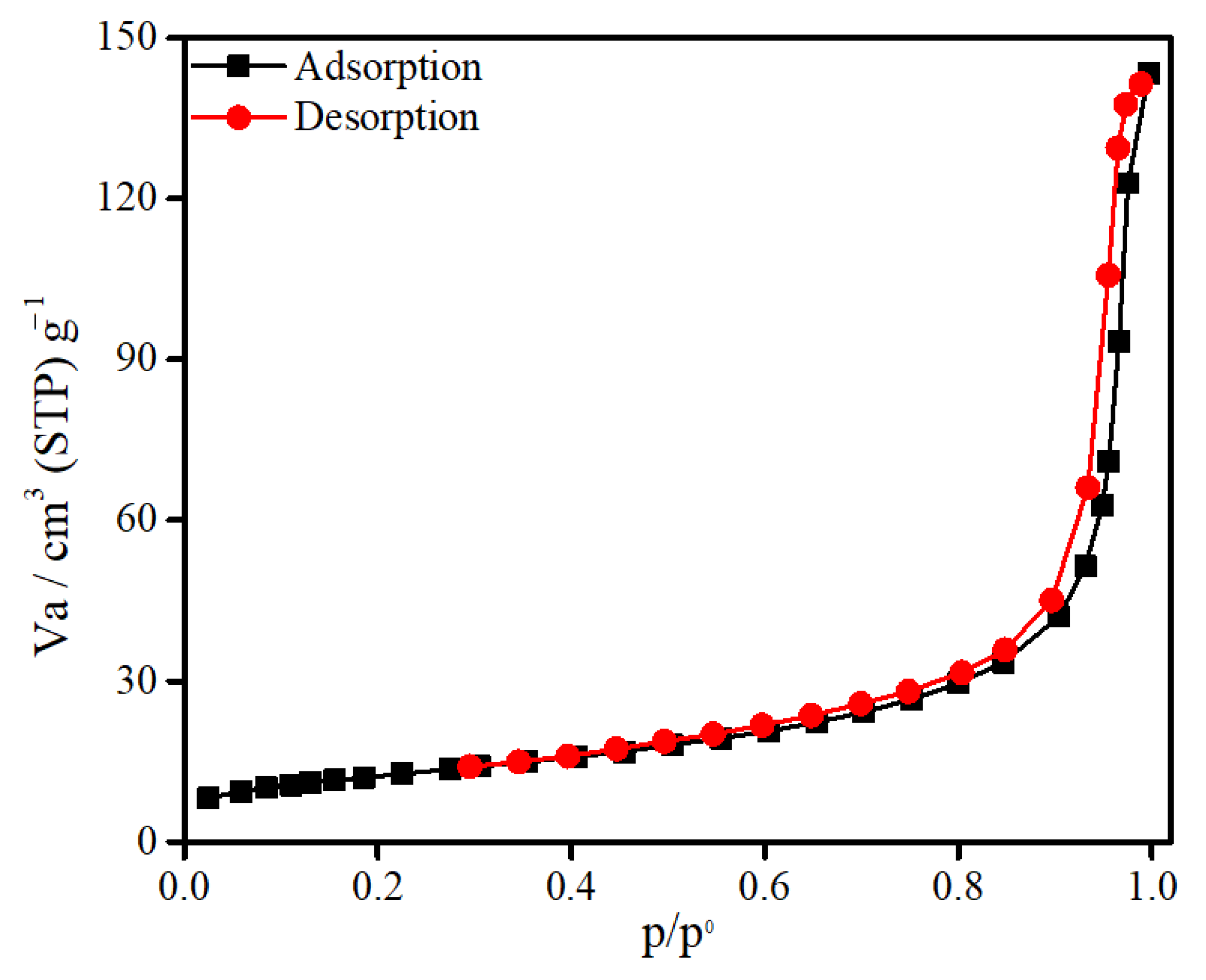
3.1. Characterization of SnFe2O4 NPs
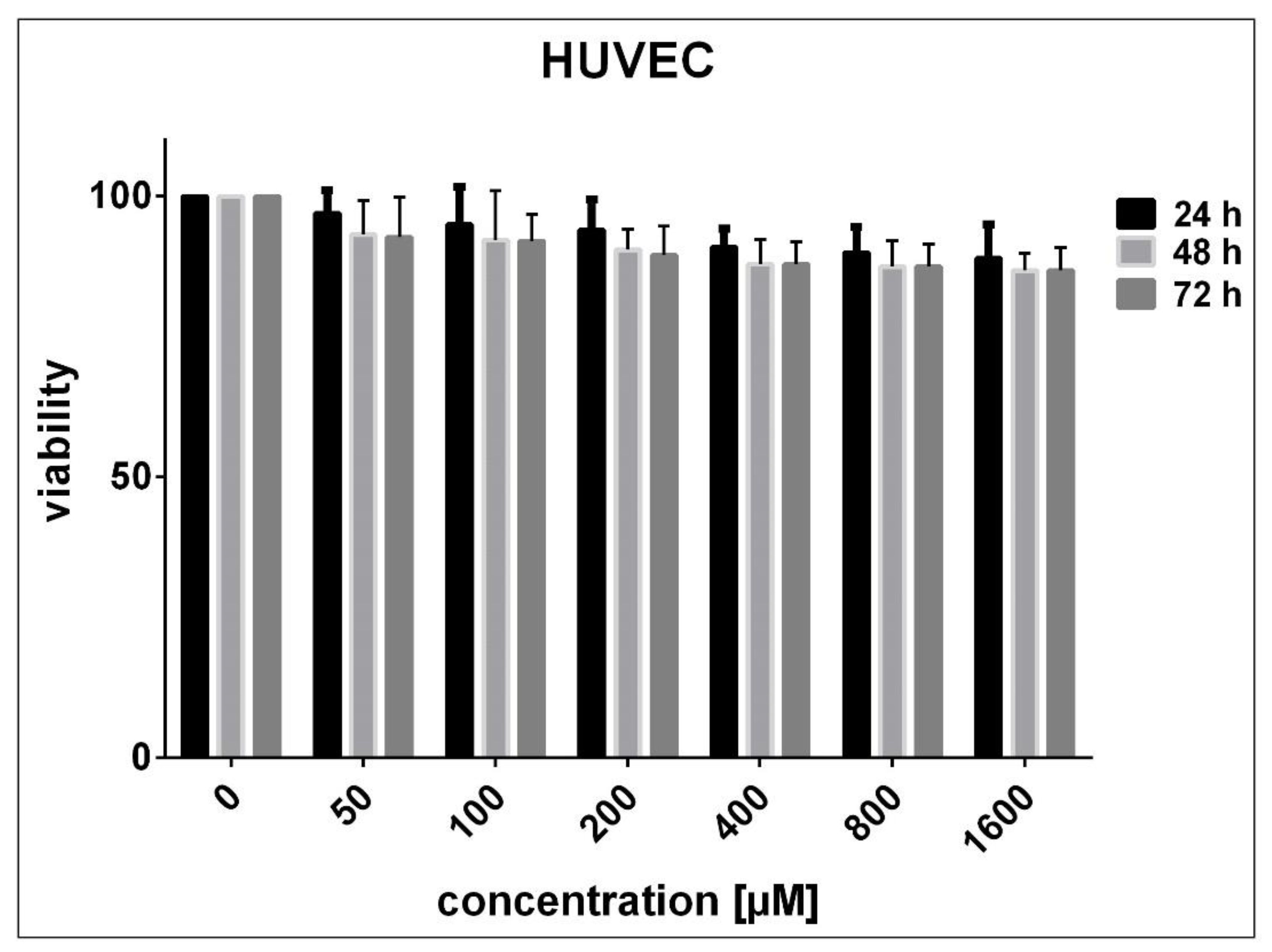
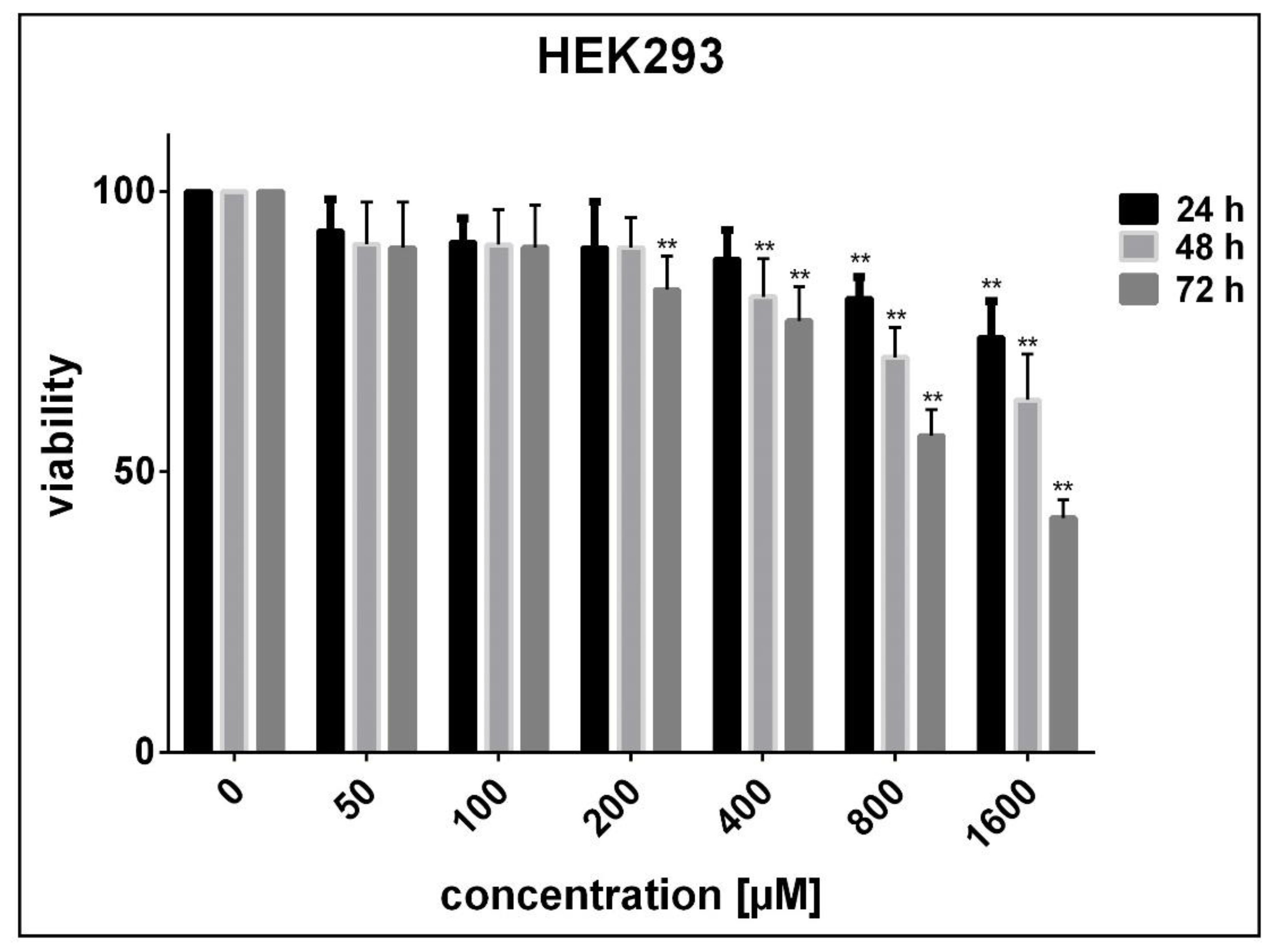
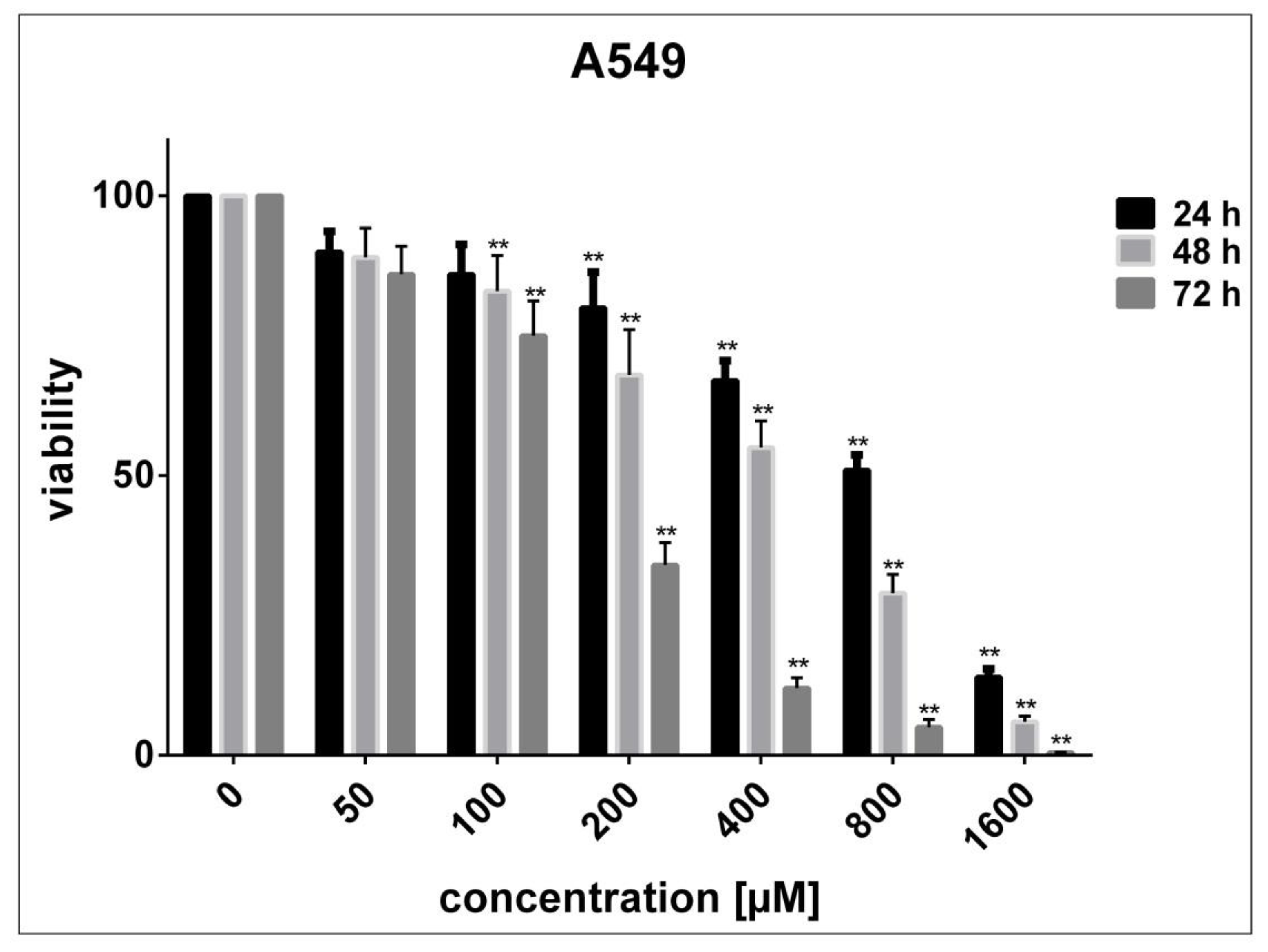
3.2. Determination of Cell Sensitivity to SnFe2O4 NPs
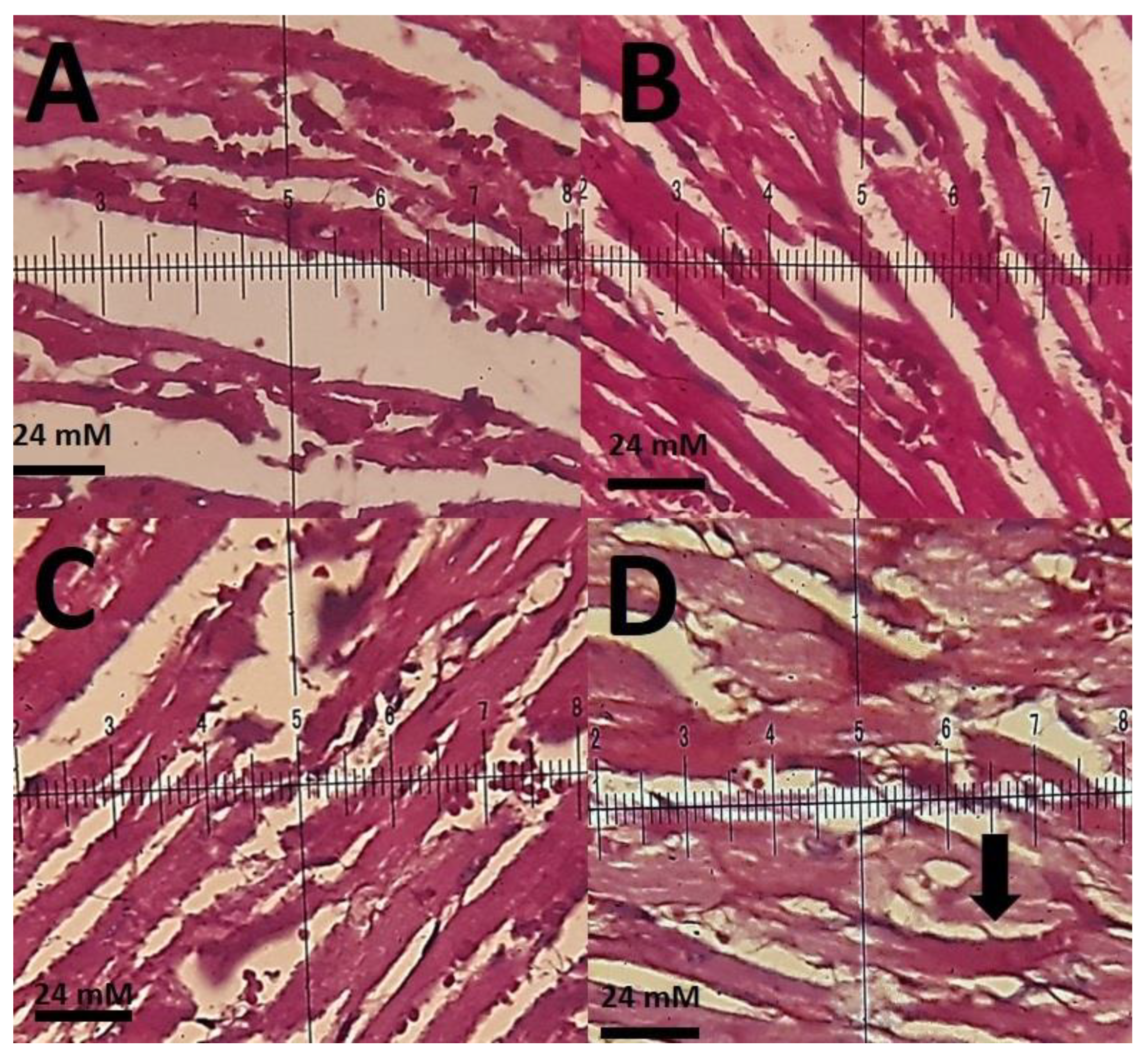
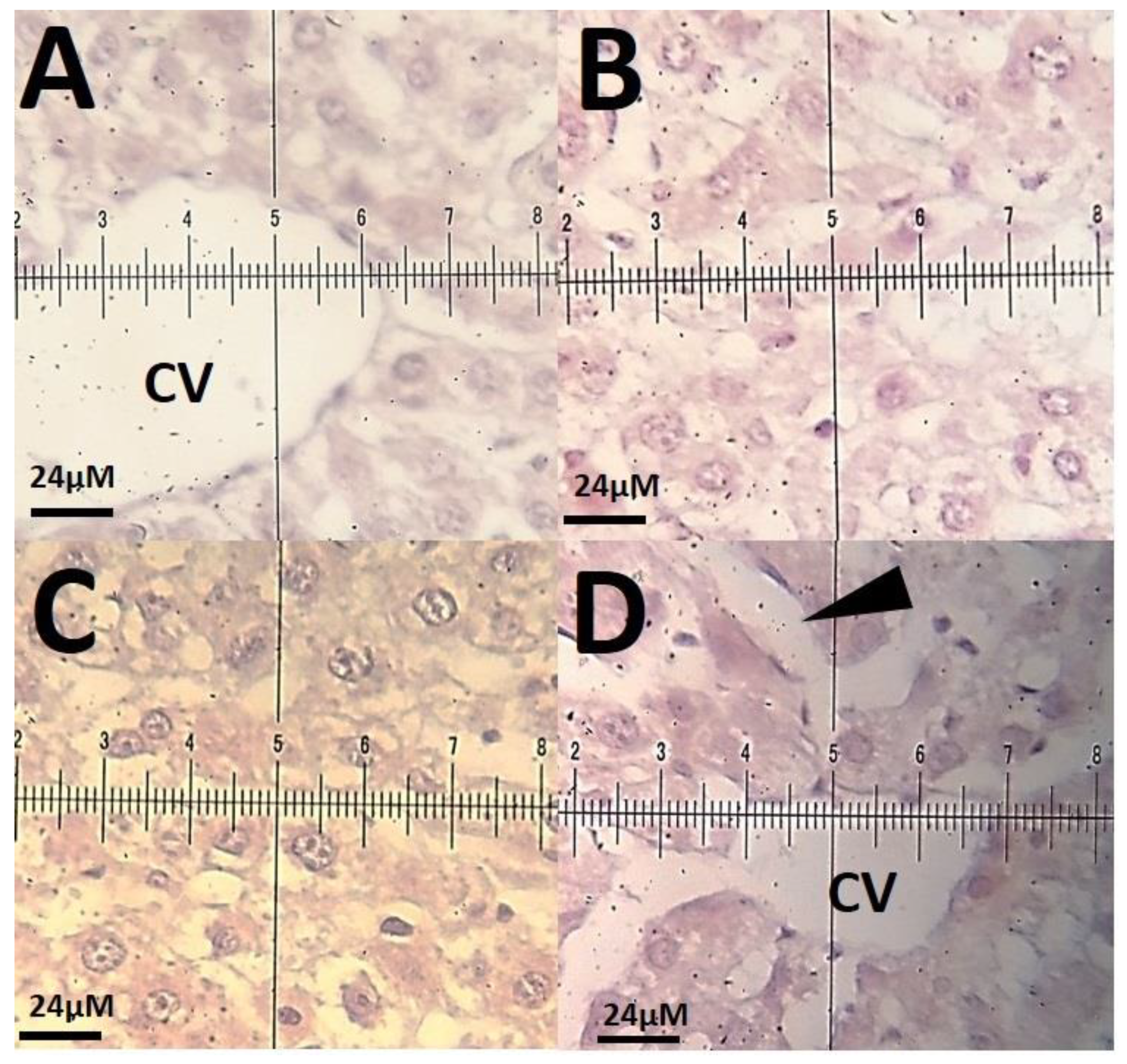
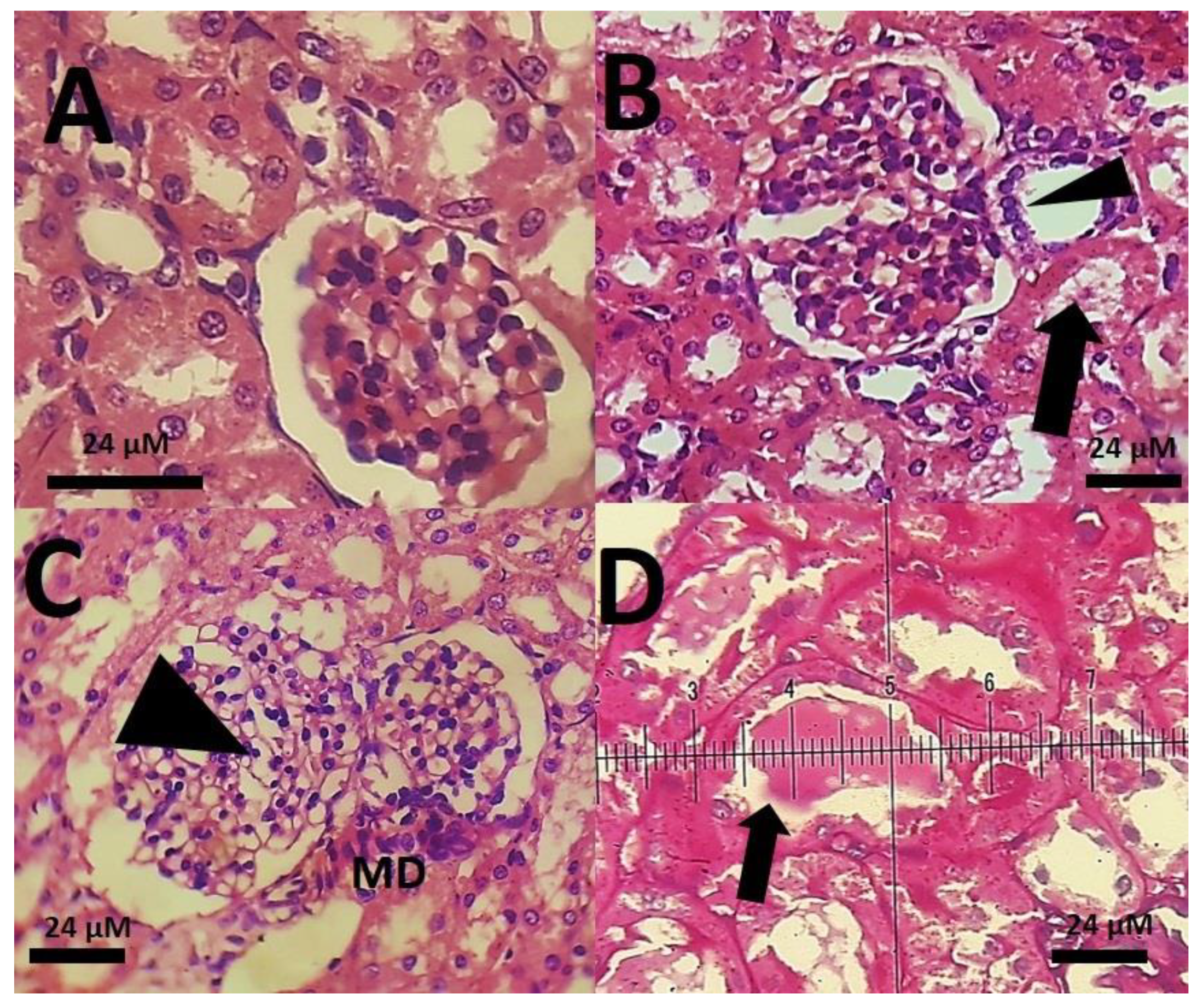
3.3. Biochemical Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farokhzad, O.C.; Langer, R. Nanomedicine: Developing smarter therapeutic and diagnostic modalities. Adv. Drug Deliv. Rev. 2006, 58, 1456–1459. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, F.; Chan, J.; Wang, A.; Langer, R.; Farokhzad, O. Nanoparticles in Medicine: Therapeutic Applications and Developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef]
- Ranji-Burachaloo, H.; Gurr, P.A.; Dunstan, D.E.; Qiao, G.G. Cancer Treatment through Nanoparticle-Facilitated Fenton Reaction. ACS Nano 2018, 12, 11819–11837. [Google Scholar] [CrossRef]
- Etminan, M.; Nabiyouni, G.; Ghanbari, D. Preparation of tin ferrite–tin oxide by hydrothermal, precipitation and auto-combustion: Photo-catalyst and magnetic nanocomposites for degradation of toxic azo-dyes. J. Mater. Sci. Mater. Electron. 2018, 29, 1766–1776. [Google Scholar] [CrossRef]
- Ahmad, I.; Shah, S.M.; Zafar, M.N.; Ullah, S.; Ul-Hamid, A.; Ashiq, M.N.; Jabeen, U.; Shafa, M.; Rahdar, A. Fabrication of highly resistive La–Zn co-substituted spinel strontium nanoferrites for high frequency devices applications. Mater. Chem. Phys. 2021, 259, 124031. [Google Scholar] [CrossRef]
- Lafta, S.H. Effect of pH on Structural, Magnetic and FMR Properties of Hydrothermally Prepared Nano Ni Ferrite. Open Chem. 2017, 15, 53–60. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Yao, G.; Zu, G.; Hao, Y. Synthesis and Characterization of NiFe2O4Nanoparticles via Solid-State Reaction. Int. J. Appl. Ceram. Technol. 2013, 10, 142–149. [Google Scholar] [CrossRef]
- Lee, K.-T.; Lu, Y.-J.; Mi, F.-L.; Burnouf, T.; Wei, Y.-T.; Chiu, S.-C.; Chuang, E.-Y.; Lu, S.-Y. Catalase-Modulated Heterogeneous Fenton Reaction for Selective Cancer Cell Eradication: SnFe2O4 Nanocrystals as an Effective Reagent for Treating Lung Cancer Cells. ACS Appl. Mater. Interfaces. 2017, 9, 1273–1279. [Google Scholar] [CrossRef]
- Agarwal, H.; Nakara, A.; Shanmugam, V.K. Anti-inflammatory mechanism of various metal and metal oxide nanoparticles synthesized using plant extracts: A review. Biomed. Pharmacother. 2019, 109, 2561–2572. [Google Scholar] [CrossRef]
- Liu, W.; Zeb, A.; Lian, J.; Wu, J.; Xiong, H.; Tang, J.; Zheng, S. Interactions of metal-based nanoparticles (MBNPs) and metal-oxide nanoparticles (MONPs) with crop plants: A critical review of research progress and prospects. Environ. Rev. 2020, 28, 294–310. [Google Scholar] [CrossRef]
- Elmoussaoui, H.; Hamedoun, M.; Mounkachi, O.; Benyoussef, A.; Masrour, R.; Hlil, E.K. New results on Magnetic Properties of Tin-Ferrite Nanoparticles. J. Supercond. Nov. Magn. 2012, 25, 1995–2002. [Google Scholar] [CrossRef]
- Sargazi, S.; Saravani, R.; Reza, J.Z.; Jaliani, H.Z.; Galavi, H.; Moudi, M.; Abtahi, N.A. Novel poly (adenosine di-phosphate-ribose) polymerase (PARP) inhibitor, AZD2461, down-regulates VEGF and induces apoptosis in prostate cancer cells. Iran Biomed. J. 2019, 23, 312–323. [Google Scholar] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cyto-toxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Zhou, H.; Xiao, L.; Luo, Y.; Chen, J.H.; Xu, J.H.; Zeng, Y.; Zhong, M.Z. Facile synthesis of monodispersed Fe2O3 nanoparticles and its cellular uptake and cytotoxicity studies. J. Nanosci. Nanotechnol. 2013, 136, 560–6565. [Google Scholar]
- Ramalingam, V.; Harshavardhan, M.; Dinesh Kumar, S.; Malathi devi, S. Wet chemical mediated hematite α-Fe2O3 nanoparticles synthesis: Preparation, characterization and anticancer activity against human meta-static ovarian cancer. J. Alloys Compd. 2020, 834, 155118. [Google Scholar] [CrossRef]
- Zerboni, A.; Bengalli, R.; Baeri, G.; Fiandra, L.; Catelani, T.; Mantecca, P. Mixture Effects of Diesel Exhaust and Metal Oxide Nanoparticles in Human Lung A549 Cells. Nanomaterials 2019, 9, 1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Yu, H.; Ding, D.; Chen, Z.; Wang, Y.; Wang, S.; Li, X.; Keidar, M.; Zhang, W. Cold atmospheric plasma and iron oxide-based magnetic nanoparticles for synergetic lung cancer therapy. Free. Radic. Biol. Med. 2019, 130, 71–81. [Google Scholar] [CrossRef]
- Ghasemi, A.; Jafari, S.; Saeidi, J.; Mohtashami, M.; Salehi, I. Synthesis and characterization of polyglycerol coated superparamagnetic iron oxide nanoparticles and cytotoxicity evaluation on normal human cell lines. Colloids Surf. A Physicochem. Eng. Asp. 2018, 551, 128–136. [Google Scholar] [CrossRef]
- Aliahmad, M.; Rahdar, A.; Sadeghfar, F.; Bagheri, S.; Hajinezhad, M.R. Synthesis and Biochemical effects of magnetite nanoparticle by surfactant-free electrochemical method in an aqueous system: The current density effect. Nanomed. Res. J. 2016, 1, 39–46. [Google Scholar]
- Rahdar, A.; Hajinezhad, M.R.; Sivasankarapillai, V.S.; Askari, F.; Noura, M.; Kyzas, G.Z. Synthesis, characteriza-tion, and intraperitoneal biochemical studies of zinc oxide nanoparticles in Rattus norvegicus. Appl. Phys. A 2020, 126, 347. [Google Scholar] [CrossRef]
- Sadidi, H.; Hooshmand, S.; Ahmadabadi, A.; Hosseini, S.J.; Baino, F.; Vatanpour, M.; Kargozar, S. Cerium Oxide Nanoparticles (Nanoceria): Hopes in Soft Tissue Engineering. Molecules 2020, 25, 4559. [Google Scholar] [CrossRef]
- Taimoory, S.M.; Rahdar, A.; Aliahmad, M.; Sadeghfar, F.; Hajinezhad, M.R.; Jahantigh, M.; Shahbazi, P.; Trant, J.F. The synthesis and characterization of a magnetite nanoparticle with potent antibacterial activity and low mammalian toxicity. J. Mol. Liq. 2018, 265, 96–104. [Google Scholar] [CrossRef]
- Rahdar, A.; Aliahmad, M.; Hajinezhad, M.R.; Samani, M. Xanthan gum-stabilized nano-ceria: Green chemistry based synthesis, characterization, study of biochemical alterations induced by intraperitoneal doses of nano-particles in rat. J. Mol. Struc. 2018, 1173, 166–172. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Gao, Y.; Mruk, D.; Chen, H.; Lui, W.; Lee, W.M.; Cheng, C.Y. Regulation of the blood-testis barrier by a local axis in the testis: Role of laminin α2 in the basement membrane. FASEB J. 2017, 31, 584–597. [Google Scholar] [CrossRef]
- Al Dine, E.J.; Ferjaoui, Z.; Ghanbaja, J.; Roques-Carmes, T.; Meftah, A.; Hamieh, T.; Toufaily, J.; Schneider, R.; Marchal, S.; Gaffet, E.; et al. Thermo-responsive magnetic Fe3O4@ P (MEO2MAX-OEGMA100-X and their applications as drug delivery systems. Int. J. Pharm. 2017, 532, 738–747. [Google Scholar] [CrossRef]
- Oshima, A.; Kanda, K.; Fujiwara, K.; Ide, T.; Takano-Kasuya, M.; Ichiyanagi, Y. PEGylation of Co–Zn Ferrite Nanoparticles for Theranostics. J. Nanosci. Nanotechnol. 2020, 20, 7255–7262. [Google Scholar] [CrossRef]
- Raval, Y.S.; Fellows, B.D.; Murbach, J.; Cordeau, Y.; Mefford, O.T.; Tzeng, T.R. Multianchored Glycoconjugate-Functionalized Magnetic Nanoparticles: A Tool for Selective Killing of Targeted Bacteria via Alternating Magnetic Fields. Adv. Funct. Mater. 2017, 27, 1701473. [Google Scholar] [CrossRef]





| Item | Treatment | |||
|---|---|---|---|---|
| Control | SnFe2O4 Nanoparticles 0.1 mg/kg | SnFe2O4 Nanoparticles 1 mg/kg | SnFe2O4 Nanoparticles 10 mg/kg | |
| MDA (nmol/mL) | 39 ± 10.7 | 37.5 ± 8.6 | 35 ± 5.3 | 52 ± 8 * |
| AST (U/L) | 65.6 ± 12.4 | 66.7 ± 15.7 | 77.6 ± 12.9 | 68.7 ± 8.8 |
| ALT (U/L) | 31.5 ± 8 | 37.8 ± 9.2 | 31.2 ± 8.6 | 39.1 ± 7.7 |
| BUN (mg/dL) | 11.2 ± 3.3 | 12.1 ± 2.6 | 13.2 ± 2.6 | 16.3 ± 4.1 * |
| Creatinine (mg/dL) | 0.75 ± 0.15 | 0.81 ± 0.24 | 0.87 ± 0.17 | 1.1 ± 0.38 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sargazi, S.; Hajinezhad, M.R.; Rahdar, A.; Zafar, M.N.; Awan, A.; Baino, F. Assessment of SnFe2O4 Nanoparticles for Potential Application in Theranostics: Synthesis, Characterization, In Vitro, and In Vivo Toxicity. Materials 2021, 14, 825. https://doi.org/10.3390/ma14040825
Sargazi S, Hajinezhad MR, Rahdar A, Zafar MN, Awan A, Baino F. Assessment of SnFe2O4 Nanoparticles for Potential Application in Theranostics: Synthesis, Characterization, In Vitro, and In Vivo Toxicity. Materials. 2021; 14(4):825. https://doi.org/10.3390/ma14040825
Chicago/Turabian StyleSargazi, Saman, Mohammad Reza Hajinezhad, Abbas Rahdar, Muhammad Nadeem Zafar, Aneesa Awan, and Francesco Baino. 2021. "Assessment of SnFe2O4 Nanoparticles for Potential Application in Theranostics: Synthesis, Characterization, In Vitro, and In Vivo Toxicity" Materials 14, no. 4: 825. https://doi.org/10.3390/ma14040825








