Investigation on the Microporosity Formation of IN718 Alloy during Laser Cladding Based on Cellular Automaton
Abstract
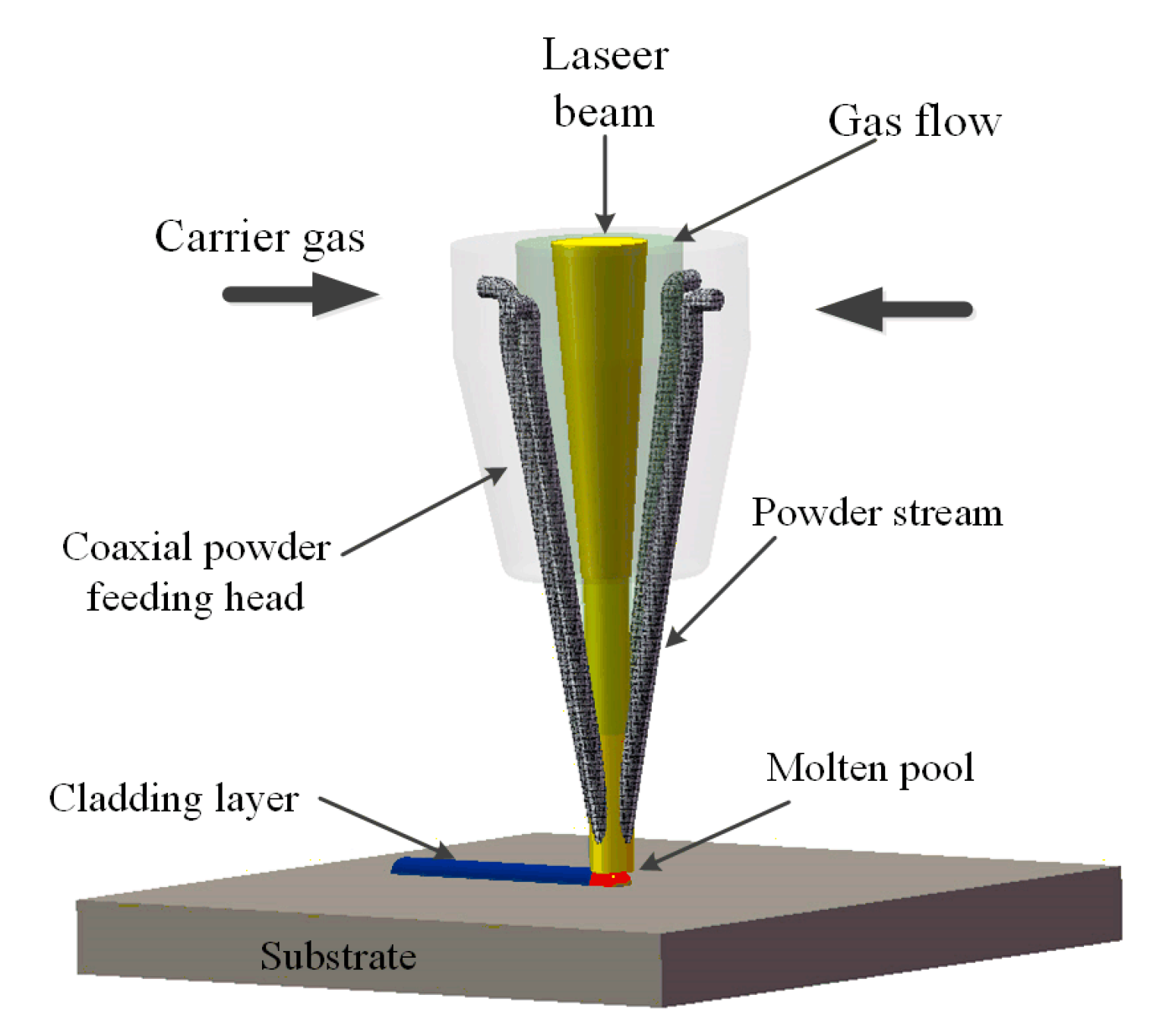
:1. Introduction
2. Model Description and Numerical Algorithm
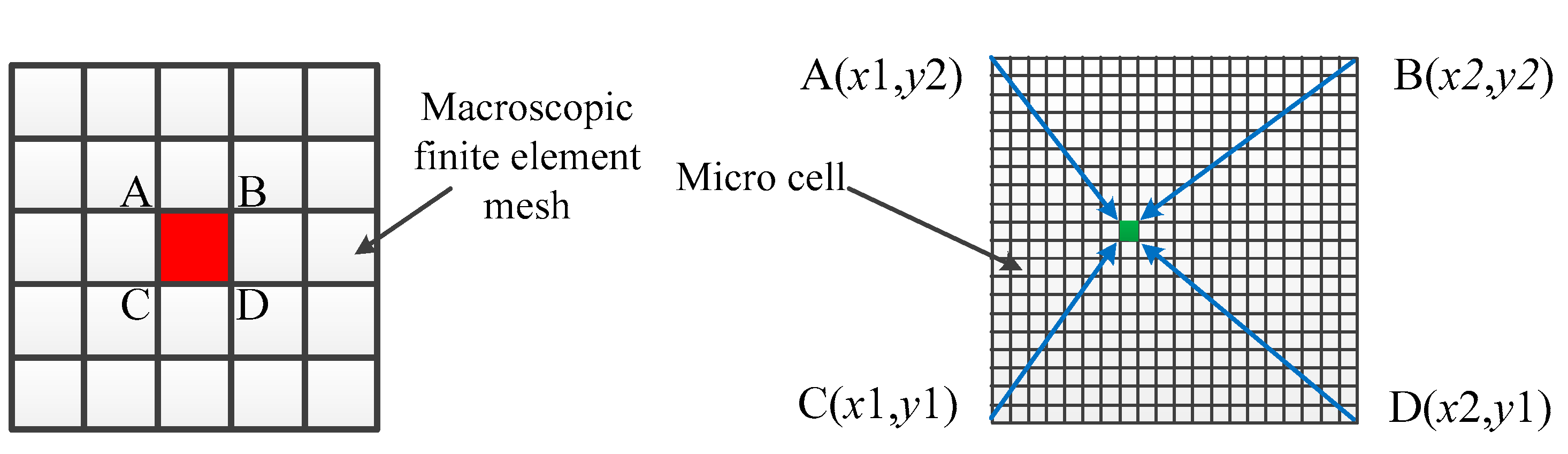
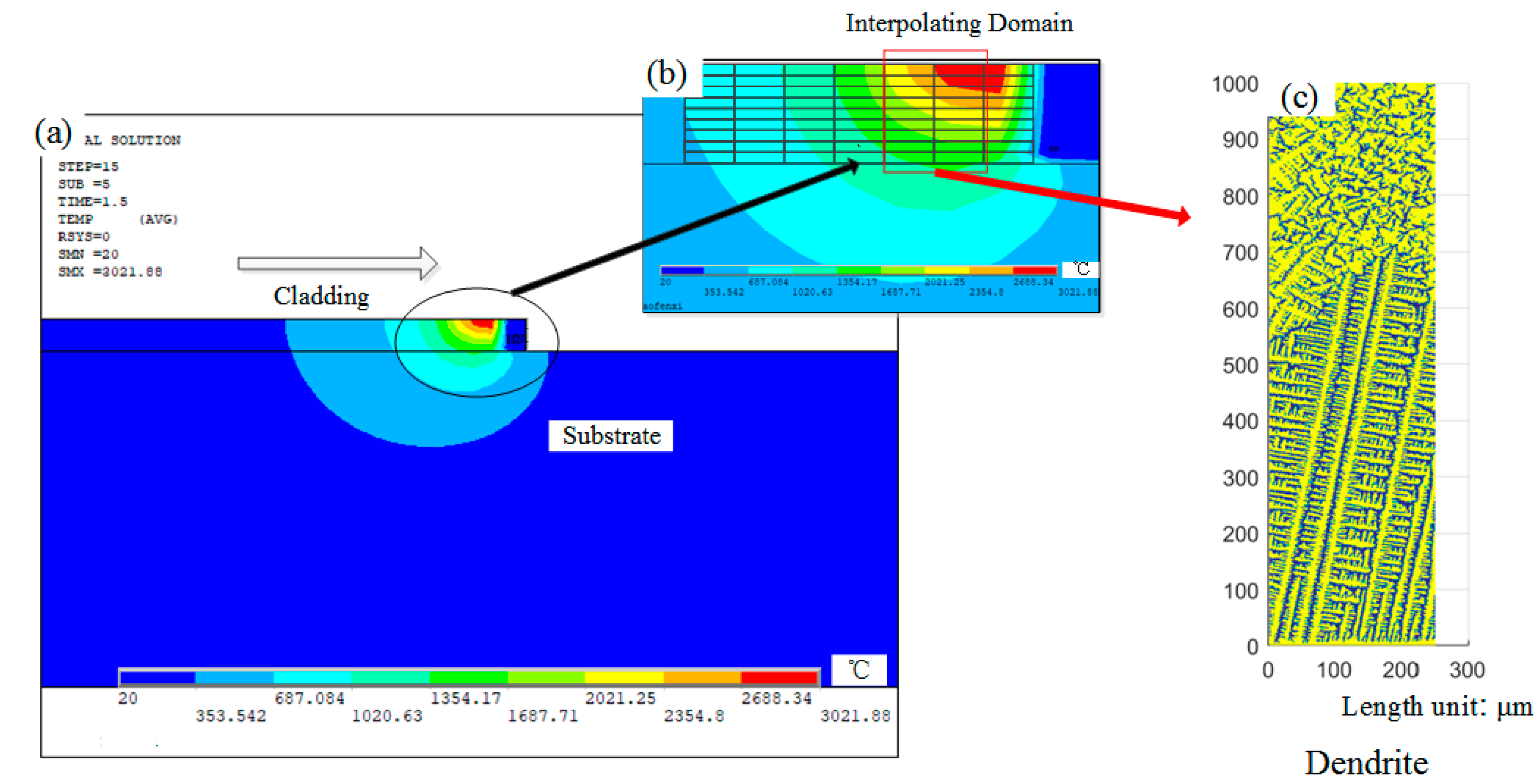
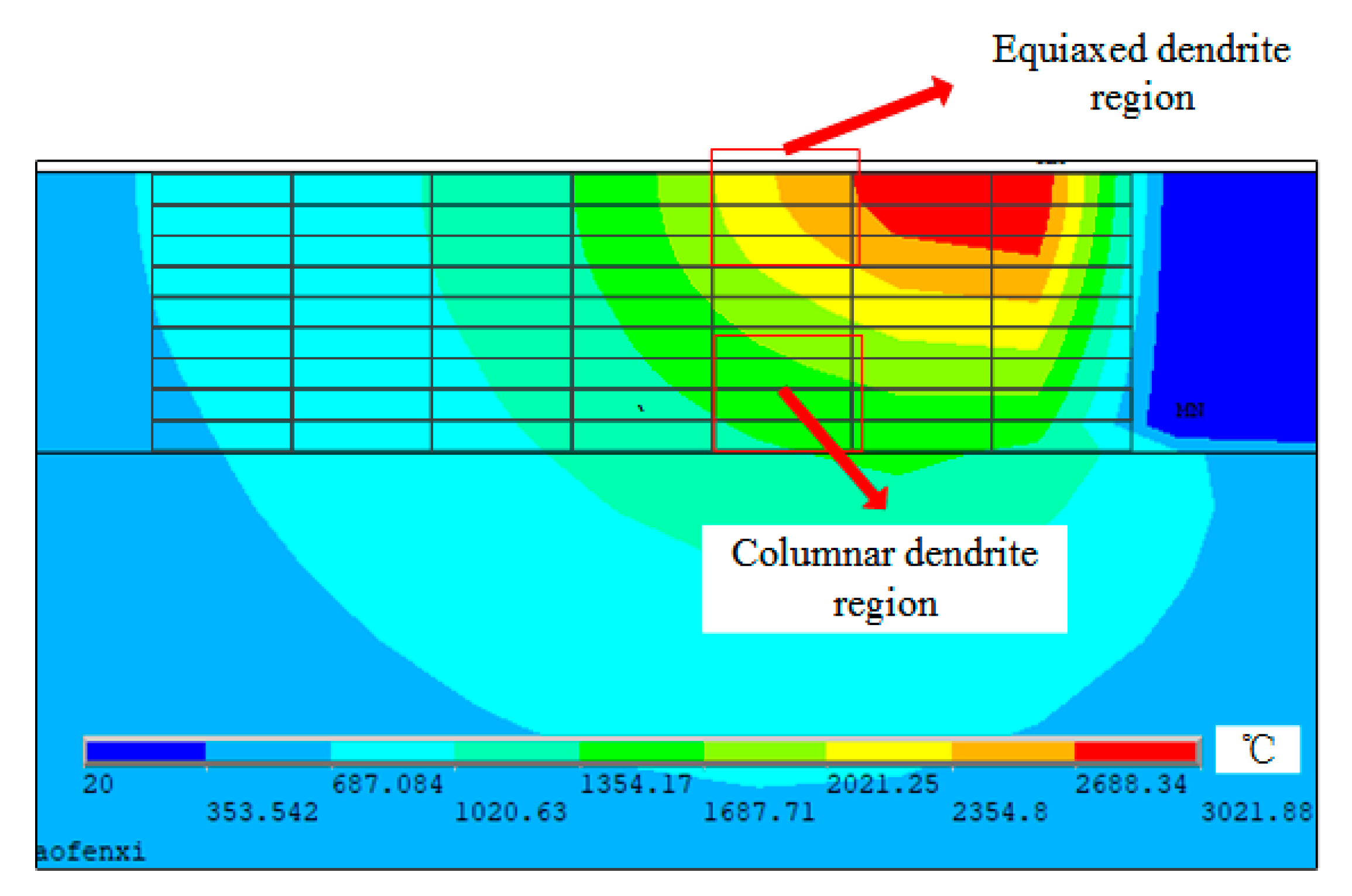
2.1. Macro-Scale Transient Temperature Field Simulation by the Finite Element Model (FEM) Model
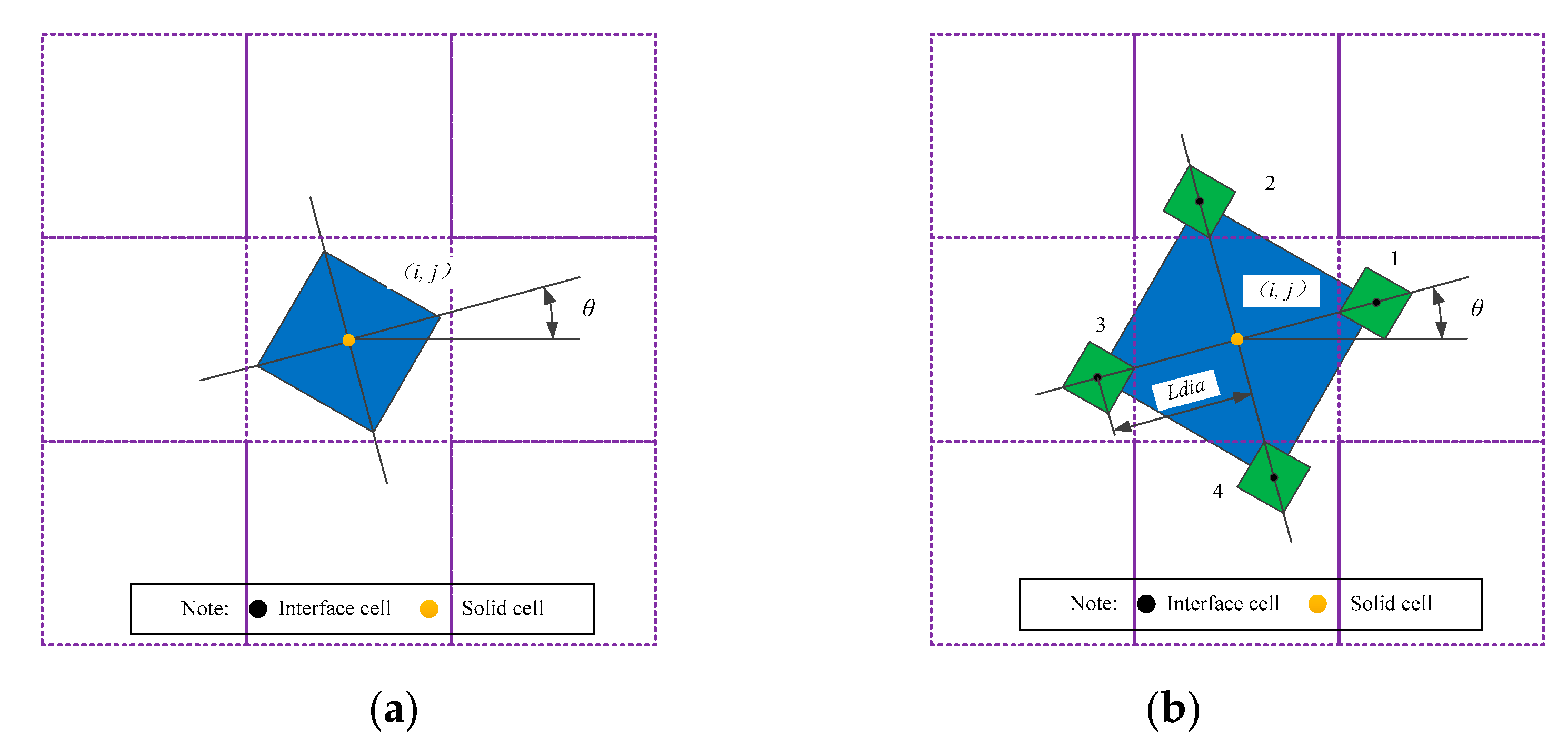
2.2. Mathematical Model of Dendrite Growth
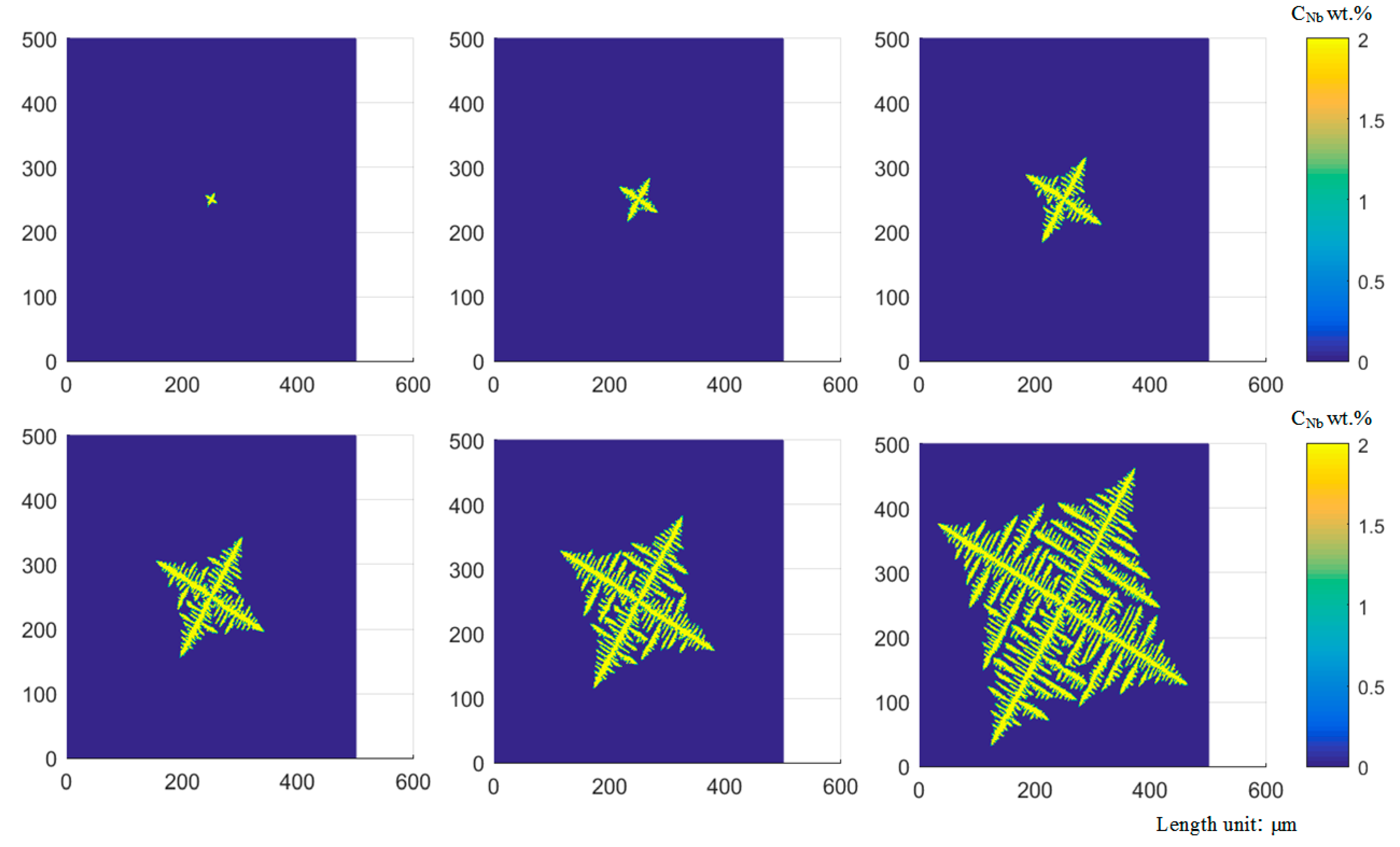
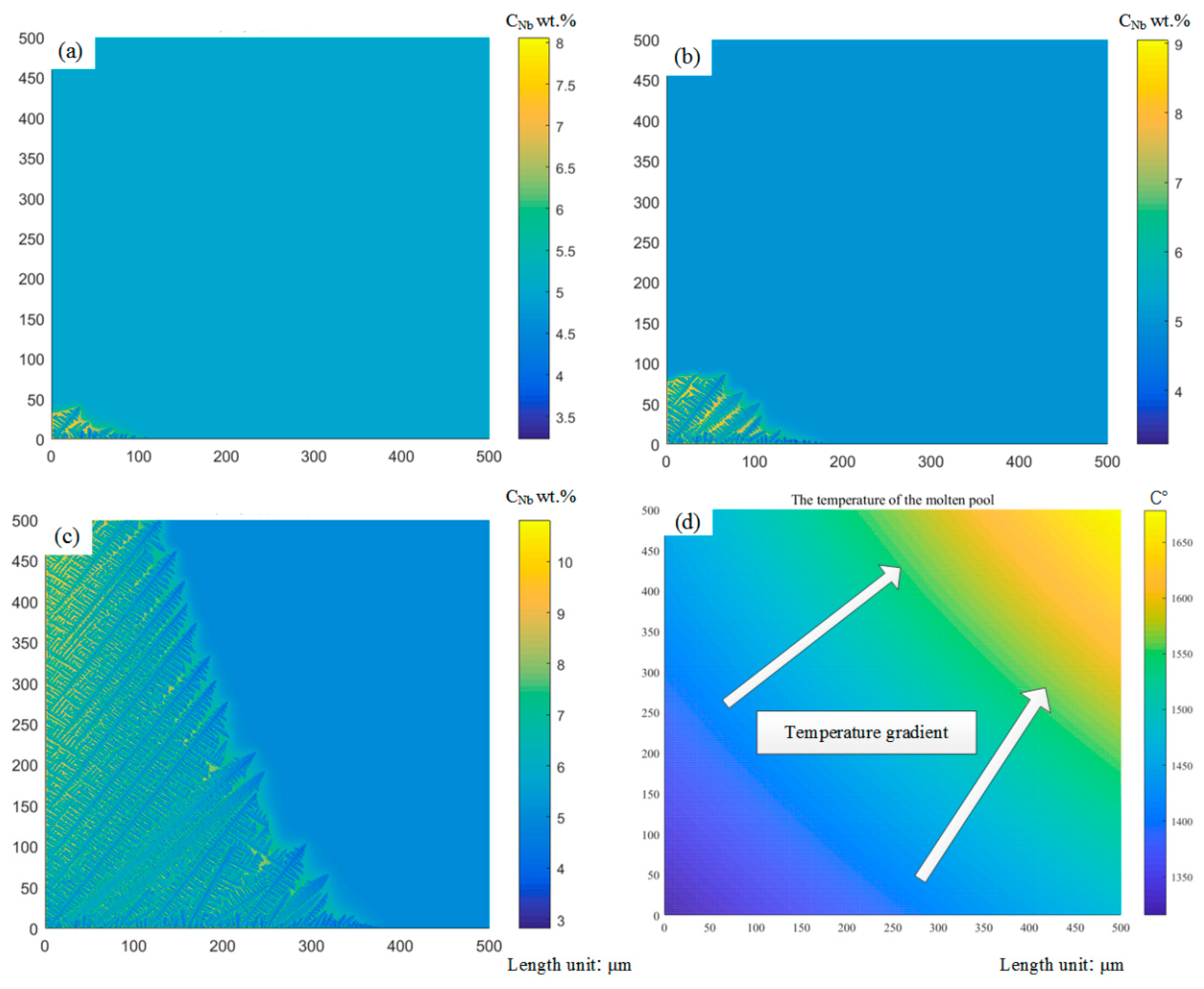
2.3. Analysis of Dendrite Growth Simulation Results
3. Mathematical Model of Porosity Evolution
3.1. Nucleation Model of Porosity
3.2. Growth of Microporosity
3.3. Coupling Growth of Porosities and Dendrites
4. Experimental Results and Discussion
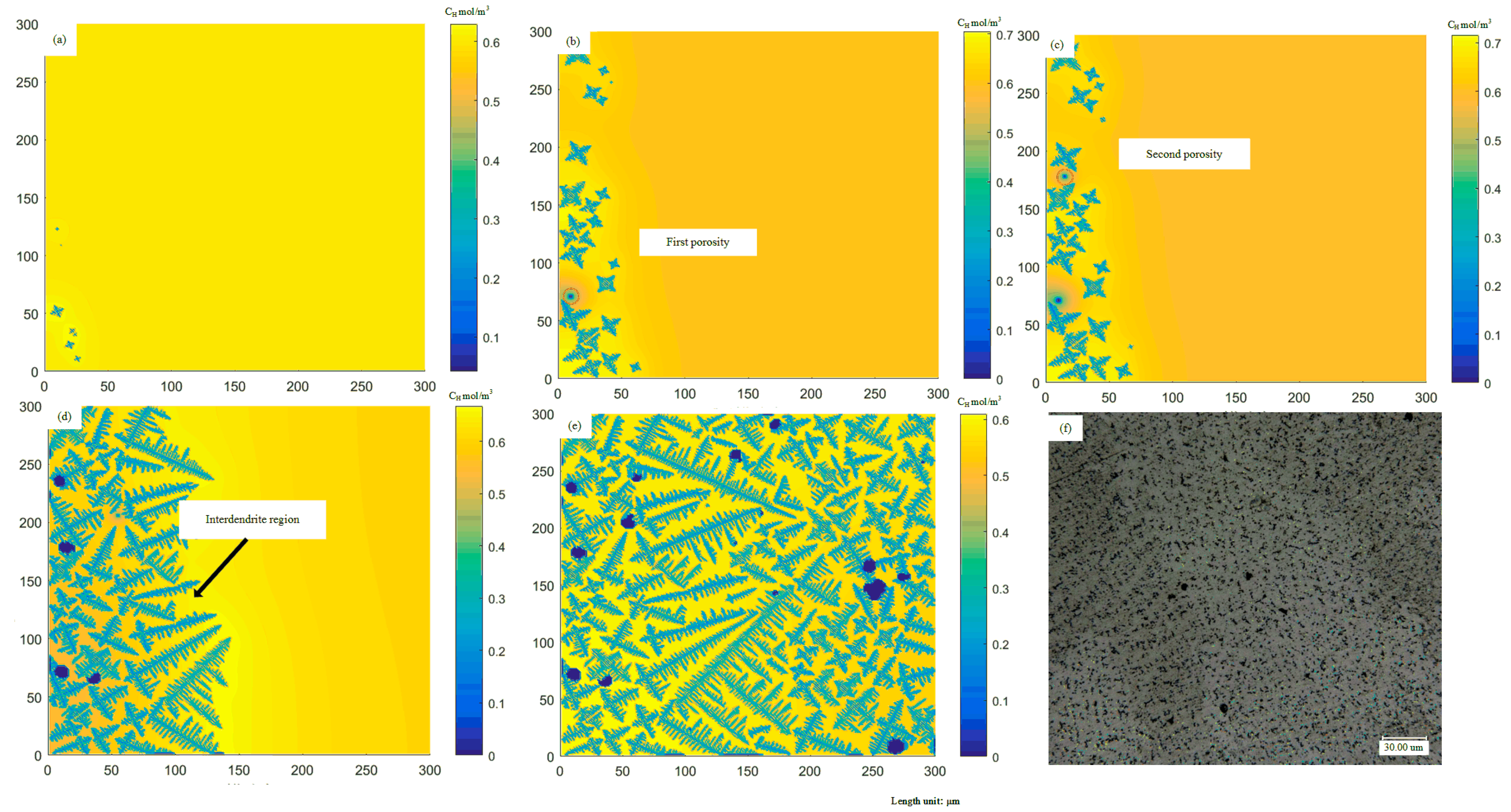
4.1. Evolution of Microstructure during Solidification
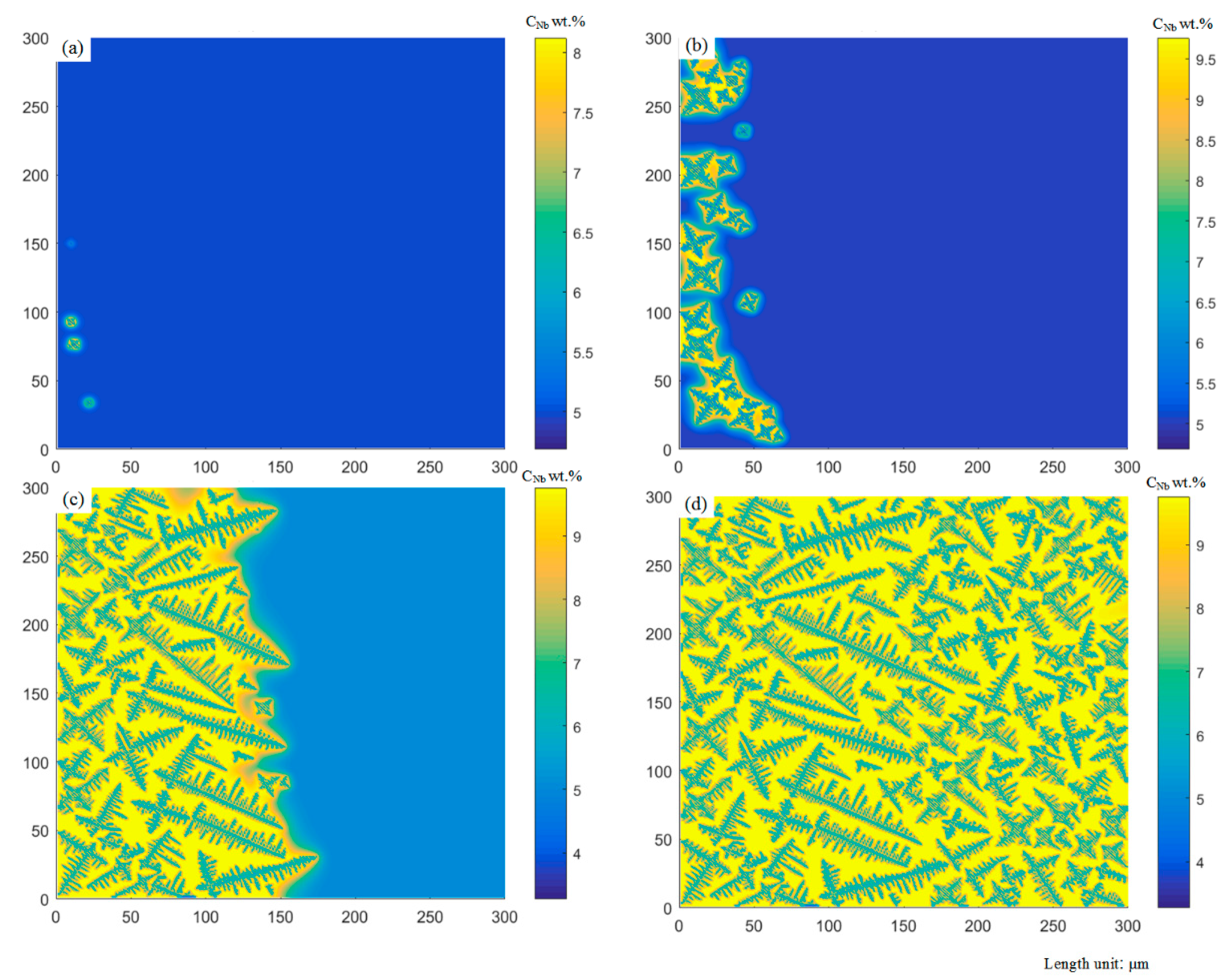
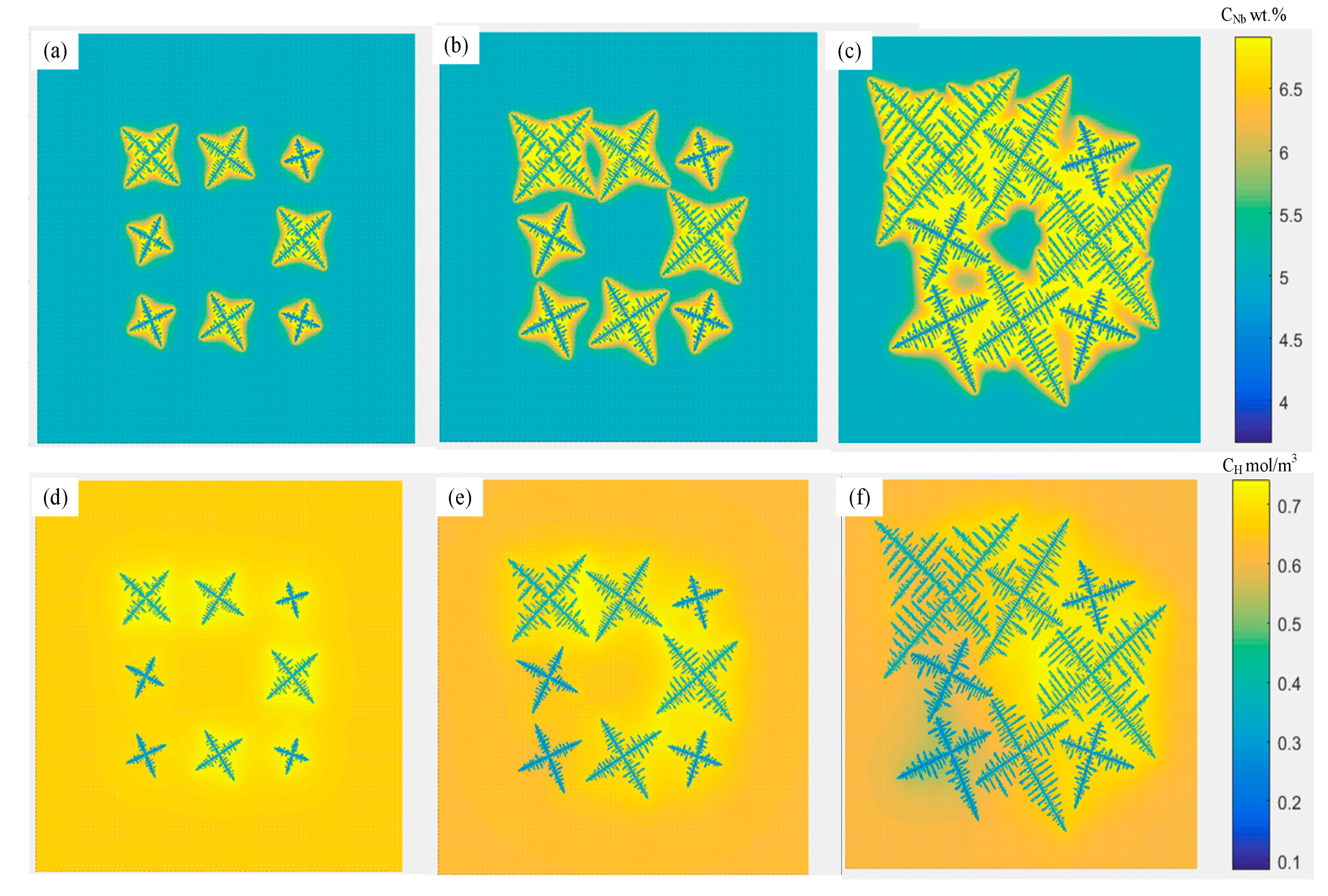
4.2. Dendrites and Multi-Bubble Interaction Evolution
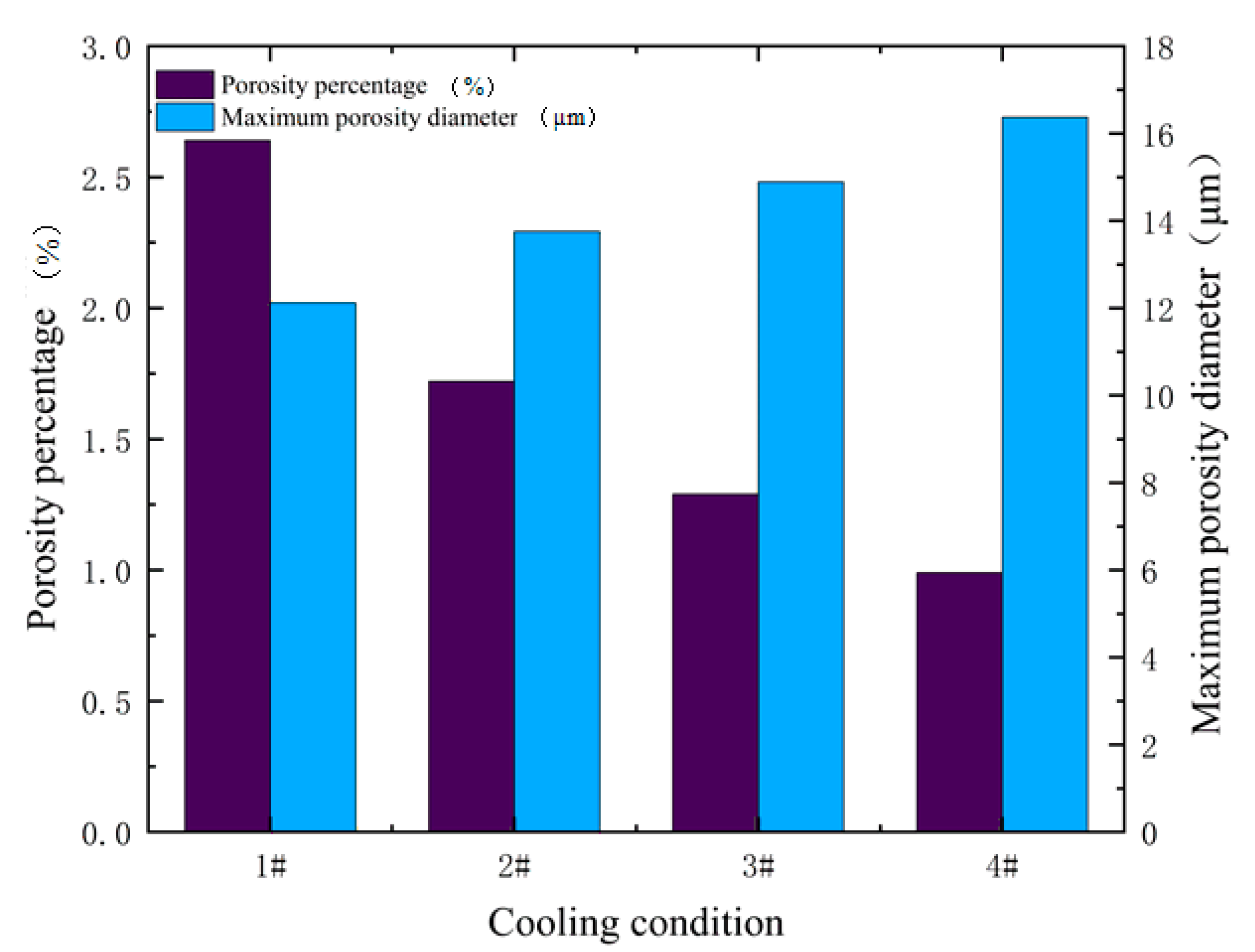
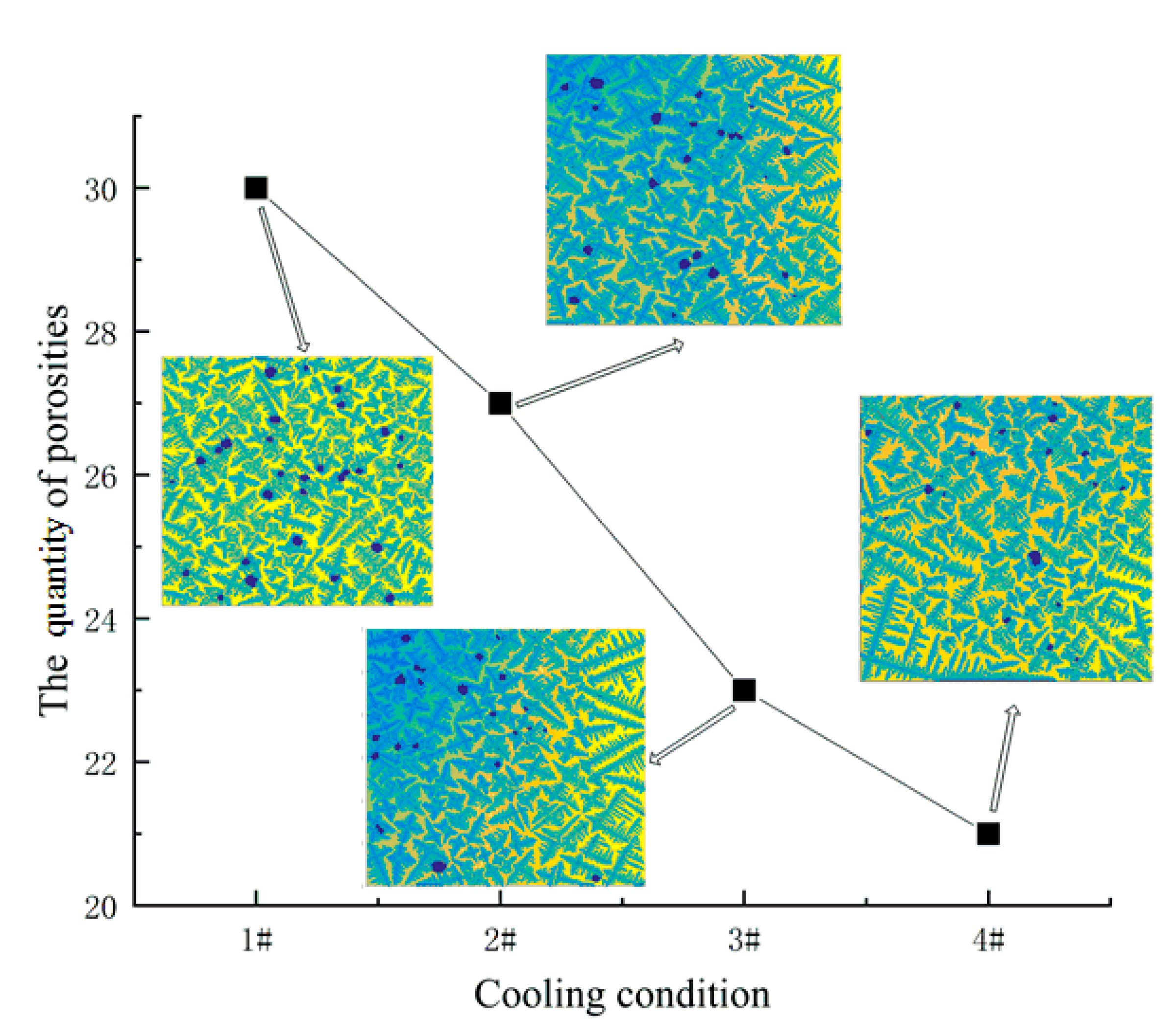
4.3. Influence of Solidification Conditions on the Morphology of Porosities
5. Conclusions
- CA-FEM is adopted to simulate the microstructure of laser cladding, which predicts the competitive grain growth under the dendrite tip of behavior and interaction between grain growth kinetics. According to the total supercooling degree, the grain cells can grow faster in the preferential growth direction, which can reflect the growth rate of the grain cell and the growth behavior of the granule. Only when the preferred orientation of dendrite growth is consistent with the direction of the maximum temperature gradient can it continue to grow. The preferential growth direction of dendrite also has an essential influence on the competition process between dendrites.
- The established model for the coupled growth of dendrites and porosities comprehensively considers the nucleation and growth of dendrites and porosities in the liquid phase, solute redistribution and diffusion and interface curvature effects. It can demonstrate the microstructure morphology of the coupled growth of dendrites and porosities in good agreement with the experimental observations. It indicates that the porosities are affected by the growth of dendrites, and the presence of porosities will also hinder the growth of dendrite.
- The simulation results were reasonably compared with experimental data in different typical regions. In the equiaxed region, for porosity, the average porosity of experimental results is 1.381%, and the simulation data is 1.574%. For the quantity of porosities, the average value of the experimental results is 21.8, and the average value of the simulation is 22. In the columnar region, it has a relatively large error.
- The competitive growth of dendrites and porosities was simulated, and the influence of different cooling conditions on the formation of porosities was analyzed. It is concluded that as the cooling rate increases, the quantity of porosities is increasing, and the porosity increases, while the maximum of the porosities is decreasing. The model can be applied to optimize the additive manufacturing process in order to obtain the desired results.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xin, B.; Ren, J.; Wang, X.; Zhu, L.; Gong, Y. Effect of laser remelting on cladding layer of inconel 718 Superalloy Formed by Laser Metal Deposition. Materials 2020, 13, 4927. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Li, A. Progress on rapid solidification laser processing for advanced materials and components. World Sci. Tech. 2004, 26, 27–31. [Google Scholar]
- Yang, K.; Xie, H.; Sun, C.; Zhao, X.; Li, F. Influence of vanadium on the microstructure of IN718 Alloy by Laser cladding. Materials 2019, 12, 3839. [Google Scholar] [CrossRef] [Green Version]
- Zhan, M.J.; Sun, G.; Wang, Z.; Shen, X.; Yan, Y.; Ni, Z. Numerical and experimental investigation on laser metal deposition as repair technology for 316L stainless steel. Opt. Laser Technol. 2019, 118, 84–92. [Google Scholar] [CrossRef]
- Sun, G.; Shen, X.; Wang, Z.; Zhan, M.; Yao, S.; Zhou, R.; Ni, Z. Laser metal deposition as repair technology for 316L stainless steel: Influence of feeding powder compositions on microstructure and mechanical properties. Opt. Laser Technol. 2019, 109, 71–83. [Google Scholar] [CrossRef]
- Oh, W.J.; Lee, W.J.; Kim, M.S.; Jeon, J.B.; Shim, D.S. Repairing additive-manufactured 316L stainless steel using direct energy deposition. Opt. Laser Technol. 2019, 117, 6–17. [Google Scholar] [CrossRef]
- Barros, R.; Silva, F.J.G.; Gouveia, R.M.; Saboori, A.; Marchese, G.; Biamino, S.; Salmi, A.; Atzeni, E. Laser powder bed fusion of inconel 718: Residual stress analysis before and after heat treatment. Metals 2019, 9, 1290. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Zuo, Z.; Liu, J. Numerical analysis for the influence of casting micro porosity on fatigue life. Eng. Fail. Anal. 2015, 48, 11–20. [Google Scholar] [CrossRef]
- Sheridan, L.; Scott-Emuakpor, O.E.; George, T.; Gockel, J.E. Relating porosity to fatigue failure in additively manufactured alloy 718. Mater. Sci. Eng. A 2018, 727, 170–176. [Google Scholar] [CrossRef]
- Lee, P.D.; Chirazi, A.; See, D. Modeling microporosity in aluminum–silicon alloys: A review. J. Light Met. 2001, 1, 15–30. [Google Scholar] [CrossRef]
- Stefanescu, D.M. Computer simulation of shrinkage related defects in metal castings—A review. Int. J. Cast Met. Res. 2005, 18, 129–143. [Google Scholar] [CrossRef]
- Xu, J.; Sun, X.K.; Liu, Q.Q.; Chen, W.X. Hydrogen permeation behavior in IN718 and GH761 superalloys. Metall. Mater. Trans. A 1994, 25, 539–544. [Google Scholar] [CrossRef]
- Latanision, R.M.; Kurkela, M. Hydrogen permeability and diffusivity in nickel and Ni-base alloys. Corrosion 1983, 39, 174–181. [Google Scholar] [CrossRef]
- Durandet, Y.C.; Brandt, M.; Liu, Q. Challenges of laser cladding Al 7075 alloy with Al-12Si alloy powder. Materials 2005, 29, 136–142. [Google Scholar]
- Yang, Z.; Wang, A.; Weng, Z.; Xiong, D.; Ye, B.; Qi, X. Porosity elimination and heat treatment of diode laser-clad homogeneous coating on cast aluminum-copper alloy. Surf. Coat. Technol. 2017, 321, 26–35. [Google Scholar] [CrossRef]
- Pei, Y.; De Hosson, J. Functionally graded materials produced by laser cladding. Acta Mater. 2000, 48, 2617–2624. [Google Scholar] [CrossRef] [Green Version]
- Lashkari, O.; Yao, L.; Cockcroft, S.; Maijer, D. X-ray microtomographic characterization of porosity in aluminum alloy A356. Met. Mater. Trans. A 2009, 40, 991–999. [Google Scholar] [CrossRef]
- Zhang, P.; Zhou, X.; Cheng, X.; Sun, H.; Ma, H.; Li, Y. Elucidation of bubble evolution and defect formation in directed energy deposition based on direct observation. Addit. Manuf. 2020, 32, 101026. [Google Scholar] [CrossRef]
- Ng, G.K.L.; Jarfors, A.E.; Bi, G.; Zheng, H.Y. Porosity formation and gas bubble retention in laser metal deposition. Appl. Phys. A 2009, 97, 641–649. [Google Scholar] [CrossRef]
- Panwisawas, C.; Qiu, C.L.; Sovani, Y.; Brooks, J.W.; Attallah, M.M.; Basoalto, H.C. On the role of thermal fluid dynamics into the evolution of porosity during selective laser melting. Scr. Mater. 2015, 105, 14–17. [Google Scholar] [CrossRef]
- Niz’ev, V.G.E.; Khomenko, M.D.; Mirzade, F.K. Process planning and optimisation of laser cladding considering hydro-dynamics and heat dissipation geometry of parts. Quan. Elec. 2018, 48, 743. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, W.; Deng, D.; Tang, Z.; Liu, X.; Yan, Z.; Zhang, H. Effect of electric-magnetic compound field on the pore distribution in laser cladding process. Opt. Laser Technol. 2018, 108, 247–254. [Google Scholar] [CrossRef]
- Ferreira, I.L.; Jefferson, F.C.; Lins, D.J. Numerical and experimental investigation of microporosity formation in a ternary Al–Cu–Si alloy. J. Alloy. Com. 2010, 503, 31–39. [Google Scholar] [CrossRef]
- Carlson Kent, D.; Lin, Z.; Beckermann, C. Modeling the effect of finite-rate hydrogen diffusion on porosity formation in Aluminum alloys. Metall. Mater. Trans. B 2007, 38, 541–555. [Google Scholar] [CrossRef]
- Gu, C.; Ridgeway, C.D.; Cinkilic, E.; Lu, Y.; Luo, A.A. Predicting gas and shrinkage porosity in solidification micro-structure: A coupled three-dimensional cellular automaton model. J. Mater. Sci. Tech. 2020, 49, 91–105. [Google Scholar] [CrossRef]
- Wang, T.; An, D.; Zhang, Q. Modeling of microporosity formation during solidification of aluminum alloys. Mater. Sci. Eng. 2015, 84, 012046. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Sun, D.; Pan, S.; Zhu, M. Microporosity formation and dendrite growth during solidification of aluminum alloys: Modeling and experiment. Int. J. Heat Mass Trans. 2020, 146, 118838. [Google Scholar] [CrossRef]
- Lee, P.D.; Chirazi, A.; Atwood, R.C.; Wang, W. Multi-scale modelling of solidification microstructures, including microsegregation and microporosity, in an Al–Si–Cu alloy. Mater. Sci. Eng. A 2004, 365, 57–65. [Google Scholar] [CrossRef]
- Dong, S.Y.; Xiong, S.M.; Liu, B.C. Numerical simulation of microporosity evolution of Aluminum alloy castings. Mater. Sci. Tech. 2004, 20, 23–26. [Google Scholar]
- Zhu, M.; Li, Z.; An, D.; Zhang, Q.; Dai, T. Celular automaton modeling of microporosity formation during solidification of aluminum alloys. ISIJ Int. 2014, 54, 384–391. [Google Scholar] [CrossRef] [Green Version]
- Gu, C.; Wei, Y.; Yu, F.; She, L. Celular automaton study of hydrogen porosity evolution coupled with dendrite growth during solidification in the molten pool of Al-Cu alloys. Metall. Mater. Transf. A 2017, 48, 4314–4323. [Google Scholar] [CrossRef]
- Wu, W.; Zhu, M.F.; Sun, D.K.; Dai, T.; Han, Q.Y.; Raabe, D. Modelling of dendritic growth and bubble formation. Mater. Sci. Eng. 2012, 33, 012103. [Google Scholar] [CrossRef]
- Wu, W.; Sun, D.K.; Dai, T.; Zhu, M.F. Numerical simulation of dendrite growth and bubble formation. Acta Phys. Sin. 2012, 61, 39–47. (In Chinese) [Google Scholar]
- Sun, D.; Zhu, M.; Wang, J.; Sun, B. Lattice Bolzmann modeling of bubble formation and dendritic growth in solidification of binary alloys. Int. J. Heat Mass Transf. 2016, 94, 474–487. [Google Scholar] [CrossRef]
- Du, L.; Wang, L.; Zheng, B.; Du, H. Phase-field modeling of microstructure evolution during solidification in presence of gas bubble. Comput. Mater. Sci. 2016, 114, 94–98. [Google Scholar] [CrossRef]
- Goldak, J.; Chakravarti, A.; Bibby, M. A new finite element model for welding heat sources. Metall. Trans. B 1984, 15, 299–305. [Google Scholar] [CrossRef]
- Gao, S.Y.; Li, J.; Li, C.G.; Zhou, Y.F. Study on the change law of the cross-section morphology of single-pass laser cladding deposition track. Chin. Laser. 2013, 5, 119–125. [Google Scholar]
- Xie, H.; Yang, K.; Li, F.; Sun, C.; Yu, Z. Investigation on the Laves phase formation during laser cladding of IN718 alloy by CA-FE. J. Manuf. Process. 2020, 52, 132–144. [Google Scholar] [CrossRef]
- Cai, C.; Han, B. Numerical simulation of laser cladding stress field on the surface of gate. China Petrol. Mach. 2016, 44, 90–93. [Google Scholar]
- Zhang, M.; Zhou, Y.; Huang, C.; Chu, Q.; Zhang, W.; Li, J. Simulation of Temperature distribution and microstructure evolution in the molten pool of GTAW Ti-6Al-4V Alloy. Materials 2018, 11, 2288. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Lee, P.D.; McLean, M. A model of solidification microstructures in nickel-based superalloys: Predicting primary dendrite spacing selection. Acta. Mater. 2003, 51, 2971–2987. [Google Scholar] [CrossRef]
- Nie, P.; Ojo, O.A.; Li, Z. Numerical modeling of microstructure evolution during laser additive manufacturing of a nickel-based superalloy. Acta Mater. 2014, 77, 85–95. [Google Scholar] [CrossRef]
- Nastac, L.; Ştefănescu, D.M. Stochastic modelling of microstructure formation in solidification processes. Modell. Simul. Mater. Sci. Eng. 1997, 5, 391–420. [Google Scholar] [CrossRef]
- Kothe, D.B.; Mjolsness, R.C.; Torrey, M.D. RIPPLE: A Computer Progarm for Incompressible Flows with Free Surface; Technical Prepared for Los Alamos National Laboratory: Los Almos, NM, USA, 1991. [Google Scholar]
- Gandin, C.A. A coupled finite element-cellular automaton model for the prediction of dendritic grain structures in solidification processes. Acta. Metall. Mater. 1994, 42, 2233–2246. [Google Scholar] [CrossRef]
- Zinoviev, A.; Zinovieva, O.; Ploshikhin, V.; Romanova, V.; Balokhonov, R. Evolution of grain structure during laser additive manufacturing. Simulation by a cellular automata method. Mater. Des. 2016, 106, 321–329. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Nie, P.; Wu, Y. Carbide and nitride precipitation during laser cladding of Inconel 718 alloy coatings. Opt. Laser Technol. 2013, 52, 30–36. [Google Scholar] [CrossRef]
- Lee, P.; Hunt, J. Measuring the nucleation of hydrogen porosity during the solidification of aluminium-copper alloys. Scr. Mater. 1997, 36, 399–404. [Google Scholar] [CrossRef]
- Lee, P.D.; Hunt, J.D. Hydrogen porosity in direction solidified aluminum-copper alloys: In situ observation. Acta Mater. 1997, 45, 4155–4169. [Google Scholar] [CrossRef]
- See, D.; Atwood, R.C.; Lee, P.D. A comparison of three modeling approaches for the prediction of microporosity in aluminum-silicon alloys. J. Mater. Sci. 2001, 36, 3423–3435. [Google Scholar] [CrossRef]
- Atwood, R.; Sridhar, S.; Zhang, W.; Lee, P.D. Diffusion-controlled growth of hydrogen pores in aluminium–silicon castings: In situ observation and modelling. Acta Mater. 2000, 48, 405–417. [Google Scholar] [CrossRef]
- Zhou, S.; Zeng, X.; Hu, Q.; Huang, Y. Analysis of crack behavior for Ni-based WC composite coatings by laser cladding and crack-free realization. Appl. Surf. Sci. 2008, 255, 1646–1653. [Google Scholar] [CrossRef]
- Hunt, J. Steady state columnar and equiaxed growth of dendrites and eutectic. Mater. Sci. Eng. 1984, 65, 75–83. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Li, Z.G.; Nie, P.L.; Chengwu, Y. Effect of ultrarapid cooling on microstructure of laser cladding IN718 coating. Surf. Eng. 2013, 29, 414–418. [Google Scholar] [CrossRef]
- Yang, S.; Chang, B.; Xing, B.; Du, D. The effect of forced cooling on the microstructure and hardness of IC10 alloy laser cladding. Trans. China Weld. Inst. 2018, 39, 31–35. [Google Scholar]
- Cai, C.; Li, M.; Han, B.; Wang, Y.; Li, J. Numerical simulation of broadband laser cladding iron-based coatings at different preheating temperatures. App. Laser 2017, 37, 66–71. [Google Scholar]
- Zhong, M.; Liu, W.; Ning, G.; Yang, L.; Chen, Y. Laser direct manufacturing of tungsten nickel collimation component. J. Mater. Process. Technol. 2004, 147, 167–173. [Google Scholar] [CrossRef]



| Parameters | Laser Power (W) | Scanning Speed (mm/s) | Powder Federate (g·min−1) | Shield Gas Flow (L·min−1) |
|---|---|---|---|---|
| - | 1500 | 10 | 18 | 15 |
| Parameter | Symbol | Units | Value | Ref. |
|---|---|---|---|---|
| Liquidus temperature | Tl | K | 1678 | [42] |
| Eutectic temperature | Teu | K | 1643 | [42] |
| Slope of the liquidus line | mL | K/(mass)% | −10.5 | [42] |
| Diffusion coefficient of Nb in liquid | DL(Nb) | m2/s | 3 × 10−9 | [38] |
| Diffusion coefficient of Nb in solid | DS(Nb) | m2/s | 1 × 10−12 | [38] |
| Diffusion coefficient of H in liquid | DH(Nb) | m2/s | 2.8 × 10−7 | [12] |
| Diffusion coefficient of H in solid | DH(Nb) | m2/s | 6.7 × 10−10 | [12] |
| The partition coefficient of Nb | k0 | / | 0.48 | [42] |
| The partition coefficient of H | kH | / | 0.069 | [25] |
| Maximum pore nucleation saturation | / | 2.0 | [25] | |
| Minimum pore nucleation saturation | / | 1.4 | [25] | |
| Timestep of Nb | t | s | 0.6 × 10−4 | / |
| Timestep of H | t | s | 0.1 × 10−5 | / |
| Sample Number | 1# | 2# | 3# | 4# | 5# |
|---|---|---|---|---|---|
| Porosity of experiment (%) | 1.08 | 0.335 | 1.14 | 1.535 | 2.813 |
| The quantity of porosities in the experiment | 26 | 25 | 20 | 15 | 23 |
| Porosity of model (%) | 1.51 | 1.31 | 1.59 | 1.51 | 1.95 |
| The quantity of porosities in the model | 22 | 21 | 20 | 23 | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, H.; Li, Z.; Li, X.; Yang, K.; Li, F.; Xie, H. Investigation on the Microporosity Formation of IN718 Alloy during Laser Cladding Based on Cellular Automaton. Materials 2021, 14, 837. https://doi.org/10.3390/ma14040837
Lv H, Li Z, Li X, Yang K, Li F, Xie H. Investigation on the Microporosity Formation of IN718 Alloy during Laser Cladding Based on Cellular Automaton. Materials. 2021; 14(4):837. https://doi.org/10.3390/ma14040837
Chicago/Turabian StyleLv, Hao, Zhijie Li, Xudong Li, Kun Yang, Fei Li, and Hualong Xie. 2021. "Investigation on the Microporosity Formation of IN718 Alloy during Laser Cladding Based on Cellular Automaton" Materials 14, no. 4: 837. https://doi.org/10.3390/ma14040837






