Plasmonic Nanocomposite Implants for Interstitial Thermotherapy: Experimental and Computational Analysis
Abstract
:1. Introduction
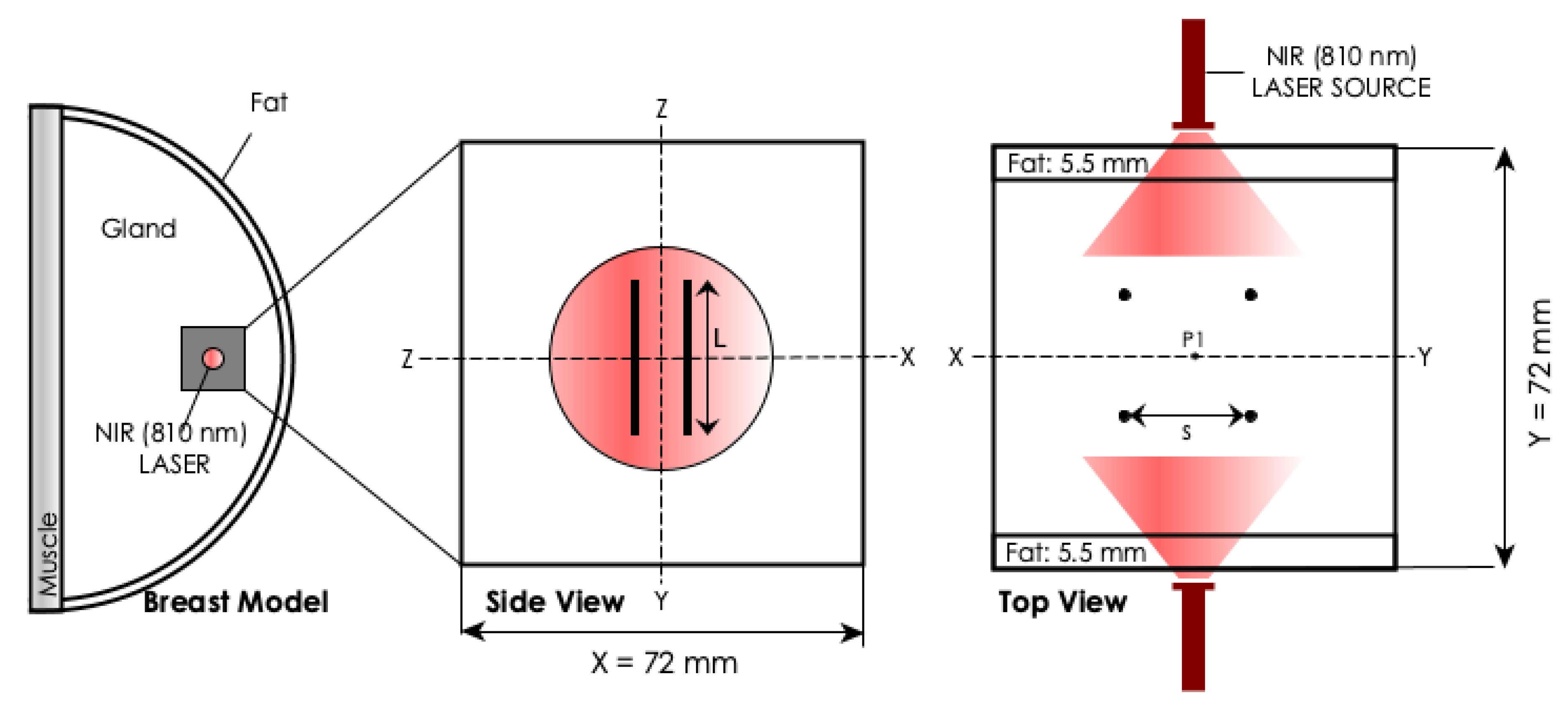
2. Methods
2.1. Experimental Analysis
2.2. Computational Analysis
3. Results
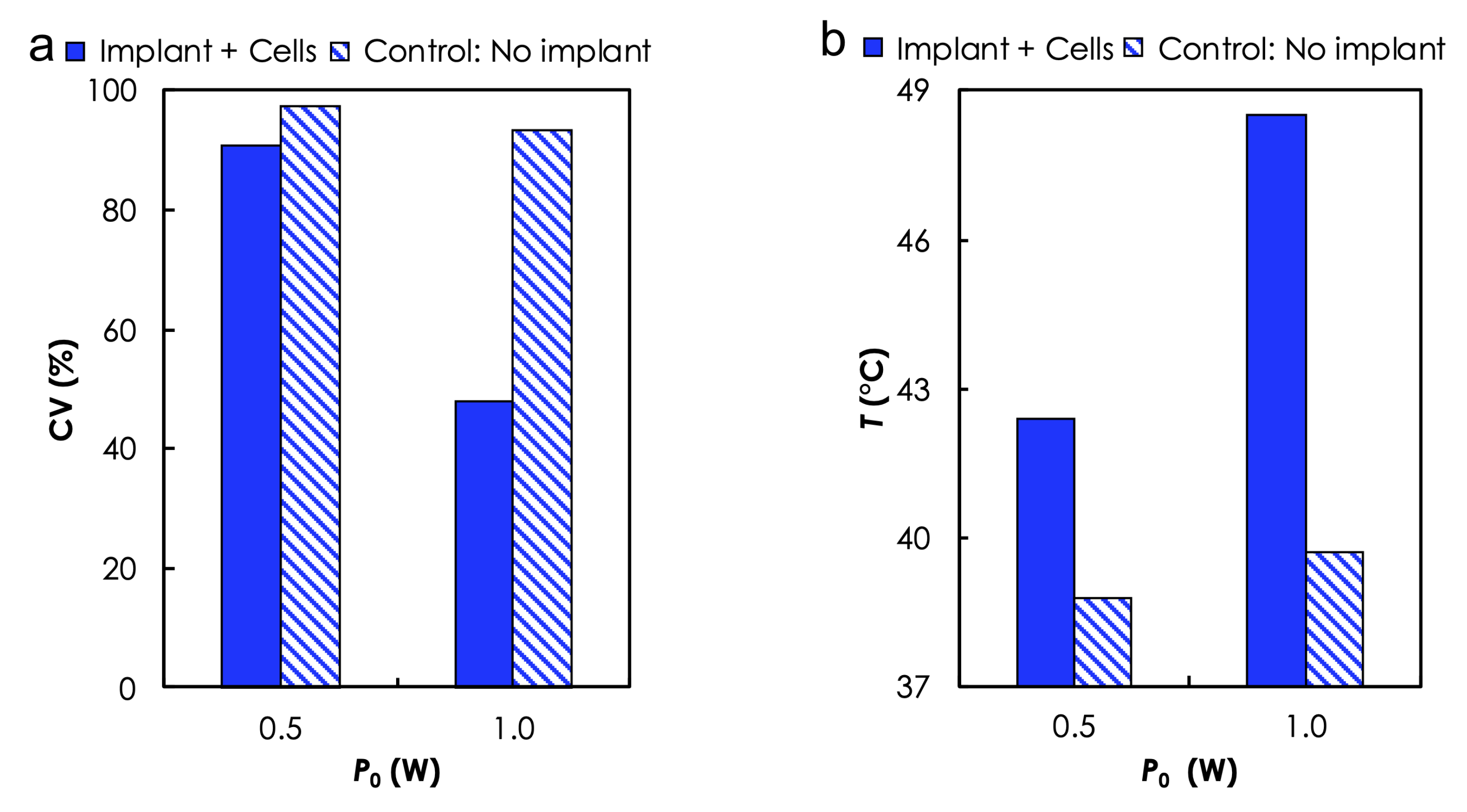
3.1. Experimental Analysis
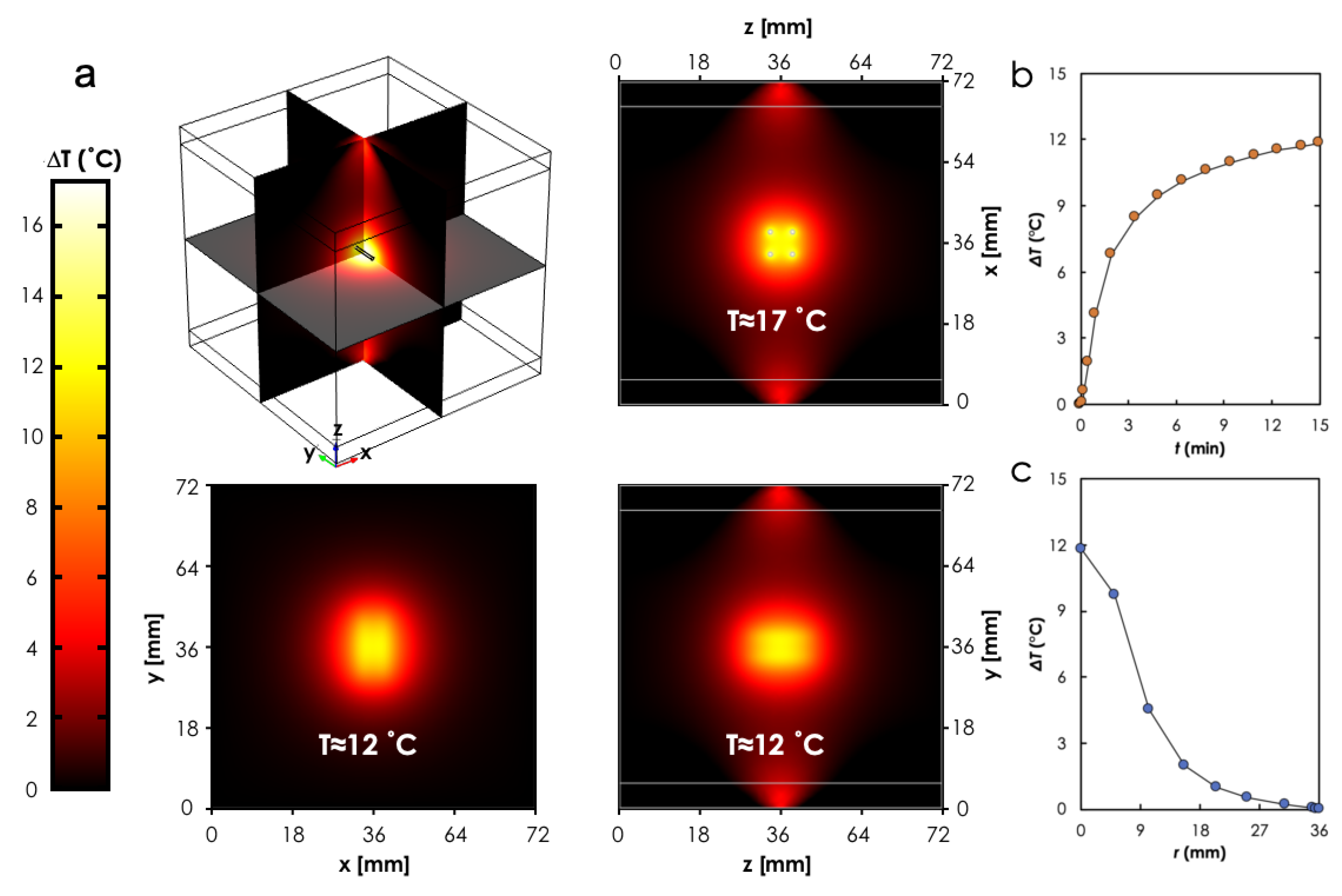
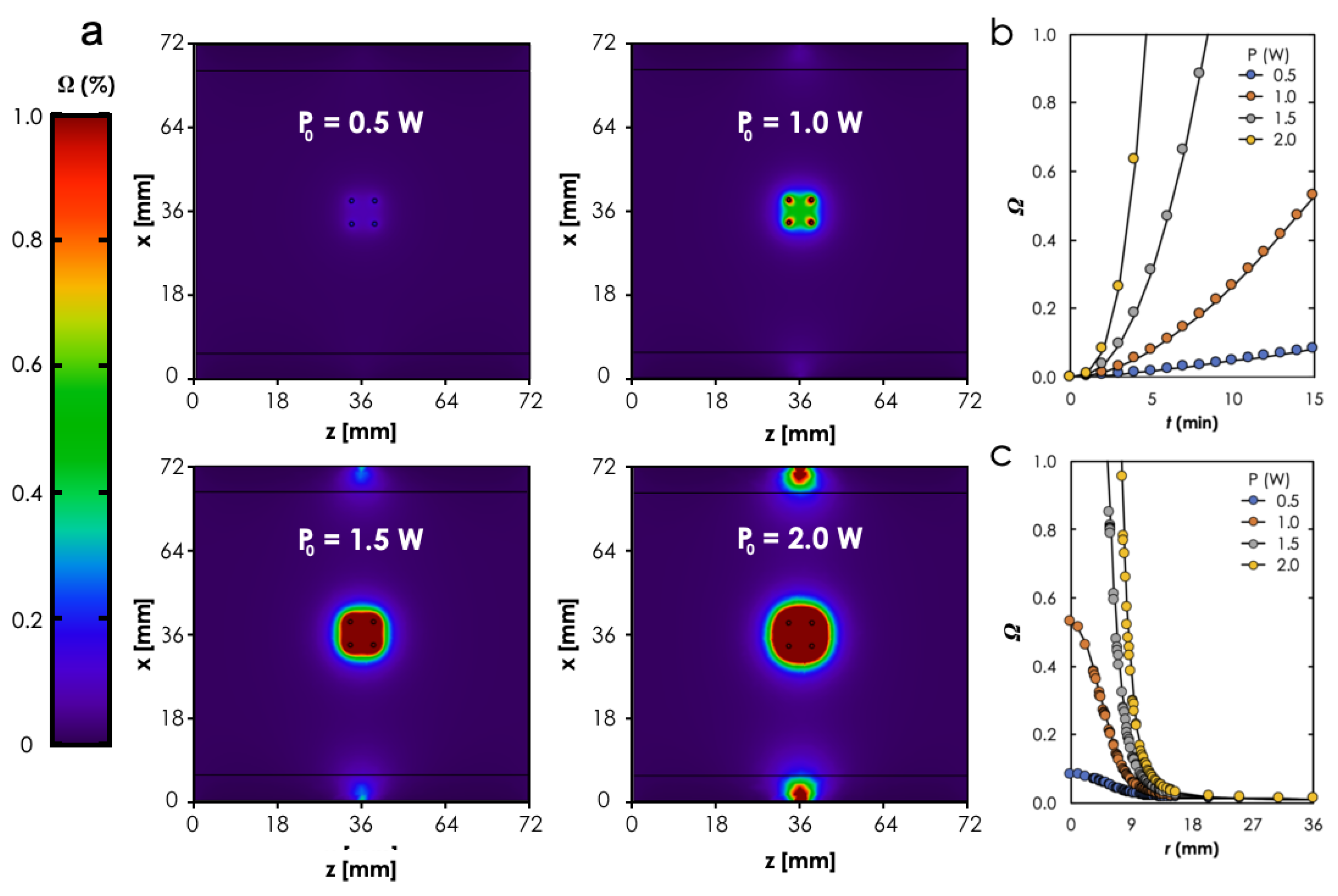
3.2. Computational Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Milligan, A.J.; Dobelbower, R.R., Jr. Interstitial Hyperthermia. Med. Instrum. 1984, 18, 175–180. [Google Scholar]
- Luo, S.; Wang, L.F.; Ding, W.J.; Wang, H.; Zhou, J.M.; Jin, H.K.; Su, S.F.; Ouyang, W.W. Clinical trials of magnetic induction hyperthermia for treatment of tumours. OA Canc. 2014, 2, 2. [Google Scholar]
- Dughiero, F.; Corazza, S. Numerical simulation of thermal disposition with induction heating used for oncological hyperthermic treatment. Med. Biol. Eng. Comput. 2005, 43, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, P.R.; Cetas, T.C.; Jones, R.C. Magnetic induction heating of ferromagnetic implants for inducing localized hyperthermia in deep-seated tumors. IEEE Trans. Biomed. Eng. 1984, 31, 235–251. [Google Scholar] [CrossRef]
- Brezovich, I.A.; Atkinson, W.J. Temperature distributions in tumor models heated by self-regulating nickel-copper alloy thermoseeds. Med. Phys. 1984, 11, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Roemer, R.B.; Cetas, T.C. Interstitial Hyperthermia using Self-Regulating Thermoseeds Combined with Conformal Radiation Therapy. Eur. Urol. 2002, 42, 147–153. [Google Scholar]
- Paulus, J.A.; Parida, G.R.; Tucker, R.D.; Park, J.B. Corrosion analysis of NiCu and PdCo thermal seed alloys used as interstitial hyperthermia implants. Biomaterials 1997, 18, 1609–1614. [Google Scholar] [CrossRef]
- Brezovich, I.A.; Meredith, R.F. Practical aspects of ferromagnetic thermoseeds hyperthermia. Radiol. Clin. North Am. 1989, 27, 589–602. [Google Scholar]
- Demer, L.J.; Chen, J.S.; Buechler, D.N.; Damento, M.A.; Poirier, D.R.; Cetas, T.C. Ferromagnetic thermoseed materials for tumor hyperthermia. IEEE Annu. Conf. Eng. Med. Biol. Soc. 1986, 8, 1448. [Google Scholar]
- Tucker, R.D.; Platz, C.E.; Huidobro, C.; Larson, T. Interstitial thermal therapy in patients with localized prostrate cancer: Histologic analysis. Urology 2002, 60, 166–169. [Google Scholar] [CrossRef]
- Tucker, R.D. Use of Interstitial temperature self-regulating thermal rods in the treatement of prostrate cancer. J. Endourol. 2003, 17, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Hergt, R.; Dutz, S.; Müller, R.; Zeisberger, M. Magnetic particle hyperthermia: Nanoparticle magnetism and materials development for cancer therapy. J. Phys. Condens. Matt. 2006, 18, 2919–2934. [Google Scholar] [CrossRef]
- Ruiz-Hernaàndez, E.; Serrano, M.; Arcos, D.; Vallet-Regí, M. Glass–Glass Ceramic thermoseeds for treatment of bone tumors. J. Biomed. Mater. Res. Part A 2006, 18, 2919–2934. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, H.; Xiao, D.; Yin, S.; Ji, W.; Jiang, S.; Luo, D.; Wang, B.; Liu, Y. Recent Advances of Plasmonic Nanoparticles and their Applications. Materials 2018, 11, 1833. [Google Scholar] [CrossRef] [Green Version]
- Doughty, A.C.V.; Hoover, A.R.; Layton, E.; Murray, C.K.; Howard, E.W.; Chen, W.R. Nanomaterial Applications in Photothermal Therapy for Cancer. Materials 2019, 12, 779. [Google Scholar] [CrossRef] [Green Version]
- Jaufred, L.; Samadi, A.; Klingberg, H.; Bendix, P.M.; Oddershede, L.B. Plasmonic heating of nanostructures. Chem. Rev. 2019, 119, 8087–8130. [Google Scholar] [CrossRef]
- Nehl, C.L.; Hafner, J.H. Shape-dependent plasmon resonances of gold nanoparticles. J. Mater. Chem. 2008, 18, 2415–2419. [Google Scholar] [CrossRef] [Green Version]
- Chu, M.; Shao, Y.; Peng, J.; Dai, X.; Li, H.; Wu, Q.; Shi, D. Near-infrared laser light mediated cancer therapy by photothermal effect of Fe3O4 magnetic nanoparticles. Biomaterials 2013, 34, 4078–4088. [Google Scholar] [CrossRef]
- Espinosa, A.; Corato, R.D.; Kolosnjaj-Tabi, J.; Flaud, P.; Pellegrino, T.; Wilhelm, C. Duality of Iron Oxide Nanoparticles in Cancer Therapy: Amplification of Heating Efficiency by Magnetic Hyperthermia and Photothermal Bimodal Treatment. ACS Nano 2016, 10, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Blumstein, A. A Review of In-Situ Grown Nanocomposite Coatings for Titanium Alloy Implants. J. Compos. Sci. 2020, 4, 41. [Google Scholar]
- Yiu, H.H.P.; Niu, H.-j.; Biermans, E.; van Tendeloo, G.; Rosseinsky, M.J. Designed Multifunctional Nanocomposites forBiomedical Applications. Adv. Funct. Mater. 2010, 20, 1599–1609. [Google Scholar] [CrossRef]
- Merino, S.; Martín, C.; Kostarelos, K.; Prato, M.; Vázquez, E. Nanocomposite Hydrogels: 3D Polymer Nanoparticle Synergies for On-Demand Drug Delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar] [CrossRef] [Green Version]
- Konku, Y.; Kutor, J.; Yaya, A.; Kan-Dapaah, K. Ablation of Hepatic Tumors through the Use of a Novel Magnetic Nanocomposite Probe: Magnetic Characterization and Finite Element Method Analysis. J. Nanotechnol. 2019, 2019, 6802125. [Google Scholar] [CrossRef] [Green Version]
- Bakhtiari, S.S.E.; Bakhsheshi-Rad, H.R.; Karbasi, S.; Tavakoli, M.; Razzaghi, M.; Ismail, A.F.; RamaKrishna, S.; Berto, F. Polymethyl Methacrylate-Based Bone Cements Containing Carbon Nanotubes and Graphene Oxide: An Overview of Physical, Mechanical, and Biological Properties. Polymers 2020, 12, 1469. [Google Scholar] [CrossRef] [PubMed]
- Bonyár, A.; Izsold, Z.; Borók, A.; Csarnovics, I.; Himics, L.; Veres, M.; Harsányi, G. PDMS-Au/Ag Nanocomposite Films as Highly Sensitive SERS Substrates. Proceedings 2018, 2, 1060. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.S.; Lee, S.Y.; Kim, K.S.; Han, D.-W. State of the Art Biocompatible Gold Nanoparticles for Cancer Theragnosis. Pharmaceutics 2020, 12, 701. [Google Scholar] [CrossRef]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold Nanoshell-Localized Photothermal Ablation of Prostate Tumors in a Clinical Pilot Device Study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590–18596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, L.-W.; Lee, J.; Razu, M.E.; Jensen, E.C.; Kim, J. Fabrication of PDMS Nanocomposite Materials and Nanostructures for Biomedical Nanosystems. IEEE Trans. Nanobioscience 2015, 14, 841–849. [Google Scholar] [CrossRef]
- Roper, D.K.; Ahn, W.; Hoepfner, M. Microscale heat transfer transduced by surface plasmon resonant gold nanoparticles. J. Phys. Chem. C 2007, 111, 3636–3641. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Roemer, R.B.; Cetas, T.C. Three dimensional simulations of ferromagnatic implant hyperthermia. Med. Phys. 1992, 19, 989–997. [Google Scholar] [CrossRef]
- Balleyguier, C.; Bidault, F.; Mathieu, M.C.; Ayadi, S.; Couanet, D.; Sigal, R. BIRADSTM mammography: Exercises. Eur. J. Radiol. 2007, 61, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Ziadan, K.M.; Shswiand, A.; AL-Alattar, A.L. The electrochemical polymerization of conducting polymer PPy/PTFE. Iraqi J. Polym. 1998, 2, 95–102. [Google Scholar]
- Kim, A.D. Transport theory for light propagation in biological tissue. J. Opt. Soc. Am. A 2004, 21, 820–827. [Google Scholar] [CrossRef]
- Sarkar, S.; Gurjarpadhye, A.A.; Rylander, C.G.; Rylander, M.N. Optical properties of breast tumor phantoms containing carbon nanotubes and nanohorns. J. Biomed. Opt. 2011, 16, 051304. [Google Scholar] [CrossRef] [Green Version]
- Pennes, H.H. Analysis of tissue and arterial blood temperatures in the resting human forearm. J. Appl. Physiol. 1948, 1, 93–122. [Google Scholar] [CrossRef] [PubMed]
- Duck, F.A. Physical Properties of Tissue: A Comprehensive Reference Book; Academic Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Sundeep, S.; Repaka, R. Parametric sensitivity analysis of critical factors affecting the thermal damage during RFA of breast tumor. Int. J. Therm. Sci. 2018, 124, 366–374. [Google Scholar]
- Paruch, M. Mathematical Modeling of Breast Tumor Destruction Using Fast Heating during Radiofrequency Ablation. Materials 2020, 13, 136. [Google Scholar] [CrossRef] [Green Version]
- Wahab, A.A.; Salim, M.I.M.; Ahamat, M.A.; Manaf, N.A.; Yunus, J.; Lai, K.W. Thermal distribution analysis of three-dimensional tumor-embedded breast models with different breast density compositions. Med. Biol. Eng. Comput. 2016, 54, 1363–1373. [Google Scholar] [CrossRef]
- Rylander, M.N.; Feng, Y.; Bass, J.; Diller, K.R. Heat shock protein expression and damage optimization for laser therapy design. Lasers Surg. Med. 2007, 39, 734–746. [Google Scholar] [CrossRef]
- Vorotnikova, E.; Ivkov, R.; Foreman, A.; Tries, M.; Braunhut, S.J. The magnitude and time-dependence of the apoptotic response of normal and malignant cells subjected to ionizing radiation versus hyperthermia. Int. J. Radiat. Biol. 2006, 82, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Boolbol, S.K.; Cate, S.P. Role of ablation in the treatment of breast cancer: A review. World J. Surg. Proced. 2015, 5, 106–110. [Google Scholar] [CrossRef]
- Mooney, R.; Schena, E.; Saccomandi, P.; Zhumkhawala, A.; Aboody, K.; Berlin, J.M. Gold nanorod-mediated near-infrared laser ablation: In vivo experiments on mice and theorectical analysis at different settings. Int. J. Hyperth 2017, 33, 150–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kannadorai, R.K.; Liu, Q. Optimization in interstitial plasmonic photothermal therapy for treatment planning. Med. Phys. 2013, 40, 103301. [Google Scholar] [CrossRef]
- Qin, Z.; Wang, Y.; Randrianalisoa, J.; Raeesi, V.; Chan, W.C.W.; Lipiński, W.; Bischof, J.C. Quantitative Comparison of Photothermal Heat Generation between Gold Nanospheres and Nanorods. Sci. Rep. 2016, 6, 29836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, L.C.; Bickford, L.R.; Lewinski, N.A.; Coughlin, A.J.; Hu, Y.; Day, E.S.; West, J.L.; Drezek, R.A. A New Era for Cancer Treatment: Gold-Nanoparticle- Mediated Thermal Therapies. Small 2010, X, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Beik, J.; Hashemian, R.; Laurent, S.; Farashahi, A.; Mobini, M.; Ghaznavi, H.; Shakeri-Zadeh, A. MRI-based numerical modeling strategy for simulation and treatment planning of nanoparticle-assisted photothermal therapy. Phys. Med. 2019, 66, 124–132. [Google Scholar] [CrossRef]
- Yao, A.; Ai, F.; Wang, D.; Huang, W.; Zhang, X. Synthesis, characterization and in vitro cytotoxicity of self-regulating magnetic implant material for hyperthermia application. Mater. Sci. Eng. C 2009, 29, 2525–2529. [Google Scholar] [CrossRef]
- Zhang, W.; Zuo, X.; Niu, Y.; Wu, C.; Wang, S.; Guanb, S.; Ravi, S.; Silva, P. Novel nanoparticles with Cr3+ substituted ferrite for self-regulating temperature hyperthermia. Nanoscale 2017, 9, 13929. [Google Scholar] [CrossRef] [PubMed]




| Tissue | Coefficients, (m) | |
|---|---|---|
| Absorption, | Reduced Scattering, | |
| Fat | 3 | 950 |
| Gland | 6 | 1100 |
| Tissue | c [J (kg K)] | [W (mK)] | [kg m] | [W m] | [s] |
|---|---|---|---|---|---|
| Fat | 2348 | 0.21 | 911 | 400 | 0.0002 |
| Gland | 2960 | 0.48 | 1041 | 700 | 0.0005 |
| Blood | 3617 | - | 1050 | - | - |
| Sample | [Au] (wt.%) | W | W | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ddt (°C/s) | (°C) | (%) | ddt (°C/s) | (°C) | (%) | ||||
| In Aqueous Solution | |||||||||
| AuNP-5 | 5 | 0.03 | 8.32 | 29.61 | 0.08 | 17.84 | 63.47 | ||
| AuNP-10 | 10 | 0.06 | 15.20 | 26.38 | 0.09 | 27.46 | 47.66 | ||
| No photoseed | - | - | 1.30 | - | - | 2.70 | - | ||
| In-vitro Suspension | |||||||||
| AuNP-10 + Cells | 10 | - | 5.40 | - | - | 11.5 | - | ||
| Cells only | - | - | 1.80 | - | - | 2.70 | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konku-Asase, Y.K.; Kan-Dapaah, K. Plasmonic Nanocomposite Implants for Interstitial Thermotherapy: Experimental and Computational Analysis. Materials 2021, 14, 841. https://doi.org/10.3390/ma14040841
Konku-Asase YK, Kan-Dapaah K. Plasmonic Nanocomposite Implants for Interstitial Thermotherapy: Experimental and Computational Analysis. Materials. 2021; 14(4):841. https://doi.org/10.3390/ma14040841
Chicago/Turabian StyleKonku-Asase, Yvonne Kafui, and Kwabena Kan-Dapaah. 2021. "Plasmonic Nanocomposite Implants for Interstitial Thermotherapy: Experimental and Computational Analysis" Materials 14, no. 4: 841. https://doi.org/10.3390/ma14040841






