Olive Stones as Filler for Polymer-Based Composites: A Review
Abstract
1. Introduction
2. Olive Waste Management, Olive Fruits, and Lignocellulosic Materials
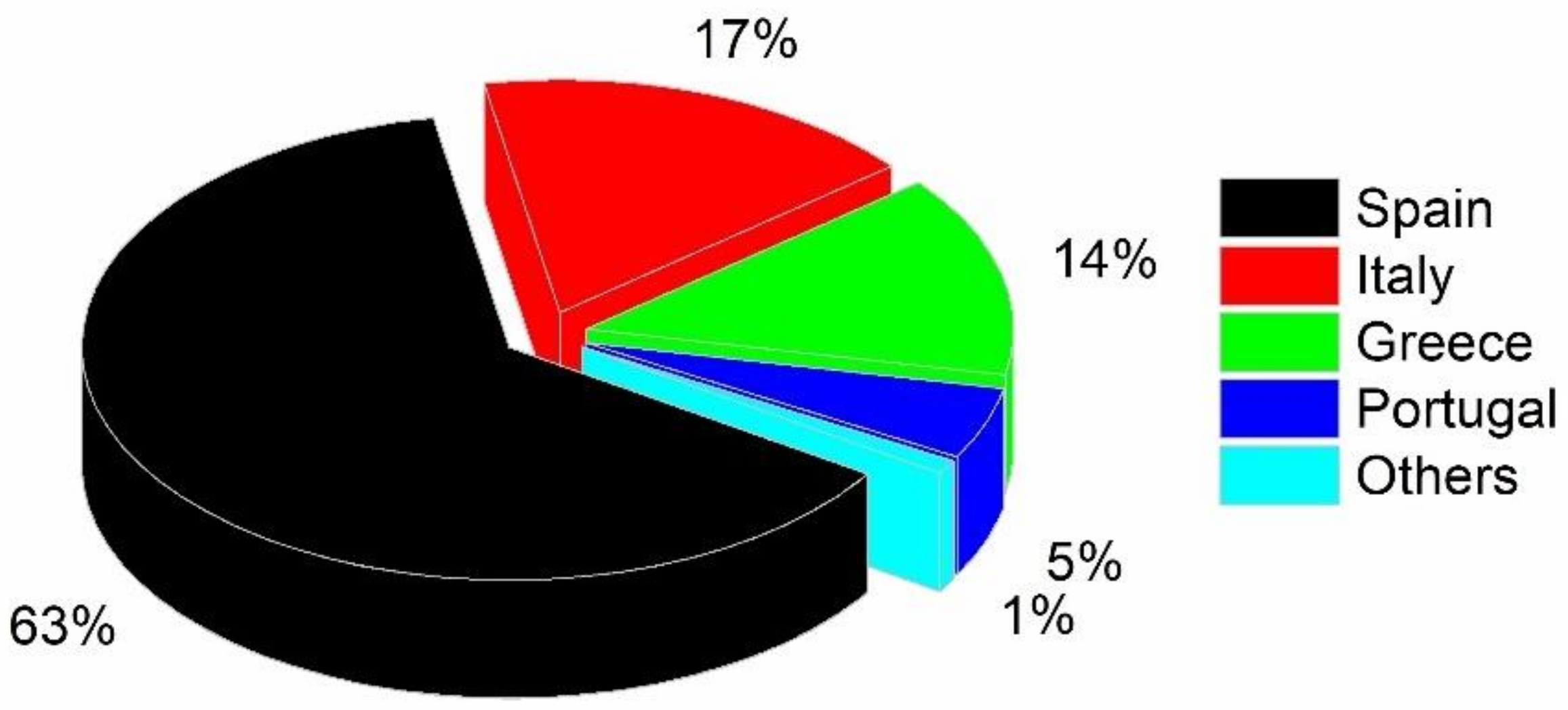
2.1. Olive Waste Management and Economic Perspective
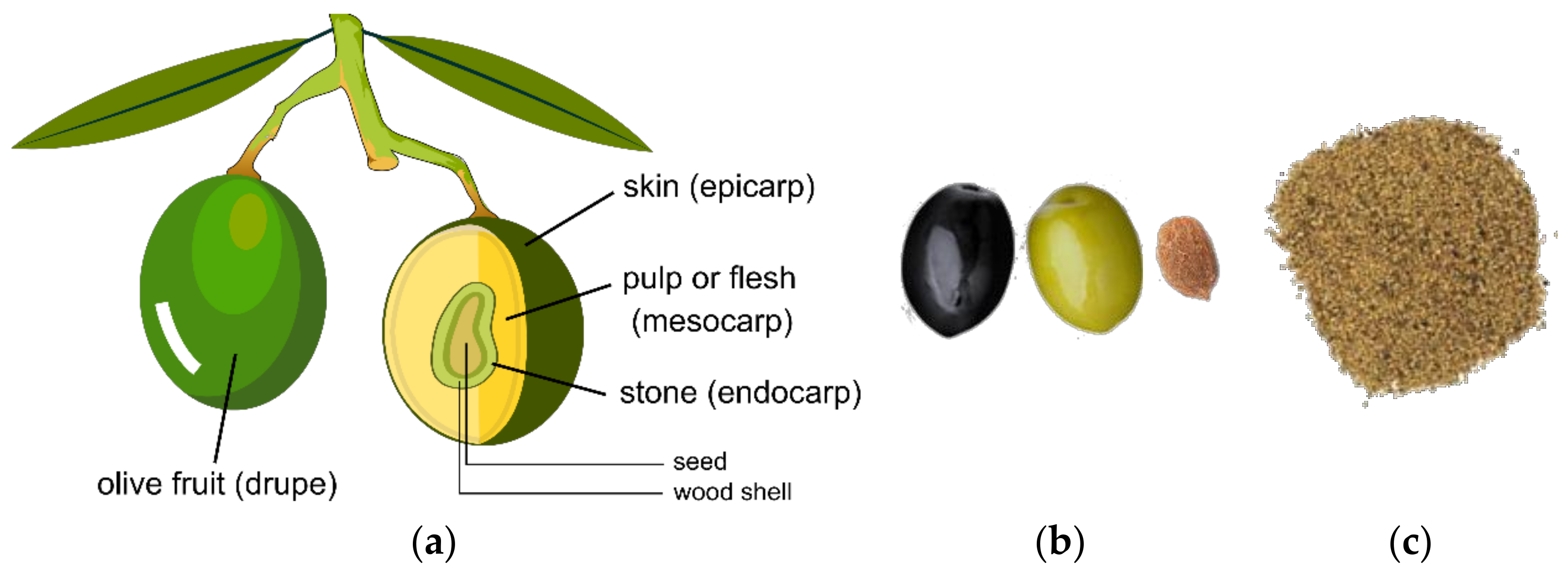
2.2. Olive Fruit Parts
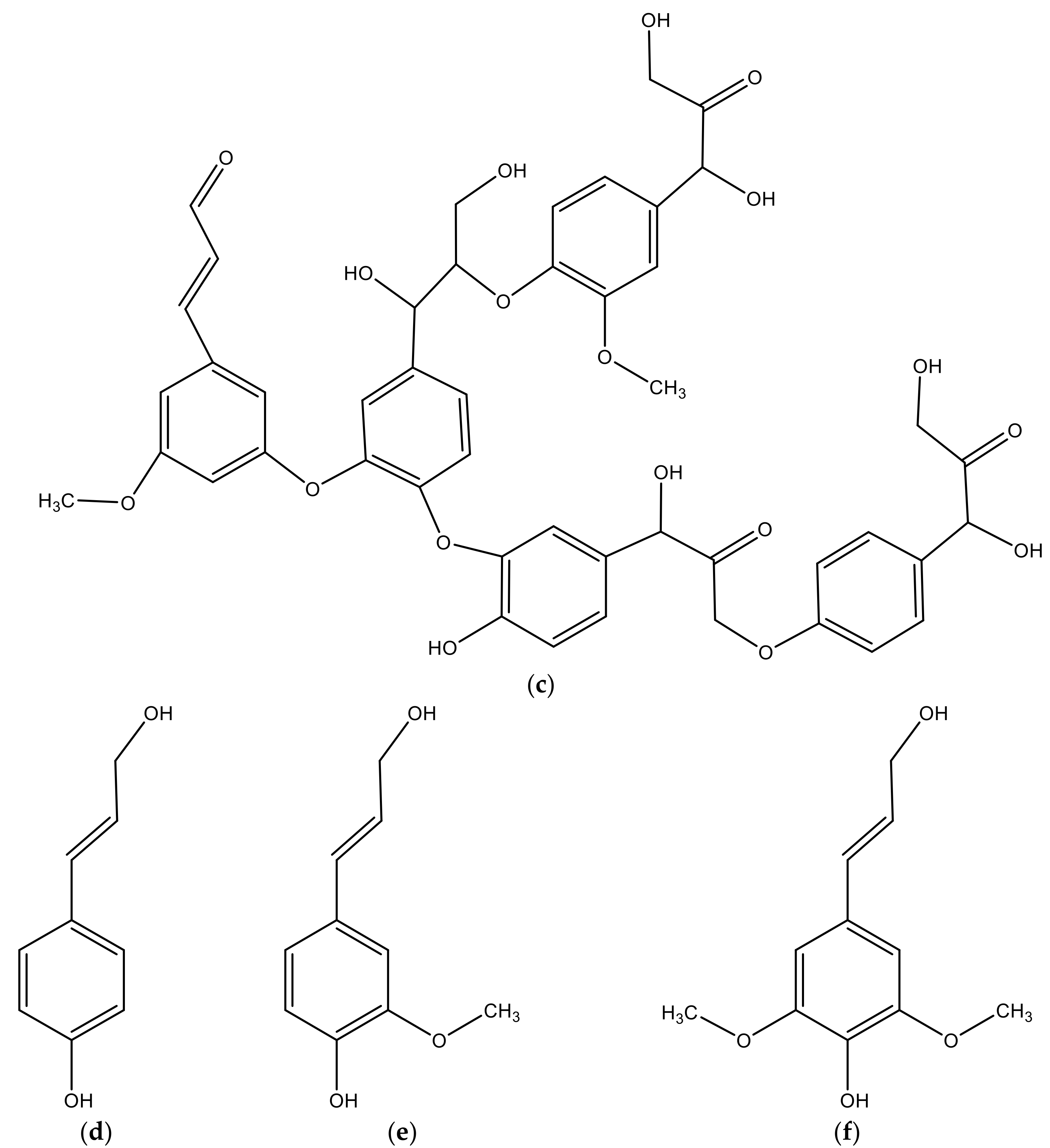
2.3. Olive Stones’ Chemical Composition
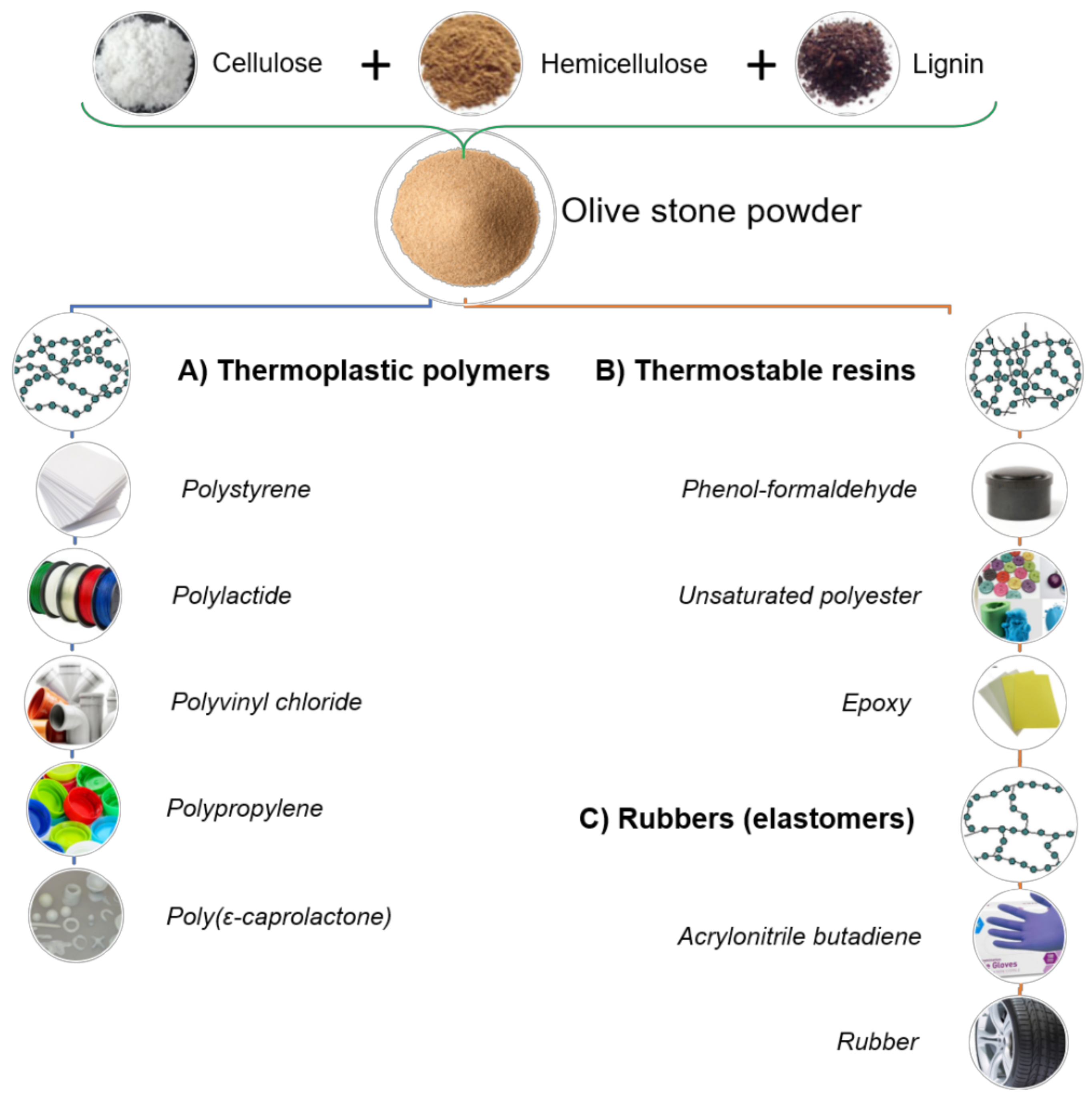
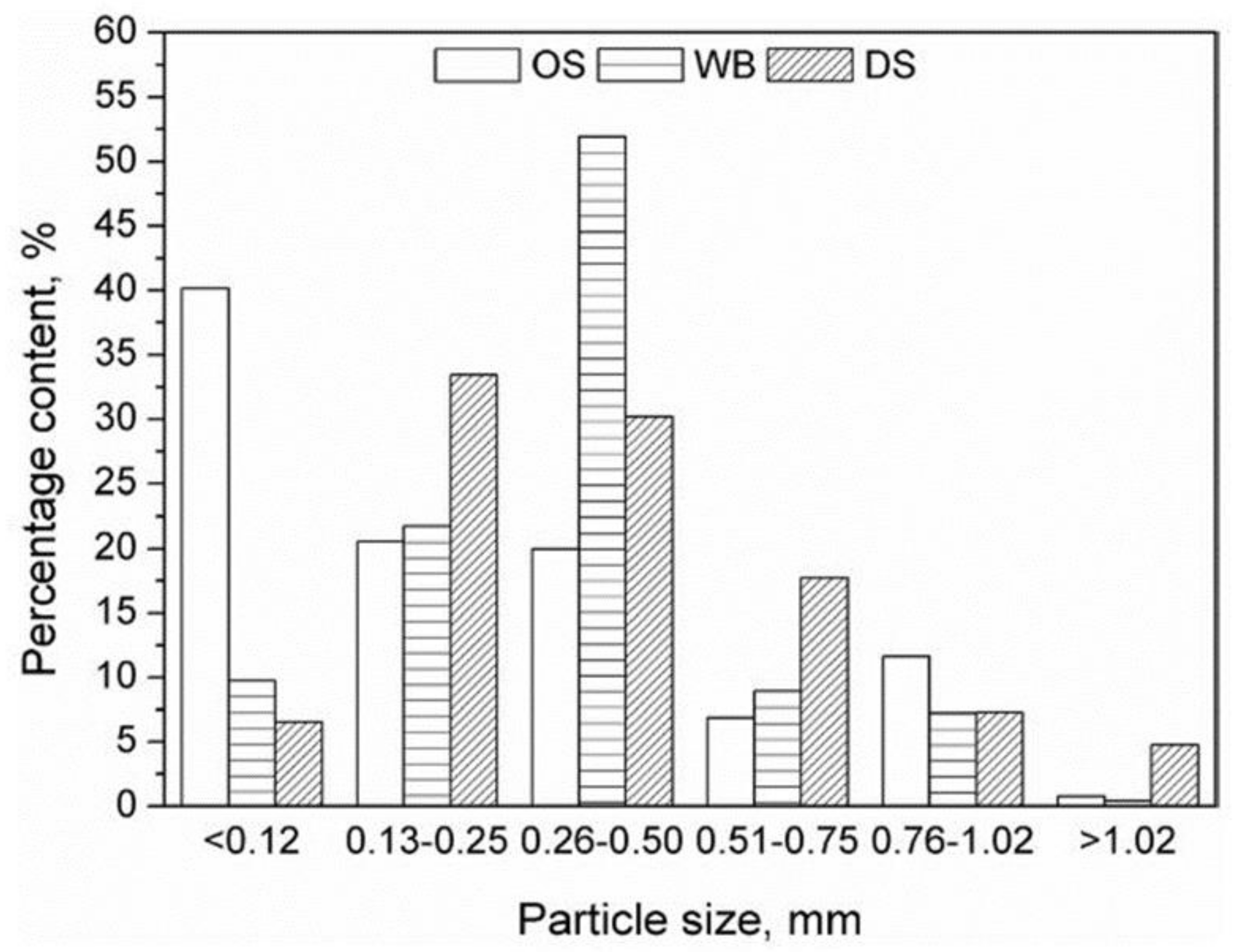
2.4. Lignocellulosic Materials as Fillers
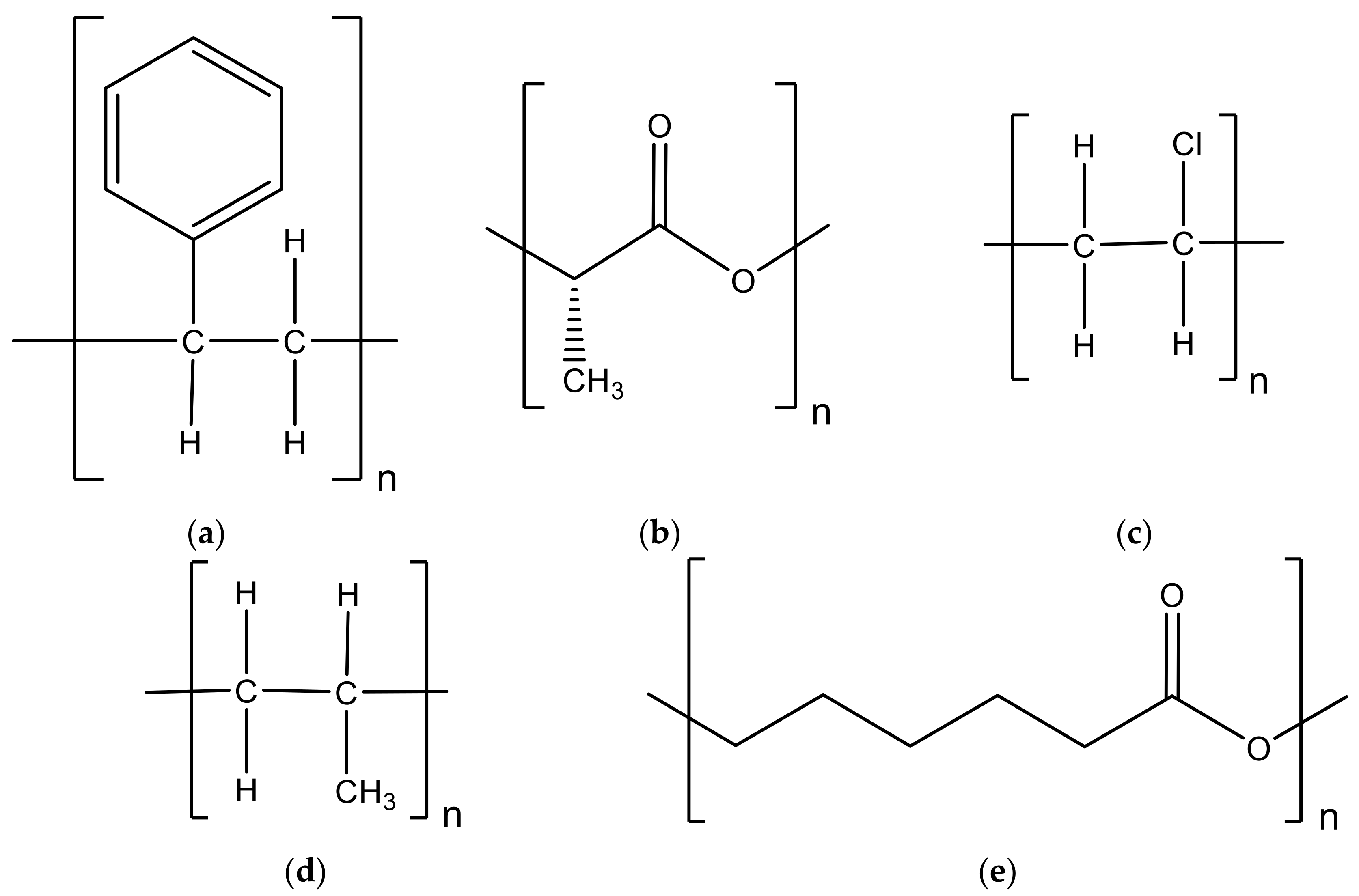
3. Thermoplastic Polymers
3.1. Polystyrene
3.2. Recycled Post-Consumer Plastic Material
3.3. Polylactide or Polylactic Acid
3.4. Polyvinyl Chloride
3.5. Polypropylene
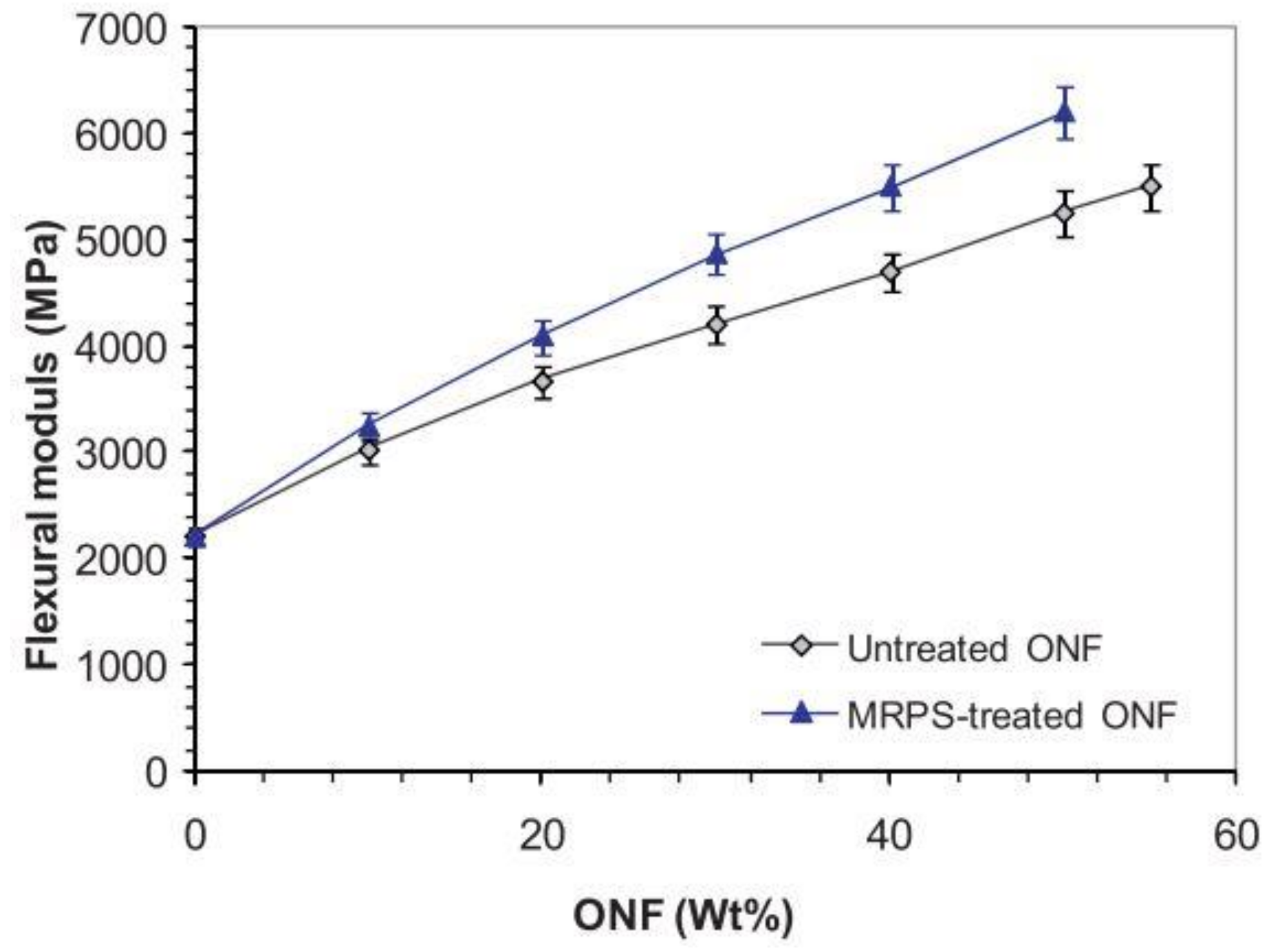
3.6. Poly(ε-Caprolactone)
4. Thermosetting Resins
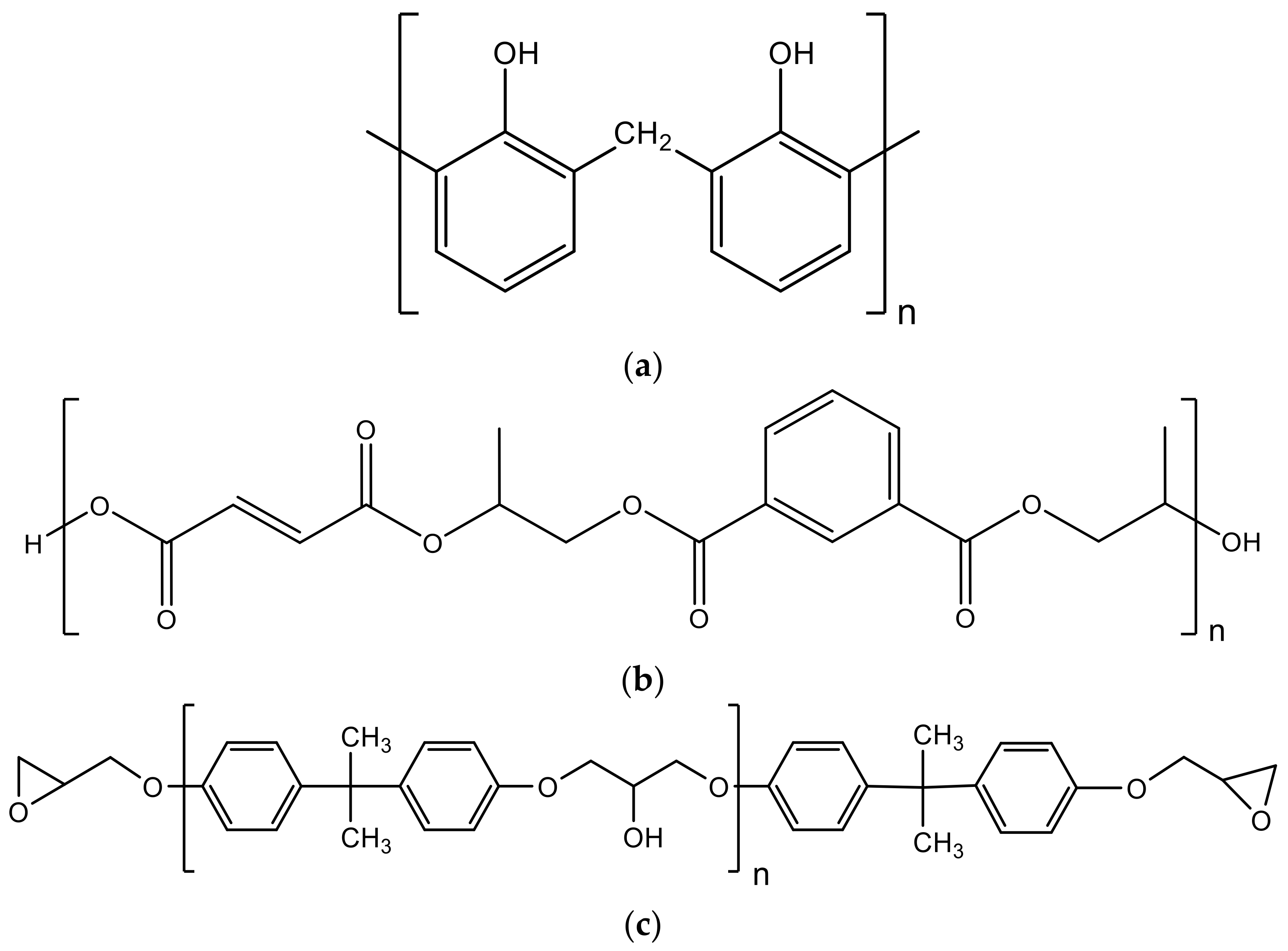
4.1. Phenol-Formaldehyde Resins
4.2. Unsaturated Polyester Resins
4.3. Epoxy Resins
5. Rubbers or Elastomeric Polymers
6. Synthetic Summary
7. Discussion
8. Conclusions
- -
- The various studies carried out with different types of polymer matrices demonstrate the interest of the scientific community in the reuse of olive stone residues in polymeric composites;
- -
- Resins filled with fillers based in olive stones present higher modulus than neat polymers, but the strength is affected by the filler content. This drawback does not provide applications for structural applications, but it is a promising solution for non-structural applications. For this purpose, studies covering the fracture and fatigue behavior are expected due to their absence in the literature;
- -
- The developed studies reveal that the mechanical properties depend on the filler/matrix interface. The functionalization of the olive stone particles with functionalization or coupling agents showed significant improvements, however, for each type of polymeric matrix, the functionalization agent must be different due to its distinct polarity and physical-chemical interactions. In this context, a complete study on the best functionalization agent and an optimized experimental procedure is highly recommended and should be a priority in future studies;
- -
- Olive stone residues constitute a cheap and useful source for reinforcing composites, however, several challenges must still be overcome. Therefore, more research is needed to achieve structural properties and, at same time, to valorize the olive oil industry.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kapellakis, I.E.; Tsagarakis, K.P.; Crowther, J.C. Olive oil history, production and by-product management. Rev. Environ. Sci. Bio/Technol. 2008, 7, 1–26. [Google Scholar] [CrossRef]
- Russo, G.; Beritognolo, I.; Bufacchi, M.; Stanzione, V.; Pisanelli, A.; Ciolfi, M.; Lauteri, M.; Brush, S.B. Advances in biocultural geography of olive tree (Olea europaea L.) landscapes by merging biological and historical assays. Sci. Rep. 2020, 10, 7673. [Google Scholar] [CrossRef]
- Cimato, A.; Attilio, C. Worldwide diffusion and relevance of olive culture. In Olive Diseases and Disorders; Schena, L., Agosteo, G.E., Cacciola, S.O., Eds.; Transworld Research Network: Kerala, India, 2011. [Google Scholar]
- Producing 69% of the World’s Production, the EU is the Largest Producer of Olive Oil. Available online: https://ec.europa.eu/info/news/producing-69-worlds-production-eu-largest-producer-olive-oil-2020-feb-04_en (accessed on 16 December 2020).
- Abu Tayeh, H.N.; Azaizeh, H.; Gerchman, Y. Circular economy in olive oil production—Olive mill solid waste to ethanol and heavy metal sorbent using microwave pretreatment. Waste Manag. 2020, 113, 321–328. [Google Scholar] [CrossRef] [PubMed]
- De la Casa, J.A.; Castro, E. Recycling of washed olive pomace ash for fired clay brick manufacturing. Constr. Build. Mater. 2014, 61, 320–326. [Google Scholar] [CrossRef]
- Stamatelatou, K.; Blika, P.S.; Ntaikou, I.; Lyberatos, G. Integrated Management Methods for the Treatment and/or Valorization of Olive Mill Wastes. In Novel Technologies in Food Science: Their Impact on Products, Consumer Trends and the Environment; McElhatton, A., do Amaral Sobral, P.J., Eds.; Springer: New York, NY, USA, 2012; pp. 65–118. [Google Scholar] [CrossRef]
- Banat, R. Olive Pomace Flour as Potential Organic Filler in Composite Materials: A Brief Review. Am. J. Polym. Sci. 2019, 9, 10–15. [Google Scholar] [CrossRef]
- Reis, P.N.B.; Ferreira, J.A.M.; Antunes, F.V.; Costa, J.D.M. Flexural behaviour of hybrid laminated composites. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1612–1620. [Google Scholar] [CrossRef]
- Saheb, D.N.; Jog, J.P. Natural fiber polymer composites: A review. Adv. Polym. Technol. J. Polym. Process. Inst. 1999, 18, 351–363. [Google Scholar] [CrossRef]
- Folkes, M.J. Short Fiber Reinforced Thermoplastic Composites; John Wiley and Sons: New York, NY, USA, 1982. [Google Scholar]
- Bledzki, A.K.; Gassan, J. Natural fiber reinforced plastics. In Handbook of Engineering Polymeric Materials; Marcel Dekker Inc.: New York, NY, USA, 1997; pp. 787–810. [Google Scholar]
- Joseph, P.V.; Joseph, K.; Thomas, S. Effect of processing variables on the mechanical properties of sisal-fiber-reinforced polypropylene composites. Compos. Sci. Technol. 1999, 59, 1625–1640. [Google Scholar] [CrossRef]
- Jayaraman, K. Manufacturing sisal-polypropylene composites with minimum fibre degradation. Compos. Sci. Technol. 2003, 63, 367–374. [Google Scholar] [CrossRef]
- Van de Velde, K.; Kiekens, P. Thermoplastic pultrusion of natural fibre reinforced composites. Compos. Struct. 2001, 54, 355–360. [Google Scholar] [CrossRef]
- Reis, P.N.B.; Ferreira, J.A.M.; Silva, P.A.A. Mechanical behaviour of composites filled by agro-waste materials. Fibers Polym. 2011, 12, 240–246. [Google Scholar] [CrossRef]
- Nourbakhsh, A.; Ashori, A. Wood plastic composites from agro-waste materials: Analysis of mechanical properties. Bioresour. Technol. 2010, 101, 2525–2528. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.N.B.; Silva, M.P.; Santos, P.; Parente, J.M.; Bezazi, A. Viscoelastic behaviour of composites with epoxy matrix filled by cork powder. Compos. Struct. 2020, 234, 111669. [Google Scholar] [CrossRef]
- Fountoulakis, M.S.; Dokianakis, S.N.; Kornaros, M.E.; Aggelis, G.G.; Lyberatos, G. Removal of phenolics in olive mill wastewaters using the white-rot fungus Pleurotus ostreatus. Water Res. 2002, 36, 4735–4744. [Google Scholar] [CrossRef]
- Sabbah, I.; Marsook, T.; Basheer, S. The effect of pretreatment on anaerobic activity of olive mill wastewater using batch and continuous systems. Process Biochem. 2004, 39, 1947–1951. [Google Scholar] [CrossRef]
- Paredes, C.; Cegarra, J.; Roig, A.; Sánchez-Monedero, M.A.; Bernal, M.P. Characterization of olive mill wastewater (alpechin) and its sludge for agricultural purposes. Bioresour. Technol. 1999, 67, 111–115. [Google Scholar] [CrossRef]
- Otles, S.; Selek, I. Treatment of olive mill wastewater and the use of polyphenols obtained after treatment. Int. J. Food Stud. 2012, 1. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Moreno, J.L.; Doula, M. Olive oil waste management EU legislation: Current situation and policy recommendations. Int. J. Chem. Environ. Eng. Syst. 2012, 3, 65–77. [Google Scholar]
- Dutournié, P.; Jeguirim, M.; Khiari, B.; Goddard, M.-L.; Jellali, S. Olive Mill Wastewater: From a Pollutant to Green Fuels, Agricultural Water Source, and Bio-Fertilizer. Part 2: Water Recovery. Water 2019, 11, 768. [Google Scholar] [CrossRef]
- Mantzavinos, D.; Kalogerakis, N. Treatment of olive mill effluents: Part I. Organic matter degradation by chemical and biological processes—An overview. Environ. Int. 2005, 31, 289–295. [Google Scholar] [CrossRef]
- Azbar, N.; Bayram, A.; Filibeli, A.; Muezzinoglu, A.; Sengul, F.; Ozer, A. A review of waste management options in olive oil production. Crit. Rev. Environ. Sci. Technol. 2004, 34, 209–247. [Google Scholar] [CrossRef]
- Rodriguez, G.; Lama, A.; Rodríguez, R.; Jiménez, A.; Guillén, R. Fernández-Bolaños. Olive stone an attractive source of bioactive and valuable compounds. J. Bioresour. Technol. 2008, 99, 5261–5269. [Google Scholar] [CrossRef]
- Cristofaro, D. A Process for the Realization of Plates and Panels Consisting of Exhausted Olive Husks of Crushed Olive Stones and Polypropylene, and Derived Product. International Patent No. WO 9738834, 23 October 1997. [Google Scholar]
- Bianchi, G. Lipids and phenols in table olives. Eur. J. Lipid Sci. Technol. 2003, 105, 229–242. [Google Scholar] [CrossRef]
- Hernández-Beltrán, J.U.; Hernández-de Lira, I.O.; Cruz-Santos, M.M.; Saucedo-Luevanos, A.; Hernández-Terán, F.; Balagurusamy, N. Insight into Pretreatment Methods of Lignocellulosic Biomass to Increase Biogas Yield: Current State, Challenges, and Opportunities. Appl. Sci. 2019, 9, 3721. [Google Scholar] [CrossRef]
- Matos, M.; Barreiro, M.F.; Gandini, A. Olive stone as a renewable source of biopolyols. Ind. Crop. Prod. 2010, 32, 7–12. [Google Scholar] [CrossRef]
- Ioelovich, M. Cellulose as a nanostructured polymer: A short review. BioResources 2008, 3, 1403–1418. [Google Scholar]
- Puls, J. Chemistry and biochemistry of hemicelluloses: Relationship between hemicellulose structure and enzymes required for hydrolysis. Macromol. Symp. 1997, 120, 183–196. [Google Scholar] [CrossRef]
- Feofilova, E.P.; Mysyakina, I.S. Lignin: Chemical structure, biodegradation, and practical application (a review). Appl. Biochem. Microbiol. 2016, 52, 573–581. [Google Scholar] [CrossRef]
- Nasrullah, A.; Bhat, A.H.; Khan, A.S.; Ajab, H. Comprehensive approach on the structure, production, processing, and application of lignin. Lignocellul. Fibre Biomass Based Compos. Mater. 2017, 165–178. [Google Scholar] [CrossRef]
- Monties, B. Plant cell walls as fibrous lignocellulosic composites: Relations with lignin structure and function. Anim. Feed Sci. Technol. 1991, 32, 159–175. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Michelin, M.; Vicente, A.A.; Teixeira, J.A.; Cerqueira, M.Â. Lignocellulosic Materials: Sources and Processing Technologies. In Lignocellulosic Materials and Their Use in Bio-Based Packaging; Ballesteros, L.F., Michelin, M., Vicente, A.A., Teixeira, J.A., Cerqueira, M.Â., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 13–33. [Google Scholar] [CrossRef]
- Kamel, S. Nanotechnology and its applications in lignocellulosic composites, a mini review. Express Polym. Lett. 2007, 1, 546–575. [Google Scholar] [CrossRef]
- Părpăriţă, E.; Darie, R.N.; Popescu, C.-M.; Uddin, M.A.; Vasile, C. Structure–morphology–mechanical properties relationship of some polypropylene/lignocellulosic composites. Mater. Des. 2014, 56, 763–772. [Google Scholar] [CrossRef]
- Georgopoulos, S.T.; Tarantili, P.A.; Avgerinos, E.; Andreopoulos, A.G.; Koukios, E.G. Thermoplastic polymers reinforced with fibrous agricultural residues. Polym. Degrad. Stab. 2005, 90, 303–312. [Google Scholar] [CrossRef]
- Hamida, B.; Ahmed, M.; Nadia, N.; Samira, M. Mechanical properties of polystyrene/olive stone flour composites. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 127–132. [Google Scholar]
- La Mantia, F.; Dintcheva, N.T.; Morreale, M.; Vaca-Garcia, C. Green composites of organic materials and recycled post-consumer polyethylene. Polym. Int. 2004, 53, 1888–1891. [Google Scholar] [CrossRef]
- Koutsomitopoulou, A.F.; Bénézet, J.C.; Bergeret, A.; Papanicolaou, G.C. Preparation and characterization of olive pit powder as a filler to PLA-matrix bio-composites. Powder Technol. 2014, 255, 10–16. [Google Scholar] [CrossRef]
- Perinović, S.; Andričić, B.; Erceg, M. Thermal properties of poly(l-lactide)/olive stone flour composites. Thermochim. Acta 2010, 510, 97–102. [Google Scholar] [CrossRef]
- Perinović Jozić, S.; Jozić, D.; Erceg, M.; Andričić, B.; Bernstorff, S. Nonisothermal crystallization of poly(l-lactide) in poly(l-lactide)/olive stone flour composites. Thermochim. Acta 2020, 683, 178440. [Google Scholar] [CrossRef]
- Naghmouchi, I.; Mutjé, P.; Boufi, S. Polyvinyl chloride composites filled with olive stone flour: Mechanical, thermal, and water absorption properties. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Naghmouchi, I.; Mutjé, P.; Boufi, S. Olive stones flour as reinforcement in polypropylene composites: A step forward in the valorization of the solid waste from the olive oil industry. Ind. Crop. Prod. 2015, 72, 183–191. [Google Scholar] [CrossRef]
- Naghmouchi, I.; Espinach, F.X.; Mutjé, P.; Boufi, S. Polypropylene composites based on lignocellulosic fillers: How the filler morphology affects the composite properties. Mater. Des. 2015, 65, 454–461. [Google Scholar] [CrossRef]
- Tasdemir, M. Effects of Olive Pit and Almond Shell Powder on Polypropylene. Key Eng. Mater. 2017, 733, 65–68. [Google Scholar] [CrossRef]
- Tasdemir, M. Polypropylene/olive pit & almond shell polymer composites: Wear and friction. IOP Conf. Ser. Mater. Sci. Eng. 2017, 204, 012015. [Google Scholar] [CrossRef]
- Gümüş, B.E.; Yağci, Ö.; Erdoğan, D.C.; Taşdemir, M. Dynamical Mechanical Properties of Polypropylene Composites Filled with Olive Pit Particles. J. Test. Eval. 2019, 47, 2551–2561. [Google Scholar] [CrossRef]
- Hejna, A.; Sulyman, M.; Przybysz, M.; Saeb, M.R.; Klein, M.; Formela, K. On the Correlation of Lignocellulosic Filler Composition with the Performance Properties of Poly(ε-Caprolactone) Based Biocomposites. Waste Biomass Valorization 2020, 11, 1467–1479. [Google Scholar] [CrossRef]
- Chong, T.Y.; Law, M.C.; Chan, Y.S. The Potentials of Corn Waste Lignocellulosic Fibre as an Improved Reinforced Bioplastic Composites. J. Polym. Environ. 2021, 29, 363–381. [Google Scholar] [CrossRef]
- Montesdeoca-Contreras, J.V.; Paltán-Zhingre, C.A.; Muñoz-Cuenca, T.F.; Fajardo-Seminario, J.I.; López-López, L.M.; Lasso-Lazo, D.R. Study of natural fibers as filler in a polymeric matrix to make environment friendly materials. Proceedings of 2015 IEEE NW Russia Young Researchers in Electrical and Electronic Engineering Conference (EIConRusNW), St. Petersburg, Russia, 2–4 February 2015; pp. 332–335. [Google Scholar]
- Simitzis, J.; Sfyrakis, J. Adsorption of phenols on carbonaceous adsorbents produced from phenol-formaldehyde-resin mixed with olive stones. Die Angew. Makromol. Chem. 1988, 163, 47–61. [Google Scholar] [CrossRef]
- Simitzis, J.; Sfyrakis, J.; Faliagas, A. Adsorption properties and microporous structure of adsorbents produced from phenolic resin and biomass. J. Appl. Polym. Sci. 1995, 58, 541–550. [Google Scholar] [CrossRef]
- Simitzis, J.; Sfyrakis, J.; Faliagas, A. Characterization of pore structure by porosimetry and sorption on adsorbents produced from novolac-biomass. Mater. Chem. Phys. 1995, 41, 245–250. [Google Scholar] [CrossRef]
- Sfyrakis, J.; Faliagas, A.; Simitzis, J. Influence of pyrolysis temperature on the adsorptive properties of adsorbents produced from novolac and biomass. J. Appl. Polym. Sci. 1995, 55, 1739–1746. [Google Scholar] [CrossRef]
- Gil, M.V.; Martínez, M.; García, S.; Rubiera, F.; Pis, J.J.; Pevida, C. Response surface methodology as an efficient tool for optimizing carbon adsorbents for CO2 capture. Fuel Process. Technol. 2013, 106, 55–61. [Google Scholar] [CrossRef]
- Elsahli, E.; Elhrari, W.; Klash, A.; Shebani, A. The use of olive stone waste for production of particleboard using commercial polyster sealer as a binding agent. In Proceedings of the 1st International Conference on Chemical, Petroleum, and Gas Engineering, Alkhoms, Libya, 25–30 January 2016. [Google Scholar]
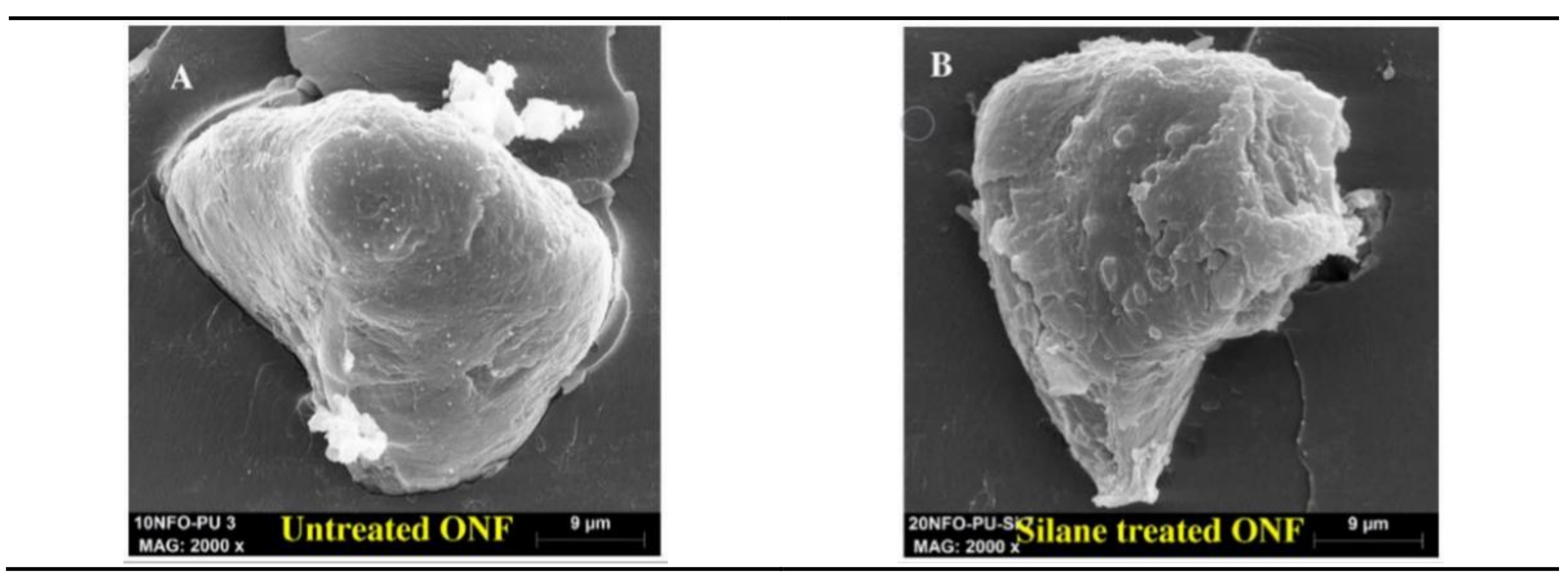
- Gharbi, A.; Hassen, R.B.; Boufi, S. Composite materials from unsaturated polyester resin and olive nuts residue: The effect of silane treatment. Ind. Crop. Prod. 2014, 62, 491–498. [Google Scholar] [CrossRef]
- Papanicolaou, G.C.; Koutsomitopoulou, A.F.; Sfakianakis, A. Effect of thermal fatigue on the mechanical properties of epoxy matrix composites reinforced with olive pits powder. J. Appl. Polym. Sci. 2012, 124, 67–76. [Google Scholar] [CrossRef]
- Papanicolaou, G.C.; Anastasiou, D.E. Development of environmentally friendly epoxy and composite adhesives and applications in single and mixed-modulus joints. J. Adhes. Sci. Technol. 2020, 1–16. [Google Scholar] [CrossRef]
- Papanicolaou, G.C.; Xepapadaki, A.G.; Angelakopoulos, G.C.; Zabaniotou, A.; Ioannidou, O. Use of solid residue from olive kernel pyrolysis for polymer matrix composite manufacturing: Physical and mechanical characterization. J. Appl. Polym. Sci. 2011, 119, 2167–2173. [Google Scholar] [CrossRef]
- Erkliğ, A.; Bulut, M.; Shihan, A. An experimental investigation on dynamic and mechanical characterization of olive pomace-filled glass/epoxy composite laminates. J. Braz. Soc. Mech. Sci. Eng. 2020, 42, 499. [Google Scholar] [CrossRef]
- Abd Mohammed, A. The experimental study and statistical evaluation of the wear and hardness of epoxy reinforced with natural materials. J. Eng. Sustain. Dev. 2016, 20, 14–24. [Google Scholar]
- Zhou, Y.; Fan, M.; Chen, L.; Zhuang, J. Lignocellulosic fibre mediated rubber composites: An overview. Compos. Part. B Eng. 2015, 76, 180–191. [Google Scholar] [CrossRef]
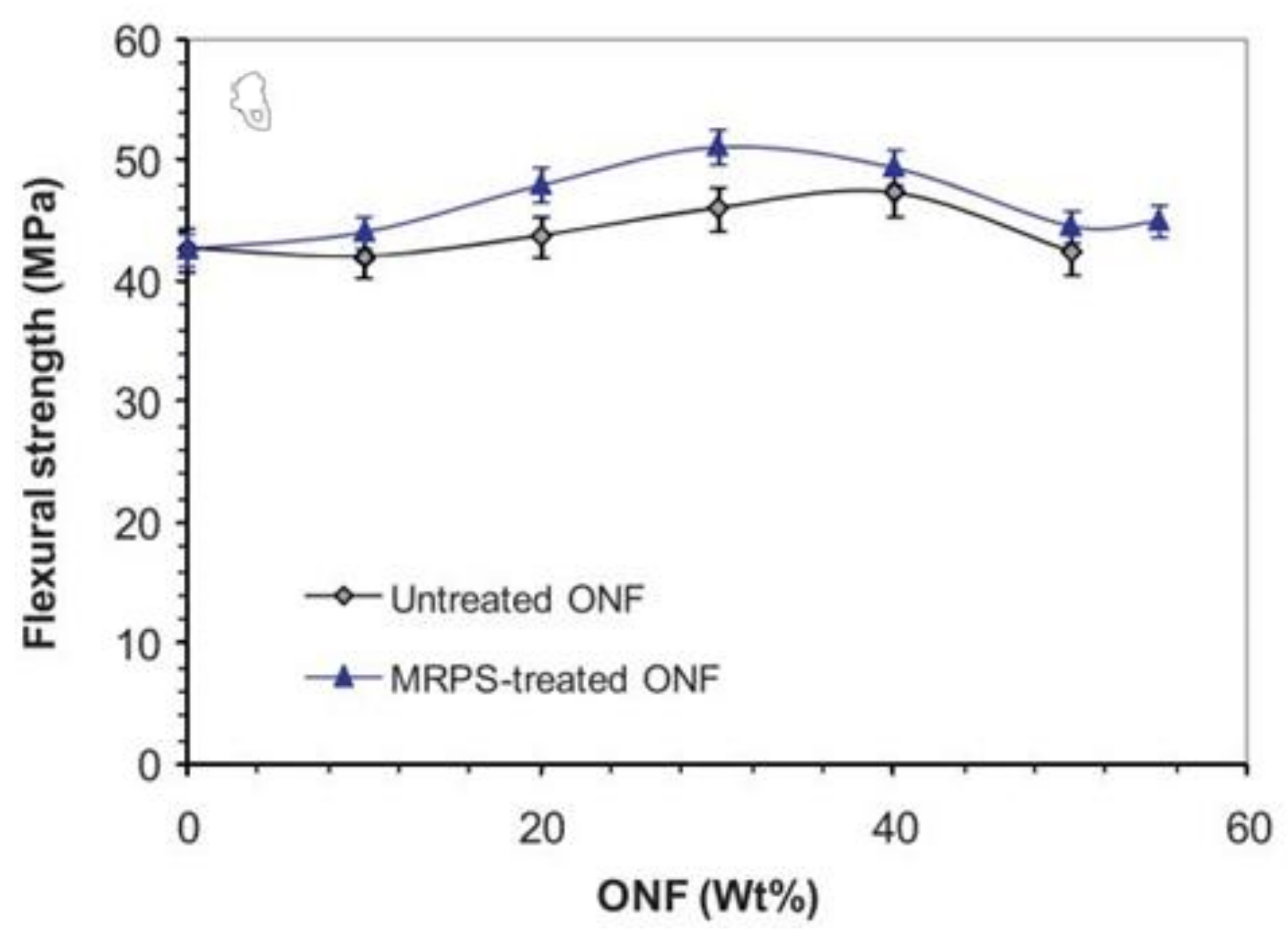
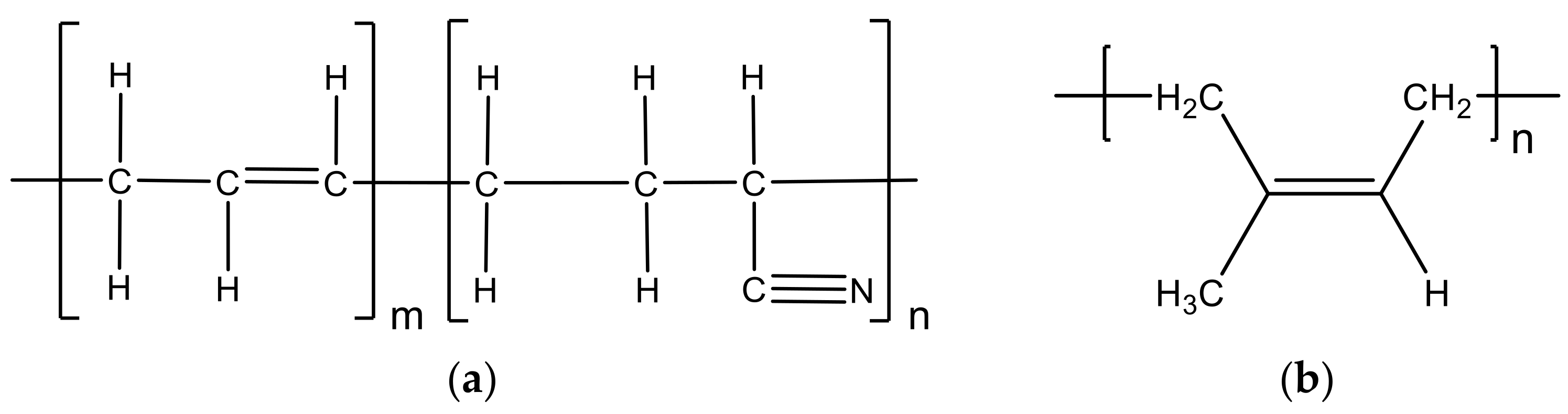
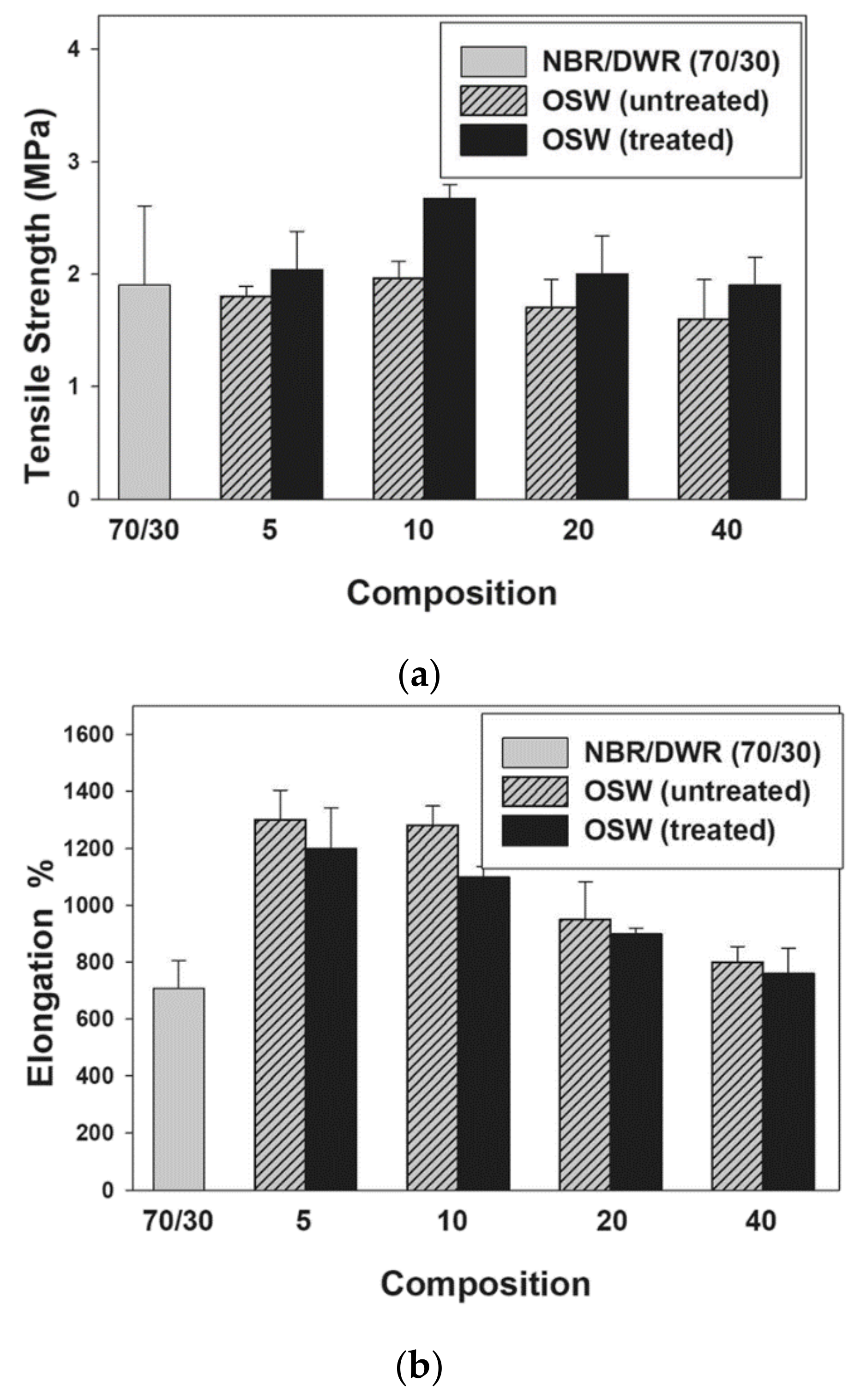
- Khalil, A.M.; El-Nemr, K.F.; Hassan, M.L. Acrylate-modified gamma-irradiated olive stones waste as a filler for acrylonitrile butadiene rubber/devulcanized rubber composites. J. Polym. Res. 2019, 26, 249. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Astray, G.; Carpena, M.; Fraga-Corral, M.; Lage, M.P.; Simal-Gandara, J. Valorization of by-products from olive oil industry and added-value applications for innovative functional foods. Food Res. Int. 2020, 109683. [Google Scholar] [CrossRef]
- Siracusa, G.; La Rosa, A.D.; Siracusa, V.; Trovato, M. Eco-compatible use of olive husk as filler in thermoplastic composites. J. Polym. Environ. 2001, 9, 157–161. [Google Scholar] [CrossRef]
- Costa, J.D.M.; Capela, C.; Ferreira, J.A.M. Mechanical behaviour of PVC/CaCO3 Particulate Composites–Influence of Temperature. Strain 2011, 47, e292–e304. [Google Scholar] [CrossRef]
- Chand, N.; Dan, T.K.; Verma, S.; Rohatgi, P.K. Rice husk ash filled polyester resin composites. J. Mater. Sci. Lett. 1987, 6, 733–735. [Google Scholar] [CrossRef]



| Application | Raw Transformed | Application Sector |
|---|---|---|
| Combustion | Electric or heat | All industries residential and commercial |
| Activated Carbon | Activated carbon | Food, chemical, petroleum, nuclear, mining, pharmacological industry |
| Bio-oil | Liquid and gas production | Wide field of industries |
| Olive seed oil | Olive seed oil | Food, pharmacological, and cosmetic industry |
| Furfural | Furfural | Wide field of industries as solvent |
| Plastic filled | Composite | Different industrial applications |
| Abrasive | Powder | Cleaning |
| Cosmetic | Cosmetic products | Cosmetic |
| Biosorbent | Granulated or powder stone | Metallurgy and food |
| Animal feed | Animal food | Food |
| Resins | Phenol–formaldehyde | Electrochemical |
| Fractionation | Soluble phenols and hemicellulose, lignin, and cellulose | Food, cosmetic, pharmaceutical, alcohol |
| General Chemical Compounds | Percentage (%) | CHCl3–EtOH Extractable Compounds | Percentage (%) |
|---|---|---|---|
| Cellulose | 31.29 | Alkanes | 1.7 |
| Hemicellulose | 21.9 | Triacylglycerols | 78 |
| Lignin | 26.5 | Free fatty acids | 7 |
| Moisture | 9.79 | Aliphatics alcohols | 0.1 |
| Fat | 5.53 | Triterpene alcohols | 1.5 |
| Proteins | 3.20 | Triterpene acids | 0.6 |
| Free sugars | 0.48 | Free sterols | traces |
| Others | 1.31 | Steryl esters | 1.1 |
| Unidentified | 10 |
| Type of Matrix | Polymer | Properties/Characterization | Application/Objectives | Ref. |
|---|---|---|---|---|
| Thermoplastic polymer | Polystyrene (PS) | Tensile strength, elongation at break, hardness | Manufacture composites with biodegradable properties, light weight, less expensive resources, easy processing, high specific modulus, and environmentally friendly. | [7] |
| Recycled post-consumer plastic material | Viscosity, tensile properties (elastic modulus, tensile strength and elongation at break), impact strength | Use very cheap filler in composite manufacture | [8] | |
| Polylactide (PLA) | Physical, thermal, mechanical properties, tensile modulus | [29] | ||
| differential scanning calorimetry (DSC), thermogravimetric analysis (TGA) | [27] | |||
| Filler | [30] | |||
| Polyvinyl chloride (PVC) | TGA | In plastic-based materials | [31] | |
| Polypropylene (PP) | In building, automotive industry, and outdoor products, such as deck floors, and furniture, park benches | [32] | ||
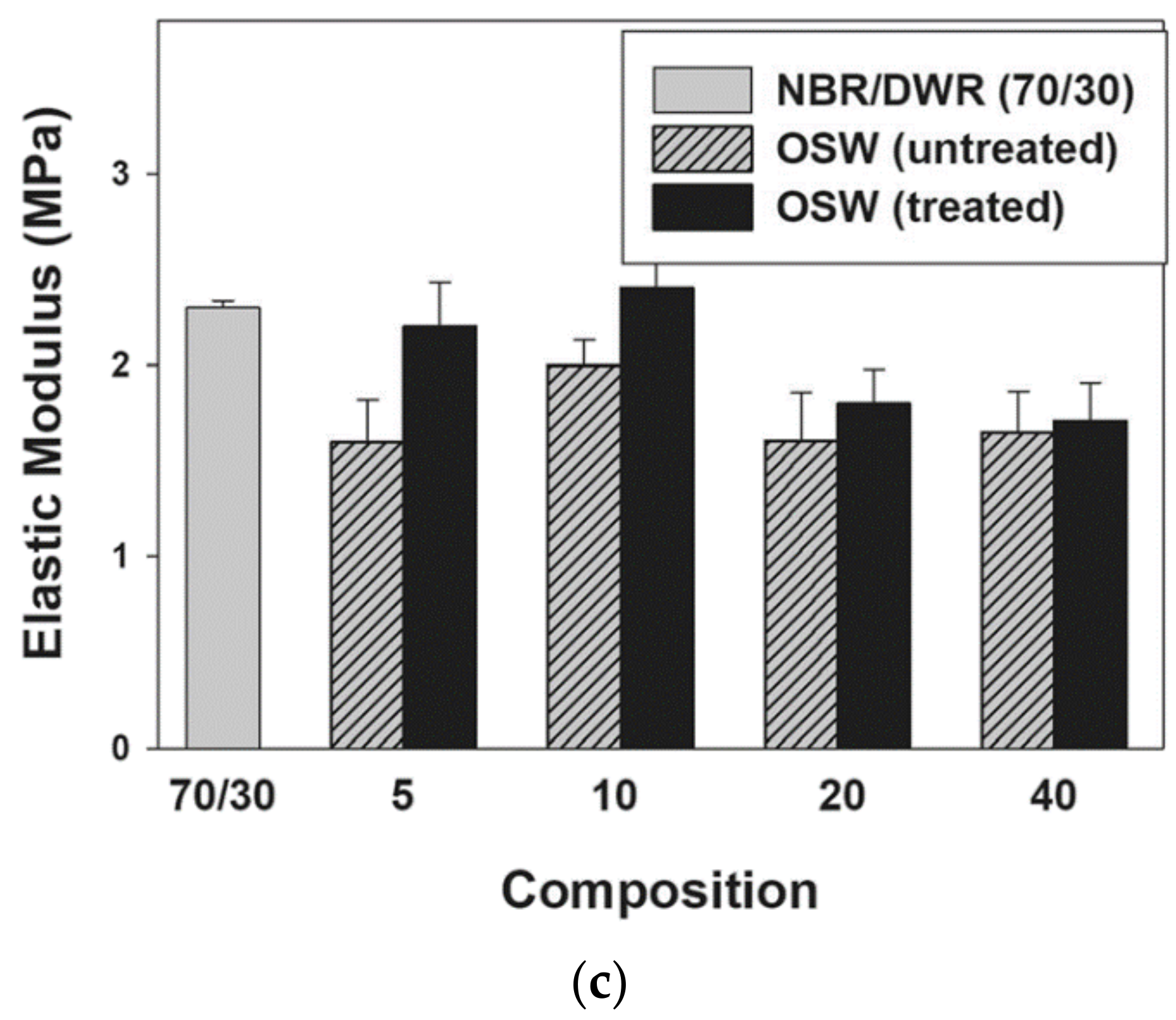
| Elasticity modulus | [33,34] | |||
| Industrial applications | [35] | |||
| Polycaprolactone (PCL) | Flexural modulus | [3] | ||
| Thermosetting resins | Phenol-formaldehyde | Adsorption | Adsorbents | [36] |
| [37] | ||||
| [38] | ||||
| [39] | ||||
| [40] | ||||
| Unsaturated polyester | Porosity, particle size, permeability | Particleboards | [4] | |
| Engineering applications | [5] | |||
| Epoxy | Bending modulus | Adhesive system | [41] | |
| [42] | ||||
| Advanced uses | [43] | |||
| [44] | ||||
| Wear rate, hardness | [45] | |||
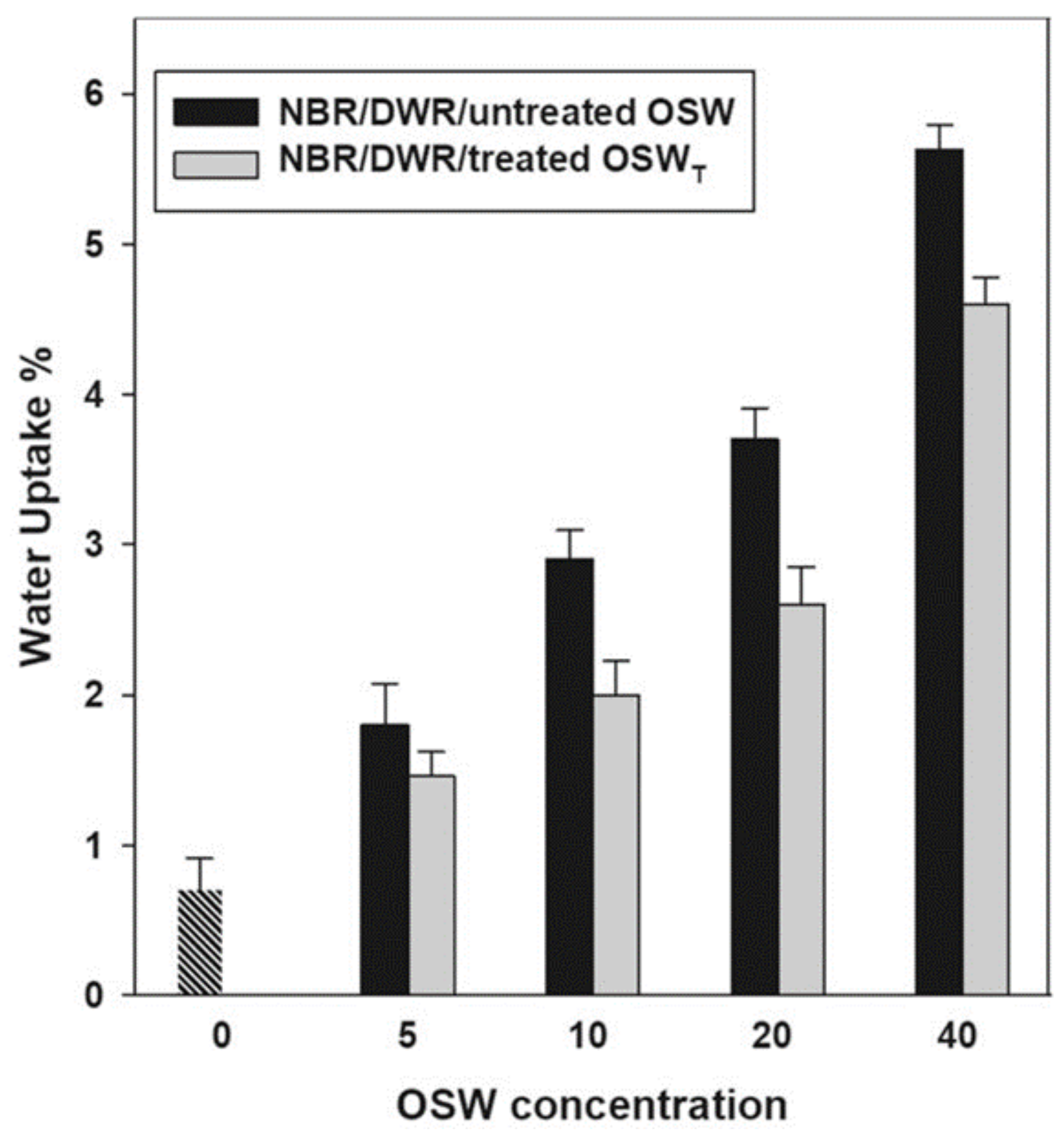
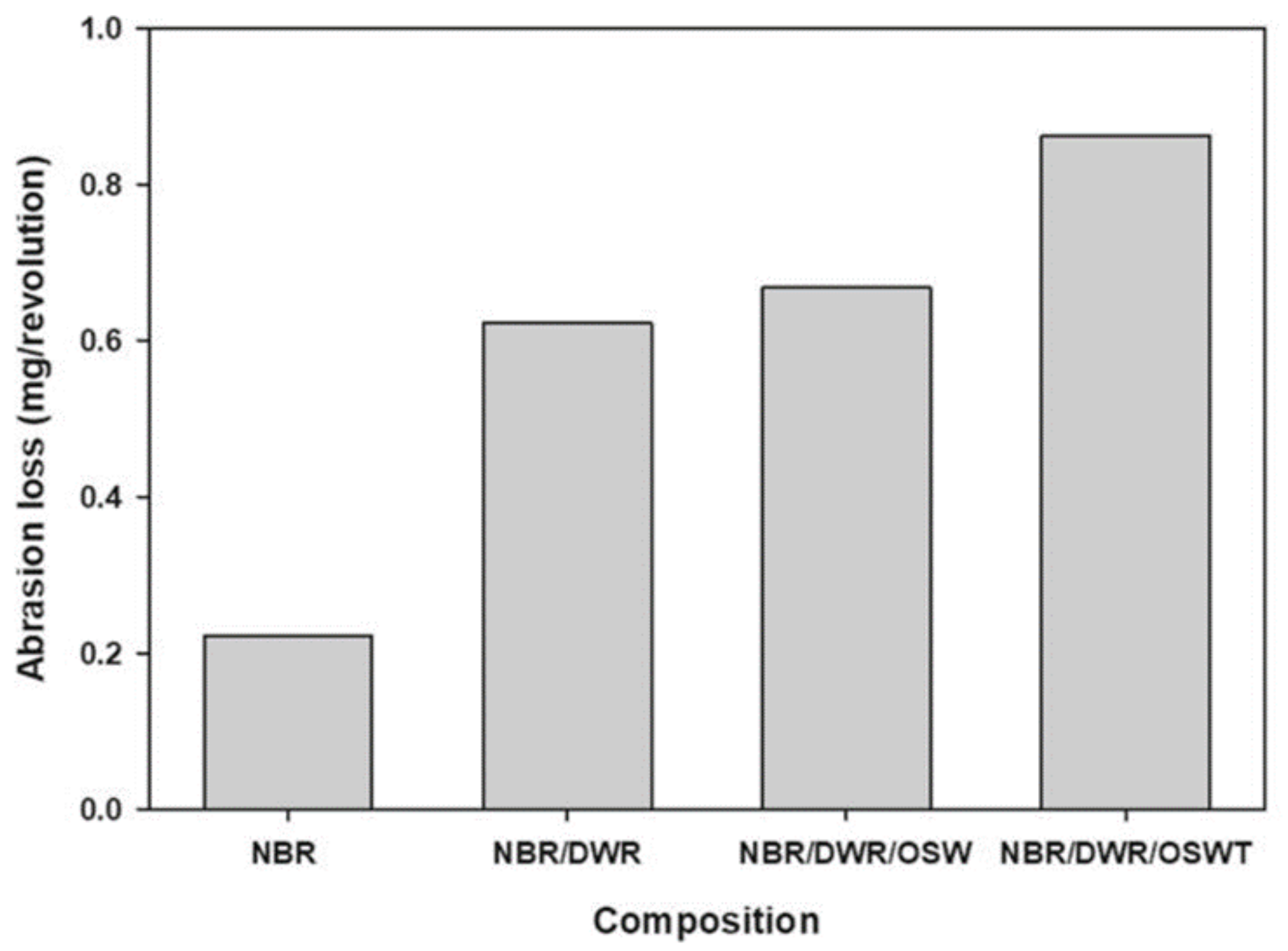
| Rubber or elastomer | NBR/DWR blends | Tensile strength, modulus of elasticity | Rubber industry as lining in fuel tanks and as rubber fuel hoses | [6] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valvez, S.; Maceiras, A.; Santos, P.; Reis, P.N.B. Olive Stones as Filler for Polymer-Based Composites: A Review. Materials 2021, 14, 845. https://doi.org/10.3390/ma14040845
Valvez S, Maceiras A, Santos P, Reis PNB. Olive Stones as Filler for Polymer-Based Composites: A Review. Materials. 2021; 14(4):845. https://doi.org/10.3390/ma14040845
Chicago/Turabian StyleValvez, Sara, Alberto Maceiras, Paulo Santos, and Paulo N. B. Reis. 2021. "Olive Stones as Filler for Polymer-Based Composites: A Review" Materials 14, no. 4: 845. https://doi.org/10.3390/ma14040845
APA StyleValvez, S., Maceiras, A., Santos, P., & Reis, P. N. B. (2021). Olive Stones as Filler for Polymer-Based Composites: A Review. Materials, 14(4), 845. https://doi.org/10.3390/ma14040845








