A Review on the Adaption of Alginate-Gelatin Hydrogels for 3D Cultures and Bioprinting
Abstract
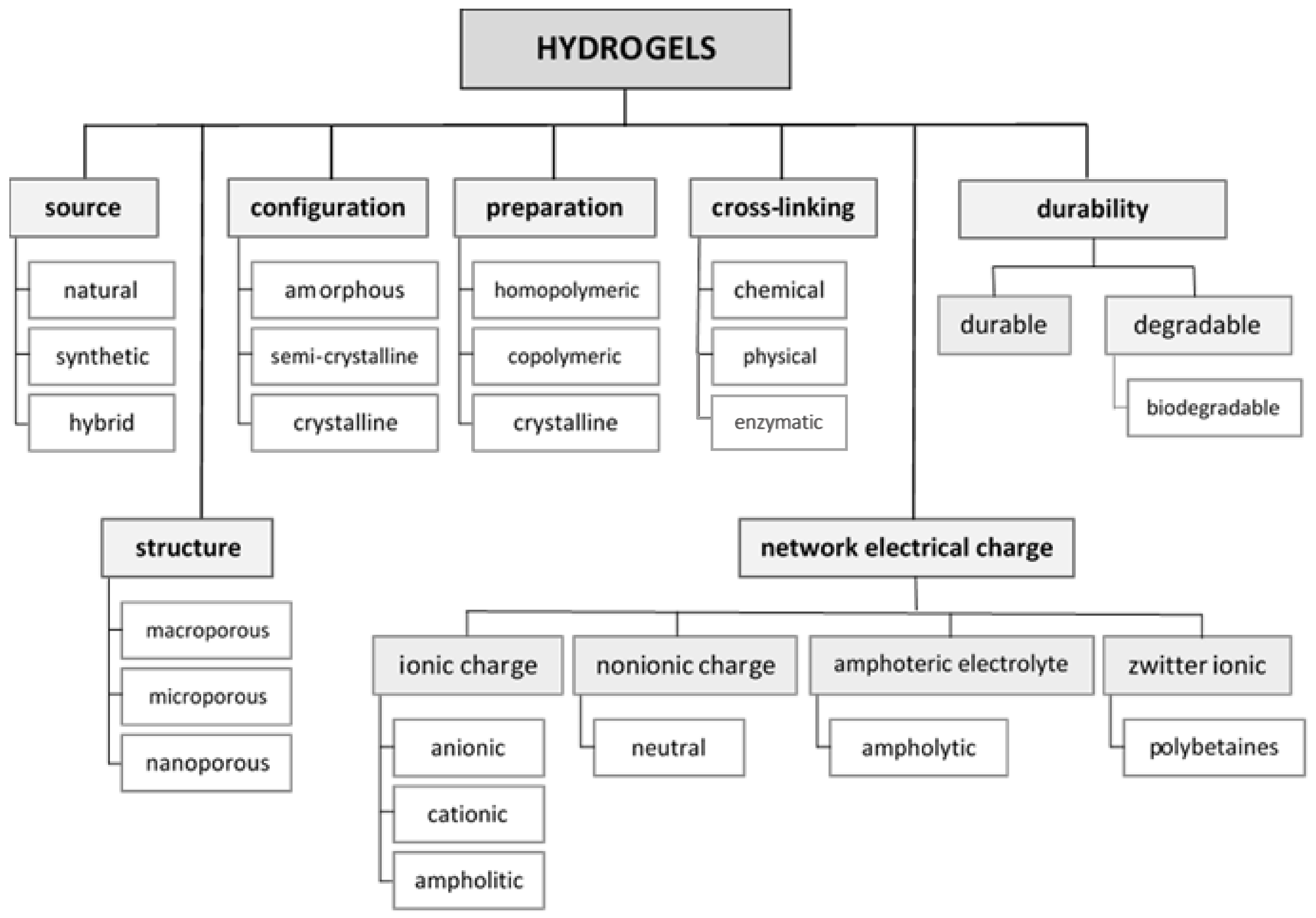
:1. Introduction
2. Materials and Methods for the Preparation and Characterization of Hydrogel Substrates
2.1. Alginate
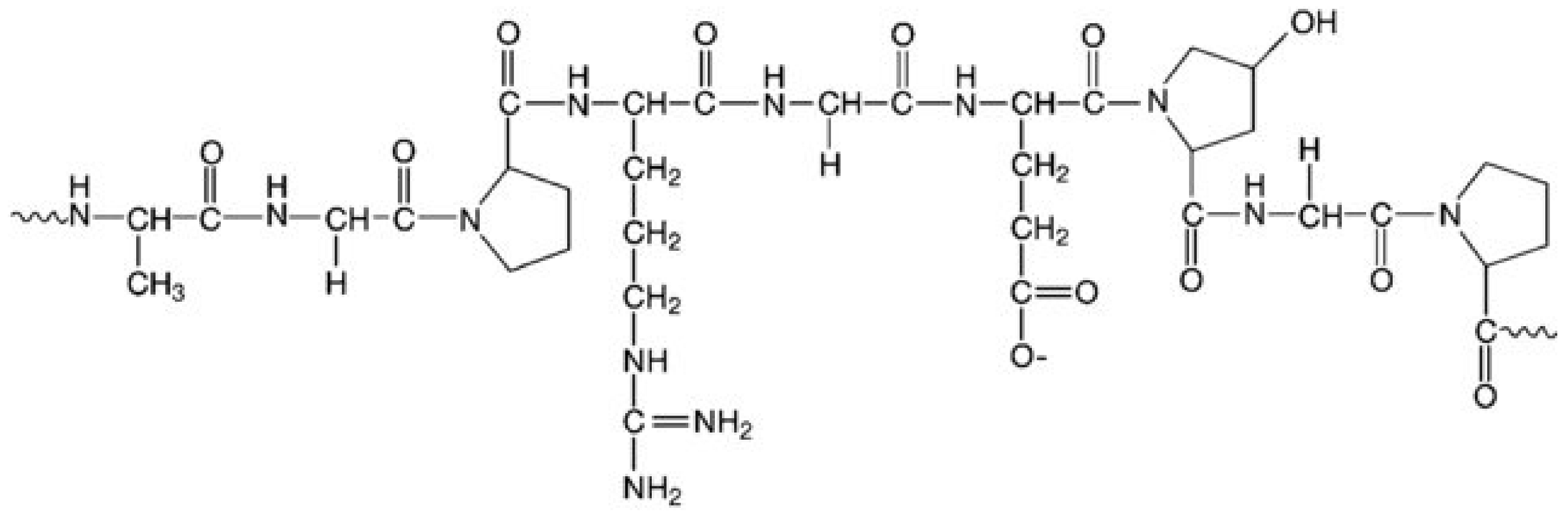
2.2. Gelatin
2.3. Alginate Dialdehyde-Gelatin Hydrogels
3. Material Additives Used in Gel Systems
3.1. Hydrogel Fabrication from Materials Useful for 3D Cell Culture
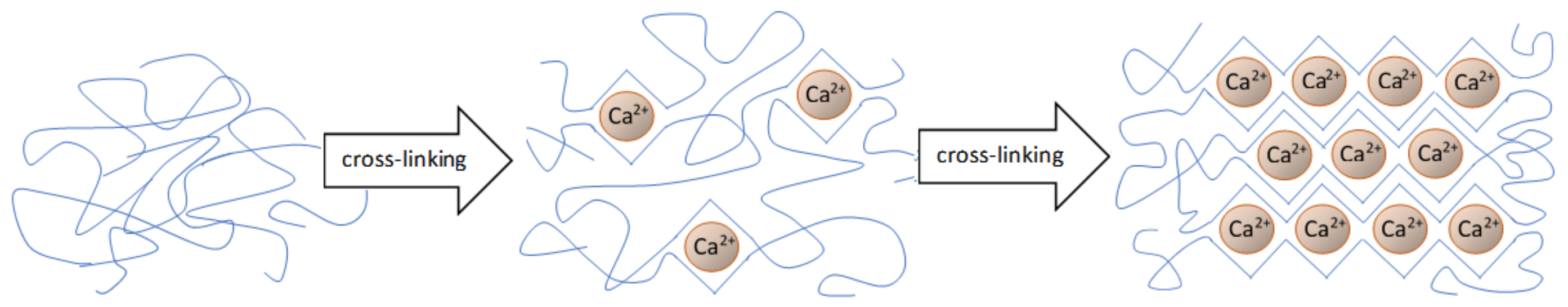
3.2. Hydrogel Cross-Linking Methods
3.3. 3D Cell Cultures
4. Mechanical Properties of Alginate-Based Hydrogels
5. Alginate-Gelatin Hydrogel as an Ink in 3D Bioprinting
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lapidot, S.A.; Kost, J. Hydrogels. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, Netherlands, 2001; pp. 3878–3882. [Google Scholar]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Bahram, M.; Mohseni, N.; Moghtader, M. An introduction to hydrogels and some recent applications. In Emerging Concepts in Analysis and Applications of Hydrogels; Majee, S.B., Ed.; IntechOpen: London, UK, 2016; pp. 9–38. [Google Scholar]
- Aljohani, W.J.; Wenchao, L.; Ullah, M.W.; Zhang, X.; Yang, G. Application of sodium alginate hydrogel. IOSR J. Biotechnol. Biochem. 2017, 3, 19–31. [Google Scholar] [CrossRef]
- Gun’ko, V.; Savina, I.; Mikhalovsky, S. Properties of water bound in hydrogels. Gels 2017, 3, 37. [Google Scholar] [CrossRef]
- Aswathy, S.H.; Uttamchand, N.K.; Inderchand, M. Commercial hydrogels for biomedical applications. Heliyon 2020, 6, e03719. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R. Cross-linked hydrogel for pharmaceutical applications: A review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Borzacchiello, A.; Ambrosio, L. Structure-Property relationships in hydrogels. In Hydrogels: Biological Properties and Applications; Springer: Berlin/Heidelberg, Germany, 2010; pp. 9–19. [Google Scholar]
- Singh, S.K.; Dhyani, A.; Juyal, D. Hydrogel: Preparation, characterization and applications. Pharma Innov. 2017, 6, 25–32. [Google Scholar]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. A mini review on hydrogels classification and recent developments in miscellaneous applications. Mater. Sci. Eng. C 2017, 79, 958–971. [Google Scholar] [CrossRef]
- Lowman, A.M.; Dziubla, T.D.; Bures, P.; Peppas, N.A. Structural and dynamic response of neutral and intelligent networks in biomedical environments. In Advances in Chemical Engineering: Molecular and Cellular Foundations of Biomaterials; Academic Press: Cambridge, MA, USA, 2004; pp. 75–130. [Google Scholar]
- Khansari, M.M.; Sorokina, L.V.; Mukherjee, P.; Mukhtar, F.; Shirdar, M.R.; Shahidi, M.; Shokuhfar, T. Classification of hydrogels based on their source: A review and application in stem cell regulation. JOM 2017, 69, 1340–1347. [Google Scholar] [CrossRef]
- Pande, P.P. Polymer hydrogels and their applications. Int. J. Mater. Sci. 2017, 12, 11–14. [Google Scholar]
- Singhal, R.; Gupta, K. A Review: Tailor-made hydrogel structures (Classifications and synthesis parameters). Polym. Plast. Technol. Eng. 2016, 55, 54–70. [Google Scholar] [CrossRef]
- Baker, J.P.; Blanch, H.W.; Prausnitzt, J.M. Swelling properties of acrylamide-based ampholytic hydrogels: Comparison of experiment with theory. Polymer 1995, 36, 1061–1069. [Google Scholar] [CrossRef]
- Maitz, M.F. Applications of synthetic polymers in clinical medicine. Biosurface Biotribology 2015, 1, 161–176. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D printing of hydrogels: Rational design strategies and emerging biomedical applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Joseph, J.; Deshmukh, K.; Tung, T.; Chidambaram, K.; Khadheer Pasha, S.K. 3D printing technology of polymer composites and hydrogels for artificial skin tissue implementations. In Polymer Nanocomposites in Biomedical Engineering; Springer: Berlin, Germany, 2019; pp. 205–233. [Google Scholar]
- Nair, A.V.; Raman, M.; Doble, M. Polysaccharide-based hydrogels for targeted drug delivery. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 343–382. [Google Scholar]
- Cascone, S.; Lamberti, G. Hydrogel-based commercial products for biomedical applications: A review. Int. J. Pharm. 2020, 573, 118803. [Google Scholar] [CrossRef]
- Ouyang, L.; Yao, R.; Chen, X.; Na, J.; Sun, W. 3D printing of HEK 293FT cell-laden hydrogel into macroporous constructs with high cell viability and normal biological functions. Biofabrication 2015, 7, 015010. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, R.; Ouyang, L.; Ding, H.; Zhang, T.; Zhang, K.; Cheng, S.; Sun, W. Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication 2014, 6, 035001. [Google Scholar] [CrossRef] [PubMed]
- Naghieh, S.; Sarker, M.; Sharma, N.K.; Barhoumi, Z.; Chen, X. Printability of 3D printed hydrogel scaffolds: Influence of hydrogel composition and printing parameters. Appl. Sci. 2019, 10, 292. [Google Scholar] [CrossRef] [Green Version]
- Gulrez, S.; Al-Assaf, S.; Philips, G.O. Hydrogels: Methods of preparation, characterisation and applications. In Progress in Molecular and Environmental Bioengineering—From Analysis and Modeling to Technology Applications; IntechOpen: London, UK, 2011. [Google Scholar]
- Kolodynska, D.; Skiba, A.; Gorecka, B.; Hubicki, Z. Hydrogels from fundaments to application. In Emerging Concepts in Analysis and Applications of Hydrogels; IntechOpen: London, UK, 2016; pp. 69–100. [Google Scholar]
- Syed, S.S.; Kulkarni, D.; Todkar, R.; Bagul, R.S.; Parekh, K.; Bhujbal, N. A novel method of coating orthodontic archwires with nanoparticles. J Int Oral Heal. 2015, 7, 30–33. [Google Scholar]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Andersen, T.; Auk-Emblem, P.; Dornish, M. 3D cell culture in alginate hydrogels. Microarrays 2015, 4, 133–161. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, G.; Toumpaniari, S.; Popov, A.; Duan, P.; Chen, J.; Dalgarno, K.; Scott, W.E.; Ferreira, A.M. Synthesis of bioinspired collagen/alginate/fibrin based hydrogels for soft tissue engineering. Mater. Sci. Eng. C 2018, 91, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Ohi, H.; Taya, M. Gelatin/hyaluronic acid content in hydrogels obtained through blue light-induced gelation affects hydrogel properties and adipose stem cell behaviors. Biomolecules 2019, 9, 342. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Su, X.; Xu, Y.; Kong, B.; Sun, W.; Mi, S. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci. Rep. 2016, 6, 24474. [Google Scholar] [CrossRef] [Green Version]
- Bakhshayesh, A.R.D.; Annabi, N.; Khalilov, R.; Akbarzadeh, A.; Samiei, M.; Alizadeh, E.; Alizadeh-Ghodsi, M.; Davaran, S.; Montaseri, A. Recent advances on biomedical applications of scaffolds in wound healing and dermal tissue engineering. Artif. Cells Nanomed. Biotechnol. 2018, 46, 691–705. [Google Scholar] [CrossRef]
- Ekerdt, B.L.; Segalman, R.A.; Schaffer, D.V. Spatial organization of cell-adhesive ligands for advanced cell culture. Biotechnol. J. 2013, 8, 1411–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinman, H.K.; Luckenbill-Edds, L.; Cannon, F.W.; Sephel, G.C. Use of extracellular matrix components for cell culture. Anal. Biochem. 1987, 166, 1–13. [Google Scholar] [CrossRef]
- Shan, Y.; Li, C.; Wu, Y.; Li, Q.; Liao, J. Hybrid cellulose nanocrystal/alginate/gelatin scaffold with improved mechanical properties and guided wound healing. Rsc Adv. 2019, 9, 22966–22979. [Google Scholar] [CrossRef] [Green Version]
- Kleinübing, S.J.; Gai, F.; Bertagnolli, C.; da Silva, M.G.C. Extraction of alginate biopolymer present in marine alga Sargassum filipendula and bioadsorption of metallic ions. Mater. Res. 2013, 16, 481–488. [Google Scholar] [CrossRef] [Green Version]
- Lewicki, J.; Bergman, J.; Kerins, C.; Hermanson, O. Optimization of 3D bioprinting of human neuroblastoma cells using sodium alginate hydrogel. Bioprinting 2019, 16, e00053. [Google Scholar] [CrossRef]
- Maciel, B.; Oelschlaeger, C.; Willenbacher, N. Chain flexibility and dynamics of alginate solutions in different solvents. Colloid Polym. Sci. 2020, 298, 791–801. [Google Scholar] [CrossRef]
- Fertah, M.; Belfkira, A.; Dahmane, E.M.; Taourirte, M.; Brouillette, F. Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arab. J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef] [Green Version]
- Fenoradosoa, T.A.; Ali, G.; Delattre, C.; Laroche, C.; Petit, E.; Wadouachi, A.; Michaud, P. Extraction and characterization of an alginate from the brown seaweed Sargassum turbinarioides Grunow. J. Appl. Phycol. 2010, 22, 131–137. [Google Scholar] [CrossRef]
- Zhang, Z.; Ortiz, O.; Goyal, R.; Kohn, J. Biodegradable polymers. In Handbook of Polymer Applications in Medicine and Medical Devices; Modjarrad, K., Ebnesajjad, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 303–335. [Google Scholar]
- Raja, M.M.; Lim, P.Q.; Wong, Y.S.; Xiong, G.M.; Zhang, Y.; Venkatraman, S.; Huang, Y. Polymeric nanomaterials: Methods of preparation and characterization. In Nanocarriers for Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 557–653. [Google Scholar]
- Gomathi, T.; Susi, S.; Abirami, D.; Sudha, P.N. Size optimization and thermal studies on calcium alginate nanoparticles. IOSR J. Pharm. 2017, 1–7. [Google Scholar]
- Aroguz, A.Z.; Baysal, K.; Adiguzel, Z.; Baysal, B.M. Alginate/Polyoxyethylene and alginate/gelatin hydrogels: Preparation, characterization, and application in tissue engineering. Appl. Biochem. Biotechnol. 2014, 173, 433–448. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Tan, H. Alginate-based biomaterials for regenerative medicine applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Batista, P.S.P.; de Morais, A.M.M.B.; Pintado, M.M.E.; de Morais, R.M.S.C. Alginate: Pharmaceutical and medical applications. Biologically-inspired systems. In Extracellular Sugar-Based Biopolymers Matrices; Cohen, E., Merzendorfer, H., Eds.; Springer: Cham, Switzerland, 2019; Volume 12, pp. 649–691. [Google Scholar]
- Nawaz, S.; Khan, S.; Farooq, U.; Haider, M.S.; Ranjha, N.M.; Rasul, A.; Nawaz, A.; Arshad, N.; Hameed, R. Biocompatible hydrogels for the controlled delivery of anti-hypertensive agent: Development, characterization and in vitro evaluation. Des. Monomers Polym. 2018, 21, 18–32. [Google Scholar] [CrossRef] [Green Version]
- Boccafoschi, F.; Ramella, M.; Fusaro, L.; Catoira, M.C.; Casella, F. Biological grafts: Surgical use and vascular tissue engineering options for peripheral vascular implants. In Encyclopedia of Biomedical Engineering; Narayan, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1–3, pp. 310–321. [Google Scholar]
- Kommareddy, S.; Shenoy, D.B.; Amiji, M.M. Gelatin nanoparticles and their biofunctionalization. In Nanotechnologies for the Life Sciences; Kumar, C.S.S.R., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007. [Google Scholar]
- Sarker, B.; Papageorgiou, D.G.; Silva, R.; Zehnder, T.; Gul-E-Noor, F.; Bertmer, M.; Kaschta, J.; Chrissafis, K.; Detsch, R.; Boccaccini, A.R. Fabrication of alginate-gelatin crosslinked hydrogel microcapsules and evaluation of the microstructure and physico-chemical properties. J. Mater. Chem. B 2014, 2, 1470–1482. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Tian, Z.; Menard, F.; Kim, K. Comparative study of gelatin methacrylate hydrogels from different sources for biofabrication applications. Biofabrication 2017, 9, 044101. [Google Scholar] [CrossRef]
- Yoon, H.J.; Shin, S.R.; Cha, J.M.; Lee, S.-H.; Kim, J.-H.; Do, J.T.; Song, H.; Bae, H. Cold water fish gelatin methacryloyl hydrogel for tissue engineering application. PLoS ONE 2016, 11, e0163902. [Google Scholar] [CrossRef] [Green Version]
- Boontheekul, T.; Kong, H.J.; Mooney, D.J. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials 2005, 26, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Lee, B.T. Fabrication of oxidized alginate-gelatin-BCP hydrogels and evaluation of the microstructure, material properties and biocompatibility for bone tissue regeneration. J. Biomater. Appl. 2011, 27, 311–321. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Jayakrishnan, A. Self-cross-linking biopolymers as injectable in situ forming biodegradable scaffolds. Biomaterials 2005, 26, 3941–3951. [Google Scholar] [CrossRef]
- Grigore, A.; Sarker, B.; Fabry, B.; Boccaccini, A.R.; Detsch, R. Behavior of encapsulated MG-63 cells in RGD and gelatine-modified alginate hydrogels. Tissue Eng. Part A 2014, 20, 2140–2150. [Google Scholar] [CrossRef]
- Dranseikiene, D.; Schrüfer, S.; Schubert, D.W.; Reakasame, S.; Boccaccini, A.R. Cell-laden alginate dialdehyde-gelatin hydrogels formed in 3D printed sacrificial gel. J. Mater. Sci. Mater. Med. 2020, 31, 31. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Wu, Y.; Fang, J.; Wei, X.; Gu, Q.; El-Hamshary, H.; Al-Deyab, S.S.; Morsi, Y.; Mo, X. Modified alginate and gelatin cross-linked hydrogels for soft tissue adhesive. Artif. CellsNanomed. Biotechnol. 2017, 45, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Ruther, F.; Distler, T.; Boccaccini, A.R.; Detsch, R. Biofabrication of vessel-like structures with alginate di-aldehyde-gelatin (ADA-GEL) bioink. J. Mater. Sci. Mater. Med. 2019, 30, 8. [Google Scholar] [CrossRef]
- Manju, S.; Muraleedharan, C.V.; Rajeev, A.; Jayakrishnan, A.; Joseph, R. Evaluation of alginate dialdehyde cross-linked gelatin hydrogel as a biodegradable sealant for polyester vascular graft. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 98B, 139–149. [Google Scholar] [CrossRef]
- Piras, C.C.; Smith, D.K. Multicomponent polysaccharide alginate-based bioinks. J. Mater. Chem. B 2020, 8, 8171–8188. [Google Scholar] [CrossRef] [PubMed]
- Utech, S.; Boccaccini, A.R. A review of hydrogel-based composites for biomedical applications: Enhancement of hydrogel properties by addition of rigid inorganic fillers. J. Mater. Sci. 2016, 51, 271–310. [Google Scholar] [CrossRef]
- Visser, J.; Melchels, F.P.W.; Jeon, J.E.; Van Bussel, E.M.; Kimpton, L.S.; Byrne, H.M.; Dhert, W.J.A.; Dalton, P.D.; Hutmacher, D.W.; Malda, J. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat. Commun. 2015, 6, 6933. [Google Scholar] [CrossRef]
- Petrova, V.A.; Elokhovskiy, V.Y.; Raik, S.V.; Poshina, D.N.; Romanov, D.P.; Skorik, Y.A. Alginate gel reinforcement with chitin nanowhiskers modulates rheological properties and drug release profile. Biomolecules 2019, 9, 291. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Huang, J.; Chang, P.R.; Li, J.; Chen, Y.; Wang, D.; Yu, J.; Chen, J. Structure and properties of polysaccharide nanocrystal-doped supramolecular hydrogels based on cyclodextrin inclusion. Polymer 2010, 51, 4398–4407. [Google Scholar] [CrossRef]
- Kinneberg, K.R.C.; Nelson, A.; Stender, M.E.; Aziz, A.H.; Mozdzen, L.C.; Harley, B.A.C.; Bryant, S.J.; Ferguson, V.L. Reinforcement of mono- and bi-layer Poly(Ethylene Glycol) hydrogels with a fibrous collagen scaffold. Ann. Biomed. Eng. 2015, 43, 2618–2629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, M.; Lee, D.; Hyun, J. Nanocellulose-alginate hydrogel for cell encapsulation. Carbohydr. Polym. 2015, 116, 223–228. [Google Scholar] [CrossRef]
- Siqueira, P.; Siqueira, É.; de Lima, A.E.; Siqueira, G.; Pinzón-Garcia, A.D.; Lopes, A.P.; Segura, M.E.C.; Isaac, A.; Pereira, F.V.; Botaro, V.R. Three-dimensional stable alginate-nanocellulose gels for biomedical applications: Towards tunable mechanical properties and cell growing. Nanomaterials 2019, 9, 78. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, H.; Shirley, S.G.; Zimmermann, U. Alginate-based encapsulation of cells: Past, present, and future. Curr. Diab. Rep. 2007, 7, 314–320. [Google Scholar] [CrossRef]
- Dvir-Ginzberg, M.; Elkayam, T.; Cohen, S. Induced differentiation and maturation of newborn liver cells into functional hepatic tissue in macroporous alginate scaffolds. Faseb J. 2008, 22, 1440–1449. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Gu, L.; Kwee, B.; Li, W.A.; Dellacherie, M.; Celiz, A.D.; Mooney, D.J. Hydrogel substrate stress-relaxation regulates the spreading and proliferation of mouse myoblasts. Acta Biomater. 2017, 62, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Alsberg, E.; Hsiong, S.; Comisar, W.; Linderman, J.; Ziff, R.; Mooney, D. Nanoscale adhesion ligand organization regulates osteoblast proliferation and differentiation. Nano Lett. 2004, 4, 1501–1506. [Google Scholar] [CrossRef] [Green Version]
- De Melo, B.A.G.; Jodat, Y.A.; Mehrotra, S.; Calabrese, M.A.; Kamperman, T.; Mandal, B.B.; Santana, M.H.A.; Alsberg, E.; Leijten, J.; Shin, S.R. 3D printed cartilage-like tissue constructs with spatially controlled mechanical properties. Adv. Funct. Mater. 2019, 29, 1906330. [Google Scholar] [CrossRef]
- Chung, J.H.Y.; Naficy, S.; Yue, Z.; Kapsa, R.; Quigley, A.; Moulton, S.E.; Wallace, G.G. Bio-ink properties and printability for extrusion printing living cells. Biomater. Sci. 2013, 1, 763–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wang, X.; Wang, X.; Chen, H.; Zhang, X.; Zhou, L.; Xu, T. 3D bioprinted rat Schwann cell-laden structures with shape flexibility and enhanced nerve growth factor expression. 3 Biotech 2018, 8, 342. [Google Scholar] [CrossRef]
- Demirtaş, T.T.; Irmak, G.; Gümüşderelioğlu, M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication 2017, 9, 035003. [Google Scholar] [CrossRef]
- Schwarz, S.; Kuth, S.; Distler, T.; Gögele, C.; Stölzel, K.; Detsch, R.; Boccaccini, A.R.; Schulze-Tanzil, G. 3D printing and characterization of human nasoseptal chondrocytes laden dual crosslinked oxidized alginate-gelatin hydrogels for cartilage repair approaches. Mater. Sci. Eng. C 2020, 116, 111189. [Google Scholar] [CrossRef]
- He, Y.; Yang, F.; Zhao, H.; Gao, Q.; Xia, B.; Fu, J. Research on the printability of hydrogels in 3D bioprinting. Sci. Rep. 2016, 6, 29977. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Gebeyehu, A.; Miranda, M.; Bahadur, D.; Patel, N.; Ramakrishnan, S.; Rishi, A.K.; Singh, M. Characterization and printability of sodium alginate -gelatin hydrogel for bioprinting NSCLC co-culture. Sci. Rep. 2019, 9, 19914. [Google Scholar] [CrossRef] [PubMed]
- Giuseppe, M.D.; Law, N.; Webb, B.; Macrae, R.A.; Liew, L.J.; Sercombe, T.B.; Dilley, R.J.; Doyle, B.J. Mechanical behaviour of alginate-gelatin hydrogels for 3D bioprinting. J. Mech. Behav. Biomed. Mater. 2018, 79, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Cuadros, T.R.; Erices, A.A.; Aguilera, J.M. Porous matrix of calcium alginate/gelatin with enhanced properties as scaffold for cell culture. J. Mech. Behav. Biomed. Mater. 2015, 46, 331–342. [Google Scholar] [CrossRef]
- Cheng, L.; Yao, B.; Hu, T.; Cui, X.; Shu, X.; Tang, S.; Wang, R.; Wang, Y.; Liu, Y.; Song, W.; et al. Properties of an alginate-gelatin-based bioink and its potential impact on cell migration, proliferation, and differentiation. Int. J. Biol. Macromol. 2019, 135, 1107–1113. [Google Scholar] [CrossRef]
- Hiller, T.; Berg, J.; Elomaa, L.; Röhrs, V.; Ullah, I.; Schaar, K.; Dietrich, A.-C.; Al-Zeer, M.; Kurtz, A.; Hocke, A.; et al. Generation of a 3D liver model comprising human extracellular matrix in an alginate/gelatin-based bioink by extrusion bioprinting for infection and transduction studies. Int. J. Mol. Sci. 2018, 19, 3129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soltan, N.; Ning, L.; Mohabatpour, F.; Papagerakis, P.; Chen, X. Printability and cell viability in bioprinting alginate dialdehyde-gelatin scaffolds. Acs Biomater. Sci. Eng. 2019, 5, 2976–2987. [Google Scholar] [CrossRef] [PubMed]
- Majidi, S.S.; Slemming-Adamsen, P.; Hanif, M.; Zhang, Z.; Wang, Z.; Chen, M. Wet electrospun alginate/gelatin hydrogel nanofibers for 3D cell culture. Int. J. Biol. Macromol. 2018, 118, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef]
- Salo, T.; Sutinen, M.; Hoque Apu, E.; Sundquist, E.; Cervigne, N.K.; de Oliveira, C.E.; Akram, S.U.; Ohlmeier, S.; Suomi, F.; Eklund, L.; et al. A novel human leiomyoma tissue derived matrix for cell culture studies. Bmc Cancer 2015, 15, 981. [Google Scholar] [CrossRef] [Green Version]
- Zarembinski, T.I.; Aleksander, S. HyStem®: A unique clinical grade hydrogel for present and future medical applications. In Hydrogels—Smart Materials for Biomedical Applications; Popa, L., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Wang, J.; Zhao, L.; Zhang, A.; Huang, Y.; Tavakoli, J.; Tang, Y. Novel bacterial cellulose/gelatin hydrogels as 3D scaffolds for tumor cell culture. Polymers 2018, 10, 581. [Google Scholar] [CrossRef] [Green Version]
- Zimoch, J.; Padial, J.S.; Klar, A.S.; Vallmajo-Martin, Q.; Meuli, M.; Biedermann, T.; Wilson, C.J.; Rowan, A.; Reichmann, E. Polyisocyanopeptide hydrogels: A novel thermo-responsive hydrogel supporting pre-vascularization and the development of organotypic structures. Acta Biomater. 2018, 70, 129–139. [Google Scholar] [CrossRef]
- Hsieh, F.Y.; Tao, L.; Wei, Y.; Hsu, S.H. A novel biodegradable self-healing hydrogel to induce blood capillary formation. Npg Asia Mater. 2017, 9, e363. [Google Scholar] [CrossRef] [Green Version]
- Dinescu, S.; Albu Kaya, M.; Chitoiu, L.; Ignat, S.; Kaya, D.A.; Costache, M. Collagen-based hydrogels and their applications for tissue engineering and regenerative medicine. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M.I.H., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1643–1664. [Google Scholar]
- Maitra, J.; Shukla, V.K. Cross-linking in hydrogels—A review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar] [CrossRef]
- Chen, Q.; Tian, X.; Fan, J.; Tong, H.; Ao, Q.; Wang, X. An interpenetrating alginate/gelatin network for three-dimensional (3D) cell cultures and organ bioprinting. Molecules 2020, 25, 756. [Google Scholar] [CrossRef] [Green Version]
- Sarker, M.; Izadifar, M.; Schreyer, D.; Chen, X. Influence of ionic crosslinkers (Ca2+ /Ba2+ /Zn2+) on the mechanical and biological properties of 3D bioplotted hydrogel scaffolds. J. Biomater. Sci. Polym. Ed. 2018, 29, 1126–1154. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Hermida, M.A.; Leslie, N.R.; Shu, W. Three-dimensional bioprinting of complex cell laden alginate hydrogel structures. Biofabrication 2015, 7, 045012. [Google Scholar] [CrossRef]
- Bajpai, M.; Shukla, P.; Bajpai, S.K. Enhancement in the stability of alginate gels prepared with mixed solution of divalent ions using a diffusion through dialysis tube (DTDT) approach. J. Macromol. Sci. Part A Pure Appl. Chem. 2017, 54, 301–310. [Google Scholar] [CrossRef]
- Tanaka, H.; Sato, Y. Photosensitivity of polyvinylesters of substituted cinnamylideneacetic acids. J. Polym. Sci. Part A-1 Polym. Chem. 1972, 10, 3279–3287. [Google Scholar] [CrossRef]
- Zhao, S.; Cao, M.; Li, H.; Li, L.; Xu, W. Synthesis and characterization of thermo-sensitive semi-IPN hydrogels based on poly(ethylene glycol)-co-poly(ε-caprolactone) macromer, N-isopropylacrylamide, and sodium alginate. Carbohydr. Res. 2010, 345, 425–431. [Google Scholar] [CrossRef]
- Lee, K.Y.; Kong, H.J.; Larson, R.G.; Mooney, D.J. Hydrogel formation via cell crosslinking. Adv. Mater. 2003, 15, 1828–1832. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, A.; Ye, R.; Wang, W.; Li, X. Transglutaminase-induced crosslinking of gelatin-calcium carbonate composite films. Food Chem. 2015, 166, 414–422. [Google Scholar] [CrossRef]
- Ofner, C.M.; Bubnis, W.A. Chemical and swelling evaluations of amino group crosslinking in gelatin and modified gelatin matrices. Pharm. Res. 1996, 13, 1821–1827. [Google Scholar] [CrossRef]
- Hasturk, O.; Jordan, K.E.; Choi, J.; Kaplan, D.L. Enzymatically crosslinked silk and silk-gelatin hydrogels with tunable gelation kinetics, mechanical properties and bioactivity for cell culture and encapsulation. Biomaterials 2020, 232, 119720. [Google Scholar] [CrossRef] [PubMed]
- Pathak, T.S.; Kim, J.S.; Lee, S.J.; Baek, D.J.; Paeng, K.J. Preparation of alginic acid and metal alginate from algae and their comparative study. J. Polym. Env. 2008, 16, 198–204. [Google Scholar] [CrossRef]
- Dong, Z.; Wang, Q.; Du, Y. Alginate/gelatin blend films and their properties for drug controlled release. J. Memb. Sci. 2006, 280, 37–44. [Google Scholar] [CrossRef]
- West, E.R.; Xu, M.; Woodruff, T.K.; Shea, L.D. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials 2007, 28, 4439–4448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drury, J.L.; Dennis, R.G.; Mooney, D.J. The tensile properties of alginate hydrogels. Biomaterials 2004, 25, 3187–3199. [Google Scholar] [CrossRef] [PubMed]
- Dhoot, N.O.; Tobias, C.A.; Fischer, I.; Wheatley, M.A. Peptide-modified alginate surfaces as a growth permissive substrate for neurite outgrowth. J. Biomed. Mater. Res. Part A 2004, 71, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cuddihy, M.J.; Kotov, N.A. Three-dimensional cell culture matrices: State of the art. Tissue Eng. Part B Rev. 2008, 14, 61–86. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Gu, Y.; Du, K.T.; Mihardja, S.; Sievers, R.E.; Lee, R.J. The effect of injected RGD modified alginate on angiogenesis and left ventricular function in a chronic rat infarct model. Biomaterials 2009, 30, 751–756. [Google Scholar] [CrossRef]
- Lam, J.; Segura, T. The modulation of MSC integrin expression by RGD presentation. Biomaterials 2013, 34, 3938–3947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emmermacher, J.; Spura, D.; Cziommer, J.; Kilian, D.; Wollborn, T.; Fritsching, U.; Steingroewer, J.; Walther, T.; Gelinsky, M.; Lode, A. Engineering considerations on extrusion-based bioprinting: Interactions of material behavior, mechanical forces and cells in the printing needle. Biofabrication 2020, 12, 025022. [Google Scholar] [CrossRef]
- Hsiong, S.X.; Boontheekul, T.; Huebsch, N.; Mooney, D.J. Cyclic arginine-glycine-aspartate peptides enhance three-dimensional stem cell osteogenic differentiation. Tissue Eng. Part A 2008, 15, 263–272. [Google Scholar] [CrossRef]
- Ning, L.; Xu, Y.; Chen, X.; Schreyer, D.J. Influence of mechanical properties of alginate-based substrates on the performance of Schwann cells in culture. J. Biomater. Sci. Polym. Ed. 2016, 27, 898–915. [Google Scholar] [CrossRef]
- Lim, K.S.; Abinzano, F.; Bernal, P.N.; Albillos Sanchez, A.; Atienza-Roca, P.; Otto, I.A.; Peiffer, Q.C.; Matsusaki, M.; Woodfield, T.B.F.; Malda, J.; et al. One-step photoactivation of a dual-functionalized bioink as cell carrier and cartilage-binding glue for chondral regeneration. Adv. Healthc. Mater. 2020, 1901792. [Google Scholar] [CrossRef]
- Ojansivu, M.; Rashad, A.; Ahlinder, A.; Massera, J.; Mishra, A.; Syverud, K.; Finne-Wistrand, A.; Miettinen, S.; Mustafa, K. Wood-based nanocellulose and bioactive glass modified gelatin-alginate bioinks for 3D bioprinting of bone cells. Biofabrication 2019, 11, 035010. [Google Scholar] [CrossRef]
- Jia, W.; Gungor-Ozkerim, P.S.; Zhang, Y.S.; Yue, K.; Zhu, K.; Liu, W.; Pi, Q.; Byambaa, B.; Dokmeci, M.R.; Shin, S.R.; et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 2016, 106, 58–68. [Google Scholar] [CrossRef] [Green Version]
- Willerth, S.M.; Sakiyama-Elbert, S.E. Combining stem cells and biomaterial scaffolds for constructing tissues and cell delivery. StemJournal 2019, 1, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Awad, H.A.; Wickham, M.Q.; Leddy, H.A.; Gimble, J.M.; Guilak, F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials 2004, 25, 3211–3222. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.; Khosravizadeh, Z.; Bahramian, H.; Kazemi, M. Time-dependent effect of encapsulating alginate hydrogel on neurogenic potential. Cell J. 2015, 17, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Rödel, P.; Anamur, C.; Seeliger, C.; Imhoff, A.B.; Herbst, E.; Vogt, S.; van Griensven, M.; Winter, G.; Engert, J. Calcium alginate gels as stem cell matrix—Making paracrine stem cell activity available for enhanced healing after surgery. PLoS ONE 2015, 10, e0118937. [Google Scholar] [CrossRef]
- Pandolfi, V.; Pereira, U.; Dufresne, M.; Legallais, C. Alginate-based cell microencapsulation for tissue engineering and regenerative medicine. Curr. Pharm. Des. 2017, 23, 3833–3844. [Google Scholar] [CrossRef] [PubMed]
- Forte, A.E.; Galvan, S.; Manieri, F.; Rodriguez y Baena, F.; Dini, D. A composite hydrogel for brain tissue phantoms. Mater. Des. 2016, 112, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-based hydrogels for organ 3D bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbah, S.A.; Liu, J.; Lam, R.W.M.; Goh, J.C.H.; Wong, H.K. In vivo bioactivity of rhBMP-2 delivered with novel polyelectrolyte complexation shells assembled on an alginate microbead core template. J. Control. Release 2012, 162, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Hsueh, H.J.; Jiang, Y.L. Light-addressable electrodeposition of cell-encapsulated alginate hydrogels for a cellular microarray using a digital micromirror device. Biomicrofluidics 2011, 5, 34109–34110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, S.; Barua, R.; Das, J. Importance of alginate bioink for 3D bioprinting in tissue engineering and regenerative medicine. In Alginates—Recent Uses of This Natural Polymer; Pereira, L., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Liberski, A.; Latif, N.; Raynaud, C.; Bollensdorff, C.; Yacoub, M. Alginate for cardiac regeneration: From seaweed to clinical trials. Glob. Cardiol. Sci. Pr. Pract. 2016, 2016, e201604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. National Library of Medicine. ClinicalTrials.gov—A Study of Autologous Neo-Kidney Augment (NKA) in Patients with Type 2 Diabetes and Chronic Kidney Disease (CKD). Available online: https://clinicaltrials.gov/ct2/show/NCT03270956 (accessed on 2 February 2021).
- U.S. National Library of Medicine. ClinicalTrials.gov—A Study of a Renal Autologous Cell Therapy (REACT) in Patients With Chronic Kidney Disease (CKD) From Congenital Anomalies of the Kidney and Urinary Tract (CAKUT). Available online: https://clinicaltrials.gov/ct2/show/NCT04115345 (accessed on 2 February 2021).
- Kong, H.J.; Lee, K.Y.; Mooney, D.J. Decoupling the dependence of rheological/mechanical properties of hydrogels from solids concentration. Polymer 2002, 43, 6239–6246. [Google Scholar] [CrossRef]
- Kaklamani, G.; Cheneler, D.; Grover, L.M.; Adams, M.J.; Bowen, J. Mechanical properties of alginate hydrogels manufactured using external gelation. J. Mech. Behav. Biomed. Mater. 2014, 36, 135–142. [Google Scholar] [CrossRef]
- Mancini, M.; Moresi, M.; Rancini, R. Mechanical properties of alginate gels: Empirical characterisation. J. Food Eng. 1999, 39, 369–378. [Google Scholar] [CrossRef]
- Wen, J.H.; Vincent, L.G.; Fuhrmann, A.; Choi, Y.S.; Hribar, K.C.; Taylor-Weiner, H.; Chen, S.; Engler, A.J. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat. Mater. 2014, 13, 979–987. [Google Scholar] [CrossRef] [Green Version]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineages. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [Green Version]
- LeRoux, M.A.; Guilak, F.; Setton, L.A. Compressive and shear properties of alginate gel: Effects of sodium ions and alginate concentration. J. Biomed. Mater. Res. 1999, 47, 46–53. [Google Scholar] [CrossRef]
- Hay, I.D.; Ur Rehman, Z.; Ghafoor, A.; Rehm, B.H.A. Bacterial biosynthesis of alginates. J. Chem. Technol. Biotechnol. 2010, 85, 752–759. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Bonn, D.; Kellay, H.; Prochnow, M.; Ben-Djemiaa, K.; Meunier, J. Delayed fracture of an inhomogeneous soft solid. Science 1998, 280, 265–267. [Google Scholar] [CrossRef]
- Tonsomboon, K.; Oyen, M.L. Composite electrospun gelatin fiber-alginate gel scaffolds for mechanically robust tissue engineered cornea. J. Mech. Behav. Biomed. Mater. 2013, 21, 185–194. [Google Scholar] [CrossRef]
- Wang, K.; Nune, K.C.; Misra, R.D.K. The functional response of alginate-gelatin-nanocrystalline cellulose injectable hydrogels toward delivery of cells and bioactive molecules. Acta Biomater. 2016, 36, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Wang, X.; Ni, Z.; Ni, Y.; Huan, W.; Lv, Y.; Bai, S. Effects of nanocellulose on alginate/gelatin bio-inks for extrusion-based 3D printing. BioResources 2020, 15, 7357–7373. [Google Scholar] [CrossRef]
- Shibayama, M.; Karino, T.; Miyazaki, S.; Okabe, S.; Takehisa, T.; Haraguchi, K. Small-angle neutron scattering study on uniaxially stretched poly(N-isopropylacrylamide)-clay nanocomposite gels. Macromolecules 2005, 38, 10772–10781. [Google Scholar] [CrossRef]
- Haraguchi, K.; Farnworth, R.; Ohbayashi, A.; Takehisa, T. Compositional effects on mechanical properties of nanocomposite hydrogels composed of poly(N,N-dimethylacrylamide) and clay. Macromolecules 2003, 36, 5732–5741. [Google Scholar] [CrossRef]
- Chang, C.W.; Van Spreeuwel, A.; Zhang, C.; Varghese, S. PEG/clay nanocomposite hydrogel: A mechanically robust tissue engineering scaffold. Soft Matter 2010, 6, 5157–5164. [Google Scholar] [CrossRef]
- Kerin, A.J.; Wisnom, M.R.; Adams, M.A. The compressive strength of articular cartilage. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1998, 212, 273–280. [Google Scholar] [CrossRef]
- Armiento, A.R.; Stoddart, M.J.; Alini, M.; Eglin, D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018, 65, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.Z.; Chen, E.Y.; Ding, G.H. The structure of collagen fibers and biomechanics. Anat. Sci 1998, 4, 289–293. [Google Scholar]
- Forte, A.E.; D’Amico, F.; Charalambides, M.N.; Dini, D.; Williams, J.G. Modelling and experimental characterisation of the rate dependent fracture properties of gelatine gels. Food Hydrocoll. 2015, 46, 180–190. [Google Scholar] [CrossRef] [Green Version]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [Green Version]
- Berg, J.; Hiller, T.; Kissner, M.S.; Qazi, T.H.; Duda, G.N.; Hocke, A.C.; Hippenstiel, S.; Elomaa, L.; Weinhart, M.; Fahrenson, C.; et al. Optimization of cell-laden bioinks for 3D bioprinting and efficient infection with influenza A virus. Sci. Rep. 2018, 8, 13877. [Google Scholar] [CrossRef]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.C.; Reid, R.R. 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Celli, J.; Rizvi, I.; Moon, S.; Hasan, T.; Demirci, U. A three-dimensional in vitro ovarian cancer coculture model using a high-throughput cell patterning platform. Biotechnol. J. 2011, 6, 204–212. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Ahadian, S.; Xu, C.; Montazerian, H.; Ko, H.; Nasiri, R.; Barros, N.; Khademhosseini, A. Bioinks and bioprinting technologies to make heterogeneous and biomimetic tissue constructs. Mater. Today Bio 2019, 1, 100008. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; McAnulty, J.F.; Schurr, M.J.; Murphy, C.J.; Abbott, N.L. Polymeric materials for chronic wound and burn dressings. In Advanced Wound Repair Therapies; Woodhead Publishing Series in Biomaterials: Sawston, UK, 2011. [Google Scholar]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 2016, 8, 035020. [Google Scholar] [CrossRef] [PubMed]
- Paxton, N.; Smolan, W.; Böck, T.; Melchels, F.; Groll, J.; Jungst, T. Proposal to assess printability of bioinks for extrusion-based bioprinting and evaluation of rheological properties governing bioprintability. Biofabrication 2017, 9, 044107. [Google Scholar] [CrossRef] [PubMed]
- Bociaga, D.; Bartniak, M.; Grabarczyk, J.; Przybyszewska, K. Sodium alginate/gelatine hydrogels for direct bioprinting-the effect of composition selection and applied solvents on the bioink properties. Materials 2019, 12, 2669. [Google Scholar] [CrossRef] [Green Version]
- Gao, T.; Gillispie, G.J.; Copus, J.S.; Pr, A.K.; Seol, Y.J.; Atala, A.; Yoo, J.J.; Lee, S.J. Optimization of gelatin-alginate composite bioink printability using rheological parameters: A systematic approach. Biofabrication 2018, 10, 034106. [Google Scholar] [CrossRef] [PubMed]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and shape fidelity of bioinks in 3D bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef]
- Diamantides, N.; Dugopolski, C.; Blahut, E.; Kennedy, S.; Bonassar, L.J. High density cell seeding affects the rheology and printability of collagen bioinks. Biofabrication 2019, 11, 045016. [Google Scholar] [CrossRef]
- Luong, E.; Gerecht, S. Stem cells and scaffolds for vascularizing engineered tissue constructs. Adv. Biochem. Eng. Biotechnol. 2009, 114, 129–172. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Feng, L.; Wu, J.; Zhu, F.; Tan, Z.; Yao, R. Bioprinting of stem cells: Interplay of bioprinting process, bioinks, and stem cell properties. Acs Biomater. Sci. Eng. 2018, 4, 3108–3124. [Google Scholar] [CrossRef]
- Tibbits, S. 4D printing: Multi-material shape change. Arch. Des. 2014, 84, 116–121. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Du, Y.; Huang, R.; Wang, Q.; Wang, X.; Zhang, L. Preparation and characterization of alginate/gelatin blend fibers. J. Appl. Polym. Sci. 2005, 96, 1625–1629. [Google Scholar] [CrossRef]
- Stanton, M.M.; Samitier, J.; Sánchez, S. Bioprinting of 3D hydrogels. Lab Chip 2015, 15, 3111–3115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, B.; Yang, Q.; Zhao, X.; Jin, G.; Ma, Y.; Xu, F. 4D Bioprinting for biomedical applications. Trends Biotechnol. 2016, 34, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, R.G.; Das, A.K. Four dimensional printing in healthcare. In 3D Printing in Medicine; Woodhead Publishing: Sawston, UK, 2017; pp. 207–218. ISBN 978-0-08-100717-4. [Google Scholar]





| Hydrogel | Type of Cells | Sodium Alginate Concentrations (w/v%) | Gelatin/Other Additive Concentrations (w/v%) | The Aim of Research | Reference |
|---|---|---|---|---|---|
| sodium alginate | C2C12 mouse myoblasts | 2.5 | testing the influence of substrate stress–relaxation on the regulation of muscle cell (myoblast) proliferation and spreading in vitro | [72] | |
| sodium alginate-gelatin | BL6 primary mouse myoblasts | 1, 2, 4 | 10 | optimization of the bioink consistency and investigation of printability with live cells | [75] |
| sodium alginate-gelatin | rat Schwann cell line RSC96s | 4 | 20 | examination of the cell behavior in the created microenvironment | [76] |
| sodium alginate-gelatin | L929, mouse fibroblast cell line | 1 | 4 | evaluation of the cell viability and possibility to develop in the created microenvironment | [44] |
| 2–2.5 | 4–8 | analysis of the printability with the cell-filled bioink and optimization of the parameters to obtain structures for cell cultures | [79] | ||
| 2.5 | 8 | ||||
| sodium alginate-gelatin | Non-Small Cell Lung Cancer (NSCLC) PDX (EGFR T790M) cell line | 1–6 | 3–8 | research on the cells growth on the manufactured hydrogel; modification of the alginate-gelatin concentration to achieve good printability | [80] |
| sodium alginate-gelatin | mesenchymal stem cells (MSCs) from adult sheep adipose tissue | 1, 3, 5, 7, 9 | 6 | examination of the printing possibilities according to the individual component concentrations | [81] |
| 5 | 2, 4, 6, 8, 10 | ||||
| sodium alginate-gelatin | human adipose-derived mesenchymal stem cells (MSCs) | 3 | 10 | evaluation of the cell development on produced substrates | [82] |
| sodium alginate-gelatin with mouse plantar dermis (PD) | mouse mesenchymal stem cell (sMSCs) | 1 | 3 | investigation of the chemical and physical properties of the Alg-Gel-PD bioink, and its effect on embedded mouse mesenchymal stem cells (MSCs) | [83] |
| sodium alginate-gelatin mixed with hECM | HepaRG human bipotent hepatic progenitor cells; A549 human epithelial lung carcinoma cells | 2 | 3 | the optimization of a bioink composed of hydrogel and human extracellular matrix (hECM) to print human HepaRG liver cells and testing of its suitability for the study of transduction using an adeno-associated virus (AAV) vector and infection with human adenovirus 5 (hAdV5) | [84] |
| hECM | |||||
| 0; 0.25; 0.5; 1; 2 | |||||
| alginate dialdehyde-gelatin (ADA-GEL) | osteosarcoma cells MG-63 | 5 | 5 | evaluation of the cell encapsulation possibility in prepared hydrogel and monitoring of cell activity | [58] |
| alginate dialdehyde–gelatin (ADA-GEL) | HUVECs human umbilical vein endothelial cells; rat Schwann cells | 2; 3; 6 | 2 | characterization of the printability and cell viability of various concentrations of alginate dialdehyde (ADA)–gelatin (Gel) hydrogels for bioprinting | [85] |
| 2 | 3; 6 | ||||
| sodium alginate with d-gluconic acid | rat liver cell | 1 | 0.2 | analysis of the cell growth on the created substrate | [71] |
| sodium alginate/PEO-bisamine (polyoxyethylene bis(amine)) | L929, mouse fibroblast cell line | 2 | 5 and 20 | evaluation of the cell viability and possibility of developing in created microenvironment | [44] |
| sodium alginate/PEO with gelatin | mesenchymal stem cells (MSCs) | 2, 4, 5 | PEO 1:1 with alginate; gelatin: 10% | examination of the cell viability and possibility of proliferating in created microenvironment | [86] |
| Cell Line | Concentration (w/v%) | Cross-Linking Method | Modification | Reference | ||
|---|---|---|---|---|---|---|
| Sodium Alginate | Gelatin | Sodium Alginate | Gelatin | |||
| NB SH-SY5Y * | 0.50–0.75 | 2.00–5.00 | Ionic (CaCl2) | Covalent (transglutaminase) | n.a. | [95] |
| 1) Hbmsc *; 2) D1 stem cells *; 3) MC3T3-E1 * | 2.00 | n.a. | n.a. | n.a. | 1) linear RGD 2) cyclic RGD | [114] |
| RSC96 * | 1.00–2.50 | n.a. | Ionic (CaCl2) | n.a. | 1) added poly-L-lysine 2) added fibronectin 3) added RGD 4) covalent binding RGD | [115] |
| ACPCs * | n.a. | 8.00 | n.a. | 1) Free radical polymerization 2) Free radical polymerization and covalent | 1) methacrylic anhydride (GelMA) 2) methacrylic anhydride and tyramine (GelMA-Tyr) | [116] |
| 1) HepaRG * 2) A549 * | 2.00 | 3.00 | 1) Ionic (CaSO4)—before printing 2) Ionic (CaCl2)—after printing 3) Ionic (CaCl2)—during incubation | n.a. | human extracellular matrix (hECM) | [84] |
| 1) Saos-2 * 2) hBMSCs * | 4.00 | 5.00 | Ionic (CaCl2) | n.a. | 1) cellulose nanofiber (CNF) 2) bioactive glass (BaG) | [117] |
| 1) HUVECs * 2) MSCs * | 1.00; 2.00; 3.00 | GelMA:5.00; 7.00 | Ionic (CaCl2) | Free-radical polymerization | 1) 4-arm poly(-ethylene glycol)-tetra-acrylate (PEGTA) 2) methacrylic anhydride | [118] |
| Material | Example | Tensile Strength | Tensile Modulus | Compressive Strength | Compressive Modulus | Reference |
|---|---|---|---|---|---|---|
| Traditional hydrogel | (PVA), PEG | 1 ~ 100 kPa | <100 kPa | 10 ~ 100 kPa | 1 ~ 100 kPa | [140,141] |
| Alginate hydrogel | n.a. | ~20 kPa | ~78 kPa | n.a. | n.a. | [142] |
| Alginate-gelatin hydrogel | n.a. | ~0.5 MPa | ~1 MPa | 2 ~ 12 MPa | 30 ~ 50 kPa | [81,142] |
| Alginate-gelatin-nanocellulose hydrogel | n.a. | n.a. | ~220 kPa | ~320 kPa | 60 ~ 110 kPa | [143,144] |
| Nanocomposite hydrogel | PEG/clay | 255 kPa | 16 kPa | 3.7 MPa | 38 kPa | [145,146,147] |
| Cartilage | n.a. | ~3 MPa | ~9 MPa | ~35 MPa | ~15 MPa | [148,149] |
| Collagen fiber | n.a. | ~75 MPa | ~1000 MPa | n.a. | n.a. | [140,150] |
| Gelatin hydrogel | n.a. | n.a. | 3–25 kPa | n.a. | n.a. | [151] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łabowska, M.B.; Cierluk, K.; Jankowska, A.M.; Kulbacka, J.; Detyna, J.; Michalak, I. A Review on the Adaption of Alginate-Gelatin Hydrogels for 3D Cultures and Bioprinting. Materials 2021, 14, 858. https://doi.org/10.3390/ma14040858
Łabowska MB, Cierluk K, Jankowska AM, Kulbacka J, Detyna J, Michalak I. A Review on the Adaption of Alginate-Gelatin Hydrogels for 3D Cultures and Bioprinting. Materials. 2021; 14(4):858. https://doi.org/10.3390/ma14040858
Chicago/Turabian StyleŁabowska, Magdalena B., Karolina Cierluk, Agnieszka M. Jankowska, Julita Kulbacka, Jerzy Detyna, and Izabela Michalak. 2021. "A Review on the Adaption of Alginate-Gelatin Hydrogels for 3D Cultures and Bioprinting" Materials 14, no. 4: 858. https://doi.org/10.3390/ma14040858
APA StyleŁabowska, M. B., Cierluk, K., Jankowska, A. M., Kulbacka, J., Detyna, J., & Michalak, I. (2021). A Review on the Adaption of Alginate-Gelatin Hydrogels for 3D Cultures and Bioprinting. Materials, 14(4), 858. https://doi.org/10.3390/ma14040858











