Fragmented α-Amylase into Microporous Metal-Organic Frameworks as Bioreactors
Abstract
:1. Introduction
2. Materials and Methods
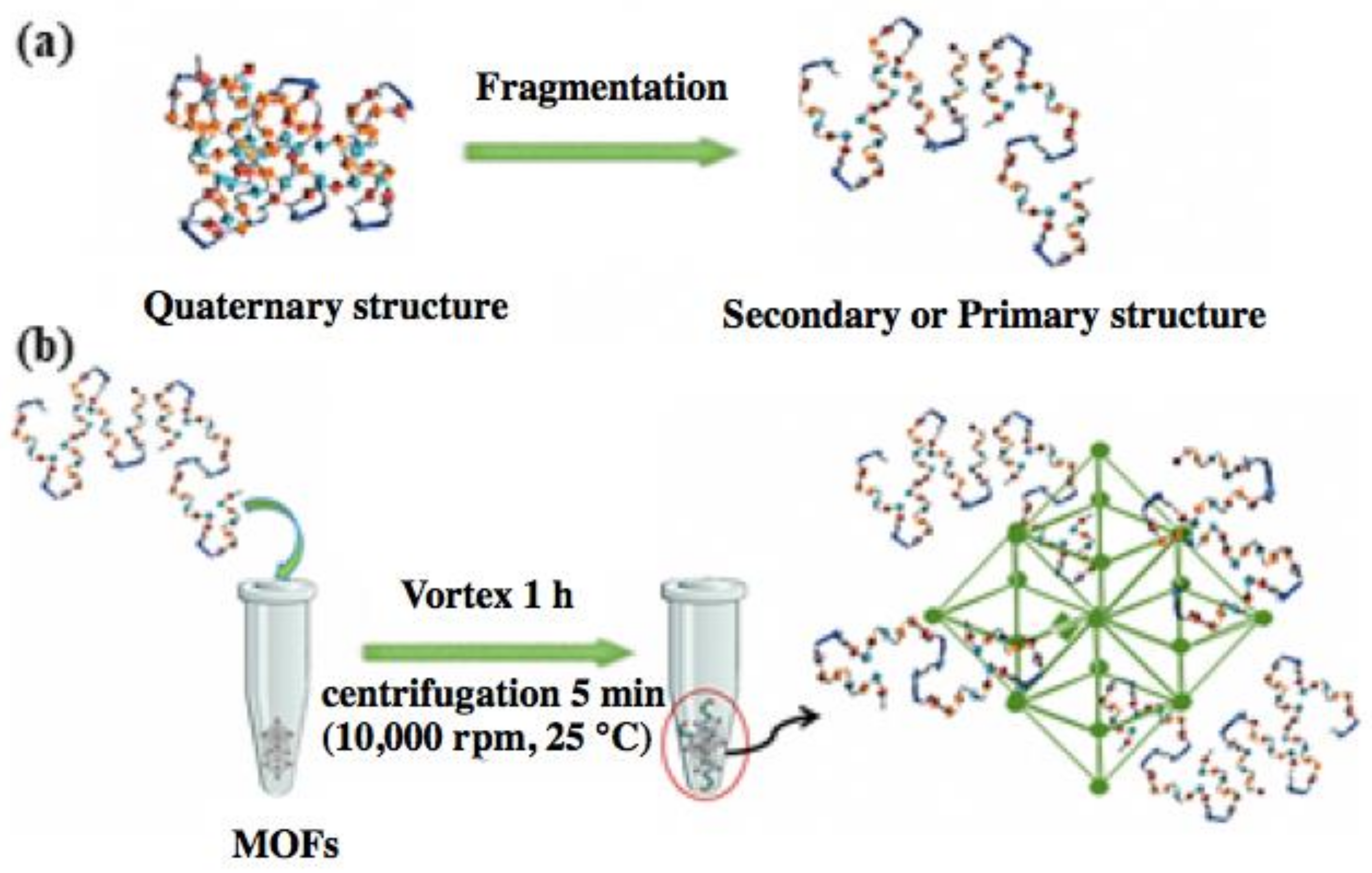
2.1. Fragmentation Process for α-Amylase
2.2. Immobilization Procedure
2.3. Starch Hydrolysis via Fragmented α-Amylase@MOFs
2.4. Determination of α-Amylase Loading Capacity
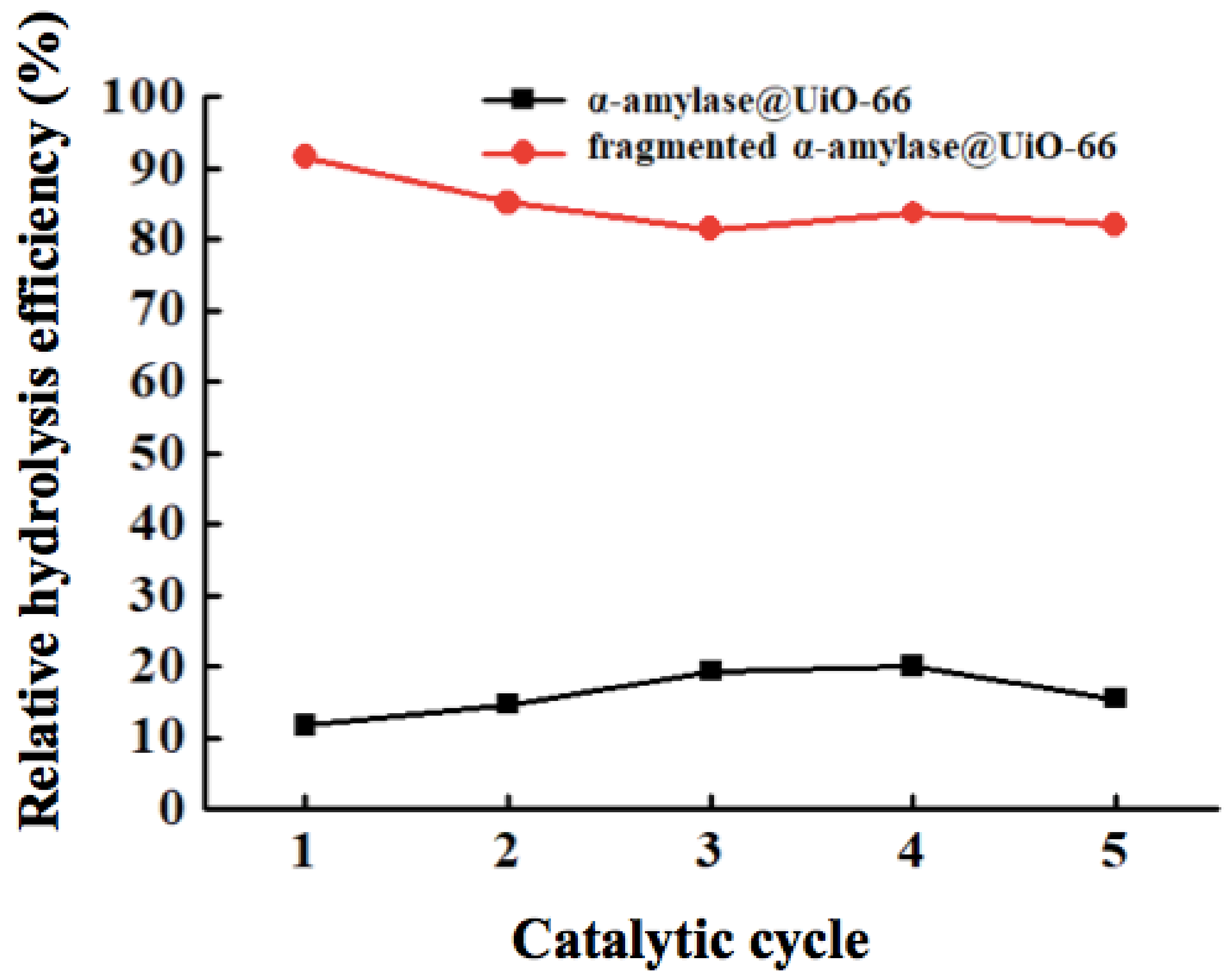
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sá, A.G.A.; Meneses, A.C.d.; Araújo, P.H.H.d.; Oliveira, D.d. A review on enzymatic synthesis of aromatic esters used as flavor ingredients for food, cosmetics and pharmaceuticals industries. Trends Food Sci. Technol. 2017, 69, 95–105. [Google Scholar] [CrossRef]
- Bornscheuer, U.T.; Huisman, G.W.; Kazlauskas, R.J.; Lutz, S.; Moore, J.C.; Robins, K. Engineering the third wave of biocatalysis. Nature 2012, 485, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Hönig, M.; Sondermann, P.; Turner, N.J.; Carreira, E.M. Enantioselective Chemo- and Biocatalysis: Partners in Retrosynthesis. Angew. Chem. Int. Ed. 2017, 56, 8942–8973. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [Green Version]
- Matsuno, H.; Nagasaka, Y.; Kurita, K.; Serizawa, T. Superior Activities of Enzymes Physically Immobilized on Structurally Regular Poly(methyl methacrylate) Surfaces. Chem. Mater. 2007, 19, 2174–2179. [Google Scholar] [CrossRef]
- Mubarak, N.M.; Wong, J.R.; Tan, K.W.; Sahu, J.N.; Abdullah, E.C.; Jayakumar, N.S.; Ganesan, P. Immobilization of cellulase enzyme on functionalized multiwall carbon nanotubes. J. Mol. Catal. B Enzym. 2014, 107, 124–131. [Google Scholar] [CrossRef]
- Han, H.; Zhou, Y.; Li, S.; Wang, Y.; Kong, X.Z. Immobilization of Lipase from Pseudomonas fluorescens on Porous Polyurea and Its Application in Kinetic Resolution of Racemic 1-Phenylethanol. ACS Appl. Mater. Interfaces 2016, 8, 25714–25724. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal-Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Sumby, C.J.; Doonan, C.J. Post-synthetic metalation of metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 5933–5951. [Google Scholar] [CrossRef] [PubMed]
- Bobbitt, N.S.; Mendonca, M.L.; Howarth, A.J.; Islamoglu, T.; Hupp, J.T.; Farha, O.K.; Snurr, R.Q. Metal-organic frameworks for the removal of toxic industrial chemicals and chemical warfare agents. Chem. Soc. Rev. 2017, 46, 3357–3385. [Google Scholar] [CrossRef]
- Liu, W.-L.; Yang, N.-S.; Chen, Y.-T.; Lirio, S.; Wu, C.-Y.; Lin, C.-H.; Huang, H.-Y. Lipase-Supported Metal-Organic Framework Bioreactor Catalyzes Warfarin Synthesis. Chem. Eur. J. 2015, 21, 115–119. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, Z.; Wang, T.; Xiao, Y.; Huo, Q.; Liu, Y. Immobilization of Bacillus subtilis lipase on a Cu-BTC based hierarchically porous metal-organic framework material: A biocatalyst for esterification. Dalton Trans. 2016, 45, 6998–7003. [Google Scholar] [CrossRef] [Green Version]
- Shih, Y.-H.; Lo, S.-H.; Yang, N.-S.; Singco, B.; Cheng, Y.-J.; Wu, C.-Y.; Chang, I.-H.; Huang, H.-Y.; Lin, C.-H. Trypsin-Immobilized Metal-Organic Framework as a Biocatalyst In Proteomics Analysis. ChemPlusChem 2012, 77, 982–986. [Google Scholar] [CrossRef]
- Liu, W.-L.; Lo, S.-H.; Singco, B.; Yang, C.-C.; Huang, H.-Y.; Lin, C.-H. Novel trypsin–FITC@MOF bioreactor efficiently catalyzes protein digestion. J. Mater. Chem. B 2013, 1, 928–932. [Google Scholar] [CrossRef]
- Liu, W.-L.; Wu, C.-Y.; Chen, C.-Y.; Singco, B.; Lin, C.-H.; Huang, H.-Y. Fast Multipoint Immobilized MOF Bioreactor. Chem. Eur. J. 2014, 20, 20–8923. [Google Scholar] [CrossRef]
- Lyu, F.; Zhang, Y.; Zare, R.N.; Ge, J.; Liu, Z. One-Pot Synthesis of Protein-Embedded Metal–Organic Frameworks with Enhanced Biological Activities. Nano Lett. 2014, 14, 5761–5765. [Google Scholar] [CrossRef]
- Liang, K.; Ricco, R.; Doherty, C.M.; Styles, M.J.; Bell, S.; Kirby, N.; Mudie, S.; Haylock, D.; Hill, A.J.; Doonan, C.J.; et al. Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat. Commun. 2015, 6, 7240. [Google Scholar] [CrossRef] [Green Version]
- Shieh, F.-K.; Wang, S.-C.; Yen, C.-I.; Wu, C.-C.; Dutta, S.; Chou, L.-Y.; Morabito, J.V.; Hu, P.; Hsu, M.-H.; Wu, K.C.W.; et al. Imparting Functionality to Biocatalysts via Embedding Enzymes into Nanoporous Materials by a de Novo Approach: Size-Selective Sheltering of Catalase in Metal–Organic Framework Microcrystals. J. Am. Chem. Soc. 2015, 137, 4276–4279. [Google Scholar] [CrossRef]
- Wu, X.; Ge, J.; Yang, C.; Hou, M.; Liu, Z. Facile synthesis of multiple enzyme-containing metal–organic frameworks in a biomolecule-friendly environment. Chem. Commun. 2015, 51, 13408–13411. [Google Scholar] [CrossRef]
- Wu, X.; Yang, C.; Ge, J.; Liu, Z. Polydopamine tethered enzyme/metal–organic framework composites with high stability and reusability. Nanoscale 2015, 7, 18883–18886. [Google Scholar] [CrossRef]
- He, H.; Han, H.; Shi, H.; Tian, Y.; Sun, F.; Song, Y.; Li, Q.; Zhu, G. Construction of Thermophilic Lipase-Embedded Metal–Organic Frameworks via Biomimetic Mineralization: A Biocatalyst for Ester Hydrolysis and Kinetic Resolution. ACS Appl. Mater. Interfaces 2016, 8, 24517–24524. [Google Scholar] [CrossRef]
- Lin, C.-H.; Lirio, S.; Shih, Y.-H.; So, P.B.; Liu, L.-H.; Yen, Y.-T.; Furukawa, S.; Liu, W.-L.; Huang, H.-Y. Fast Multipoint Immobilization of Lipase through Chiral L-Proline on MOF as Chiral Bioreactor. Dalton Trans. 2021. [Google Scholar] [CrossRef]
- Swarnalatha, V.; Aluri Esther, R.; Dhamodharan, R. Immobilization of α-amylase on gum acacia stabilized magnetite nanoparticles, an easily recoverable and reusable support. J. Mol. Catal. B Enzym. 2013, 96, 6–13. [Google Scholar] [CrossRef]
- Baştürk, E.; Demir, S.; Danış, Ö.; Kahraman, M.V. Covalent immobilization of α-amylase onto thermally crosslinked electrospun PVA/PAA nanofibrous hybrid membranes. J. Appl. Polym. Sci. 2013, 127, 349–355. [Google Scholar] [CrossRef]
- Garrett, R.H.; Grisham, C.M. Biochemistry; Brooks/Cole Cengage Learning: Boston, MA, USA, 2010. [Google Scholar]
- Boja, E.S.; Fales, H.M. Overalkylation of a Protein Digest with Iodoacetamide. Anal. Chem. 2001, 73, 3576–3582. [Google Scholar] [CrossRef] [PubMed]
- Kinter, M.; Sherman, N. The Preparation of Protein Digests for Mass Spectrometric Sequencing Experiments. In Protein Sequencing and Identification Using Tandem Mass Spectrometry; Matthiesen, R., Ed.; John Wiley & Sons Inc.: New York, NY, USA, 2000. [Google Scholar]
- Sreerama, N.; Venyaminov, S.Y.U.; Woody, R.W. Estimation of the number of α-helical and β-strand segments in proteins using circular dichroism spectroscopy. Protein Sci. 1999, 8, 370–380. [Google Scholar] [CrossRef]
- Yu, Z.-L.; Zeng, W.-C.; Zhang, W.-H.; Liao, X.-P.; Shi, B. Effect of ultrasonic pretreatment on kinetics of gelatin hydrolysis by collagenase and its mechanism. Ultrason. Sonochem. 2016, 29, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Gigras, P.; Mohapatra, H.; Goswami, V.K.; Chauhan, B. Microbial α-amylases: A biotechnological perspective. Process Biochem. 2003, 38, 1599–1616. [Google Scholar] [CrossRef]
- Bernfeld, P. Amylases, α and β. In Methods Enzymology; Academic Press: New York, NY, USA, 1955; Volume 1, pp. 149–158. [Google Scholar]
- Cordeiro, A.L.; Lenk, T.; Werner, C. Immobilization of Bacillus licheniformis α-amylase onto reactive polymer films. J. Biotechnol. 2011, 154, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Bárcia, P.S.; Guimarães, D.; Mendes, P.A.P.; Silva, J.A.C.; Guillerm, V.; Chevreau, H.; Serre, C.; Rodrigues, A.E. Reverse shape selectivity in the adsorption of hexane and xylene isomers in MOF UiO-66. Microporous Mesoporous Mater. 2011, 139, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef]
- Férey, G.; Serre, C.; Mellot-Draznieks, C.; Millange, F.; Surblé, S.; Dutour, J.; Margiolaki, I. A Hybrid Solid with Giant Pores Prepared by a Combination of Targeted Chemistry, Simulation, and Powder Diffraction. Angew. Chem. Int. Ed. 2004, 43, 6296–6301. [Google Scholar] [CrossRef]
- Loiseau, T.; Serre, C.; Huguenard, C.; Fink, G.; Taulelle, F.; Henry, M.; Bataille, T.; Férey, G. A Rationale for the Large Breathing of the Porous Aluminum Terephthalate (MIL-53) Upon Hydration. Chem. Eur. J. 2004, 10, 1373–1382. [Google Scholar] [CrossRef]
- Horcajada, P.; Serre, C.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Metal-Organic Frameworks as Efficient Materials for Drug Delivery. Angew. Chem. Int. Ed. 2006, 45, 5974–5978. [Google Scholar] [CrossRef] [PubMed]

| MOFs | Window/Channel Size (nm) | BET Surface Area (m2 g−1) | Catalytic Efficiency (%) [a] | |
|---|---|---|---|---|
| 1st Catalysis | 2nd Catalysis | |||
| UiO-66 | 0.7 [36] | 1060 | 91.6 | 82.0 |
| MIL-101(Cr) | 0.86, 1.2, 1.47, 1.6 [37] | 2452 | 68.6 | 63.1 |
| MIL-100(Al) | 0.48, 0.58, 0.86 [38] | 1300 | 76.8 | 52.7 |
| MIL-53(Al) | 0.85 [39] | 1353 | 53.6 | 26.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.-H.; Chiu, R.-Y.; So, P.B.; Lirio, S.; Huang, H.-Y.; Liu, W.-L.; Lin, C.-H. Fragmented α-Amylase into Microporous Metal-Organic Frameworks as Bioreactors. Materials 2021, 14, 870. https://doi.org/10.3390/ma14040870
Liu L-H, Chiu R-Y, So PB, Lirio S, Huang H-Y, Liu W-L, Lin C-H. Fragmented α-Amylase into Microporous Metal-Organic Frameworks as Bioreactors. Materials. 2021; 14(4):870. https://doi.org/10.3390/ma14040870
Chicago/Turabian StyleLiu, Li-Hao, Ru-Yin Chiu, Pamela Berilyn So, Stephen Lirio, Hsi-Ya Huang, Wan-Ling Liu, and Chia-Her Lin. 2021. "Fragmented α-Amylase into Microporous Metal-Organic Frameworks as Bioreactors" Materials 14, no. 4: 870. https://doi.org/10.3390/ma14040870







