Dynamic Crosslinking: An Efficient Approach to Fabricate Epoxy Vitrimer
Abstract
:1. Introduction
2. Experimental
2.1. Material
2.2. Preparation of Epoxy Vitrimer by Dynamic Crosslinking
2.3. Preparation of Epoxy Vitrimer by Static Curing
2.4. Gel Fraction Measurement
2.5. Fourier Transform Infrared (FT-IR) Spectroscopy
2.6. Mechanical Properties
2.7. Stress Relaxation
2.8. Dynamic Mechanical Analysis (DMA)
3. Results and Discussion
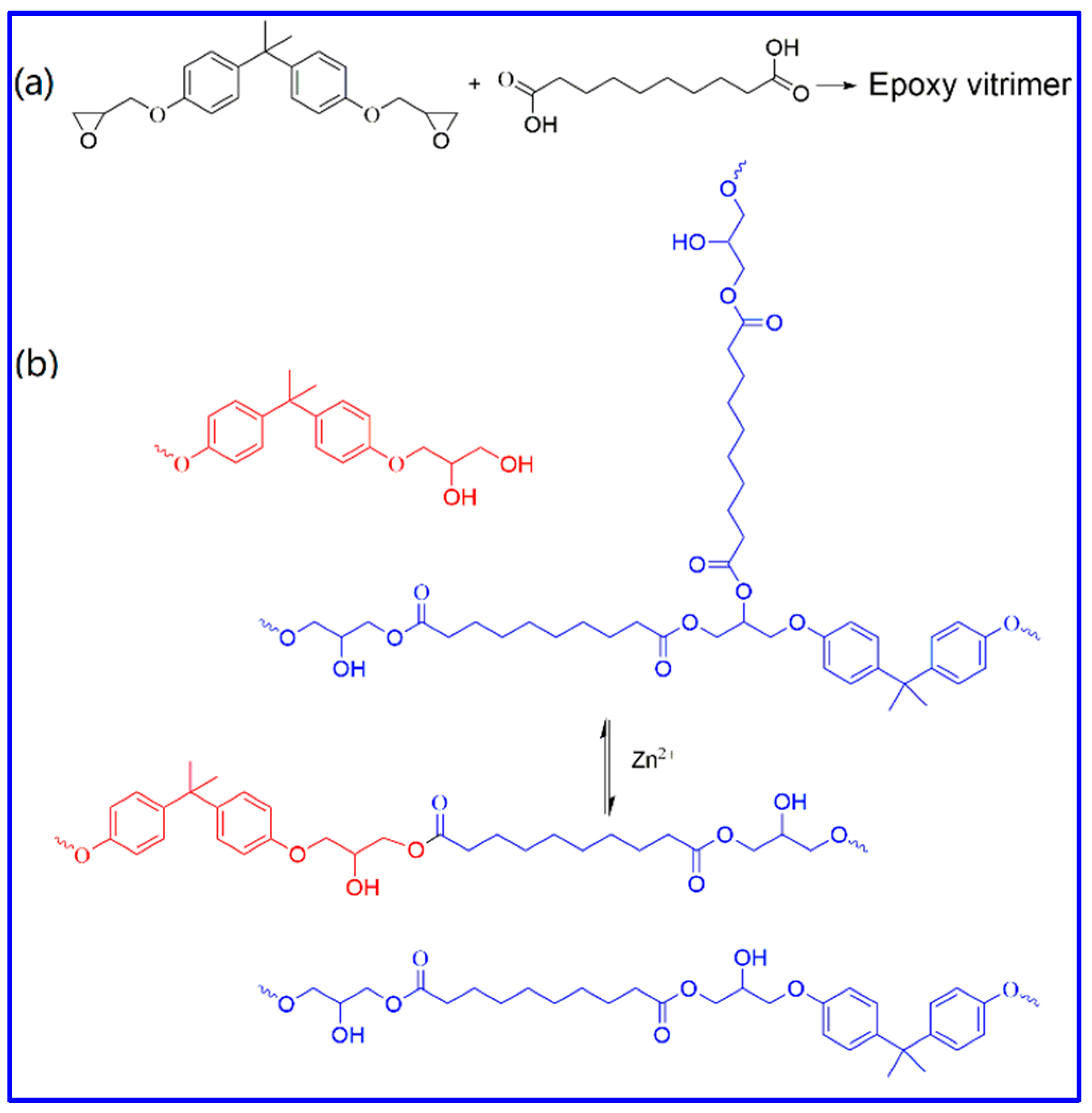
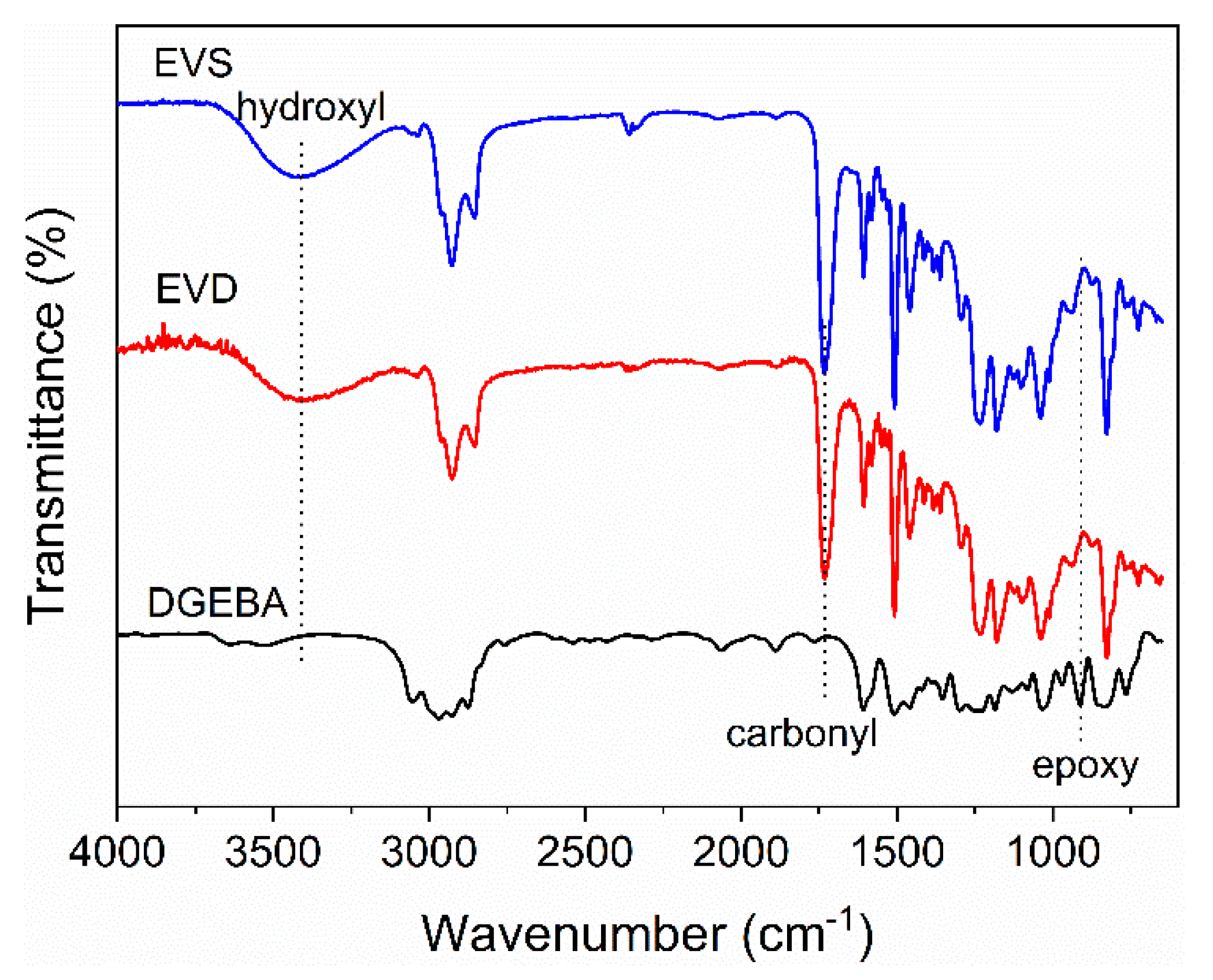
3.1. Epoxy Vitrimer Fabrication
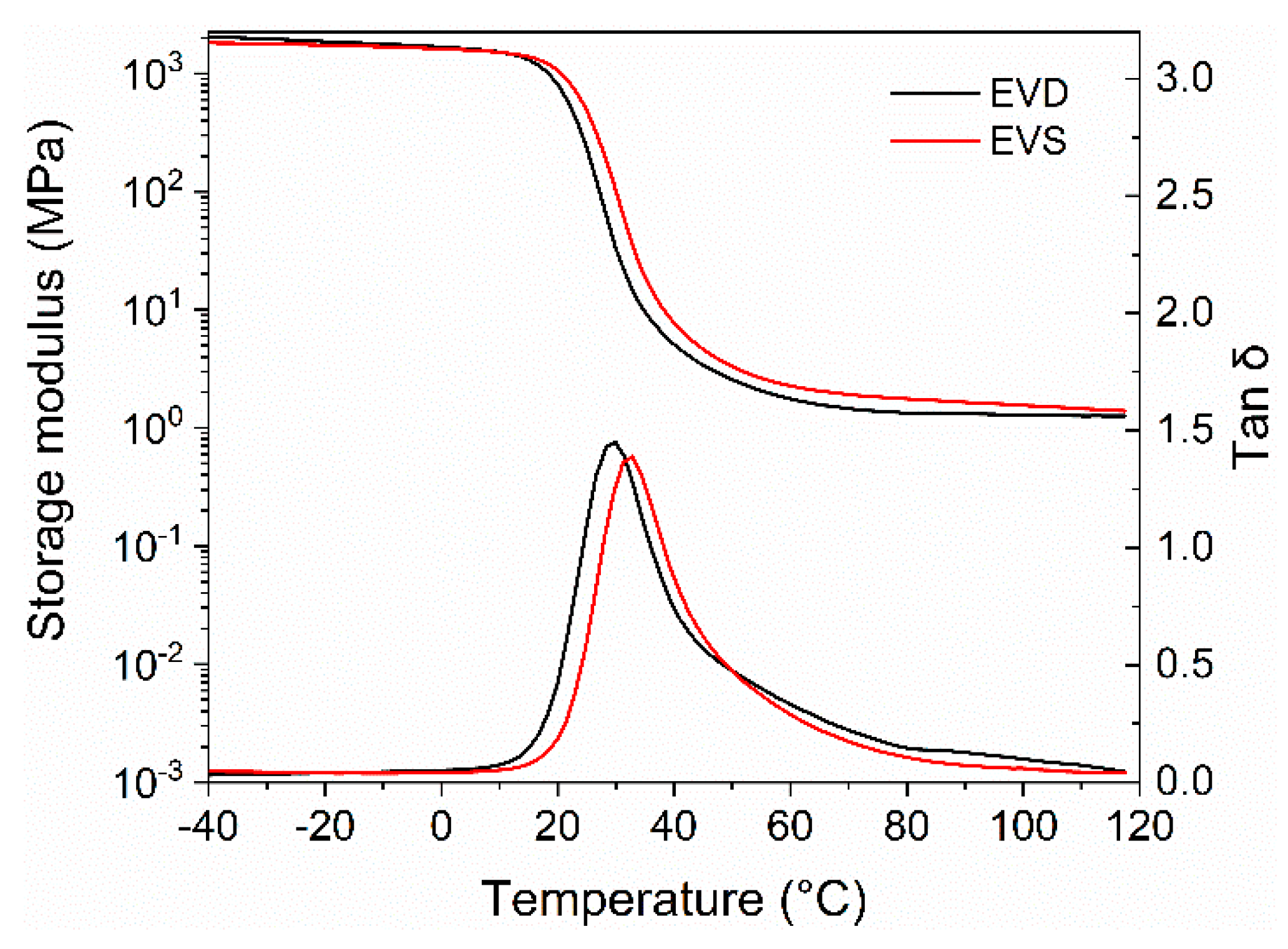
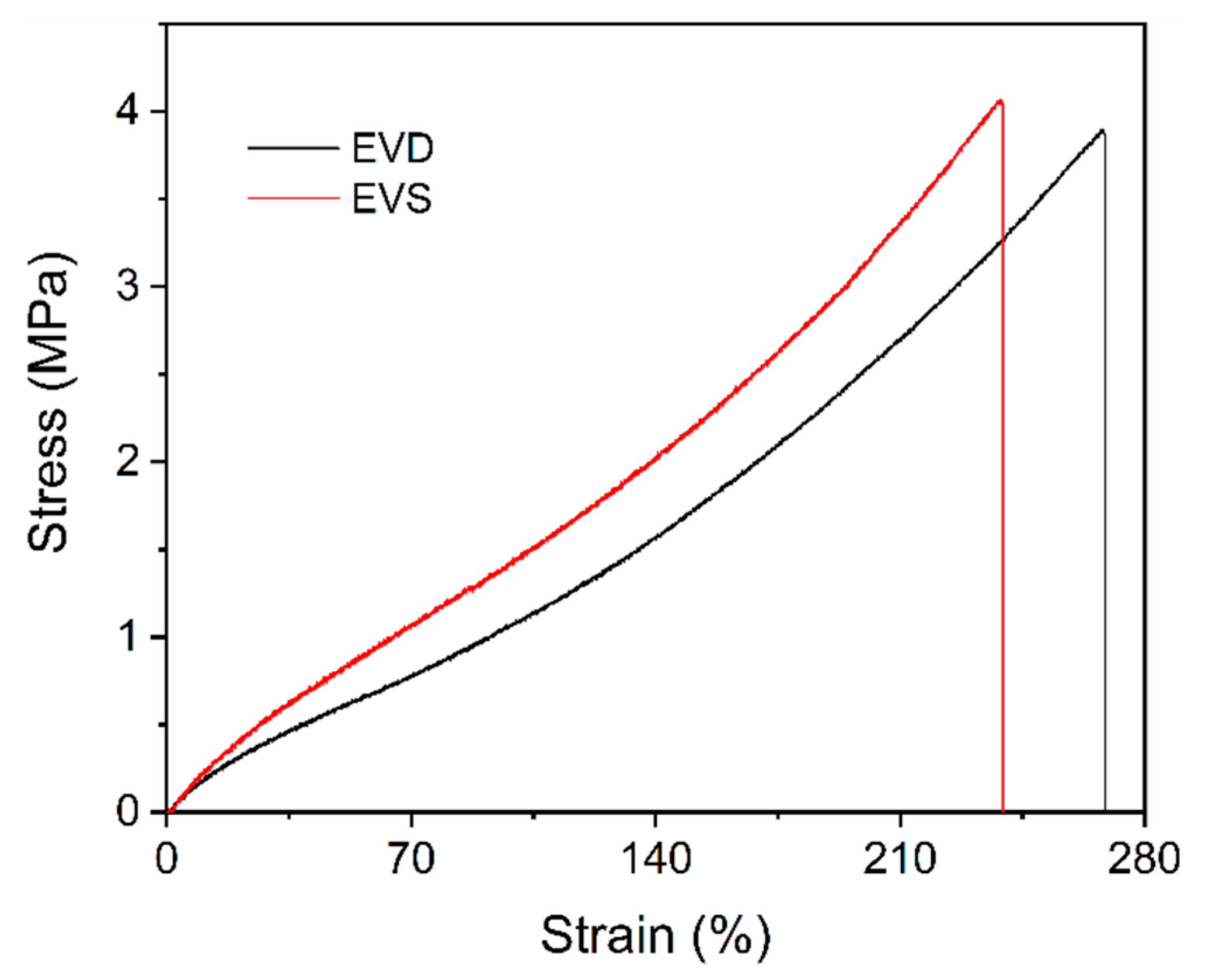
3.2. Mechanical Properties
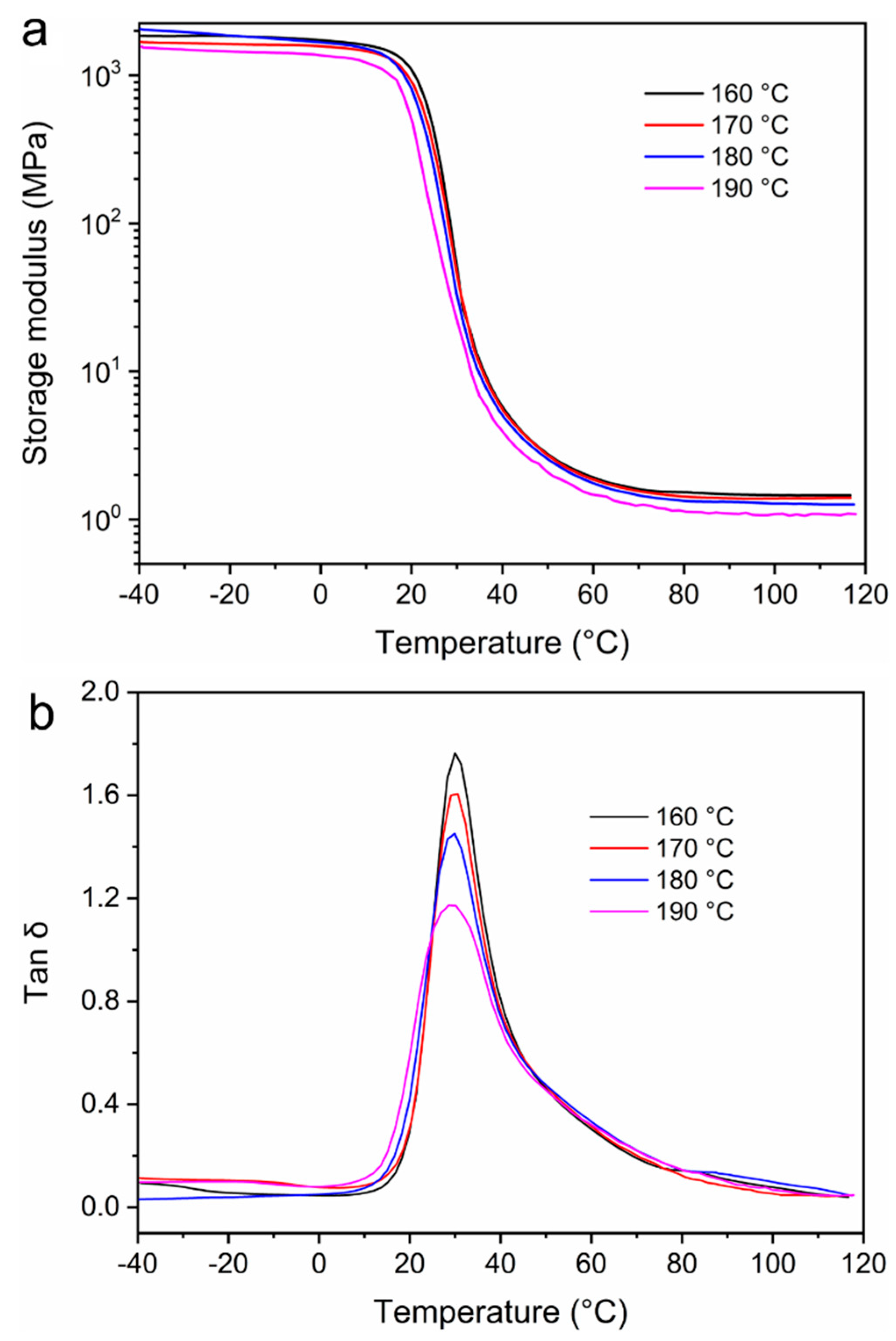
3.3. Stress Relaxation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Du, H.; Liu, L.; Leng, J. Shape memory polymers and their composites in aerospace applications: A review. Smart Mater. Struct. 2014, 23, 023001. [Google Scholar] [CrossRef]
- Auvergne, R.; Caillol, S.; David, G.; Boutevin, B.; Pascault, J.-P. Biobased Thermosetting Epoxy: Present and Future. Chem. Rev. 2013, 114, 1082–1115. [Google Scholar] [CrossRef] [PubMed]
- Kondyurin, A.; Klyachkin, Y. Adhesion of UV-treated rubbers to epoxy adhesives. J. Appl. Polym. Sci. 1996, 62, 1–8. [Google Scholar] [CrossRef]
- Rimdusit, S.; Ishida, H. Development of new class of electronic packaging materials based on ternary systems of benzoxazine, epoxy, and phenolic resins. Polymer 2000, 41, 7941–7949. [Google Scholar] [CrossRef]
- Nagarajan, K.J.; Balaji, A.N.; Basha, K.S.; Ramanujam, N.R.; Kumar, R.A. Effect of agro waste α-cellulosic micro filler on mechanical and thermal behavior of epoxy composites. Int. J. Biol. Macromol. 2020, 152, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Liu, F.; Mekala, S.; Patel, A.; Gross, R.A.; Manas-Zloczower, I. High Performance Biobased Epoxy Nanocomposite Reinforced with a Bacterial Cellulose Nanofiber Network. ACS Sustain. Chem. Eng. 2019, 7, 5986–5992. [Google Scholar] [CrossRef]
- Rybak, A.; Jarosinski, L.; Gaska, K.; Kapusta, C. Graphene nanoplatelet-silica hybrid epoxy composites as electrical insulation with enhanced thermal conductivity. Polym. Compos. 2018, 39, E1682–E1691. [Google Scholar] [CrossRef]
- Paluvai, N.R.; Mohanty, S.; Nayak, S.K. Synthesis and Modifications of Epoxy Resins and Their Composites: A Review. Polym. Plast. Technol. Eng. 2014, 53, 1723–1758. [Google Scholar] [CrossRef]
- Maeda, T.; Otsuka, H.; Takahara, A. Dynamic covalent polymers: Reorganizable polymers with dynamic covalent bonds. Prog. Polym. Sci. 2009, 34, 581–604. [Google Scholar] [CrossRef]
- Fang, Z.; Zheng, N.; Zhao, Q.; Xie, T. Healable, Reconfigurable, Reprocessable Thermoset Shape Memory Polymer with Highly Tunable Topological Rearrangement Kinetics. ACS Appl. Mater. Interfaces 2017, 9, 22077–22082. [Google Scholar] [CrossRef]
- Niu, X.; Wang, F.; Li, X.; Zhang, R.; Wu, Q.; Sun, P. Using Zn2+ Ionomer To Catalyze Transesterification Reaction in Epoxy Vitrimer. Ind. Eng. Chem. Res. 2019, 58, 5698–5706. [Google Scholar] [CrossRef]
- Lai, J.C.; Li, L.; Wang, D.P.; Zhang, M.H.; Mo, S.R.; Wang, X.; Zeng, K.Y.; Li, C.H.; Jiang, Q.; You, X.Z.; et al. A rigid and healable polymer cross-linked by weak but abundant Zn(II)-carboxylate interactions. Nat. Commun. 2018, 9, 2725. [Google Scholar] [CrossRef] [Green Version]
- Obadia, M.M.; Mudraboyina, B.P.; Serghei, A.; Montarnal, D.; Drockenmuller, E. Reprocessing and Recycling of Highly Cross-Linked Ion-Conducting Networks through Transalkylation Exchanges of C–N Bonds. J. Am. Chem. Soc. 2015, 137, 6078–6083. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, Y.; Zhu, J.; Yu, J.; Hu, Z. Bio-based epoxy vitrimers: Reprocessibility, controllable shape memory, and degradability. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1790–1799. [Google Scholar] [CrossRef]
- Yang, Y.; Pei, Z.; Zhang, X.; Tao, L.; Wei, Y.; Ji, Y. Carbon nanotube-vitrimer composite for facile and efficient photo-welding of epoxy. Chem. Sci. 2014, 5, 3486–3492. [Google Scholar] [CrossRef]
- Brutman, J.P.; Delgado, P.A.; Hillmyer, M.A. Polylactide Vitrimers. ACS Macro Lett. 2014, 3, 607–610. [Google Scholar] [CrossRef] [Green Version]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-like malleable materials from permanent organic networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef]
- Denissen, W.; Winne, J.M.; Du Prez, F.E. Vitrimers: Permanent organic networks with glass-like fluidity. Chem. Sci. 2016, 7, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imbernon, L.; Oikonomou, E.K.; Norvez, S.; Leibler, L. Chemically crosslinked yet reprocessable epoxidized natural rubber via thermo-activated disulfide rearrangements. Polym. Chem. 2015, 6, 4271–4278. [Google Scholar] [CrossRef]
- Deng, G.; Li, F.; Yu, H.; Liu, F.; Liu, C.; Sun, W.; Jiang, H.; Chen, Y. Dynamic Hydrogels with an Environmental Adaptive Self-Healing Ability and Dual Responsive Sol-Gel Transitions. ACS Macro Lett. 2012, 1, 275–279. [Google Scholar] [CrossRef]
- Tillman, K.R.; Meacham, R.; Rolsma, A.N.; Barankovich, M.; Witkowski, A.M.; Mather, P.T.; Graf, T.; Shipp, D.A. Dynamic covalent exchange in poly(thioether anhydrides). Polym. Chem. 2020, 11, 7551–7561. [Google Scholar] [CrossRef]
- Ciaccia, M.; Cacciapaglia, R.; Mencarelli, P.; Mandolini, L.; Di Stefano, S. Fast transimination in organic solvents in the absence of proton and metal catalysts. A key to imine metathesis catalyzed by primary amines under mild conditions. Chem. Sci. 2013, 4, 2253. [Google Scholar] [CrossRef]
- Belowich, M.E.; Stoddart, J.F. Dynamic imine chemistry. Chem. Soc. Rev. 2012, 41, 2003. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-X.; Tournilhac, F.; Leibler, L.; Guan, Z. Making Insoluble Polymer Networks Malleable via Olefin Metathesis. J. Am. Chem. Soc. 2012, 134, 8424–8427. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-X.; Guan, Z. Olefin Metathesis for Effective Polymer Healing via Dynamic Exchange of Strong Carbon–Carbon Double Bonds. J. Am. Chem. Soc. 2012, 134, 14226–14231. [Google Scholar] [CrossRef] [PubMed]
- Scholl, M.; Ding, S.; Lee, C.W.; Grubbs, R.H. Synthesis and Activity of a New Generation of Ruthenium-Based Olefin Metathesis Catalysts Coordinated with 1,3-Dimesityl-4,5-dihydroimidazol-2-ylidene Ligands§. Org. Lett. 1999, 1, 953–956. [Google Scholar] [CrossRef]
- Krishnamurthy, S. The principle of vinylogy. J. Chem. Educ. 1982, 59, 543. [Google Scholar] [CrossRef]
- FusoN, R.C. The Principle of Vinylogy. Chem. Rev. 1935, 16, 1–27. [Google Scholar] [CrossRef]
- Cromwell, O.R.; Chung, J.; Guan, Z. Malleable and Self-Healing Covalent Polymer Networks through Tunable Dynamic Boronic Ester Bonds. J. Am. Chem. Soc. 2015, 137, 6492–6495. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Z.; Zhang, X.; Liu, Y.; Wu, S.; Guo, B. Covalently Cross-Linked Elastomers with Self-Healing and Malleable Abilities Enabled by Boronic Ester Bonds. ACS Appl. Mater. Interfaces 2018, 10, 24224–24231. [Google Scholar] [CrossRef]
- Cash, J.J.; Kubo, T.; Bapat, A.P.; Sumerlin, B.S. Room-Temperature Self-Healing Polymers Based on Dynamic-Covalent Boronic Esters. Macromolecules 2015, 48, 2098–2106. [Google Scholar] [CrossRef]
- Chen, J.H.; An, X.P.; Li, Y.D.; Wang, M.; Zeng, J.B. Reprocessible epoxy networks with tunable physical properties:synthesis, stress relaxation and recyclability. Chin. J. Polym. Sci. 2018, 36, 641–648. [Google Scholar] [CrossRef]
- Delahaye, M.; Winne, J.M.; Du Prez, F.E. Internal Catalysis in Covalent Adaptable Networks: Phthalate Monoester Transesterification As a Versatile Dynamic Cross-Linking Chemistry. J. Am. Chem. Soc. 2019, 141, 15277–15287. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, L.; Wu, Y.; Zhao, X.; Zhang, Y. Rapid Stress Relaxation and Moderate Temperature of Malleability Enabled by the Synergy of Disulfide Metathesis and Carboxylate Transesterification in Epoxy Vitrimers. ACS Macro Lett. 2019, 8, 255–260. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, J.-L.; Zhang, L.-J.; Peng, Y.; Wang, H.; Ling, F.-W.; Huang, G.-S.; Wu, J.-R. An Interfacial Dynamic Crosslinking Approach toward Catalyst-free and Mechanically Robust Elastomeric Vitrimer with a Segregated Structure. Chin. J. Polym. Sci. 2021, 39, 201–210. [Google Scholar] [CrossRef]
- Spiesschaert, Y.; Guerre, M.; De Baere, I.; Van Paepegem, W.; Winne, J.M.; Du Prez, F.E. Dynamic Curing Agents for Amine-Hardened Epoxy Vitrimers with Short (Re)processing Times. Macromolecules 2020, 53, 2485–2495. [Google Scholar] [CrossRef]
- Demongeot, A.; Groote, R.; Goossens, H.; Hoeks, T.; Tournilhac, F.; Leibler, L. Cross-Linking of Poly(butylene terephthalate) by Reactive Extrusion Using Zn(II) Epoxy-Vitrimer Chemistry. Macromolecules 2017, 50, 6117–6127. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-H.; Yuan, W.-Q.; Li, Y.-D.; Weng, Y.-X.; Zeng, J.-B. Malleable and Sustainable Poly(ester amide) Networks Synthesized via Melt Condensation Polymerization. ACS Sustain. Chem. Eng. 2019, 7, 15147–15153. [Google Scholar] [CrossRef]
- Vidil, T.; Tournilhac, F.; Musso, S.; Robisson, A.; Leibler, L. Control of reactions and network structures of epoxy thermosets. Prog. Polym. Sci. 2016, 62, 126–179. [Google Scholar] [CrossRef] [Green Version]
- Jian, X.-Y.; An, X.-P.; Li, Y.-D.; Chen, J.-H.; Wang, M.; Zeng, J.-B. All Plant Oil Derived Epoxy Thermosets with Excellent Comprehensive Properties. Macromolecules 2017, 50, 5729–5738. [Google Scholar] [CrossRef]
- Zhao, X.-L.; Liu, Y.-Y.; Weng, Y.; Li, Y.-D.; Zeng, J.-B. Sustainable Epoxy Vitrimers from Epoxidized Soybean Oil and Vanillin. ACS Sustain. Chem. Eng. 2020, 8, 15020–15029. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Wang, H.; Huang, X.; Huang, G.; Wu, J. Weldable, maleable and programmable epoxy vitrimers with high mechanical properties and water insensitivity. Chem. Eng. J. 2019, 368, 61–70. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, L.; Liang, G.; Gu, A. Developing Reversible Self-Healing and Malleable Epoxy Resins with High Performance and Fast Recycling through Building Cross-Linked Network with New Disulfide-Containing Hardener. Ind. Eng. Chem. Res. 2018, 57, 12397–12406. [Google Scholar] [CrossRef]
- Xu, Z.; Liang, Y.; Ma, X.; Chen, S.; Yu, C.; Wang, Y.; Zhang, D.; Miao, M. Recyclable thermoset hyperbranched polymers containing reversible hexahydro-s-triazine. Nat. Sustain. 2019, 3, 29–34. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; He, J.; Li, Y.-D.; Zhao, X.-L.; Zeng, J.-B. Biobased epoxy vitrimer from epoxidized soybean oil for reprocessable and recyclable carbon fiber reinforced composite. Compos. Commun. 2020, 22, 100445. [Google Scholar] [CrossRef]
- Legrand, A.; Soulié-Ziakovic, C. Silica-Epoxy Vitrimer Nanocomposites. Macromolecules 2016, 49, 5893–5902. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ran, Y.; Zheng, L.-J.; Zeng, J.-B. Dynamic Crosslinking: An Efficient Approach to Fabricate Epoxy Vitrimer. Materials 2021, 14, 919. https://doi.org/10.3390/ma14040919
Ran Y, Zheng L-J, Zeng J-B. Dynamic Crosslinking: An Efficient Approach to Fabricate Epoxy Vitrimer. Materials. 2021; 14(4):919. https://doi.org/10.3390/ma14040919
Chicago/Turabian StyleRan, Yin, Ling-Ji Zheng, and Jian-Bing Zeng. 2021. "Dynamic Crosslinking: An Efficient Approach to Fabricate Epoxy Vitrimer" Materials 14, no. 4: 919. https://doi.org/10.3390/ma14040919
APA StyleRan, Y., Zheng, L.-J., & Zeng, J.-B. (2021). Dynamic Crosslinking: An Efficient Approach to Fabricate Epoxy Vitrimer. Materials, 14(4), 919. https://doi.org/10.3390/ma14040919




