Preparation and Electrical Properties of Silicone Composite Films Based on Silver Nanoparticle Decorated Multi-Walled Carbon Nanotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Ag Nanoparticle Decorated MWNT
2.3. Sample Preparation
2.4. Measurements
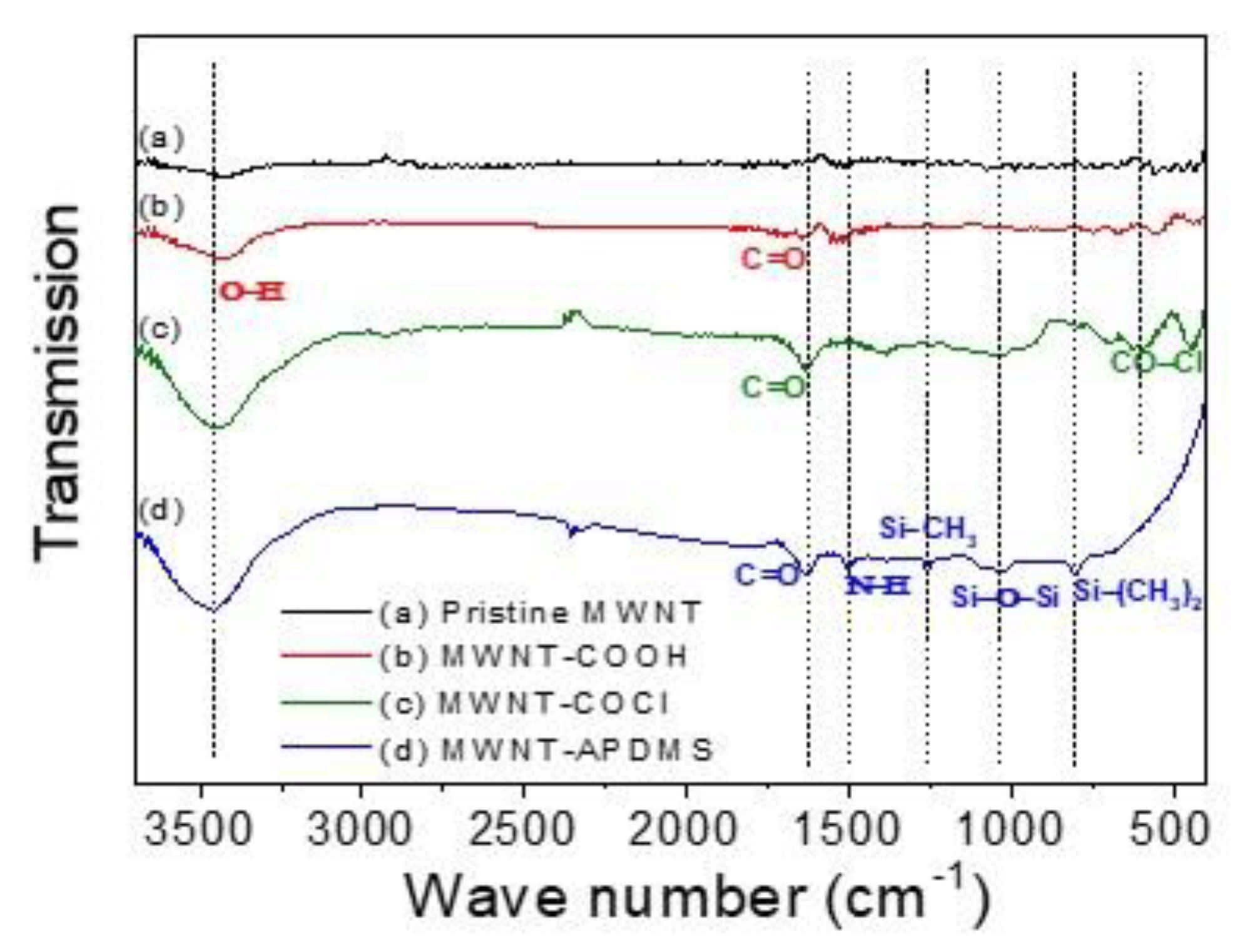
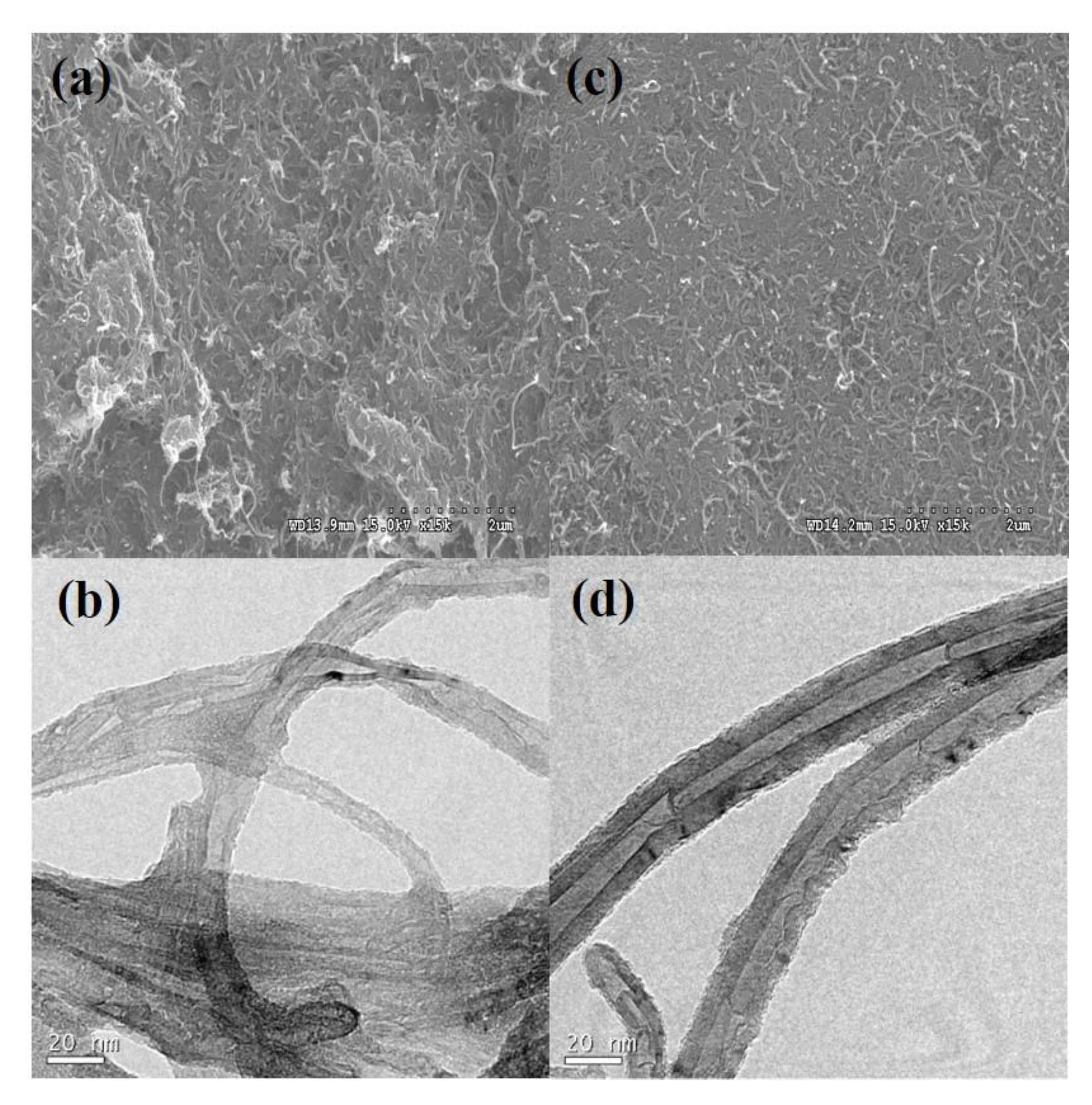
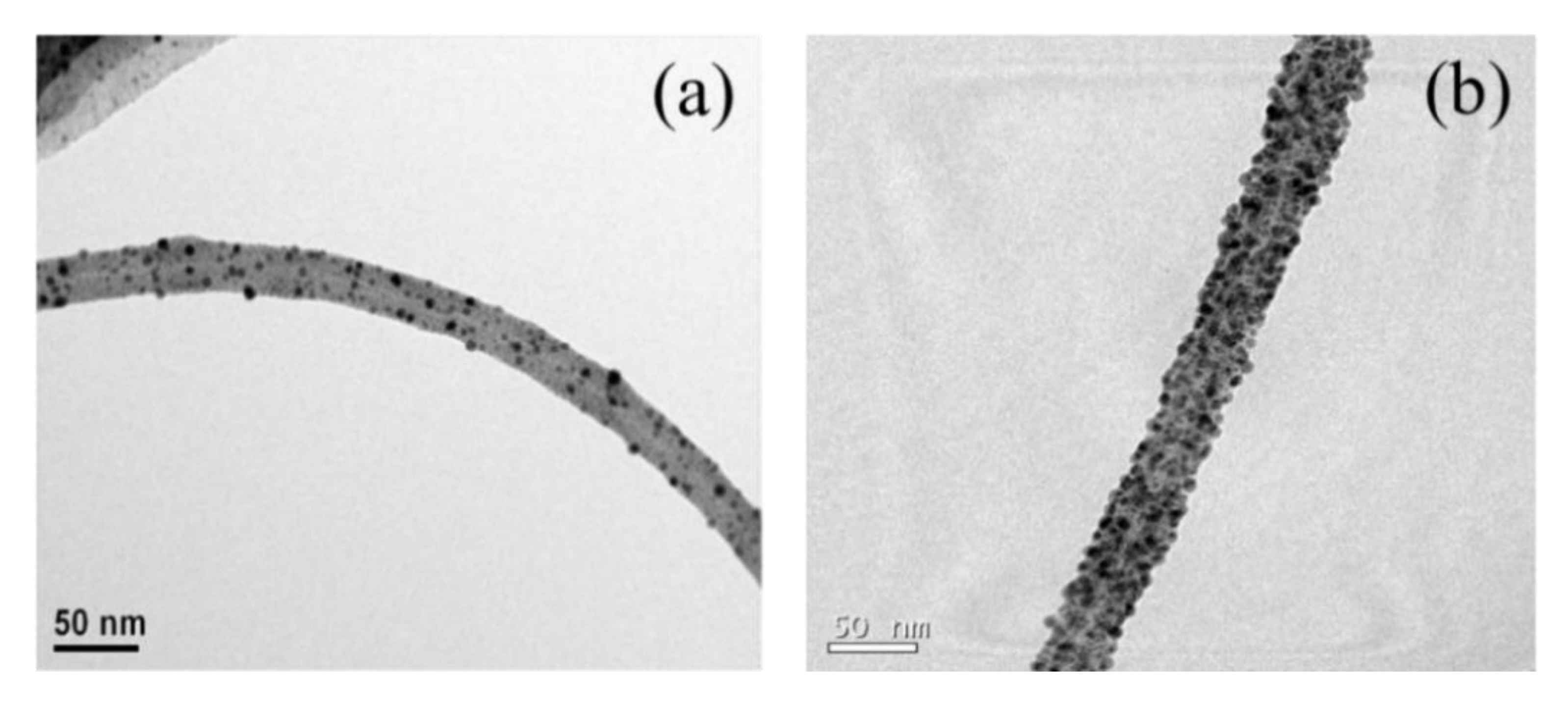
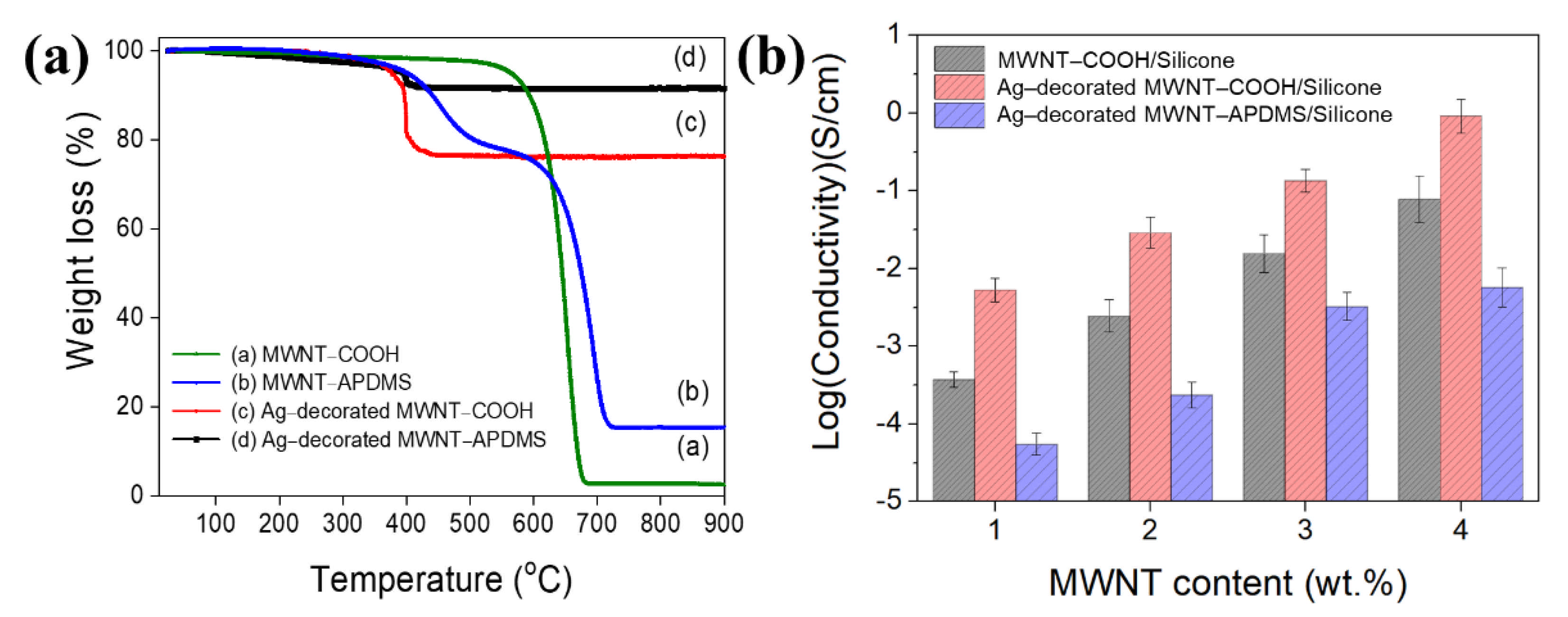
3. Results and Discussion
4. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Ghosh, S.; Polaki, S.R.; Ajikumar, P.; Krishna, N.G.; Kamruddin, M. Aging effects on vertical graphene nanosheets and their thermal stability. Indian J. Phys. 2018, 92, 337–342. [Google Scholar] [CrossRef]
- Atchudan, R.; Cha, B.G.; Lone, N.; Kim, J.; Joo, J. Synthesis of high–quality carbon nanotubes by using monodisperse spherical mesoporous silica encapsulating iron oxide nanoparticles. Korean J. Chem. Eng. 2019, 36, 157–165. [Google Scholar] [CrossRef]
- Ghosh, S.; Yong, W.D.; Jin, E.M.; Polaki, S.R.; Jeong, S.M.; Jun, H. Mesoporous carbon nanofiber engineered for improved supercapacitor performance. Korean J. Chem. Eng. 2019, 36, 312–320. [Google Scholar] [CrossRef]
- Li, W.; Liang, C.; Zhou, W.; Qiu, J.; Zhou, Z.; Sun, G.; Xin, Q. Preparation and characterization of multi–walled carbon nanotube–supported platinum for cathode catalyst of direct methanol fuel cells. J. Phys. Chem. B 2003, 107, 6292–6299. [Google Scholar] [CrossRef]
- Zhu, S.; Ni, J.; Li, Y. Carbon Nanotube–Based Electrodes for Flexible Supercapacitors. Nano Res. 2020, 13, 1825–1841. [Google Scholar] [CrossRef]
- Daksh, D.; Rawtani, D.; Agrawal, Y.K. Recent Developments in Bio–Nanoelectronics Devices: A Review. J. Bionanosci. 2016, 10, 81–93. [Google Scholar] [CrossRef]
- Biswas, C.; Lee, Y.H. Graphene Versus Carbon Nanotubes in Electronic Devices. Adv. Funct. Mater. 2011, 21, 3806–3826. [Google Scholar] [CrossRef]
- Kavitha, P.; Sarada, M.; Vijayavardhan, K.; Sudha, V.Y.; Srinivasulu, A. Carbon nanotube field effect transistors based ternary Ex–OR and Ex–NOR gates. Curr. Nanosci. 2016, 12, 520–526. [Google Scholar]
- Lee, S.W.; Campbell, E.E.B. Nanoelectromechanical devices with. carbon nanotubes. Curr. Appl. Phys. 2013, 13, 1844–1859. [Google Scholar] [CrossRef]
- Das, D.; Rahaman, H. Analysis of Crosstalk in Single and Multiwall Carbon Nanotube Interconnects and Its Impact on Gate Oxide Reliability. IEEE Trans. Nanotechnol. 2011, 10, 1362–1370. [Google Scholar] [CrossRef]
- Sharma, P.; Ahuja, P. Recent advances in carbon nanotube–based electronics. Mater. Res. Bull. 2008, 43, 2517–2526. [Google Scholar] [CrossRef]
- Liu, G.; Ling, Q.-D.; Teo, E.Y.H.; Zhu, C.-X.; Chan, D.S.-H.; Neoh, K.-G.; Kang, E.-T. Electrical Conductance Tuning and Bistable Switching in Poly(N–vinylcarbazole)−Carbon Nanotube Composite Films. ACS Nano 2009, 3, 1929–1937. [Google Scholar] [CrossRef]
- Kumar, S.; Kaur, I.; Dharamvir, K.; Bharadwaj, L.M. Controlling the density and site of attachment of gold nanoparticles onto the surface of carbon nanotubes. J. Colloid Interface Sci. 2012, 369, 23–27. [Google Scholar] [CrossRef]
- Kausar, A.; Rafique, I.; Muhammad, B. Significance of carbon nanotube in flame–retardant polymer/CNT composite: A review. Polym–Plast Technol. 2017, 56, 470–487. [Google Scholar] [CrossRef]
- Zheng, C.; Feng, M.; Du, Y.; Zhan, H. Synthesis and third–order nonlinear optical properties of a multiwalled carbon nanotube–organically modified silicate nanohybrid gel glass. Carbon 2009, 47, 2889–2897. [Google Scholar] [CrossRef]
- Sreeja, R.; Aneesh, P.M.; Hasna, K.; Jayaraj, M.K. Linear and nonlinear optical properties of multi walled carbon nanotubes with attached gold nanoparticles. J. Electrochem. Soc. 2011, 158, K187. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Terentjev, E.M. Dispersion of Carbon Nanotubes: Mixing, Sonication, Stabilization, and Composite Properties. Polymers 2012, 4, 275–295. [Google Scholar] [CrossRef]
- Raja, M.; Ryu, S.H.; Shanmugharaj, A.M. Influence of surface modified multiwalled carbon nanotubes on the mechanical and electroactive shape memory properties of polyurethane (PU)/poly (vinylidene diflouride)(PVDF) composites. Colloid. Surf. A 2014, 450, 59–66. [Google Scholar] [CrossRef]
- Kingston, C.; Zepp, R.; Andrady, A.; Boverhof, D.; Fehir, R.; Hawkins, D.; Roberts, J.; Sayre, P.; Shelton, B.; Sultan, Y.; et al. Release characteristics of selected carbon nanotube polymer composites. Carbon 2014, 68, 33–57. [Google Scholar] [CrossRef]
- Gkikas, G.; Barkoula, N.-M.; Paipetis, A.S. Effect of dispersion conditions on the thermo–mechanical and toughness properties of multi walled carbon nanotubes–reinforced epoxy. Compos. B Eng. 2012, 43, 2697–2705. [Google Scholar] [CrossRef]
- Jang, Y.K.; Jang, P.G.; Kim, J.K.; Park, M.; Yoon, H.G. Electrical properties of imidazolemodified MWNT/polyphenylenesulfide composites prepared by melt mixing. J. Nanosci. Nanotechnol. 2009, 9, 4180–4186. [Google Scholar] [CrossRef]
- Rebib, F.; Tomasella, E.; Beche, E.; Cellier, J.; Jacquet, M. FTIR and XPS investigations of a–SiOxNy thin films structure. J. Phys. Conf. Ser. 2008, 100, 082034. [Google Scholar] [CrossRef]
- Ma, P.C.; Tang, B.Z.; Kim, J.-K. Effect of CNT Decoration with Silver Nanoparticles on Electrical Conductivity of CNT–Polymer Composites. Carbon 2008, 46, 1497–1505. [Google Scholar] [CrossRef]
- Colomer, J.-F.; Stephan, C.; Lefrant, S.; Tendeloo, G.V.; Willems, I.; Konya, Z.; Fonseca, A.; Laurent, C.; Nagy, J.B. Large–scale synthesis of single–wall carbon nanotubes by catalytic chemical vapor deposition (CCVD) method. Chem. Phys. Lett. 2000, 317, 83–89. [Google Scholar] [CrossRef]
- Kong, J.; Cassell, A.M. Chemical vapor deposition of methane for single–walled carbon nanotubes. Chem. Phys. Lett. 1998, 292, 567–574. [Google Scholar] [CrossRef]
- Nishino, H.; Yasuda, S.; Namai, T.; Futaba, D.N.; Yamada, T.; Yumura, M.; Iijima, S.; Hata, K. Water–Assisted Highly Efficient Synthesis of Single–Walled Carbon Nanotubes Forests from Colloidal Nanoparticle Catalysts. J. Phys. Chem. C 2007, 111, 17961–17965. [Google Scholar] [CrossRef]
- Camino, G.; Lomakin, S.M.; Lazzari, M. Polydimethylsiloxane thermal degradation—Part 1. Kinet. Asp. Polym. 2001, 42, 2395–2402. [Google Scholar]
- Zhang, X.M.; Yang, X.L.; Wang, K.Y. Electrical Conductivity Enhancement of Epoxy by Hybrid Carbon Nanotubes and Self–Made Silver Nanoparticles. Fibers Polym. 2019, 20, 1480–1485. [Google Scholar] [CrossRef]
- Xin, F.; Li, L. Decoration of Carbon Nanotubes with Silver Nanoparticles for Advanced CNT/Polymer Nan℃omposites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 961–967. [Google Scholar] [CrossRef]
- Wang, D.; Li, D.; Muhammad, J.; Zhou, Y.; Wang, Z.; Lu, S.; Dong, X.; Zhang, Z. In Situ Synthesis and Electronic Transport of the Carbon–Coated Ag@C/MWNT Nano Composite. RSC Adv. 2018, 8, 7450–7456. [Google Scholar] [CrossRef]
- Liang, G.D.; Bao, S.P.; Tjong, S.C. Microstructure and Properties of Polypropylene Composites Filled with Silver and Carbon Nanotube Nanoparticles Prepared by Melt–Compounding. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2007, 142, 55–61. [Google Scholar] [CrossRef]
- Chua, T.P.; Mariatti, M.; Azizan, A.; Rashid, A.A. Effects of Surface–Functionalized Multi–Walled Carbon Nanotubes on the Properties of Poly(Dimethyl Siloxane) Nanocomposites. Compos. Sci. Technol. 2010, 70, 671–677. [Google Scholar] [CrossRef]

| Composite | % of CNT/Ag in Polymer Matrix | Electrical Conductivity (S cm−1) | Reference |
|---|---|---|---|
| CNT/Ag in epoxy resin | 0.10% | 30.53 | [25] |
| CNT/Ag in epoxy resin | 65% | 102.04 | [29] |
| Ag–CNT/PS (polystyrene) | 10 | 0.65 | [30] |
| Ag@C/MWNT | – | 3.85 | [31] |
| Ag/MWNT in polypropylene | 3 | ~4.5 × 10−4 | [32] |
| MWNT/PDMS | 0 | 1.83 × 10−6 | [33] |
| Ag/MWNT in silicone composite | 4% | 0.92 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.S.; Phiri, I.; Kim, S.H.; Oh, K.; Ko, J.M. Preparation and Electrical Properties of Silicone Composite Films Based on Silver Nanoparticle Decorated Multi-Walled Carbon Nanotubes. Materials 2021, 14, 948. https://doi.org/10.3390/ma14040948
Lee KS, Phiri I, Kim SH, Oh K, Ko JM. Preparation and Electrical Properties of Silicone Composite Films Based on Silver Nanoparticle Decorated Multi-Walled Carbon Nanotubes. Materials. 2021; 14(4):948. https://doi.org/10.3390/ma14040948
Chicago/Turabian StyleLee, Kwang Se, Isheunesu Phiri, Sang Hern Kim, Kyeongkeun Oh, and Jang Myoun Ko. 2021. "Preparation and Electrical Properties of Silicone Composite Films Based on Silver Nanoparticle Decorated Multi-Walled Carbon Nanotubes" Materials 14, no. 4: 948. https://doi.org/10.3390/ma14040948
APA StyleLee, K. S., Phiri, I., Kim, S. H., Oh, K., & Ko, J. M. (2021). Preparation and Electrical Properties of Silicone Composite Films Based on Silver Nanoparticle Decorated Multi-Walled Carbon Nanotubes. Materials, 14(4), 948. https://doi.org/10.3390/ma14040948






